Dimensional Engineering of 1D/2D Synergistic TiO2 Nanostructures for High-Efficiency Photocatalytic CO2 Reduction
Abstract
1. Introduction
2. Experiments
2.1. Chemical Reagents
2.2. Catalyst Synthesis
2.2.1. Preparation of 2D-TiO2
2.2.2. Preparation of 1D-TiO2
2.3. Activity Test
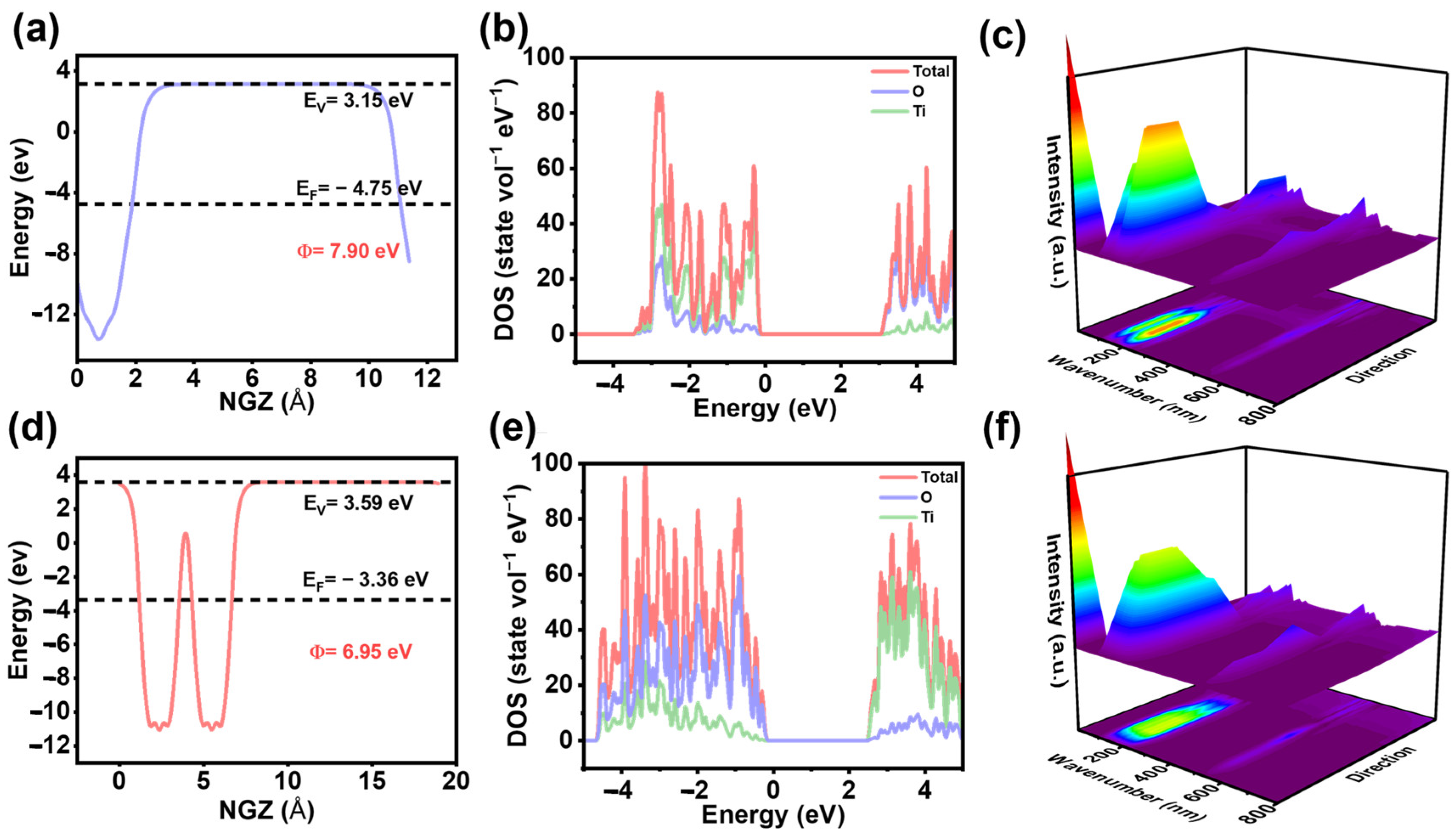
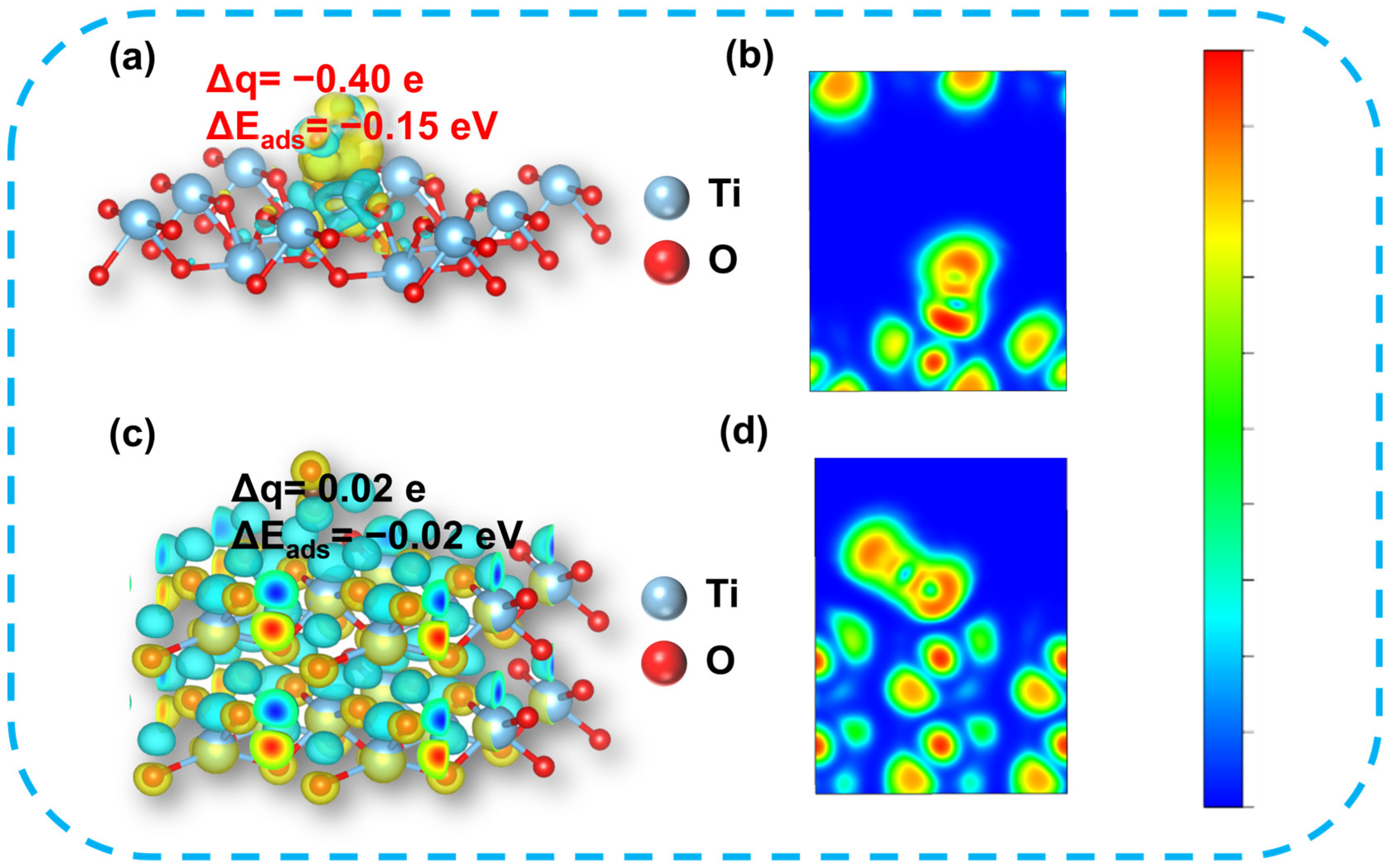
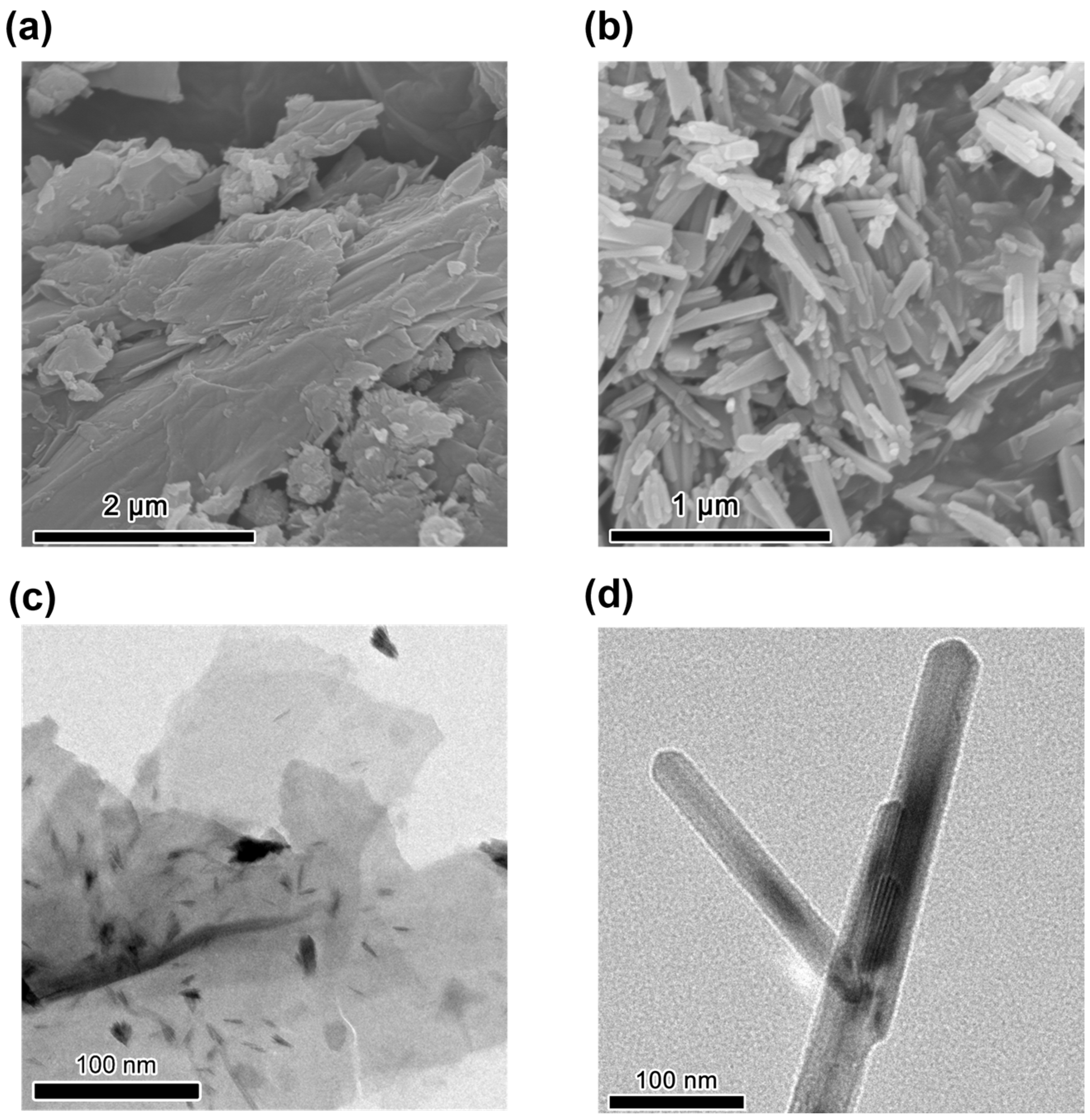
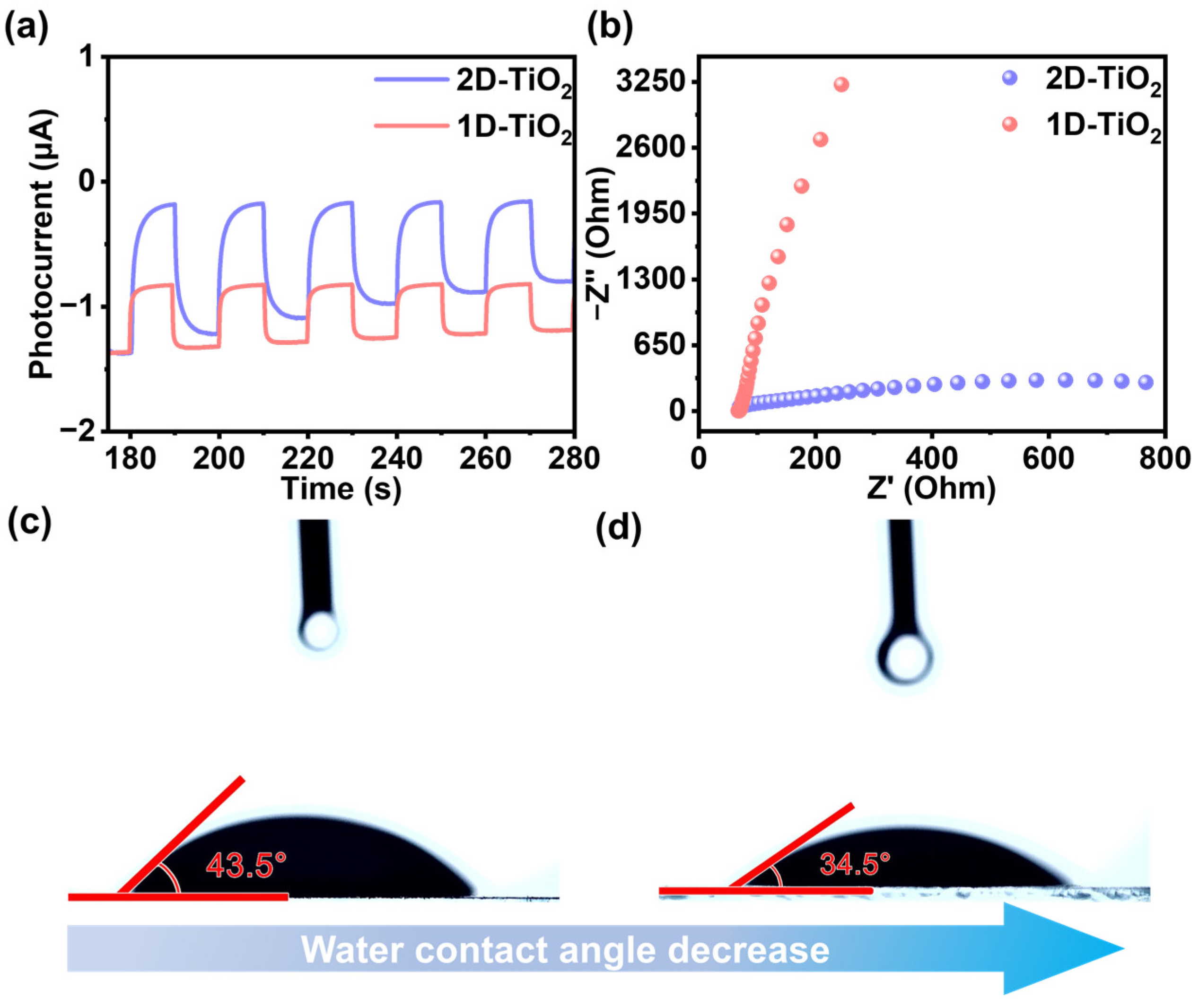
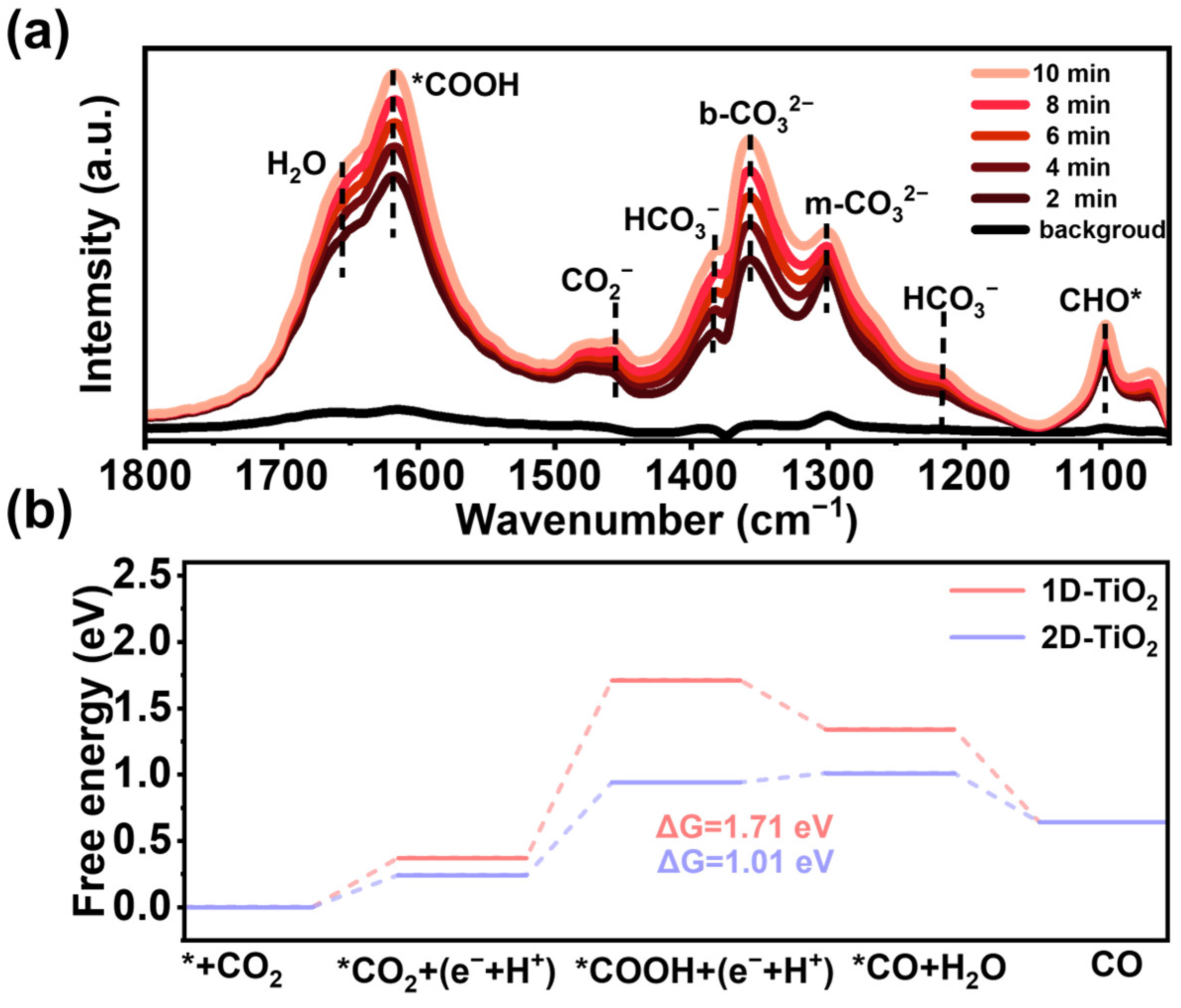
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, G.; Wong, W.S.Y.; Kraft, M.; Ager, J.W.; Vollmer, D.; Xu, R. Wetting-regulated gas-involving (photo)electrocatalysis: Biomimetics in energy conversion. Chem. Soc. Rev. 2021, 50, 10674–10699. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef]
- Wang, K.; Du, Y.; Li, Y.; Wu, X.; Hu, H.; Wang, G.; Xiao, Y.; Chou, S.; Zhang, G. Atomic-level insight of sulfidation-engineered Aurivillius-related Bi2O2SiO3 nanosheets enabling visible light low-concentration CO2 conversionconversion. Carbon Energy 2023, 5, e264. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Lin, M.; Wang, H.; Bai, Y.; Liu, C.; Lu, J.; Luo, Q.; Wang, G.; Jiang, H.-L.; et al. Photoinduced Metastable Asymmetric Cu Single Atoms for Photoreduction of CO2 to Ethylene. Adv. Energy Mater. 2023, 13, 2302692. [Google Scholar] [CrossRef]
- Ban, C.; Wang, Y.; Feng, Y.; Zhu, Z.; Duan, Y.; Ma, J.; Zhang, X.; Liu, X.; Zhou, K.; Zou, H.; et al. Photochromic single atom Ag/TiO2 catalysts for selective CO2 reduction to CH4. Energy Environ. Sci. 2024, 17, 518–530. [Google Scholar] [CrossRef]
- Das, R.; Das, K.; Ray, B.; Vinod, C.P.; Peter, S.C. Green transformation of CO2 to ethanol using water and sunlight by the combined effect of naturally abundant red phosphorus and Bi2MoO6. Energy Environ. Sci. 2022, 15, 1967–1976. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.K.; Kim, J. Revolutionary advancements in carbon dioxide valorization via metal-organic framework-based strategies. Carbon Capture Sci. Technol. 2025, 15, 100405. [Google Scholar] [CrossRef]
- Mihara, T.; Nozaki, K.; Kowaka, Y.; Jiang, M.; Yamashita, K.; Miura, H.; Ohara, S. Enhanced Photocatalysis of Electrically Polarized Titania Nanosheets. Nanomaterials 2024, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Abdellah, M.; Cao, Y.; Lin, W.; Liu, Y.; Meng, J.; Zhou, Q.; Zhao, Q.; Yan, X.; Li, Z.; et al. Ultrafast charge transfer dynamics in 2D covalent organic frameworks/Re-complex hybrid photocatalyst. Nat. Commun. 2022, 13, 845. [Google Scholar] [CrossRef]
- Gueorguiev, G.K.; Pacheco, J.M. Silicon and metal nanotemplates: Size and species dependence of structural and electronic properties. J. Chem. Phys. 2003, 119, 10313–10317. [Google Scholar] [CrossRef]
- Pela, R.R.; Hsiao, C.-L.; Hultman, L.; Birch, J.; Gueorguiev, G.K. Electronic and optical properties of core–shell InAlN nanorods: A comparative study via LDA, LDA-1/2, mBJ, HSE06, G0W0 and BSE methods. Phys. Chem. Chem. Phys. 2024, 26, 7504–7514. [Google Scholar] [CrossRef]
- Ruan, X.; Li, S.; Huang, C.; Zheng, W.; Cui, X.; Ravi, S.K. Catalyzing Artificial Photosynthesis with TiO2 Heterostructures and Hybrids: Emerging Trends in a Classical yet Contemporary Photocatalyst. Adv. Mater. 2024, 36, 2305285. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, D.; Li, Y.; Chen, G.; Ma, X. Explainable molecular simulation and machine learning for carbon dioxide adsorption on magnesium oxide. Fuel 2024, 357, 129725. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Cheng, B.; Ong, H.C. Microwave-hydrothermal preparation and visible-light photoactivity of plasmonic photocatalyst Ag-TiO2 nanocomposite hollow spheres. Chem. Asian J. 2010, 5, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Nasirian, M.; Lin, Y.P.; Bustillo-Lecompte, C.F.; Mehrvar, M. Enhancement of photocatalytic activity of titanium dioxide using non-metal doping methods under visible light: A review. Int. J. Environ. Sci. Technol. 2018, 15, 2009–2032. [Google Scholar] [CrossRef]
- Lu, W.; Yan, L.; Ye, W.; Ning, J.; Zhong, Y.; Hu, Y. Defect engineering of electrode materials towards superior reaction kinetics for high-performance supercapacitors. J. Mater. Chem. A 2022, 10, 15267–15296. [Google Scholar] [CrossRef]
- Yue, X.; Xiang, J.; Chen, J.; Li, H.; Qiu, Y.; Yu, X. High surface area, high catalytic activity titanium dioxide aerogels prepared by solvothermal crystallization. J. Mater. Sci. Technol. 2020, 47, 223–230. [Google Scholar] [CrossRef]
- Cho, D.; Ko, K.C.; Lamiel-García, O.; Bromley, S.T.; Lee, J.Y.; Illas, F. Effect of Size and Structure on the Ground-State and Excited-State Electronic Structure of TiO2 Nanoparticles. J. Chem. Theory Comput. 2016, 12, 3751–3763. [Google Scholar] [CrossRef]
- Cho, E.; Han, S.; Ahn, H.-S.; Lee, K.-R.; Kim, S.K.; Hwang, C.S. First-principles study of point defects in rutile TiO2−x. Phys. Rev. B 2006, 73, 193202. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Zhang, G.; Song, L.; Tu, Z.; Han, C. AZO work function enhanced by oxygen plasma immersion ion implantation. Vacuum 2023, 212, 112038. [Google Scholar] [CrossRef]
- Wen, C.; Ni, X.; Han, M.; Yu, Y.; Liu, C.; Zhang, Y.; Zheng, B.; Feng, S. The Function of Photocatalytic Performance and Carrier Separation Efficiency Tuned by Doping Content in Homogeneous Photocatalysts. Adv. Sci. 2025, 12, 2501026. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, W.; Yang, Y.; Zhang, A.; Zhang, S.; Guo, Y.; Guo, Y. Morphology-controlled preparation and enhanced simulated sunlight and visible-light photocatalytic activity of Pt/Bi5Nb3O15 heterostructures. Phys. Chem. Chem. Phys. 2013, 15, 8342–8351. [Google Scholar] [CrossRef]
- Mishra, S.B.; Nanda, B.R.K. Facet dependent catalytic activities of anatase TiO2 for CO2 adsorption and conversion. Appl. Surf. Sci. 2020, 531, 147330. [Google Scholar] [CrossRef]
- Álvarez, A.; Borges, M.; Corral-Pérez, J.J.; Olcina, J.G.; Hu, L.; Cornu, D.; Huang, R.; Stoian, D.; Urakawa, A. CO2 Activation over Catalytic Surfaces. ChemPhysChem 2017, 18, 3135–3141. [Google Scholar] [CrossRef]
- Xu, H.; Song, H.; Bi, C.; Zhou, G.; Liu, X.; Zhong, K.; Jiang, W.; Yang, J.; Shen, W.; Hao, N.; et al. Breaking the intrinsic activity barriers of bilayer metal oxides for catalytic CO2 reduction. J. Colloid Interface Sci. 2024, 675, 419–428. [Google Scholar] [CrossRef]
- Alosfur, F.K.M.; Ouda, A.A.; Ridha, N.J.; Abud, S.H. High photocatalytic activity of TiO2 nanorods prepared by simple method. Mater. Res. Express 2019, 6, 065028. [Google Scholar] [CrossRef]
- Manseki, K.; Saka, K.; Matsui, M.; Vafaei, S.; Sugiura, T. Structure identification of Ti(iv) clusters in low-temperature TiO2 crystallization: Creating high-surface area brush-shaped rutile TiO2. CrystEngComm 2017, 19, 5844–5848. [Google Scholar] [CrossRef]
- Wu, S.; Lee, W.P.C.; Thenuwara, H.N.; Li, X.; Wu, P. Enhancing CO2 Adsorption on MgO: Insights into Dopant Selection and Mechanistic Pathways. Biomimetics 2025, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Grinter, D.C.; Graciani, J.; Palomino, R.M.; Xu, F.; Waluyo, I.; Sanz, J.F.; Senanayake, S.D.; Rodriguez, J.A. Adsorption and activation of CO2 on Pt/CeOx/TiO2(110): Role of the Pt-CeOx interface. Surf. Sci. 2021, 710, 121852. [Google Scholar] [CrossRef]
- Zang, Y.; Cai, J.; Han, Y.; Wu, H.; Zhu, W.; Shi, S.; Zhang, H.; Ran, Y.; Yang, F.; Ye, M.; et al. CO2 Activation on Ni(111) and Ni(110) Surfaces in the Presence of Hydrogen. J. Phys. Chem. Lett. 2023, 14, 4381–4387. [Google Scholar] [CrossRef]
- Millet, M.-M.; Algara-Siller, G.; Wrabetz, S.; Mazheika, A.; Girgsdies, F.; Teschner, D.; Seitz, F.; Tarasov, A.; Levchenko, S.V.; Schlögl, R.; et al. Ni Single Atom Catalysts for CO2 Activation. J. Am. Chem. Soc. 2019, 141, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.-H.; Zeng, M.; Jiang, H.; Xiong, C.-R. Photocatalytic Reduction of Carbon Dioxide to Methanol over N-doped TiO2 Nanofibers under Visible Irradiation. J. Inorg. Mater. 2018, 33, 839–844. [Google Scholar]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Wang, J.; Liao, W.; Tan, Y.; Henrotte, O.; Kang, Y.; Liu, K.; Fu, J.; Lin, Z.; Chai, L.; Cortes, E.; et al. Transfer dynamics of photo-generated carriers in catalysis. Chem. Soc. Rev. 2025, 54, 6553–6596. [Google Scholar] [CrossRef]
- Li, Q.; Ni, C.; Cui, J.; Li, C.; Fan, F. Impact of Reaction Environment on Photogenerated Charge Transfer Demonstrated by Sequential Imaging. J. Am. Chem. Soc. 2025, 147, 9103–9110. [Google Scholar] [CrossRef]
- Liu, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Surface Engineering of g-C3N4 by Stacked BiOBr Sheets Rich in Oxygen Vacancies for Boosting Photocatalytic Performance. Angew. Chem. Int. Ed. 2020, 59, 4519–4524. [Google Scholar] [CrossRef]
- Zhai, R.; Wang, Z.; Gu, M.; Liu, H.; Zhao, X.; Li, J.; Su, Y.; Cheng, Y.; Wang, S.; Zhang, J. Electron gathering at violet phosphorene-Ag interface for photoreduction of CO2 to ethylene. Appl. Catal. B Environ. Energy 2025, 361, 124603. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, H.; Liu, J.; Bi, C.; Tian, J.; Zhong, K.; Wang, B.; Ding, P.; Wang, X.; Chu, P.K.; et al. Stacking Engineering of Heterojunctions in Half-Metallic Carbon Nitride for Efficient CO2 Photoreduction. Adv. Sci. 2023, 10, 2307192. [Google Scholar] [CrossRef]
- Yang, J.; Yang, K.; Zhu, X.; Wang, Z.; Yang, Z.; Ding, X.; Zhong, K.; He, M.; Li, H.; Xu, H. Band engineering of non-metal modified polymeric carbon nitride with broad spectral response for enhancing photocatalytic CO2 reduction. Chem. Eng. J. 2023, 461, 141841. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Xu, Q.; Duan, L.; Guo, H. Understanding inclusive quantum dots hollow CN@CIZS heterojunction for enhanced photocatalytic CO2 reduction. Appl. Surf. Sci. 2022, 604, 154601. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, G.; Yi, J.; Ding, P.; Yang, J.; Zhong, K.; Song, Y.; Hua, Y.; Zhu, X.; Yuan, J.; et al. Accelerated Photoreduction of CO2 to CO over a Stable Heterostructure with a Seamless Interface. ACS Appl. Mater. Interfaces 2021, 13, 39523–39532. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, X.; Mei, Z.; Cai, S.; An, Q.; Fu, Y.; Wang, H.; Liu, T.; Guo, H. Understanding rich oxygen vacant hollow CeO2@MoSe2 heterojunction for accelerating photocatalytic CO2 reduction. J. Colloid Interface Sci. 2022, 611, 644–653. [Google Scholar] [CrossRef]
- Li, M.; Idros, M.N.; Wu, Y.; Burdyny, T.; Garg, S.; Zhao, X.S.; Wang, G.; Rufford, T.E. The role of electrode wettability in electrochemical reduction of carbon dioxide. J. Mater. Chem. A 2021, 9, 19369–19409. [Google Scholar] [CrossRef]
- Shi, R.; Guo, J.; Zhang, X.; Waterhouse, G.I.N.; Han, Z.; Zhao, Y.; Shang, L.; Zhou, C.; Jiang, L.; Zhang, T. Efficient wettability-controlled electroreduction of CO2 to CO at Au/C interfaces. Nat. Commun. 2020, 11, 3028. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hou, H.; Yang, M.; Zhu, Z.; Fu, H.; Zhang, D.; Luo, Y.; Yang, W. Engineering the S-scheme heterojunction between NiO microrods and MgAl-LDH nanoplates for efficient and selective photoreduction of CO2 to CH4. Chem. Eng. J. 2023, 474, 145813. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.; Yu, Q.; He, M.; Zhang, W.; Mo, Z.; Yuan, J.; She, Y.; Xu, H.; Li, H. Multidimensional In2O3/In2S3 heterojunction with lattice distortion for 2 photoconversion. Chin. J. Catal. 2022, 43, 1286–1294. [Google Scholar] [CrossRef]
- Zhou, A.-Q.; Yang, J.-M.; Zhu, X.-W.; Zhu, X.-L.; Liu, J.-Y.; Zhong, K.; Chen, H.-X.; Chu, J.-Y.; Du, Y.-S.; Song, Y.-H.; et al. Self-assembly construction of NiCo LDH/ultrathin g-C3N4 nanosheets photocatalyst for enhanced CO2 reduction and charge separation mechanism study. Rare Met. 2022, 41, 2118–2128. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, T.; Liu, H.; Jia, X.; Zhang, D.; Wei, L.; Xu, J.; Li, H. Electrochemical CO2 Reduction on SnO: Insights into C1 Product Dynamic Distribution and Reaction Mechanisms. ACS Catal. 2025, 15, 3173–3183. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.-X.; Gandionco, K.A.; Bond, A.M.; Zhang, J. Electrocatalytic carbon dioxide reduction: From fundamental principles to catalyst design. Mater. Today Adv. 2020, 7, 100074. [Google Scholar] [CrossRef]
- Wang, B.; Chen, H.; Zhang, W.; Liu, H.; Zheng, Z.; Huang, F.; Liu, J.; Liu, G.; Yan, X.; Weng, Y.-X.; et al. Semimetallic Bismuthene with Edge-Rich Dangling Bonds: Broad-Spectrum-Driven and Edge-Confined Electron Enhancement Boosting CO2 Hydrogenation Reduction. Adv. Mater. 2024, 36, 2312676. [Google Scholar] [CrossRef]
- Huang, M.; Liu, C.; Cui, P.; Wu, T.; Feng, X.; Huang, H.; Zhou, J.; Wang, Y. Facet-Dependent Photoinduced Transformation of Cadmium Sulfide (CdS) Nanoparticles. Environ. Sci. Technol. 2021, 55, 13132–13141. [Google Scholar] [CrossRef]
- Xiong, X.; Mao, C.; Yang, Z.; Zhang, Q.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. Photocatalytic CO2 Reduction to CO over Ni Single Atoms Supported on Defect-Rich Zirconia. Adv. Energy Mater. 2020, 10, 2002928. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.; Li, P.; Kako, T.; Ye, J. High-active anatase TiO2 nanosheets exposed with 95% {100} facets toward efficient H2 evolution and CO2 photoreduction. ACS Appl. Mater. Interfaces 2013, 5, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, W.; Wang, Z.; Zhu, W.; Chu, W.; Wu, C.; Xie, Y. Surface-adsorbed ions on TiO2 nanosheets for selective photocatalytic CO2 reduction. Nano Res. 2018, 11, 3362–3370. [Google Scholar] [CrossRef]
- Ioannidou, T.; Anagnostopoulou, M.; Vasiliadou, I.A.; Marchal, C.; Alexandridou, E.-O.; Keller, V.; Christoforidis, K.C. Mixed phase anatase nanosheets/brookite nanorods TiO2 photocatalysts for enhanced gas phase CO2 photoreduction and H2 production. J. Environ. Chem. Eng. 2024, 12, 111644. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Wan, Q.; Shi, Y.-Z.; Wang, H.; Kang, Y.-Y.; Zhu, S.-Y.; Lin, S.; Wu, L. Selective photocatalytic reduction CO2 to CH4 on ultrathin TiO2 nanosheet via coordination activation. Appl. Catal. B Environ. 2021, 288, 120000. [Google Scholar] [CrossRef]
- Dong, L.; Xiong, Z.; Zhou, Y.; Zhao, J.; Li, Y.; Wang, J.; Chen, X.; Zhao, Y.; Zhang, J. Photocatalytic CO2 reduction over postcalcinated atomically thin TiO2 nanosheets: Residual carbon removal and structure transformation. J. CO2 Util. 2020, 41, 101262. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, W.; Miao, W.; Yuan, Z.; Yang, G.; Kong, F.; Yan, T.; Chen, J.; Huang, B.; An, C.; et al. Living Atomically Dispersed Cu Ultrathin TiO2 Nanosheet CO2 Reduction Photocatalyst. Adv. Sci. 2019, 6, 1900289. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Huang, F.; Shi, X.; Xu, H.; Xu, J.; Zhu, X. Dimensional Engineering of 1D/2D Synergistic TiO2 Nanostructures for High-Efficiency Photocatalytic CO2 Reduction. Materials 2025, 18, 4148. https://doi.org/10.3390/ma18174148
Liu X, Huang F, Shi X, Xu H, Xu J, Zhu X. Dimensional Engineering of 1D/2D Synergistic TiO2 Nanostructures for High-Efficiency Photocatalytic CO2 Reduction. Materials. 2025; 18(17):4148. https://doi.org/10.3390/ma18174148
Chicago/Turabian StyleLiu, Xiang, Fujiang Huang, Xiang Shi, Hangmin Xu, Jian Xu, and Xingwang Zhu. 2025. "Dimensional Engineering of 1D/2D Synergistic TiO2 Nanostructures for High-Efficiency Photocatalytic CO2 Reduction" Materials 18, no. 17: 4148. https://doi.org/10.3390/ma18174148
APA StyleLiu, X., Huang, F., Shi, X., Xu, H., Xu, J., & Zhu, X. (2025). Dimensional Engineering of 1D/2D Synergistic TiO2 Nanostructures for High-Efficiency Photocatalytic CO2 Reduction. Materials, 18(17), 4148. https://doi.org/10.3390/ma18174148






