Enzyme Immobilization on Nanomaterials and Their Applications
Abstract
1. Introduction
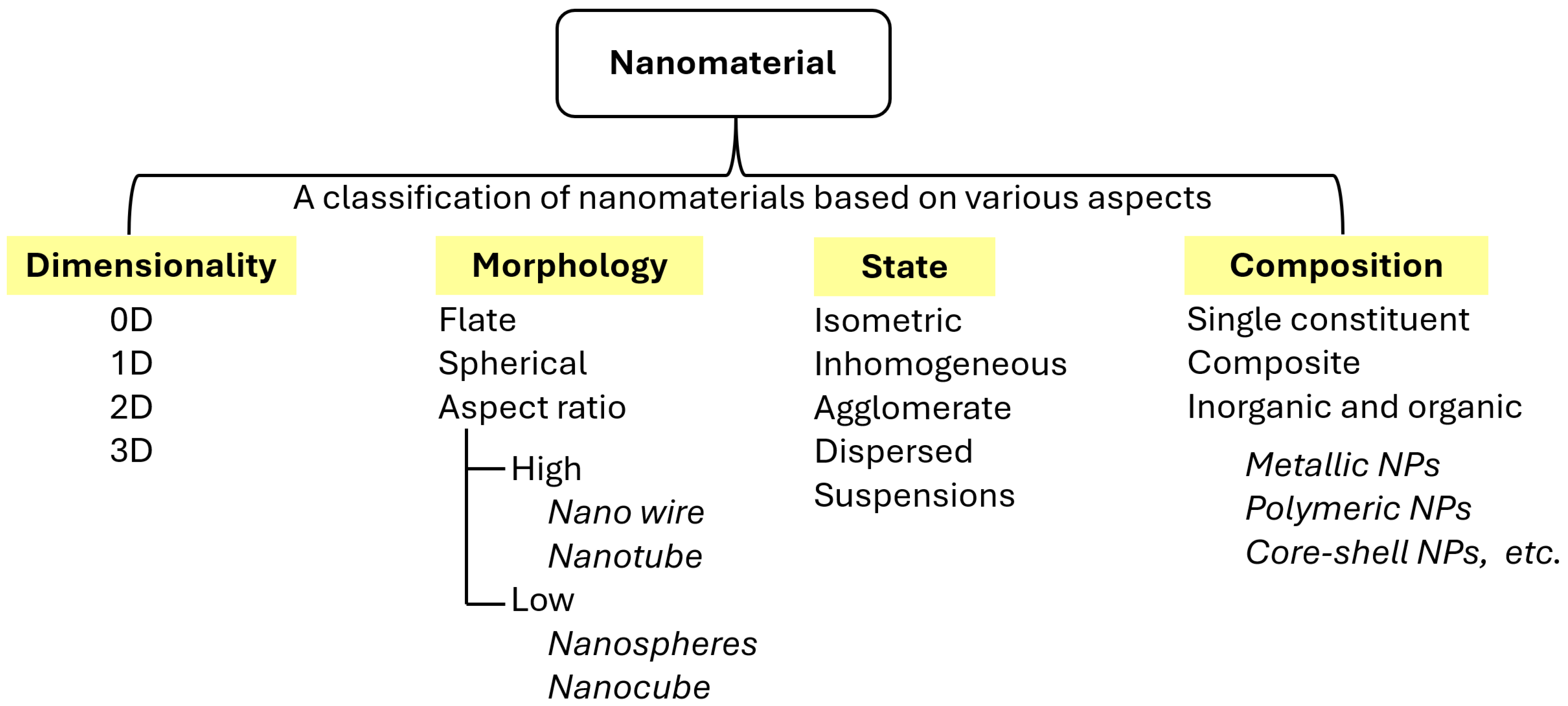
2. Nanomaterials for Immobilizing Enzymes
2.1. Carbon-Based Nanomaterials
2.2. Graphene and Derivatives
2.3. Metal and Metal–Oxide Nanoparticles
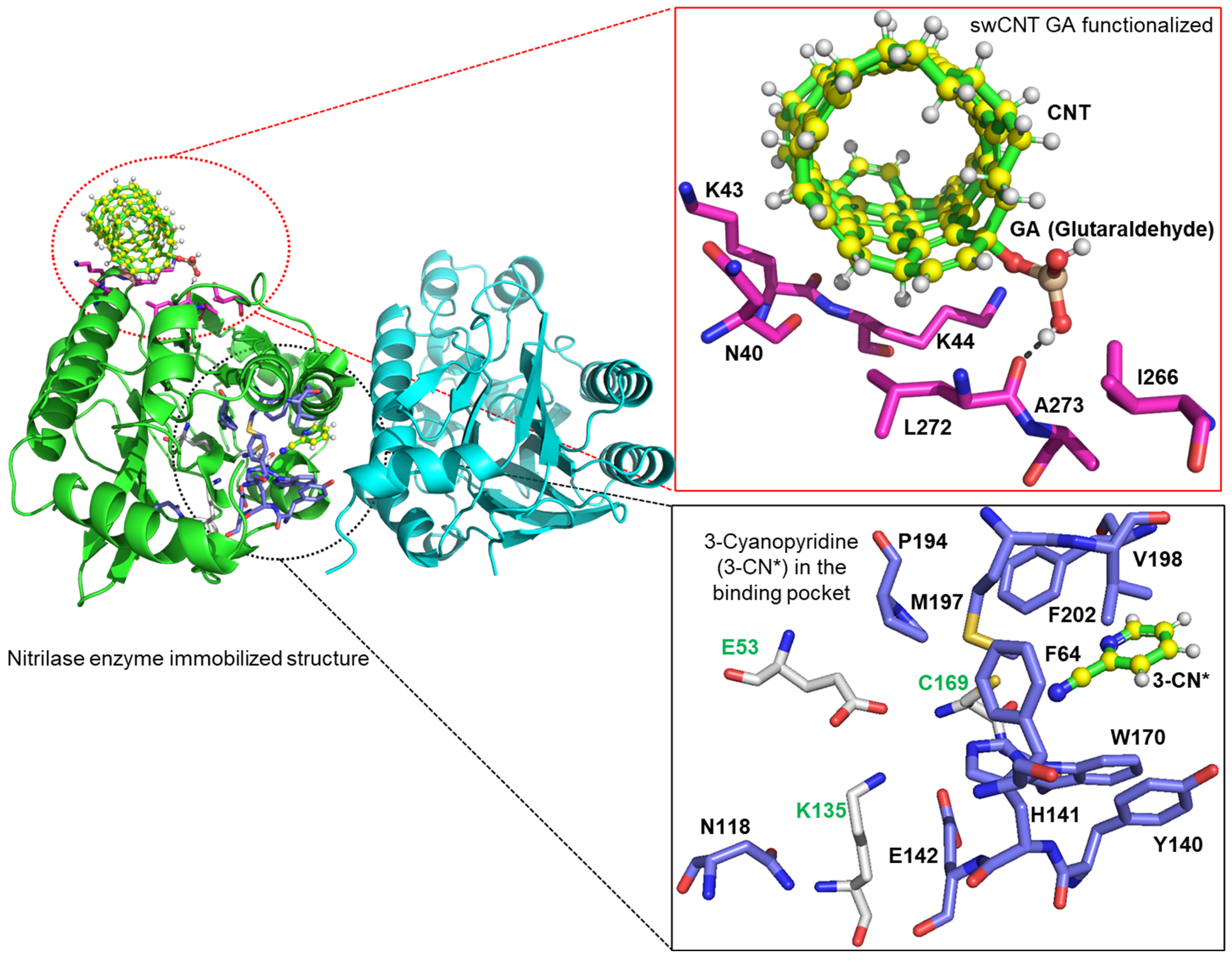
2.4. MNPs
2.5. Polymeric Nanoparticles
| Nanomaterial | Skeleton Matrix | Functionalization Groups | Enzyme | Enzyme Source | Reusability (Retained Activity) | Reference |
|---|---|---|---|---|---|---|
| Polymeric nanoparticles | Hyaluronic acid, chitin, and chitosan | Glutaraldehyde | Catalase | Sunflower seeds | 25 cycles (73.80%) (79.55%) | [169] |
| CS-ALG-Fe3O4 MNPs | Ionic gelation method | Laccase | Trametes versicolor | 10 cycles (81%) | [164] | |
| Hyaluronic acid, chitin, and chitosan | Glutaraldehyde | Catalase | Bacillus subtilis | 25 cycles (73.80%) (79.55%) | [122] | |
| Fe3O4@chitosan | Glutaraldehyde | Laccase | Rhus verniciflua | 10 cycles (75.8%) | [170] | |
| ZnO/chitosan | Glutaraldehyde, APTES | Trypsin | - | 10 cycles (50%) | [171] | |
| Silica-coated magnetic nanoparticles | Isocyanatopropyltriethoxysilane | Prolidase | Escherichia coli prolidase | 20 cycles (80%) | [172] | |
| Chitosan-Fe3O4 and chitosan-ZnO | - | α-Amylase | - | 10 cycles (50%) | [173] | |
| Chitosan-cobalt oxide beads | Cyanuric chloride | Peroxidase | Euphorbia tirucalli | 10 cycles (60%) | [174] | |
| Chitosan coated superparamagnetic nanoparticles | 1,3,5-triazine | Glucoamylase | Aspergillus niger | 10 cycles (70%) | [175] |
3. Enzyme Immobilization on Nanomaterials for Biotransformation
4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhavaniramya, S.; Vanajothi, R.; Vishnupriya, S.; Premkumar, K.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Enzyme immobilization on nanomaterials for biosensor and biocatalyst in food and biomedical industry. Curr. Pharm. Des. 2019, 25, 2661–2676. [Google Scholar] [CrossRef]
- Nawaz, A.F.; Samra Zafar, S.L.F.; Shahzadi, K.; Fatima, Z.; Siddique, I. Use of nanomaterials for the immobilization of industrially important enzymes. J. Nanotechnol. 2021, 3, 45–57. [Google Scholar]
- Cavalcante, A.L.G.; Dari, D.N.; da Silva Aires, F.I.; de Castro, E.C.; Dos Santos, K.M.; Dos Santos, J.C.S. Advancements in enzyme immobilization on magnetic nanomaterials: Toward sustainable industrial applications. RSC Adv. 2024, 14, 17946–17988. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Park, G.D.; Shanmugam, R.; Pagolu, R.; Patel, S.K.; Bisht, A.; Kim, D.R.; Kang, Y.C.; Lee, J.K. Investigating the role of metals loaded on nitrogen-doped carbon-nanotube electrodes in electroenzymatic alcohol dehydrogenation. Appl. Catal. B Environ. 2022, 307, 121195. [Google Scholar] [CrossRef]
- Borzouee, F.; Varshosaz, J.; Cohan, R.A.; Norouzian, D.; Pirposhteh, R.T. A comparative analysis of different enzyme immobilization nanomaterials: Progress, constraints and recent trends. Curr. Med. Chem. 2021, 28, 3980–4003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lovell, J.F.; Shi, J.; Zhang, Y. Nanomaterials for co-immobilization of multiple enzymes. BMEMat 2024, 3, e12080. [Google Scholar] [CrossRef]
- Sharifi, M.; Karim, A.Y.; Mustafa Qadir Nanakali, N.; Salihi, A.; Aziz, F.M.; Hong, J.; Khan, R.H.; Saboury, A.A.; Hasan, A.; Abou-Zied, O.K.; et al. Strategies of enzyme immobilization on nanomatrix supports and their intracellular delivery. J. Biomol. Struct. Dyn. 2020, 38, 2746–2762. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Mohamad, M.; Rashid, M.U.; Norizan, M.N.; Hamzah, F.; Mat, H.B. Recent advances in enzyme immobilisation strategies: An overview of techniques and composite carriers. J. Compos. Sci. 2023, 7, 488. [Google Scholar] [CrossRef]
- Onoka, I.; Makangara, J.J. Micro-and nanocarriers for immobilization of enzymes. In Marine Biopolymers, 1st ed.; Elsevier: San Diego, CA, USA, 2025; pp. 545–572. ISBN 978-0-443-15606-9. [Google Scholar]
- Ayub, J.; Saeed, M.U.; Hussain, N.; Zulfiqar, I.; Mehmood, T.; Iqbal, H.M.; Bilal, M. Designing robust nano-biocatalysts using nanomaterials as multifunctional carriers-expanding the application scope of bio-enzymes. Top. Catal. 2023, 66, 625–648. [Google Scholar] [CrossRef]
- Tan, W.Y.; Gopinath, S.C.; Anbu, P.; Yaakub, A.R.W.; Subramaniam, S.; Chen, Y.; Sasidharan, S. Bio-enzyme hybrid with nanomaterials: A potential cargo as sustainable biocatalyst. Sustainability 2023, 15, 7511. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, J.; Salmon, S. Developing enzyme immobilization with fibrous membranes: Longevity and characterization considerations. Membranes 2023, 13, 532. [Google Scholar] [CrossRef]
- Reis, C.L.B.; Sousa, E.Y.A.D.; Serpa, J.D.F.; Oliveira, R.C.; Santos, J.C.S.D. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Química Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, J.; Wang, M.; Xu, W.; Wang, Y.; Wen, L.; Dong, J. Pros and cons in various immobilization techniques and carriers for enzymes. Appl. Biochem. Biotechnol. 2024, 196, 5633–5655. [Google Scholar] [CrossRef] [PubMed]
- Bouguerra, O.M.; Wahab, R.A.; Huyop, F.; Al-Fakih, A.M.; Mahmood, W.M.A.W.; Mahat, N.A.; Sabullah, M.K. An Overview of Crosslinked Enzyme Aggregates: Concept of Development and Trends of Applications. Appl. Biochem. Biotechnol. 2024, 196, 5711–5739. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhai, S.; Xue, S.; Zhi, L. Enzyme immobilization using covalent organic frameworks: From synthetic strategy to COFs functional role. ACS Appl. Mater. Interfaces 2024, 16, 40371–40390. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yu, D.; Du, X. Review of porous microspheres for enzyme immobilization: Strategies, applications, and prospects. Int. J. Biol. Macromol. 2025, 295, 139627. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, B.; Wang, C.; Jiang, S.; Wang, H.; Yuan, Y.A.; Wei, D. Structural insights into enzymatic activity and substrate specificity determination by a single amino acid in nitrilase from Syechocystis sp. PCC6803. J. Struct. Biol. 2014, 188, 93–101. [Google Scholar] [CrossRef]
- Teixeira, S.; Sousa, S.F.; NMFSA, C. An Unsual Cys-Glu-Lys Catalytic Triad is Responsible for the Catalytic Mechanism of the Nitrilase Superfamily: A QM/MM Study on Nit2. ChemPhysChem 2021, 22, 796–804. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Pandit, S.; Lee, S.; Ebrahimi, S.B.; Samanta, D. Modulating enzyme activity using engineered nanomaterials. ChemBioChem 2025, 26, e202400520. [Google Scholar] [CrossRef]
- Mishra, S.D.; Kawadkar, J.; Joshi, P.A.; Vats, K.; Srivastava, A.; Mishra, R.K.; Rai, V. Amphoteric Cross-Linked Cellulose Nanoparticles as a Platform for Immobilization of Proteins and Cells, Enabling Bioanalyte Sensing. ACS Appl. Mater. Interfaces 2025, 17, 7262–7274. [Google Scholar] [CrossRef]
- Patil, P.D.; Gargate, N.; Tiwari, M.S.; Nadar, S.S. Defect metal-organic frameworks (D-MOFs): An engineered nanomaterial for enzyme immobilization. Coord. Chem. Rev. 2025, 531, 216519. [Google Scholar] [CrossRef]
- Zaixing, L.; Chao, L.; Qinqin, Z.; Chen, H.; Wenjing, Z.; Bingbing, X.; Xue, Q.; Guixia, L.; Zhifang, N. Magnetic nanocomposites as multifunctional carriers for enzymes immobilization: A review. Chem. Pap. 2024, 78, 1353–1365. [Google Scholar] [CrossRef]
- Dakal, T.C.; Bhushan, R.; Dhakar, R.; Kumar, A. Classification and Applications of Bio-nanomaterials. In Bio-Nanomaterials in Environmental Remediation: Industrial Applications; Wiley-VCH: Weinheim, Germany, 2025; pp. 47–73. [Google Scholar]
- Da Costa, F.P.; Cipolatti, E.P.; Furigo, A., Jr.; Oliveira Henriques, R. Nanoflowers: A new approach of enzyme immobilization. Chem. Rec. 2022, 22, e202100293. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Khatik, A.G.; Patil, P.D.; Tiwari, M.S.; Nadar, S.S.; Jain, A.K. Recent immobilization techniques for ketoreducases: Its design and their industrial application. Biocatal. Agric. Biotechnol. 2024, 56, 103027. [Google Scholar] [CrossRef]
- Heinks, T.; Montua, N.; Teune, M.; Liedtke, J.; Höhne, M.; Bornscheuer, U.T.; Fischer von Mollard, G. Comparison of Four Immobilization Methods for Different Transaminases. Catalysts 2023, 13, 300. [Google Scholar] [CrossRef]
- Anboo, S.; Lau, S.Y.; Kansedo, J.; Yap, P.S.; Hadibarata, T.; Jeevanandam, J.; Kamaruddin, A.H. Recent advancements in enzyme-incorporated nanomaterials: Synthesis, mechanistic formation, and applications. Biotechnol. Bioeng. 2022, 119, 2609–2638. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Salem, S.S.; Hammad, E.N.; Mohamed, A.A.; El-Dougdoug, W. A comprehensive review of nanomaterials: Types, synthesis, characterization, and applications. Biointerface Res. Appl. Chem. 2022, 13, 41. [Google Scholar] [CrossRef]
- Trotta, F.; Mele, A. Nanomaterials: Classification and properties. In Nanosponges: Synthesis and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 1–26. [Google Scholar]
- Rodríguez-Gómez, F.D.; Penon, O.; Monferrer, D.; Rivera-Gil, P. Classification system for nanotechnology-enabled health products with both scientific and regulatory application. Front. Med. 2023, 10, 1212949. [Google Scholar] [CrossRef]
- Patel, S.K.; Choi, H.; Lee, J.K. Multimetal-based inorganic–protein hybrid system for enzyme immobilization. ACS Sustain. Chem. Eng. 2019, 7, 13633–13638. [Google Scholar] [CrossRef]
- Kumari, A.; Rajeev, R.; Benny, L.; Sudhakar, Y.N.; Varghese, A.; Hegde, G. Recent advances in carbon nanotubes-based biocatalysts and their applications. Adv. Colloid. Interface Sci. 2021, 297, 102542. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, J.; Fu, Y.; Shi, W.; Wang, X.; Su, Y.; Wang, X.; Wang, X. Immobilization of lipase on mesoporous silica nanocarriers for efficient preparation of phytosterol esters. Int. J. Biol. Macromol. 2025, 286, 138310. [Google Scholar] [CrossRef]
- Prlainović, N.Ž.; Milovanović, J.S.; Milašinović, N.Z.; Bezbradica, D.I.; Mijin, D.Ž. Multi-walled carbon nanotubes as lipase carriers for organic synthesis: Current trends and recent update. Hem. Ind. 2024, 78, 1–16. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, M.; Chen, G.; Chi, C.; Zhao, J. A novel Affi-Cova magnetic nanoparticles for one-step covalent immobilization of His-tagged enzyme directly from crude cell lysate. Int. J. Biol. Macromol. 2024, 280, 135811. [Google Scholar] [CrossRef] [PubMed]
- Meskher, H.; Ragdi, T.; Thakur, A.K.; Ha, S.; Khelfaoui, I.; Sathyamurthy, R.; Sharshir, S.W.; Pandey, A.K.; Saidur, R.; Singh, P.; et al. A review on CNTs-based electrochemical sensors and biosensors: Unique properties and potential applications. Crit. Rev. Anal. Chem. 2024, 54, 2398–2421. [Google Scholar] [CrossRef]
- Ranjbari, S.; Bolourinezhad, M.; Kesharwani, P.; Rezayi, M.; Sahebkar, A. Applications of Carbon Nanotube Biosensors: Sensing the Future. J. Drug Deliv. Technol. 2024, 97, 105747. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.; Zhong, Y.; Li, R.; Deng, W.; Xiao, X.; Xu, Y.; Zhang, J.; Hu, X.; Wang, Y. A review of nanomaterials for biosensing applications. J. Mater. Chem. B 2024, 12, 1168–1193. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Patil, T.V.; Dutta, S.D.; Lee, J.; Ganguly, K.; Kim, H.; Randhawa, A.; Lim, K.T. Single-Walled Carbon Nanotube-Based Optical Nano/Biosensors for Biomedical Applications: Role in Bioimaging, Disease Diagnosis, and Biomarkers Detection. Adv. Mater. Technol. 2024, 9, 2400279. [Google Scholar] [CrossRef]
- Saguin, N.S.G.; Maulik, G.; Cao, X.; Luo, X.; Nag, A.; Gao, J.; Deng, S.; Wong, J.W. CNTs-based biosensors for enzyme detection. Sens. Actuators A Phys. 2024, 377, 115753. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, S.; Qin, W. Electrochemical biochemical oxygen demand biosensors and their applications in aquatic environmental monitoring. Sens. Bio-Sens. Res. 2024, 44, 100642. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhu, Z.; He, J.; Yang, Y.; Liang, Y.; Li, Z.; Zhu, M.; Xiao, M.; Zhang, Z. Mass Production of Carbon Nanotube Transistor Biosensors for Point-of-Care Tests. Nano Lett. 2024, 24, 10510–10518. [Google Scholar] [CrossRef]
- Masood, M.; Albayouk, T.; Saleh, N.I.; El-Shazly, M.; El-Nashar, H.A. Carbon nanotubes: A novel innovation as food supplements and biosensing for food safety. Front. Nutr. 2024, 11, 1381179. [Google Scholar] [CrossRef]
- Pikula, K.; Johari, S.A.; Santos-Oliveira, R.; Golokhvast, K. Toxicity and biotransformation of carbon-based nanomaterials in marine microalgae Heterosigma akashiwo. Int. J. Mol. Sci. 2023, 24, 10020. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef]
- Abdulmalek, S.A.; Li, K.; Wang, J.; Ghide, M.K.; Yan, Y. Co-immobilization of Rhizopus oryzae and Candida rugosa lipases onto mMWCNTs@ 4-arm-PEG-NH2—A novel magnetic nanotube–polyethylene glycol amine composite—And its applications for biodiesel production. Int. J. Mol. Sci. 2021, 22, 11956. [Google Scholar] [CrossRef]
- Moradpour, H.; Forootanfar, H.; Ameri, A.; Beitollahi, H. Fabrication of the carbon paste electrode modified with Trametes versicolor laccase immobilized on carboxyl functionalized multi-walled carbon nanotubes and its application for measurement of dopamine. Int. J. Biol. Macromol. 2024, 283, 137891. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Ibrahim, G.G.; Yang, M.; Yan, J.; Yan, Y. Improved performance of Pseudomonas fluorescens lipase immobilized on a magnetic mesoporous solvothermal hybrid-nanocarrier and its application in ultrasonication assisted estolide synthesis. J. Ind. Eng. Chem. 2024, 140, 258–268. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Jing, S.L.; Shao, J.L.; Chen, J.X.; Zhang, W.F.; Wan, S.Y.; Shen, Y.P.; Yang, H.; Yu, W. Purification and immobilization of β-glucosidase using surface modified mesoporous silica Santa Barbara Amorphous 15 for eco-friendly preparation of sagittatoside A. Nat. Prod. Bioprospect. 2024, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Varan, N.E.; Yildirim, D.; Toprak, A.; Fernandéz-Lafuente, R.; Alagöz, D. Effect of the activation strategy of nickel oxide-multi-walled carbon nanotubes on the immobilization of xylanase for synthesis of xylooligosaccharides. Appl. Biochem. Biotechnol. 2024, 72, 911–923. [Google Scholar] [CrossRef]
- Kaur, P.; Jana, A.K. Candida rugosa lipase immobilization on Fe3O4 coated carboxyl functionalised multiwalled carbon nanotubes for production of food flavour esters. Biotechnol. Bioprocess. Eng. 2023, 28, 310–326. [Google Scholar] [CrossRef]
- Özdemir, F.İ.; Karaaslan, B.; Tülek, A.; Yucebilgic, G.; Yildirim, D. Immobilization of recombinant L-asparaginase from Geobacillus kaustophilus on magnetic MWCNT-nickel composites. Process Biochem. 2023, 127, 10–20. [Google Scholar] [CrossRef]
- Habimana, P.; Jiang, Y.; Gao, J.; Ndayambaje, J.B.; Darwesh, O.M.; Mwizerwa, J.P.; Zheng, X.; Ma, L. Enhancing laccase stability and activity for dyes decolorization using ZIF-8@ MWCNT nanocomposite. Chin. J. Chem. Eng. 2022, 48, 66–75. [Google Scholar] [CrossRef]
- Patila, M.; Athanasiou, P.E.; Kortessis, L.; Potsi, G.; Kouloumpis, A.; Gournis, D.; Stamatis, H. Immobilization of laccase on hybrid super-structured nanomaterials for the decolorization of phenolic dyes. Processes 2022, 10, 233. [Google Scholar] [CrossRef]
- Alagöz, D.; Toprak, A.; Yildirim, D.; Tükel, S.S.; Fernandez-Lafuente, R. Modified silicates and carbon nanotubes for immobilization of lipase from Rhizomucor miehei: Effect of support and immobilization technique on the catalytic performance of the immobilized biocatalysts. Enzym. Microb. Technol. 2021, 144, 109739. [Google Scholar] [CrossRef]
- Ji, S.; Liu, W.; Su, S.; Gan, C.; Jia, C. Chitosan derivative functionalized carbon nanotubes as carriers for enzyme immobilization to improve synthetic efficiency of ethyl caproate. LWT 2021, 149, 111897. [Google Scholar] [CrossRef]
- Khan, M.; Husain, Q. Multiwalled carbon nanotubes bound beta-galactosidase: It’s activity, stability and reusability. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2020; Volume 630, pp. 365–405. [Google Scholar]
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H.; Hayati, B. Environmentally friendly novel covalently immobilized enzyme bionanocomposite: From synthesis to the destruction of pollutant. Compos. B Eng. 2020, 184, 107666. [Google Scholar] [CrossRef]
- Yadav, A.; Agrawal, D.C.; Srivastava, R.R.; Srivastava, A.; Kayastha, A.M. Nanoparticles decorated carbon nanotubes as novel matrix: A comparative study of influences of immobilization on the catalytic properties of Lens culinaris β-galactosidase (Lcβ-gal). Int. J. Biol. Macromol. 2020, 144, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, B.; Pillai, S.; Permaul, K.; Singh, S. Simultaneous removal of heavy metals and cyanate in a wastewater sample using immobilized cyanate hydratase on magnetic-multiwall carbon nanotubes. J. Hazard. Mater. 2019, 363, 73–80. [Google Scholar] [CrossRef]
- Kumar, A.; Park, G.D.; Patel, S.K.; Kondaveeti, S.; Otari, S.; Anwar, M.Z.; Kalia, V.C.; Singh, Y.; Kim, S.C.; Cho, B.K.; et al. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem. Eng. J. 2019, 359, 1252–1264. [Google Scholar] [CrossRef]
- Asmat, S.; Anwer, A.H.; Husain, Q. Immobilization of lipase onto novel constructed polydopamine grafted multiwalled carbon nanotube impregnated with magnetic cobalt and its application in synthesis of fruit flavours. Int. J. Biol. Macromol. 2019, 140, 484–495. [Google Scholar] [CrossRef]
- Li, L.J.; Xia, W.J.; Ma, G.P.; Chen, Y.L.; Ma, Y.Y. A study on the enzymatic properties and reuse of cellulase immobilized with carbon nanotubes and sodium alginate. AMB Express 2019, 9, 112. [Google Scholar] [CrossRef]
- Szelwicka, A.; Boncel, S.; Jurczyk, S.; Chrobok, A. Exceptionally active and reusable nanobiocatalyst comprising lipase non-covalently immobilized on multi-wall carbon nanotubes for the synthesis of diester plasticizers. Appl. Catal. A Gen. 2019, 574, 41–47. [Google Scholar] [CrossRef]
- Ahmad, R.; Khare, S.K. Immobilization of Aspergillus niger cellulase on multiwall carbon nanotubes for cellulose hydrolysis. Bioresour. Technol. 2018, 252, 72–75. [Google Scholar] [CrossRef]
- Bencze, L.C.; Bartha-Vári, J.H.; Katona, G.; Toşa, M.I.; Paizs, C.; Irimie, F.D. Nanobioconjugates of Candida antarctica lipase B and single-walled carbon nanotubes in biodiesel production. Bioresour. Technol. 2016, 200, 853–860. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, G.; Su, F.; Li, K.; Xu, L.; Han, X.; Yan, Y. Lipase oriented-immobilized on dendrimer-coated magnetic multi-walled carbon nanotubes toward catalyzing biodiesel production from waste vegetable oil. Fuel 2016, 178, 172–178. [Google Scholar] [CrossRef]
- Sahu, S.; Shera, S.S.; Banik, R.M. Enhanced reusability of horseradish peroxidase immobilized onto graphene oxide/magnetic chitosan beads for cost effective cholesterol oxidase assay. Open Biotechnol. J. 2019, 13, 93–104. [Google Scholar] [CrossRef]
- Banerjee, A.N. Graphene and its derivatives as biomedical materials: Future prospects and challenges. Interface Focus 2018, 8, 20170056. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Du, S. Application of graphene derivatives and their nanocomposites in tribology and lubrication: A review. RSC Adv. 2019, 9, 40642–40661. [Google Scholar] [CrossRef]
- Gao, F.; Guo, Y.; Fan, X.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y.; Wang, X. Enhancing the catalytic performance of chloroperoxidase by co-immobilization with glucose oxidase on magnetic graphene oxide. Biochem. Eng. J. 2019, 143, 101–109. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhang, H.; Yang, Y.Y.; Yang, F.Q. An online dual-enzyme co-immobilized microreactor based on capillary electrophoresis for enzyme kinetics assays and screening of dual-target inhibitors against thrombin and factor Xa. J. Chromatogr. A 2020, 1619, 460948. [Google Scholar] [CrossRef]
- Mahmood Khan, R.; Pervaiz, M.; Saeed, Z.; Majid, A.; Liaqat, M. Graphene-Based Enzymatic and Non-Enzymatic Electrochemical Glucose Sensors: Review of Current Research and Advances in Nanotechnology. ChemistrySelect 2023, 8, e202303952. [Google Scholar]
- Srivastava, N.; Singh, R.; Lal, B.; Mohammad, A.; Rai, A.K.; Ahmad, I.; Srivastava, M.; Choi, C.H.; Gupta, V.K. Graphene and its derivatives fabrication from paddy straw for improved and sustainable application in biofuels production: New Insight. Process Saf. Environ. Prot. 2024, 187, 1596–1605. [Google Scholar] [CrossRef]
- Krishna Perumal, P.; Chen, C.W.; Giri, B.S.; Singhania, R.R.; Patel, A.K.; Dong, C.D. Graphene-based functional electrochemical sensors for the detection of chlorpyrifos in water and food samples: A review. J. Food Sci. Technol. 2024, 61, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, W.; Chen, J.; Li, H.; Han, L.; Li, X.; Wang, J.; Song, W.; Xu, C.; Cai, X.; et al. Sensitivity-Enhancing Strategies of Graphene Field-Effect Transistor Biosensors for Biomarker Detection. ACS Sens. 2024, 9, 2705–2727. [Google Scholar] [CrossRef]
- Joshi, R.; Ravindran, V.; Lahiri, I. Graphene-based materials and electrochemical biosensors: An overview. J. Phys. Condens. Matter 2025, 37, 143001. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Chakrabarty, S. Graphene and its derivatives (GO, rGO and GQD): A comprehensive review of their role in combating COVID-19. J. Adv. Phys. 2025, 10, 2435278. [Google Scholar] [CrossRef]
- Shang, H.; Wang, J.; Guo, B.; Zhu, H.; Li, H. Immobilization of Phospholipase D on Fe3O4@SiO2-Graphene Oxide Nanocomposites: A Strategy to Improve Catalytic Stability and Reusability in the Efficient Production of Phosphatidylserine. Molecules 2025, 30, 912. [Google Scholar] [CrossRef]
- Monajati, M.; Ariafar, N.; Abedi, M.; Borandeh, S.; Tamaddon, A.M. Immobilization of L-Asparaginase on biofunctionalized magnetic graphene oxide nanocomposite: A promising approach for Enhanced Stability and reusability. Heliyon 2024, 10, e40072. [Google Scholar] [CrossRef]
- Hou, H.; Xu, F.; Ding, X.; Zheng, L.; Shi, J. Magnetic biocatalytic nanoreactors based on graphene oxide with graded reduction degrees for the enzymatic synthesis of phytosterol esters. Carbon. 2024, 226, 119170. [Google Scholar] [CrossRef]
- Pinto, G.C.; Lucena, G.N.; Piazza, R.D.; Costa, J.M.L.; e Silva, E.T.C.C.; Gu, Y.; de Paula, A.V.; Silva, N.J.O.; Marques, R.F.C. Evaluation of the alternating magnetic field (AMF) influence in catalytic activities of enzymes immobilized into magnetic graphene oxide: A new approach. Mater. Today Commun. 2023, 36, 106441. [Google Scholar] [CrossRef]
- Yu, D.; Li, Z.; Zhou, X.; Wang, W.; Wang, L.; Liu, T.; Du, J. Study on the modification of magnetic graphene oxide and the effect of immobilized lipase. Int. J. Biol. Macromol. 2022, 216, 498–509. [Google Scholar] [CrossRef]
- Badoei-Dalfard, A.; Tahami, A.; Karami, Z. Lipase immobilization on glutaraldehyde activated graphene oxide/chitosan/cellulose acetate electrospun nanofibrous membranes and its application on the synthesis of benzyl acetate. Colloids Surf. B Biointerfaces 2022, 209, 112151. [Google Scholar] [CrossRef] [PubMed]
- Paz-Cedeno, F.R.; Carceller, J.M.; Iborra, S.; Donato, R.K.; Godoy, A.P.; de Paula, A.V.; Monti, R.; Corma, A.; Masarin, F. Magnetic graphene oxide as a platform for the immobilization of cellulases and xylanases: Ultrastructural characterization and assessment of lignocellulosic biomass hydrolysis. Renew. Energy 2021, 164, 491–501. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Motamedi, E.; Salekdeh, G.H. Application of the immobilized enzyme on magnetic graphene oxide nano-carrier as a versatile bi-functional tool for efficient removal of dye from water. Bioresour. Technol. 2021, 319, 124228. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Wu, M.; Huan, W.; Li, J. Magnetic graphene oxide nanocomposites as an effective support for lactase immobilization with improved stability and enhanced photothermal enzymatic activity. New J. Chem. 2021, 45, 5939–5948. [Google Scholar] [CrossRef]
- Rong, J.; Zhou, Z.; Wang, Y.; Han, J.; Li, C.; Zhang, W.; Ni, L. Immobilization of horseradish peroxidase on multi-armed magnetic graphene oxide composite: Improvement of loading amount and catalytic activity. Food Technol. Biotechnol. 2019, 57, 260–271. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Song, X.; Cai, J. Hydrophilic polyethylenimine modified magnetic graphene oxide composite as an efficient support for dextranase immobilization with improved stability and recyclable performance. Biochem. Eng. J. 2019, 141, 163–172. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, Y.; Ren, H.; Wang, Y.; Wang, S.; Fang, B. Assembly of graphene oxide-formate dehydrogenase composites by nickel-coordination with enhanced stability and reusability. Eng. Life Sci. 2018, 18, 326–333. [Google Scholar] [CrossRef]
- Vineh, M.B.; Saboury, A.A.; Poostchi, A.A.; Rashidi, A.M.; Parivar, K. Stability and activity improvement of horseradish peroxidase by covalent immobilization on functionalized reduced graphene oxide and biodegradation of high phenol concentration. Int. J. Biol. Macromol. 2018, 106, 1314–1322. [Google Scholar] [CrossRef]
- Samak, N.A.; Tan, Y.; Sui, K.; Xia, T.T.; Wang, K.; Guo, C.; Liu, C. CotA laccase immobilized on functionalized magnetic graphene oxide nano-sheets for efficient biocatalysis. Mol. Catal. 2018, 445, 269–278. [Google Scholar] [CrossRef]
- Besharati Vineh, M.; Saboury, A.A.; Poostchi, A.A.; Mamani, L. Physical adsorption of horseradish peroxidase on reduced graphene oxide nanosheets functionalized by amine: A good system for biodegradation of high phenol concentration in wastewater. Int. J. Environ. Res. 2018, 12, 45–57. [Google Scholar] [CrossRef]
- Asmat, S.; Husain, Q. Exquisite stability and catalytic performance of immobilized lipase on novel fabricated nanocellulose fused polypyrrole/graphene oxide nanocomposite: Characterization and application. Int. J. Biol. Macromol. 2018, 117, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Choi, S.H.; Kang, Y.C.; Lee, J.K. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl. Mater. Interfaces 2017, 9, 2213–2222. [Google Scholar] [CrossRef]
- Gong, A.; Zhu, C.T.; Xu, Y.; Wang, F.Q.; Tsabing, D.A.K.; Wu, F.A.; Wang, J. Moving and unsinkable graphene sheets immobilized enzyme for microfluidic biocatalysis. Sci. Rep. 2017, 7, 4309. [Google Scholar] [CrossRef] [PubMed]
- Asmat, S.; Husain, Q.; Azam, A. Lipase immobilization on facile synthesized polyaniline-coated silver-functionalized graphene oxide nanocomposites as novel biocatalysts: Stability and activity insights. RSC Adv. 2017, 7, 5019–5029. [Google Scholar] [CrossRef]
- Chen, J.; Leng, J.; Yang, X.; Liao, L.; Liu, L.; Xiao, A. Enhanced performance of magnetic graphene oxide-immobilized laccase and its application for the decolorization of dyes. Molecules 2017, 22, 221. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, J.; Deng, Q.; Zheng, M.; Wan, C.; Zheng, C.; Li, Y.; Huang, F. Preparation of carriers based on ZnO nanoparticles decorated on graphene oxide (GO) nanosheets for efficient immobilization of lipase from Candida rugosa. Molecules 2017, 22, 1205. [Google Scholar] [CrossRef]
- Ding, Y.; Cui, R.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y. Well-oriented bioarchitecture for immobilization of chloroperoxidase on graphene oxide nanosheets by site-specific interactions and its catalytic performance. J. Mater. Sci. 2017, 52, 10001–10012. [Google Scholar] [CrossRef]
- Li, W.; Wen, H.; Shi, Q.; Zheng, G. Study on immobilization of (+) γ-lactamase using a new type of epoxy graphene oxide carrier. Process Biochem. 2016, 51, 270–276. [Google Scholar] [CrossRef]
- Royvaran, M.; Taheri-Kafrani, A.; Isfahani, A.L.; Mohammadi, S. Functionalized superparamagnetic graphene oxide nanosheet in enzyme engineering: A highly dispersive, stable and robust biocatalyst. Chem. Eng. J. 2016, 288, 414–422. [Google Scholar] [CrossRef]
- Khan, M.; Husain, Q.; Naqvi, A.H. Graphene based magnetic nanocomposites as versatile carriers for high yield immobilization and stabilization of β-galactosidase. RSC Adv. 2016, 6, 53493–53503. [Google Scholar] [CrossRef]
- Singh, K.; Srivastava, G.; Talat, M.; Srivastava, O.N.; Kayastha, A.M. α-Amylase immobilization onto functionalized graphene nanosheets as scaffolds: Its characterization, kinetics and potential applications in starch based industries. Biochem. Biophys. Rep. 2015, 3, 18–25. [Google Scholar] [CrossRef]
- Patel, V.; Gajera, H.; Gupta, A.; Manocha, L.; Madamwar, D. Synthesis of ethyl caprylate in organic media using Candida rugosa lipase immobilized on exfoliated graphene oxide: Process parameters and reusability studies. Biochem. Eng. J. 2015, 95, 62–70. [Google Scholar] [CrossRef]
- Shao, Y.; Jing, T.; Tian, J.; Zheng, Y. Graphene oxide-based Fe3O4 nanoparticles as a novel scaffold for the immobilization of porcine pancreatic lipase. RSC Adv. 2015, 5, 103943–103955. [Google Scholar] [CrossRef]
- Yoon, Y.; Truong, P.L.; Lee, D.; Ko, S.H. Metal-oxide nanomaterials synthesis and applications in flexible and wearable sensors. ACS Nanosci. Au 2021, 2, 64–92. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, M.H.; Upadhyay, T.K.; Khan, F.; Pandey, P.; Park, M.N.; Sharangi, A.B.; Saeed, M.; Upadhye, V.J.; Kim, B. Metallic and metal oxide-derived nanohybrid as a tool for biomedical applications. Biomed. Pharmacother. 2022, 155, 113791. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.; Adil, S.F.; Shaik, M.R.; Abdelgawad, A.; Hatshan, M.R.; Khan, M. Surface-coated magnetic nanostructured materials for robust bio-catalysis and biomedical applications-A review. J. Adv. Res. 2022, 38, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, S.; Zhao, X.; Liang, H. Co-immobilization of enzymes and magnetic nanoparticles by metal-nucleotide hydrogelnanofibers for improving stability and recycling. Molecules 2017, 22, 179. [Google Scholar] [CrossRef]
- Varshan, G.A.; Namasivayam, S.K.R. A green chemistry principle for the biotransformation of fungal biomass derived chitosan into versatile nano scale materials with high biocompatibility and potential biological activities—A review. BioNanoScience 2024, 14, 4145–4166. [Google Scholar] [CrossRef]
- Jacinto, C.; Javed, Y.; Lavorato, G.; Tarraga, W.A.; Conde, B.I.C.; Orozco, J.M.; Picco, A.S.; Garcia, J.; Dias, C.S.B.; Malik, S.; et al. Biotransformation and biological fate of magnetic iron oxide nanoparticles for biomedical research and clinical applications. Nanoscale Adv. 2025, 7, 2818–2886. [Google Scholar] [CrossRef]
- Huang, S.W.; Yao, Y.Y.; Zhang, H.X.; Guo, W.Y.; Fang, M.H.; Wang, H.B.; Sun, Y.J.; Li, M.H. Novel mechanisms for selenite biotransformation and selenium nanoparticles biogenesis in Acinetobacter sp. SX5 isolated from seleniferous soil. J. Hazard. Mater 2025, 489, 137694. [Google Scholar] [CrossRef]
- Perera, K.Y.; Pradhan, D.; Rafferty, A.; Jaiswal, A.K.; Jaiswal, S. A comprehensive review on metal oxide-nanocellulose composites in sustainable active and intelligent food packaging. Food Chem. Adv. 2023, 3, 100436. [Google Scholar] [CrossRef]
- Kyomuhimbo, H.D.; Feleni, U.; Haneklaus, N.H.; Brink, H. Recent advances in applications of oxidases and peroxidases polymer-based enzyme biocatalysts in sensing and wastewater treatment: A review. Polymers 2023, 15, 3492. [Google Scholar] [CrossRef] [PubMed]
- Mashhadban, K.F.; Gorgani, L.; Darzi, G.N. Enzymatic electrochemical biosensors for urea detection: A review. Sens. Actuators A Phys. 2024, 374, 115499. [Google Scholar] [CrossRef]
- Gupta, D.; Joseph, K.; Dixit, P.; Gupta, T.K. Industrial applications of nanoparticle immobilized enzymes. In Nano-Enzyme Incorporated Particles; Academic Press: Cambridge, MA, USA, 2024; pp. 187–220. [Google Scholar]
- Chaudhari, D.S.; Upadhyay, R.P.; Shinde, G.Y.; Gawande, M.B.; Filip, J.; Varma, R.S.; Zboril, R. A review on sustainable iron oxide nanoparticles: Synthesis and application in organic catalysis and environmental remediation. Green. Chem. 2024, 26, 7579–7655. [Google Scholar] [CrossRef]
- Sun, T.; Fu, M.; Xing, J.; Ge, Z. Magnetic nanoparticles encapsulated laccase nanoflowers: Evaluation of enzymatic activity and reusability for degradation of malachite green. Water Sci. Technol. 2020, 81, 29–39. [Google Scholar] [CrossRef]
- Atiroğlu, V.; Atiroğlu, A.; Özacar, M. Immobilization of α-amylase enzyme on a protein@ metal–organic framework nanocomposite: A new strategy to develop the reusability and stability of the enzyme. Food Chem. 2021, 349, 129127. [Google Scholar] [CrossRef]
- Salem, K.; Jabalera, Y.; Puentes-Pardo, J.D.; Vilchez-Garcia, J.; Sayari, A.; Hmida-Sayari, A.; Jimenez-Lopez, C.; Perduca, M. Enzyme storage and recycling: Nanoassemblies of α-amylase and xylanase immobilized on biomimetic magnetic nanoparticles. ACS Sustain. Chem. Eng. 2021, 9, 4054–4063. [Google Scholar] [CrossRef]
- Noma, S.A.A.; Ulu, A.; Acet, Ö.; Sanz, R.; Sanz-Pérez, E.S.; Odabaşı, M.; Ateş, B. Comparative study of ASNase immobilization on tannic acid-modified magnetic Fe3O4/SBA-15 nanoparticles to enhance stability and reusability. New J. Chem. 2020, 44, 4440–4451. [Google Scholar] [CrossRef]
- Ghannadi, S.; Abdizadeh, H.; Miroliaei, M.; Saboury, A.A. Immobilization of alcohol dehydrogenase on titania nanoparticles to enhance enzyme stability and remove substrate inhibition in the reaction of formaldehyde to methanol. Ind. Eng. Chem. Res. 2019, 58, 9844–9854. [Google Scholar] [CrossRef]
- Defaei, M.; Taheri-Kafrani, A.; Miroliaei, M.; Yaghmaei, P. Improvement of stability and reusability of α-amylase immobilized on naringin functionalized magnetic nanoparticles: A robust nanobiocatalyst. Int. J. Biol. Macromol. 2018, 113, 354–360. [Google Scholar] [CrossRef]
- Fortes, C.C.; Daniel-da-Silva, A.L.; Xavier, A.M.; Tavares, A.P. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem. Eng. Process 2017, 117, 1–8. [Google Scholar] [CrossRef]
- Singh, V.; Rakshit, K.; Rathee, S.; Angmo, S.; Kaushal, S.; Garg, P.; Chung, J.H.; Sandhir, R.; Sangwan, R.S.; Singhal, N. Metallic/bimetallic magnetic nanoparticle functionalization for immobilization of α-amylase for enhanced reusability in bio-catalytic processes. Bioresour. Technol. 2016, 214, 528–533. [Google Scholar] [CrossRef]
- Motevalizadeh, S.F.; Khoobi, M.; Sadighi, A.; Khalilvand-Sedagheh, M.; Pazhouhandeh, M.; Ramazani, A.; Faramarzi, M.A.; Shafiee, A. Lipase immobilization onto polyethylenimine coated magnetic nanoparticles assisted by divalent metal chelated ions. J. Mol. Catal. B Enzym. 2015, 120, 75–83. [Google Scholar] [CrossRef]
- Meenakshi, M.; Bhaskar, R.; Kumar, S.A.; Kumar, R.S. A Concise Review on Magnetic Nanoparticles: Their Properties, Types, Synthetic Methods, and Current Trending Applications. Curr. Nanosci. 2025, 21, 2–17. [Google Scholar] [CrossRef]
- Oraby, K.R.; Villalonga, A.; Hassan, F.S.; Zayed, M.A.; Mubarak, M.F.; Ojeda, I.; Sánchez, A.; Villalonga, R. Immobilization of laccase on Fe3O4@ SiO2 core@ shell magnetic nanoparticles for methylene blue biodegradation. Process Biochem. 2025, 148, 10–16. [Google Scholar] [CrossRef]
- Seenuvasan, M.; Vinodhini, G.; Malar, C.G.; Balaji, N.; Kumar, K.S. Magnetic nanoparticles: A versatile carrier for enzymes in bio-processing sectors. IET Nanobiotechnol. 2018, 12, 535–548. [Google Scholar] [CrossRef]
- Kritika; Roy, I. Therapeutic applications of magnetic nanoparticles: Recent advances. Mater. Adv. 2022, 3, 7425–7444. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar Yadav, D.; Rani, P.; Bhardwaj, N.; Gupta, A.; Bishnoi, N.R. Functionalized iron oxide nanoparticles for covalent immobilization of cellic CTec2 cellulase: Enabling enzyme reusability in cellulosic biomass conversion. Biofuels 2024, 15, 363–373. [Google Scholar] [CrossRef]
- Behshad, Y.; Pazhang, M.; Najavand, S.; Sabzi, M. Enhancing enzyme stability and functionality: Covalent immobilization of trypsin on magnetic gum arabic modified Fe3O4 nanoparticles. Appl. Biochem. Biotechnol. 2024, 196, 5283–5300. [Google Scholar] [CrossRef]
- Jimenez-Carretero, M.; Jabalera, Y.; Sola-Leyva, A.; Carrasco-Jimenez, M.P.; Jimenez-Lopez, C. Nanoassemblies of acetylcholinesterase and β-lactamase immobilized on magnetic nanoparticles as biosensors to detect pollutants in water. Talanta 2023, 258, 124406. [Google Scholar] [CrossRef]
- Da Silva Cavalcanti, M.H.; Alves, L.B.; Duarte, A.; Mendes, A.A.; da Silva, J.M.S.F.; da Silveira, N.J.F.; Escote, M.T.; Virtuoso, L.S. Immobilization of Thermomyces lanuginosus lipase via ionic adsorption on superparamagnetic iron oxide nanoparticles: Facile synthesis and improved catalytic performance. Chem. Eng. J. 2022, 431, 134128. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Zhou, R.; Zeng, B.; Liu, X.; Li, X.; Zhang, G. Peptide-inspired one-step synthesis of surface-functionalized Fe3O4 magnetic nanoparticles for oriented enzyme immobilization and biocatalytic applications. ACS Appl. Nano Mater. 2022, 5, 8260–8270. [Google Scholar] [CrossRef]
- Cao, X.; Xu, H.; Li, F.; Zou, Y.; Ran, Y.; Ma, X.; Cao, Y.; Xu, Q.; Qiao, D.; Cao, Y. One-step direct transesterification of wet yeast for biodiesel production catalyzed by magnetic nanoparticle-immobilized lipase. Renew. Energy 2021, 171, 11–21. [Google Scholar] [CrossRef]
- Herman, R.A.; Zhu, X.; Ayepa, E.; Khurshid, M.; Zhang, Z.P.; You, S.; Qian, J.F.; Wang, J. Magnetic Janus SiO2 nanoparticles immobilized protease mutant T70I as a novel clarification agent for juice processing. Int. J. Biol. Macromol. 2025, 292, 139327. [Google Scholar] [CrossRef]
- Farhan, L.O.; Mehdi, W.A.; Taha, E.M.; Farhan, A.M.; Mehde, A.A.; Özacar, M. Various type immobilizations of Isocitrate dehydrogenases enzyme on hyaluronic acid modified magnetic nanoparticles as stable biocatalysts. Int. J. Biol. Macromol. 2021, 182, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Han, J.; Wu, J.; Li, Y.; Wang, L.; Mao, Y.; Wang, Y. A two-step method for the synthesis of magnetic immobilized cellulase with outstanding thermal stability and reusability. New J. Chem. 2021, 45, 6144–6150. [Google Scholar] [CrossRef]
- Coutinho, T.C.; Malafatti, J.O.; Paris, E.C.; Tardioli, P.W.; Farinas, C.S. Hydroxyapatite-CoFe2O4 magnetic nanoparticle composites for industrial enzyme immobilization, use, and recovery. ACS Appl. Nano Mater. 2020, 3, 12334–12345. [Google Scholar] [CrossRef]
- Suo, H.; Xu, L.; Xue, Y.; Qiu, X.; Huang, H.; Hu, Y. Ionic liquids-modified cellulose coated magnetic nanoparticles for enzyme immobilization: Improvement of catalytic performance. Carbohydr. Polym. 2020, 234, 115914. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F. Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. J. Environ. Chem. Eng. 2019, 7, 102805. [Google Scholar] [CrossRef]
- Wang, X.Y.; Jiang, X.P.; Li, Y.; Zeng, S.; Zhang, Y.W. Preparation Fe3O4@ chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biol. Macromol. 2015, 75, 44–50. [Google Scholar] [CrossRef]
- Osuna, Y.; Sandoval, J.; Saade, H.; López, R.G.; Martinez, J.L.; Colunga, E.M.; de la Cruz, G.; Segura, E.P.; Arévalo, F.J.; Zon, M.A.; et al. Immobilization of Aspergillus niger lipase on chitosan-coated magnetic nanoparticles using two covalent-binding methods. Bioprocess. Biosyst. Eng. 2015, 38, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Rathod, V.K. Combined effect of enzyme co-immobilized magnetic nanoparticles (MNPs) and ultrasound for effective extraction and purification of curcuminoids from Curcuma longa. Ind. Crop. Prod. 2022, 177, 114385. [Google Scholar] [CrossRef]
- Liao, J.; Han, S.; Li, X.; He, J.; Secundo, F.; Liang, H. Co-immobilization of two-component hydroxylase monooxygenase by functionalized magnetic nanoparticles for preserving high catalytic activity and enhancing enzyme stability. Int. J. Biol. Macromol. 2020, 164, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, N.; Shahedi, M.; Habibi, Z.; Yousefi, M.; Ghasemi, S.; Mohammadi, M. A multi-component approach for co-immobilization of lipases on silica-coated magnetic nanoparticles: Improving biodiesel production from waste cooking oil. Bioprocess. Biosyst. Eng. 2022, 45, 2043–2060. [Google Scholar] [CrossRef]
- Gao, X.; Pan, H.; Wei, Y.; Ye, M.; Qiao, C.; Wang, J.; Liu, Q.; Zhou, C. A novel bienzymatic bioreactor based on magnetic hierarchical porous MOF for improved catalytic activity and stability: Kinetic analysis, thermodynamics properties and biocatalyst applications. Biochem. Eng. J. 2023, 197, 108995. [Google Scholar] [CrossRef]
- Giannakopoulou, A.; Patila, M.; Spyrou, K.; Chalmpes, N.; Zarafeta, D.; Skretas, G.; Gournis, D.; Stamatis, H. Development of a four-enzyme magnetic nanobiocatalyst for multi-step cascade reactions. Catalysts 2019, 9, 995. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, S.; Miao, S.; Suo, H.; Xu, H.; Hu, Y. Co-immobilization of laccase and ABTS onto amino-functionalized ionic liquid-modified magnetic chitosan nanoparticles for pollutants removal. J. Hazard. Mater. 2021, 401, 123353. [Google Scholar] [CrossRef]
- Kaur, G.; Taggar, M.S.; Kalia, A. Cellulase-immobilized chitosan-coated magnetic nanoparticles for saccharification of lignocellulosic biomass. Environ. Sci. Pollut. Res. Int. 2023, 30, 111627–111647. [Google Scholar] [CrossRef]
- Bushra, R.; Ahmad, M.; Alam, K.; Seidi, F.; Shakeel, S.; Song, J.; Jin, Y.; Xiao, H. Recent advances in magnetic nanoparticles: Key applications, environmental insights, and future strategies. Sustain. Mater. Technol. 2024, 40, e00985. [Google Scholar] [CrossRef]
- Araújo, E.V.; Carneiro, S.V.; Neto, D.M.A.; Freire, T.M.; Costa, V.M.; Freire, R.M.; Fechine, L.M.U.D.; Clemente, C.S.; Denardin, J.C.; Dos Santos, J.C.S.; et al. Advances in surface design and biomedical applications of magnetic nanoparticles. Adv. Colloid. Interface Sci. 2024, 328, 103166. [Google Scholar] [CrossRef]
- Seghiri, R.; Haounati, R.; Rbaa, M. Multifunctional magnetic nanoparticles in biocatalysis. In Multifunctional Magnetic Nanoparticles in Therapy, Biology, and Pharmacy; CRC Press: Boca Raton, FL, USA, 2025; pp. 171–198. [Google Scholar]
- Chauhan, P.; Kumar, D.; Sharma, R. Biogenic Magnetic Iron Oxide Nanoparticles for Multifunctional Applications in Environmental Remediation and Biomedical Field. J. Environ. Chem. Eng. 2025, 13, 115646. [Google Scholar] [CrossRef]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T. Immobilization of enzymes by polymeric materials. Catalysts 2021, 11, 1211. [Google Scholar] [CrossRef]
- Rodriguez-Abetxuko, A.; Sánchez-deAlcázar, D.; Muñumer, P.; Beloqui, A. Tunable polymeric scaffolds for enzyme immobilization. Front. Bioeng. Biotechnol. 2020, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Suthiwangcharoen, N.; Li, T.; Wu, L.; Reno, H.B.; Thompson, P.; Wang, Q. Facile Co-Assembly Process to Generate Core–Shell Nanoparticles with Functional Protein Corona. Biomacromolecules 2014, 15, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Pinto, M.C.C.; Lorente, G.F.; Manoel, E.A.; Guisán, J.M.; Ninow, J.L.; de Oliveira, D.; Pessela, B.C. Production of new nanobiocatalysts via immobilization of lipase B from C. antarctica on polyurethane nanosupports for application on food and pharmaceutical industries. Int. J. Biol. Macromol. 2020, 165, 2957–2963. [Google Scholar] [CrossRef]
- Lee, G.; Kim, J.; Lee, J.H. Development of magnetically separable polyaniline nanofibers for enzyme immobilization and recovery. Enzym. Microb. Technol. 2008, 42, 466–472. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; Alkabli, J.; El-Shishtawy, R.M. Improving enzyme immobilization: A new carrier-based magnetic polymer for enhanced covalent binding of laccase enzyme. Int. J. Biol. Macromol. 2024, 282, 137362. [Google Scholar] [CrossRef]
- Chen, T.; Peng, Y.; Qiu, M.; Yi, C.; Xu, Z. Heterogenization of homogeneous catalysts in polymer nanoparticles: From easier recovery and reuse to more efficient catalysis. Coord. Chem. Rev. 2023, 489, 215195. [Google Scholar] [CrossRef]
- Khan, R.S.; Rather, A.H.; Wani, T.U.; Rather, S.U.; Amna, T.; Hassan, M.S.; Sheikh, F.A. Recent trends using natural polymeric nanofibers as supports for enzyme immobilization and catalysis. Biotechnol. Bioeng. 2023, 120, 22–40. [Google Scholar] [CrossRef]
- Çalbaş, B.; Keobounnam, A.N.; Korban, C.; Doratan, A.J.; Jean, T.; Sharma, A.Y.; Wright, T.A. Protein–polymer bioconjugation, immobilization, and encapsulation: A comparative review towards applicability, functionality, activity, and stability. Biomater. Sci. 2024, 12, 2841–2864. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, X.; Zhao, J.; Zhai, X.; Xia, W.; Li, P.; Lai, K.; Wu, L. Preparation and Application of Enzyme-based Hydrogels. Biosens. Bioelectron. X 2025, 23, 100594. [Google Scholar] [CrossRef]
- Atiroğlu, V.; Atiroğlu, A.; Atiroğlu, A.; Al-Hajri, A.S.; Özacar, M. Green immobilization: Enhancing enzyme stability and reusability on eco-friendly support. Food Chem. 2024, 448, 138978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, W.; Liu, Y.; Zhang, K.; Chen, Y.; Yin, X. Laccase immobilized on chitosan-coated Fe3O4 nanoparticles as reusable biocatalyst for degradation of chlorophenol. J. Mol. Struct. 2020, 1220, 128769. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ikram, S. Zinc oxide nanoparticles-impregnated chitosan surfaces for covalent immobilization of trypsin: Stability & kinetic studies. Int. J. Biol. Macromol. 2022, 207, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.F.; Lo, H.F.; Chi, M.C.; Lai, K.L.; Lin, M.G.; Lin, L.L. Affinity immobilization of a bacterial prolidase onto metal-ion-chelated magnetic nanoparticles for the hydrolysis of organophosphorus compounds. Int. J. Mol. Sci. 2019, 20, 3625. [Google Scholar] [CrossRef]
- Bindu, V.U.; Shanty, A.A.; Mohanan, P.V. Parameters affecting the improvement of properties and stabilities of immobilized α-amylase on chitosan-metal oxide composites. Int. J. Biochem. Biophys. 2018, 6, 44–57. [Google Scholar] [CrossRef]
- Shukla, A.; Gundampati, R.K.; Jagannadham, M.V. Immobilization of Euphorbia tirucalli peroxidase onto chitosan-cobalt oxide magnetic nanoparticles and optimization using response surface methodology. Int. J. Biol. Macromol. 2017, 102, 384–395. [Google Scholar] [CrossRef]
- Amirbandeh, M.; Taheri-Kafrani, A.; Soozanipour, A.; Gaillard, C. Triazine-functionalized chitosan-encapsulated superparamagnetic nanoparticles as reusable and robust nanocarrier for glucoamylase immobilization. Biochem. Eng. J. 2017, 127, 119–127. [Google Scholar] [CrossRef]
- Thakur, K.; Attri, C.; Seth, A. Nanocarriers-based immobilization of enzymes for industrial application. 3 Biotech 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Hooe, S.L.; Breger, J.C.; Medintz, I.L. Enhancing enzymatic activity with nanoparticle display–an updated compendium and engineering outlook. Mol. Syst. Des. Eng. 2024, 9, 679–704. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on metal–organic framework classification, synthetic approaches, and influencing factors: Applications in energy, drug delivery, and wastewater treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef] [PubMed]
- Bisht, M.; Thayallath, S.K.; Bharadwaj, P.; Franklin, G.; Mondal, D. Biomass-derived functional materials as carriers for enzymes: Towards sustainable and robust biocatalysts. Green. Chem. 2023, 25, 4591–4624. [Google Scholar] [CrossRef]
- Ahmadi, S.; Pourebrahimi, S.; Malloum, A.; Pirooz, M.; Osagie, C.; Ghosh, S.; Zafar, M.N.; Dehghani, M.H. Hydrogel-based materials as super adsorbents and antibacterial agents for the remediation of emerging pollutants: A comprehensive review. Emerg. Contam. 2024, 10, 100336. [Google Scholar] [CrossRef]
- Qamar, S.A.; Junaid, M.; Riasat, A.; Jahangeer, M.; Bilal, M.; Mu, B.Z. Carrageenan-based Hybrids with Biopolymers and Nano-structured Materials for Biomimetic Applications. Starch-Stärke 2024, 76, 2200018. [Google Scholar] [CrossRef]
- Fan, Y.; Su, F.; Li, K.; Ke, C.; Yan, Y. Carbon nanotube filled with magnetic iron oxide and modified with polyamidoamine dendrimers for immobilizing lipase toward application in biodiesel production. Sci. Rep. 2017, 7, 45643. [Google Scholar] [CrossRef]
- Park, J.S.; Yang, S.Y.; Lee, J.K.; Kang, Y.C. A novel strategy for encapsulating metal sulfide nanoparticles inside hollow carbon nanosphere-aggregated microspheres for efficient potassium ion storage. J. Mater. Chem. A 2022, 10, 17790–17800. [Google Scholar] [CrossRef]
- Kim, S.J.; Hong, J.H.; Lee, J.K.; Kang, Y.C. Macroporous microspheres consisting of thickness-controlled bamboo-like CNTs and flower-like Co3O4 nanoparticles as highly efficient bifunctional oxygen electrocatalysts for Zn–air batteries. J. Mater. Chem. A 2021, 9, 25160–25167. [Google Scholar] [CrossRef]
- Hashempour, Y.; Mortezazadeh, F.; Rezaei, S.; Salehipour, M.; Gholami-Borujeni, F.; Ebrahimnejad, P.; Mogharabi-Manzari, M. Co-immobilization of laccase and zinc oxide nanoparticles onto bacterial cellulose to achieve synergistic effect of photo and enzymatic catalysis for biodegradation of favipiravir. Int. J. Biol. Macromol. 2025, 292, 139288. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; Xue, Z.; Li, H.; Shen, S. Wearable biosensor with high specific surface area PGA-CNTs electrode for sweat glucose detection. Microchem. J. 2025, 210, 112965. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, Y.J.; Chang, J.H. Comparative Study of Enzymatic Lipolysis Using Nanofructosome-Coated CalB Lipase Encapsulated in Silica and Immobilized on Silica-Coated Magnetic Nanoparticles. ACS Omega 2025, 10, 13319–13326. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Chen, C.L.; Chang, J.S. Immobilization of Burkholderia sp. lipase on a ferric silica nanocomposite for biodiesel production. J. Biotechnol. 2012, 158, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Zhao, C.; Ding, Y.; Xu, P. Biodiesel production in packed-bed reactors using lipase–nanoparticle biocomposite. Bioresour. Technol. 2011, 102, 6352–6355. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, K.; He, Y.; Wang, Y.; Han, X.; Yan, Y. Enhanced performance of lipase immobilized onto Co2+ -chelated magnetic nanoparticles and its application in biodiesel production. Fuel 2019, 255, 115794. [Google Scholar] [CrossRef]
- Hübner, J.; Brakowski, R.; Wohlgemuth, J.; Brenner-Weiß, G.; Franzreb, M. Compartmented microfluidic bioreactor system using magnetic enzyme immobilisates for fast small-scale biotransformation studies. Eng. Life Sci. 2015, 15, 721–726. [Google Scholar] [CrossRef]
- Bartha-Vári, J.H.; Moisă, M.E.; Bencze, L.C.; Irimie, F.D.; Paizs, C.; Toșa, M.I. Efficient biodiesel production catalyzed by nanobioconjugate of lipase from Pseudomonas fluorescens. Molecules 2020, 25, 651. [Google Scholar] [CrossRef]
- Neupane, S.; Patnode, K.; Li, H.; Baryeh, K.; Liu, G.; Hu, J.; Chen, B.; Pan, Y.; Yang, Z. Enhancing enzyme immobilization on carbon nanotubes via metal–organic frameworks for large-substrate biocatalysis. ACS Appl. Mater. Interfaces 2019, 11, 12133–12141. [Google Scholar] [CrossRef]
- Kujawa, J.; Głodek, M.; Li, G.; Al-Gharabli, S.; Knozowska, K.; Kujawski, W. Highly effective enzymes immobilization on ceramics: Requirements for supports and enzymes. Sci. Total Environ. 2021, 801, 149647. [Google Scholar] [CrossRef]
- Guisan, J.M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Moreno-Gamero, D. Enzyme immobilization strategies for the design of robust and efficient biocatalysts. Curr. Opin. Green. Sustain. Chem. 2022, 35, 100593. [Google Scholar] [CrossRef]
- Kotwal, N.; Pathania, D.; Singh, A.; Sheikh, Z.U.D.; Kothari, R. Enzyme immobilization with nanomaterials for hydrolysis of lignocellulosic biomass: Challenges and future Perspectives. Carbohydr. Res. 2024, 543, 109208. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Lock, S.S.M.; Yap, P.S.; Cheah, K.W.; Chan, Y.H.; Yiin, C.L.; Ku, A.Z.E.; Loy, A.C.M.; Chin, B.L.F.; Chai, Y.H. Immobilized enzyme/microorganism complexes for degradation of microplastics: A review of recent advances, feasibility and future prospects. Sci. Total Environ. 2022, 832, 154868. [Google Scholar] [CrossRef]
- Khafaga, D.S.; Muteeb, G.; Elgarawany, A.; Aatif, M.; Farhan, M.; Allam, S.; Almatar, B.A.; Radwan, M.G. Green nanobiocatalysts: Enhancing enzyme immobilization for industrial and biomedical applications. PeerJ 2024, 12, e17589. [Google Scholar] [CrossRef]
- Cervantes, F.J.; Ramírez-Montoya, L.A. Immobilized nanomaterials for environmental applications. Molecules 2022, 27, 6659. [Google Scholar] [CrossRef]
| Advantage | Disadvantage | Solution |
|---|---|---|
| High surface area and loading capacity Nanomaterials, such as CNTs, MNPs, and graphene, have a large surface area-to-volume ratio, increasing enzyme loading and catalytic efficiency [3,5,6]. | High cost of nanomaterials Fabrication and functionalization of nanomaterials are expensive; large-scale applications are economically challenging [5,7,8]. | Adopt low-cost fabrication techniques such as plasma reactors and sol–gel methods. |
| Enhanced stability Immobilizing nanomaterials increases enzyme stability against denaturation caused by environmental factors such as temperature, pH, and solvents, which are suitable for industrial applications [3,6,9]. | Diffusion limitations Diffusion of substrates and products can be restricted within the immobilization matrix at high enzyme loading densities, reducing apparent activity [5,7,9]. | Use of porous supports or optimize matrix design to increase diffusion. |
| Reusability and cost-effectiveness Enzymes immobilized on nanomaterials can be easily separated from reaction mixtures (e.g., MNPs can be recovered using external magnetic fields) and reused for multiple cycles, reducing operational costs [3,6,10]. | Enzyme leaching and instability Noncovalent immobilization methods may result in enzyme leaching over time, reducing catalytic performance and contaminating the reaction medium [7,9]. | Use covalent binding or crosslinking to enhance enzyme immobilization stability. |
| Increase catalytic activity Nanoscale interactions between enzymes and nanomaterials enhance catalytic activity due to orientation and stabilization of the enzyme’s active site [3,5]. | Complex preparation Immobilization procedures often require specialized techniques and equipment, increasing process complexity compared with traditional methods [7,8]. | Develop simplified protocols or use prefunctionalized, ready-to-use supports. |
| Versatility in applications Enzymes immobilized on nanomaterials are used in various fields such as biosensors, biofuel production, drug delivery, and industrial biocatalysis due to their functionality [3,6]. | Scalability issues and environmental sensitivity Scalability of enzyme immobilization on nanomaterials is limited due to challenges with reproducibility and uniformity during large-scale production [8,11]. Some nanomaterials are prone to degrading or aggregating under specific conditions (e.g., MNPs in acidic or oxidative environments), which can compromise enzyme stability [3,7]. | Optimization of processes and development of novel stable, environmentally resistant nanomaterials for immobilization. |
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Adsorption | Simple and cost-effective method. Minimal chemical modification of enzyme. Rapid immobilization process. | Adsorption immobilization relies on weak binding forces (van der Waals, ionic, and hydrogen bonds), making enzymes prone to desorption under shifts in pH, ionic strength, or temperature, resulting in low stability and activity loss. | [20,21] |
| Covalent Binding | Strong and stable linkage prevents enzyme leakage. High thermal and operational stability. Reusable for multiple cycles. | Covalent binding of enzymes presents significant drawbacks, primarily the risk of enzyme denaturation due to chemical modifications during immobilization. This method involves a complex process requiring expensive reagents and supports, increasing operational costs. Additionally, conformational changes induced by binding often lead to partial or complete loss of enzyme activity, reducing catalytic efficiency. | [8,14,22,23,25]. |
| Entrapment | Protects enzymes from environmental changes (e.g., pH and temperature). Suitable for thermally and mechanically stable enzymes. | Diffusion limitations restrict substrate access to enzymes, reducing reaction rates. Recovering enzymes is challenging as they are trapped within the matrix, complicating reuse and process efficiency. | [14] |
| Encapsulation | High reproducibility and protection from shear forces. | Encapsulation includes weak binding forces that can cause enzyme leakage, particularly under certain conditions such as high ionic strength. Enzyme inactivation due to mechanical stress during the encapsulation process is also a risk. Additionally, swelling of the capsules can lead to further leaching of the encapsulated enzymes. Low mechanical strength and large pore size in some encapsulation materials can also contribute to high enzyme leakage. | [15,26] |
| Crosslinking | Carrier-free immobilization with high enzyme density. High stability under industrial conditions. Easy recycling and reuse. | Disadvantages of crosslinking include possible overcrowding of enzymes, which can reduce their activity. The process is irreversible, meaning that once enzyme activity is lost, it cannot be recovered. Additionally, crosslinking often requires highly pure, crystallized enzymes, making it an expensive technique. The chemicals used can also result in enzyme denaturation, further inhibiting activity. | [23,27] |
| Nanomaterial | Skeleton Matrix | Functionalization Groups | Enzyme | Enzyme Source | Reusability (Retained Activity) | Reference |
|---|---|---|---|---|---|---|
| CNT and derivatives | Multiwalled CNT (mwCNT) | HNO3/H2SO4 | Laccase | Trametes versicolor | 10 cycles (90%) | [49] |
| mwCNT | EDC/NHS crosslinkers | Lipase | Pseudomonas fluorescens | 10 cycles (49.2%) | [50] | |
| CNT | N-aminoethyl-γ-aminopropyl trimethoxy | β-glucosidase | Terrabacter ginsenosidimutans | 14 cycles (76%) | [51] | |
| mwCNT-NiO | Glutaraldehyde, APTES | Xylanase | Thermomyces lanuginosus | 6 cycles (80%) | [52] | |
| mwCNT | HNO3/H2SO4 | Laccase | Trametes versicolor | 10 cycles (90%) | [49] | |
| Fe3O4 mwCNT | EDC/NHS | Lipase | Candida rugosa | 10 cycles (91%) | [53] | |
| Magnetic nickel oxide mwCNT | 3-aminopropyltriethoxysilane | L-asparaginase | Geobacillus kaustophilus | 10 cycles (90%) | [54] | |
| ZIF-8@MWCNT | HNO3/H2SO4 | Laccases | - | 10 cycles (68%) | [55] | |
| GO-CNT | Glutaraldehyde | Laccase | Trametes versicolor | 11 cycles (50%) | [56] | |
| Superparamagnetic mwCNT | Polyethylene glycol amine polymer | Lipases | - | 10 cycles (78.5%) | [48] | |
| CNT | Glutaraldehyde/APTES | Lipase | Rhizomucor miehei | 10 cycles (90%) | [57] | |
| CNT | Cinnamaldehyde ethanol solution | Porcine pancreatic lipase | Porcine pancreatic lipase | 7 cycles (69%) | [58] | |
| Polyaniline cobalt CNT | Glutaraldehyde | β-galactosidase | Aspergillus oryzae | 10 cycles (74%) | [59] | |
| CNT | Glutaraldehyde | Laccase | Novo Nordisk Company, Bagsværd, Denmark | 10 cycles (69%) | [60] | |
| mwCNT-MoS2 NC | Glutaraldehyde crosslinker | β-galactosidase | Lens culinaris | 21 cycles (>50%) | [61] | |
| mwCNT | Glutaraldehyde | Cyanate hydratase | Pichia pastoris | 10 cycles (>94%) | [62] | |
| Mesoporous SiO2 microparticle | Glutaraldehyde | Lipase | Thermomyces lanuginosus | 12 cycles (95.4%) | [63] | |
| mwCNT | Aminated polydopamine | Lipase | Candida rugosa | 10 cycles (84%) | [64] | |
| mwCNT | Sodium alginate | Cellulase | Trichoderma | 7 cycles (70%) | [65] | |
| mwCNT | - | Lipase | Candida antarctica | 7 cycles (95%) | [66] | |
| mwCNT | Carbodiimide coupling | Cellulase | Aspergillus niger | 10 cycles (85%) | [67] | |
| swCNT | N,N′-carbonyldiimidazole, CH2Cl2 | Lipase B | Candida antarctica | 10 cycles (>90%) | [68] | |
| Magnetic-mwCNT | Polyamidoamine | Lipase | Rhizomucor miehei | 10 cycles (94%) | [69] |
| Nanomaterial | Skeleton Matrix | Functionalization Groups | Enzyme | Enzyme Source | Reusability (Retained Activity) | Reference |
|---|---|---|---|---|---|---|
| Graphene and derivatives | Graphene oxide (GO) Fe3O4@SiO2 | Glutaraldehyde | Phospholipase D | Streptomyces chromofuscus | 10 cycles (78.3%) | [81] |
| Graphene oxide (GO-Asp-Fe3O4) | EDC/NHS | L-asparaginase | - | 8 cycles (80%) | [82] | |
| Magnetic GO | 3-Mercaptopropyl trimethoxysilane | Lipase | Candida rugosa | 8 cycles 76.5% | [83] | |
| Magnetic GO | Glutaraldehyde | Lipase | Candida rugosa | 10 cycles (50%) | [84] | |
| Magnetic GO | γ-Ureapropyltrimethoxy silane, APTES, γ-mercapto propyltriethoxysilane | Lipase | Aspergillus oryzae | 8 cycles (80.2%) | [85] | |
| Activated GO/chitosan/cellulose | Glutaraldehyde | Lipase | Bacillus licheniformis (Lipase Km12) | 10 cycles (80%) | [86] | |
| Magnetic GO | Carbodiimide | Cellulase/sylanase | - | 10 cycles (70%) | [87] | |
| Magnetic GO | Glutaraldehyde, APTES | PersiManXyn1 | - | 15 cycles (94%) | [88] | |
| Magnetic GO | Polyethylenimine | Lactase | Escherichia coli, Sangon Biotech (Shanghai, China) | 20 cycles (83.1%) | [89] | |
| Magnetic GO | Polyethylene glycol (PEG) | Horseradish peroxidase | - | 8 cycles (68.1%) | [90] | |
| Magnetic GO | Glutaraldehyde | Dextranase | - | 20 cycles (85.7%) | [91] | |
| GO | Glutaraldehyde | Horseradish Peroxidase | - | 12 cycles (90%) | [70] | |
| GO | Horseradish peroxidase and oxalate oxidase | Formate dehydrogenase | Candida boidinii | 8 cycles (63.8%) | [92] | |
| Reduced GO | Glutaraldehyde | Horseradish peroxidase | - | 10 cycles (70%) | [93] | |
| Magnetic GO | Nα, Nα-Bis(carboxymethyl)-l-lysine hydrate | Laccase | - | 10 cycles (89.4%) | [94] | |
| GO | Amine group | Horseradish peroxidase | - | 10 cycles (60%) | [95] | |
| GO | - | Lipase | Candida rugosa | 10 cycles (85%) | [96] | |
| Reduced GO | - | Laccase, horseradish peroxidase | Trametes versicolor | 10 cycles (92.6%) | [97] | |
| Graphene | - | Naringinase | 10 cycles (85.4%) | [98] | ||
| Polyaniline–silver functionalized GO nanocomposites | Glutaraldehyde | Lipase | Aspergillus niger | 11 cycles (86%) | [99] | |
| Magnetic GOx | EDC/NHS | Laccase | Trametes versicolor | 11 cycles (59.8%) | [100] | |
| GO(GO/ZnO) | - | Lipase | Candida rugosa | 14 cycles (90%) | [101] | |
| GO | EDAC, N-hydroxysulfosuccinimide sodium salt | Chloroperoxidase | Caldariomyces fumago | 8 cycles (52%) | [102] | |
| GO | Epoxy chloropropane | γ-Lactamase | Escherichia coli BL21(DE3) | 15 cycles (70%) | [103] | |
| GO nanosheets Superparamagnetic iron oxide nanoparticles | Cyanuric chloride | Xylanase | Thermomyces lanuginosus | 10 cycles (70%) | [104] | |
| Graphene–iron oxide nanocomposites (Gr@Fe3O4 NCs) | - | β-galactosidase | Aspergillus oryzae | 8 cycles (83%) | [105] | |
| Graphene sheets | Cysteamine and glutaraldehyde | α-Amylase | Triticum aestivum | 10 cycles (73%) | [106] | |
| Exfoliated graphene oxide (EGO) | Silane | Lipase | Candida rugosa | 30 cycles (50%) | [107] | |
| GO-based magnetic | 3-Chloropropyltriethoxysilane | Lipase | - | 10 cycles (87%) | [108] |
| Nanomaterial | Skeleton Matrix | Functionalization Groups | Enzyme | Enzyme Source | Reusability (Retained Activity) | Reference |
|---|---|---|---|---|---|---|
| Metal and metal oxide nanoparticles | Magnetic laccase nanoflowers | Glutaraldehyde | Laccase | Trametes versicolor | 18 times (90%) | [121] |
| Metal–organic frameworks | Glutaraldehyde | α-Amylase | Bacillus subtilis | 20 cycles (81%) | [122] | |
| Magnetic nanoparticles | Glutaraldehyde | α-Amylase and xylanase | Bacillus subtilis and Aspergillus niger | 15 cycles (82%) 11 cycles (64%) | [123] | |
| Fe3O4/SBA-15 | Tannic acid | L-asparaginase | Escherichia coli | 16 times (70%) | [124] | |
| Titania nanoparticles | Glutaraldehyde | Alcohol dehydrogenase | Saccharomyces cerevisiae | 10 times (84%) | [125] | |
| - | Naringin | α-Amylase | Bacillus subtilis | 10 times (60%) | [126] | |
| FeCl3 | APTES, Glutaraldehyde | Laccase | Aspergillus oryzae | 6 cycles (75%) | [127] | |
| Magnetic nanoparticles | 3-phosphono propionic acid | α-Amylase | Aspergillus oryzae | 10 cycles (60%) | [128] | |
| Fe3O4 magnetic nanoparticles | Polyethylenimine | Lipase | Thermomyces lanuginosa | 10 cycles (60%) | [129] |
| Nanomaterial | Skeleton Matrix | Functionalization Groups | Enzyme | Enzyme Source | Reusability (Retained Activity) | Reference |
|---|---|---|---|---|---|---|
| Magnetic nanoparticles (MNPs) | Silica-coated amine functionalized iron oxide nanoparticle (IONP@SiO2-NH2) | Glutaraldehyde | Cellulase | - | 6 cycles (80%) | [134] |
| Fe3O4, Affi-Cova beads | IDA | r-BirA | 10 cycles (76.1%) | [37] | ||
| Fe3O4, SiO2 core@shell | 3-(Triethoxysilyl) propyl isocyanate | Laccase | Trametes versicolor | 13 cycles (88%) | [131] | |
| - | Glutaraldehyde | Trypsin | - | 15 cycles (93%) | [135] | |
| Fe3O4 | N-hydroxysuccinimide, ethyl-3-(3-dimethylaminopropyl) carbodiimide | β-Lactamase | Bacillus cereus | 12 cycles (BC: 57%, IC: 65%) | [136] | |
| Fe3O4 | N-acetyl and N-methylamide | Lipase | Thermomyces lanuginosus | 12 cycles (80%) | [137] | |
| Fe3O4 | SpyCatcher-fused elastin-like polypeptides | Xylanase Lichenase | - | 10 cycles (66.3 and 72.7%) | [138] | |
| Fe3O4, SiO2-CHO | Tetraethyl orthosilicate, APTES, and glutaraldehyde | Lipase | Saitozyma podzolica | 10 cycles (90%) | [139] | |
| Fe2O3, Fe3O4 | APTES | Laccase | 10 cycles (82.9%) | [140] | ||
| Fe3O4 | Hyaluronic acid | Isocitrate dehydrogenases | - | DH/HA/MNPs, IDH/HA/MNPs-CLEAs and IDH/BSA/HA/MNPs-CLEAs 15 cycles (76%, 80%, 85%) | [141] | |
| Super paramagnetic iron oxide nanoparticles | APTES | Laccase | Thermomyces lanuginosus | 12 cycles (80%) | [137] | |
| Fe3O4@C@cellulase-SiO2 | - | Cellulase | - | 9 cycles (80%) | [142] | |
| Fe3O4 | Hydroxyapatite in cobalt ferrite (CoFe2O4) | β-glucosides | - | 10 cycle (70%) | [143] | |
| Fe3O4, carboxymethyl cellulose | PPL-MCMC PPL-IL-MCMC | Cellulose | - | 10 cycles (83.9%, 86.1%) | [144] | |
| Fe3O4, chitosan | Glutaraldehyde | Peroxidase | Pseudomonas aeruginosa | 100 cycles (95%) | [145] | |
| His6-EcPepQ@NiNTASiMNPs | Ni2+ | Lipase | Thermomyces lanuginosus | 20 cycles (80%) | [146] | |
| FeCl2, FeCl3 | Glutaraldehyde, glycidol | Chitosan | Aspergillus niger | 15 cycles (80%) | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.V.; Singh, B.; Kumar, A.; Sambyal, K.; Karuppanan, K.K.; Lee, J.-K. Enzyme Immobilization on Nanomaterials and Their Applications. Materials 2025, 18, 4106. https://doi.org/10.3390/ma18174106
Singh RV, Singh B, Kumar A, Sambyal K, Karuppanan KK, Lee J-K. Enzyme Immobilization on Nanomaterials and Their Applications. Materials. 2025; 18(17):4106. https://doi.org/10.3390/ma18174106
Chicago/Turabian StyleSingh, Rahul Vikram, Bakul Singh, Anurag Kumar, Krishika Sambyal, Karthikeyan Kugalur Karuppanan, and Jung-Kul Lee. 2025. "Enzyme Immobilization on Nanomaterials and Their Applications" Materials 18, no. 17: 4106. https://doi.org/10.3390/ma18174106
APA StyleSingh, R. V., Singh, B., Kumar, A., Sambyal, K., Karuppanan, K. K., & Lee, J.-K. (2025). Enzyme Immobilization on Nanomaterials and Their Applications. Materials, 18(17), 4106. https://doi.org/10.3390/ma18174106








