Enhanced Fire Retardancy of Epoxy Resins upon Addition of Boron Nitride Nanoparticles Using Boron Polyol Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
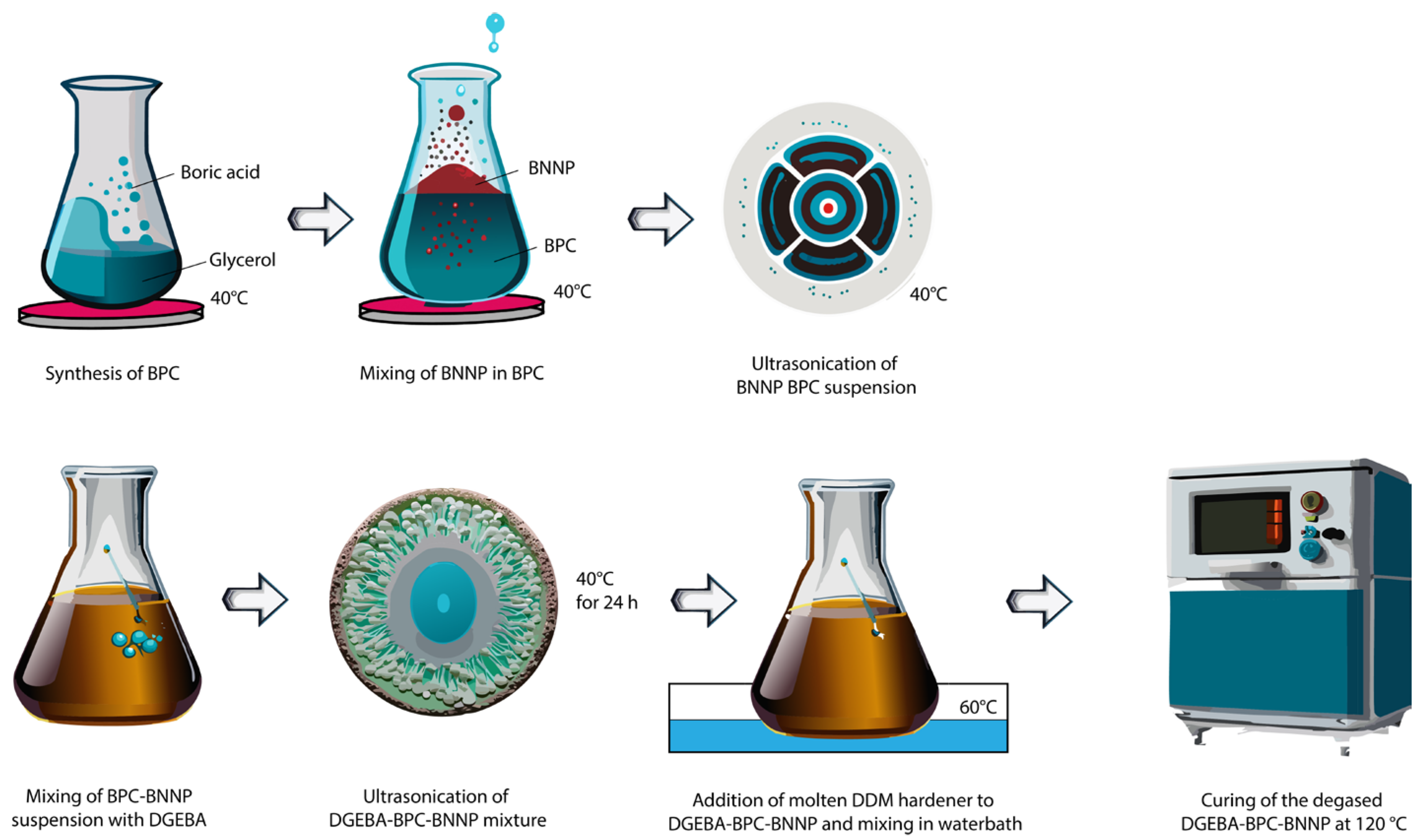
2.2. Synthesis of BPC Complex and Preparation of BNNP–BPC Colloid
2.3. Preparation of EP–BPC–BNNP Blend and Composite
2.4. Characterisations
3. Results and Discussion
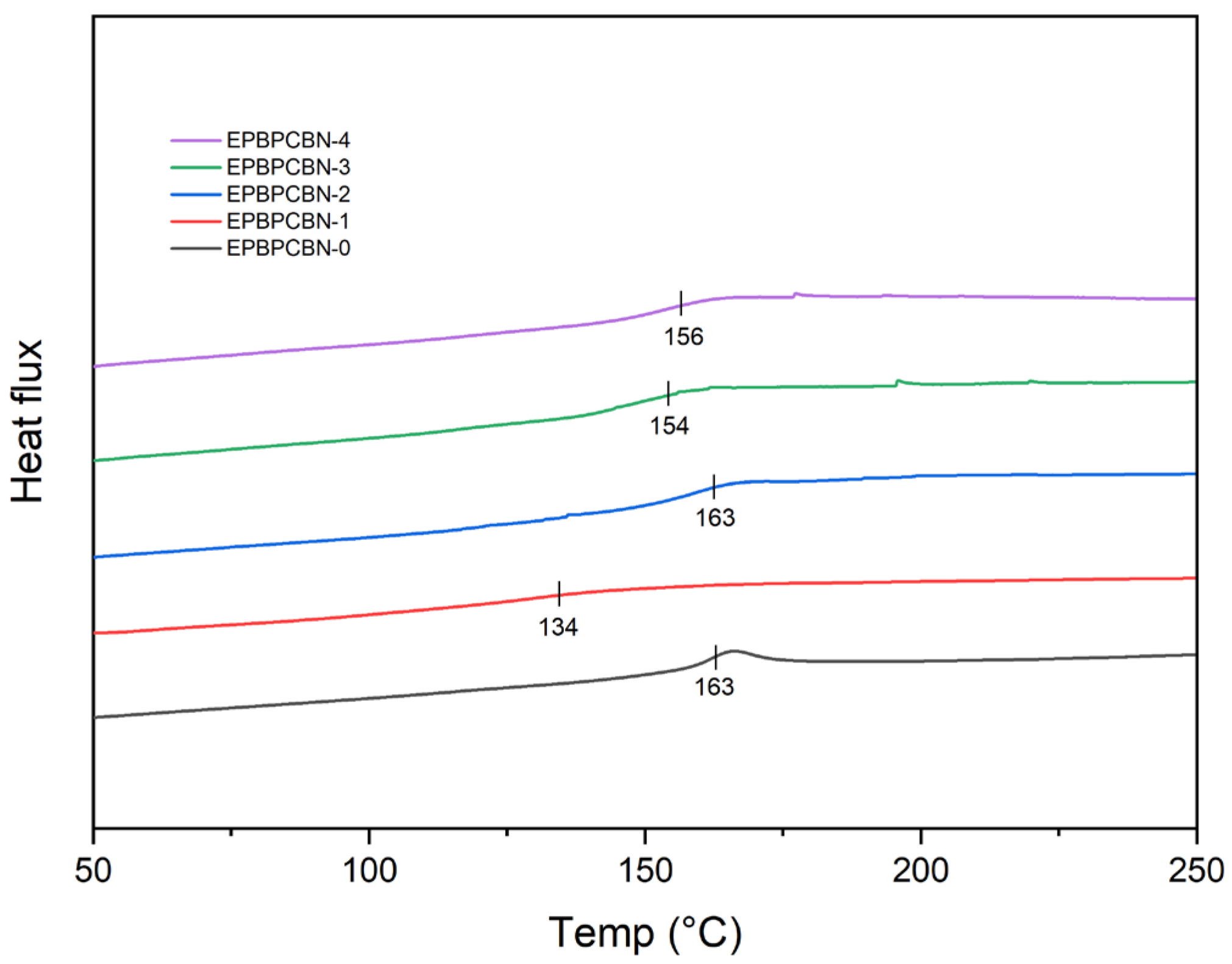
3.1. Phase Behaviour Studies of Neat Epoxy, Fire-Retardant Blend, and Composite
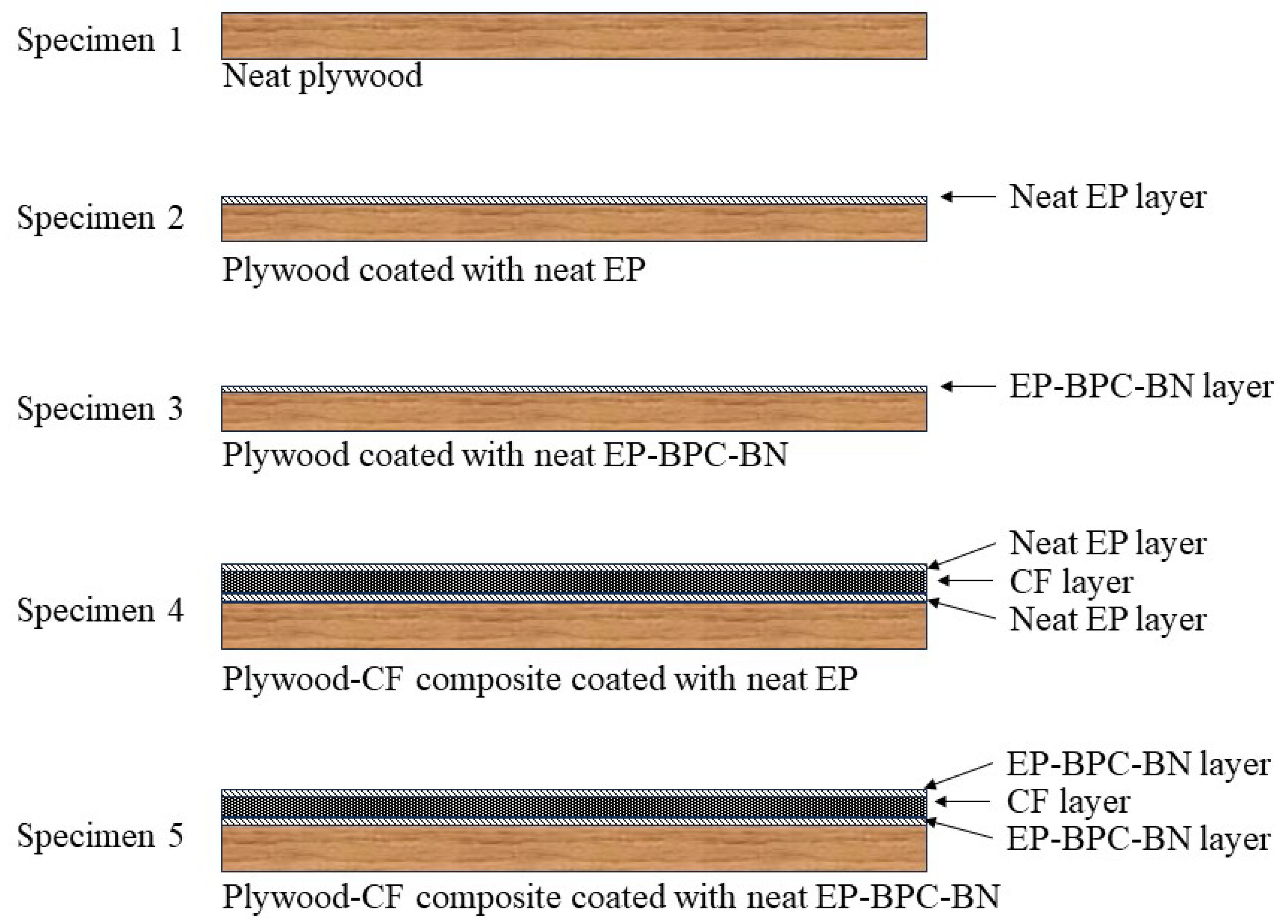
3.2. Heat Propagation Studies of Neat Epoxy, Fire-Retardant Blend, and Composite
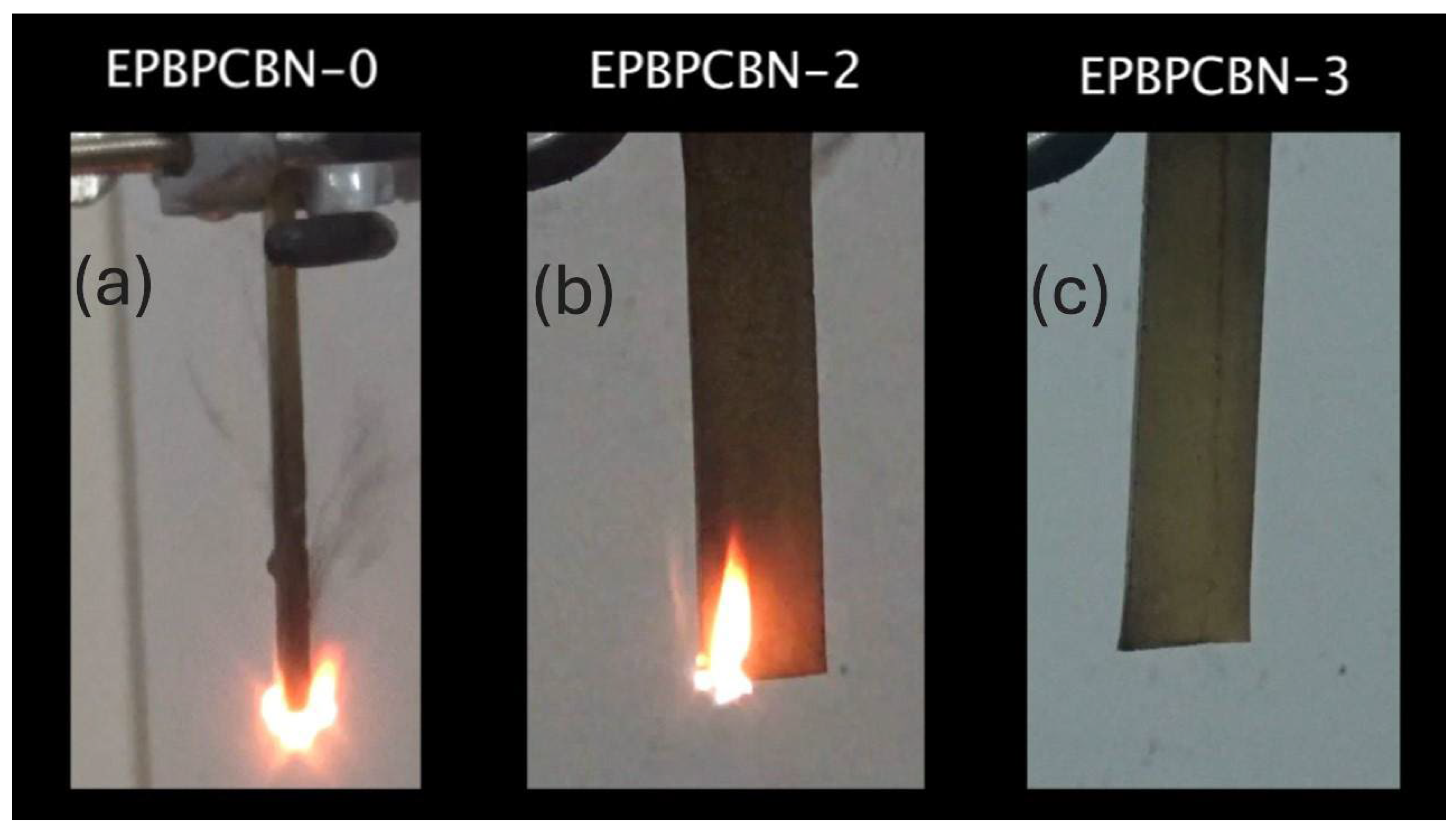
3.3. Vertical Burning Test
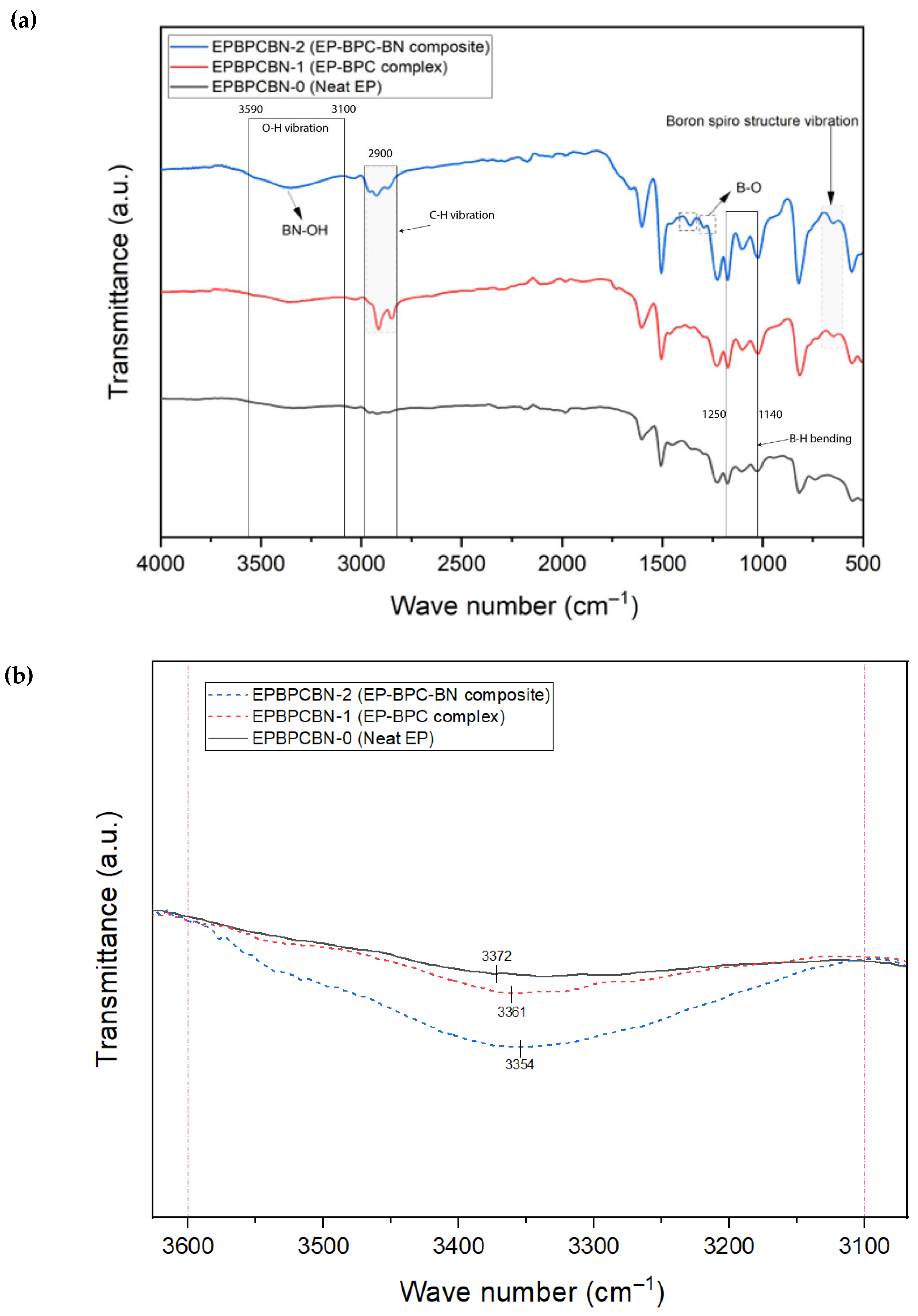
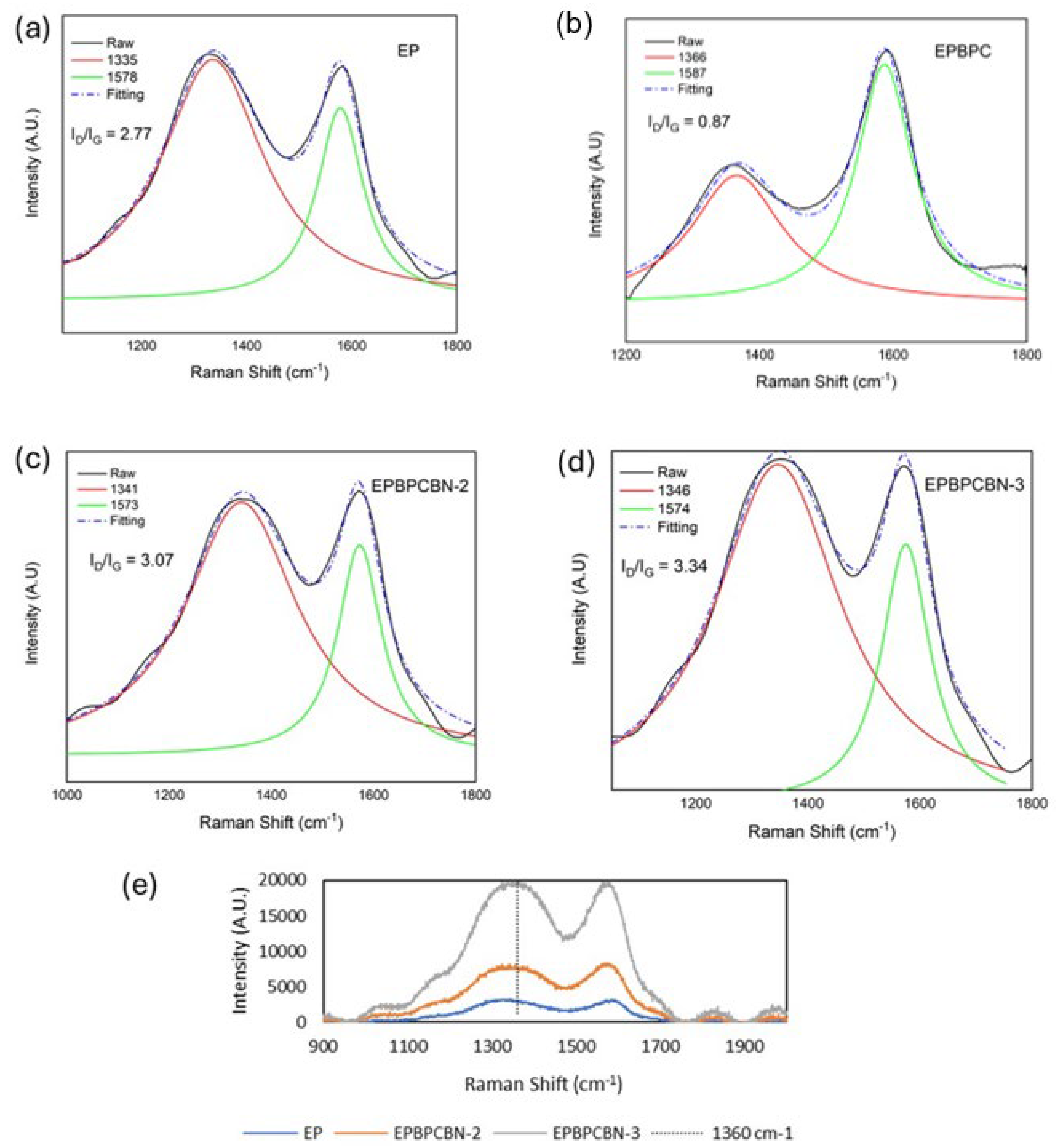
3.4. Chemical Structural Analysis of the Burned Composite
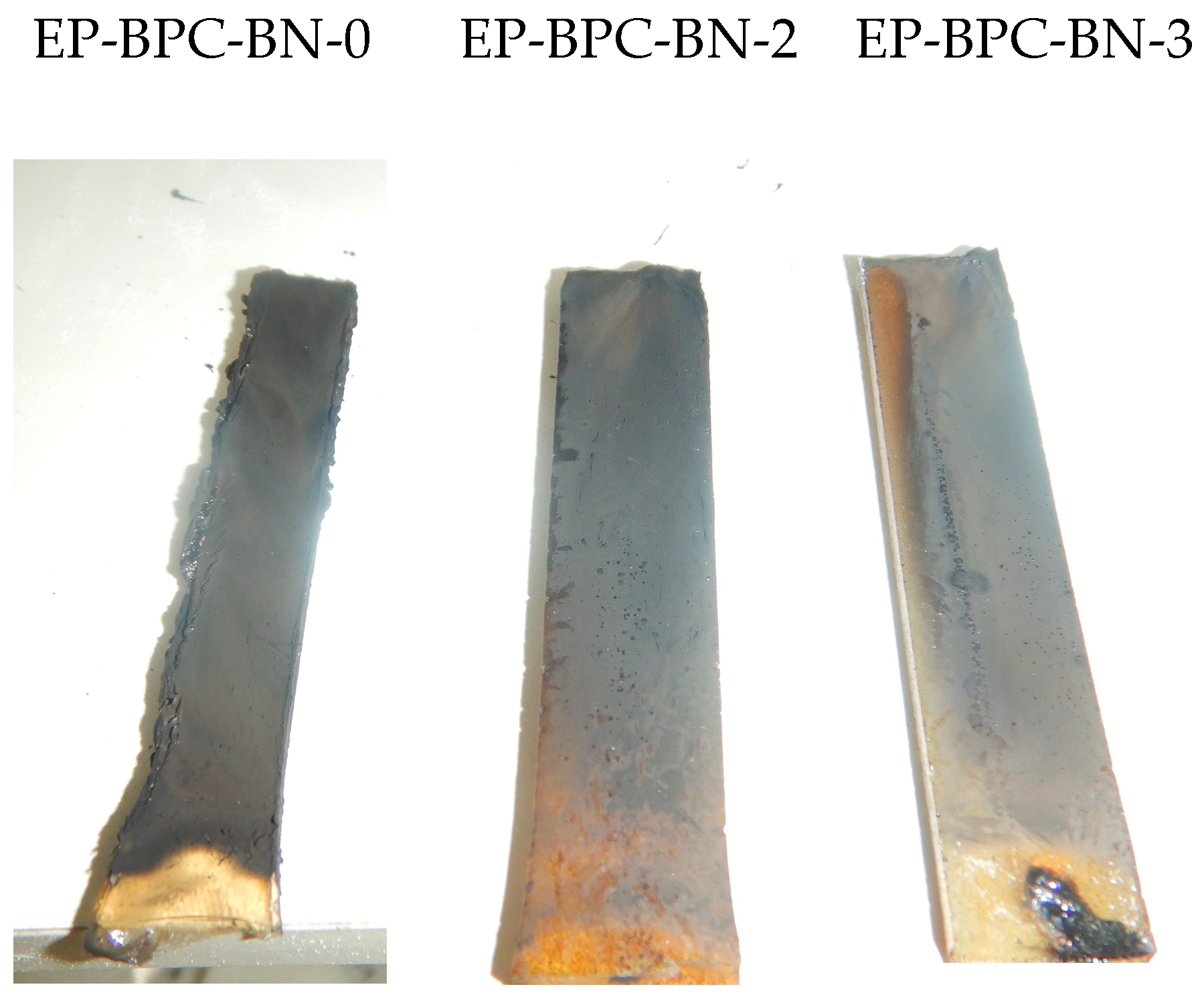
3.5. Char Residue Analysis
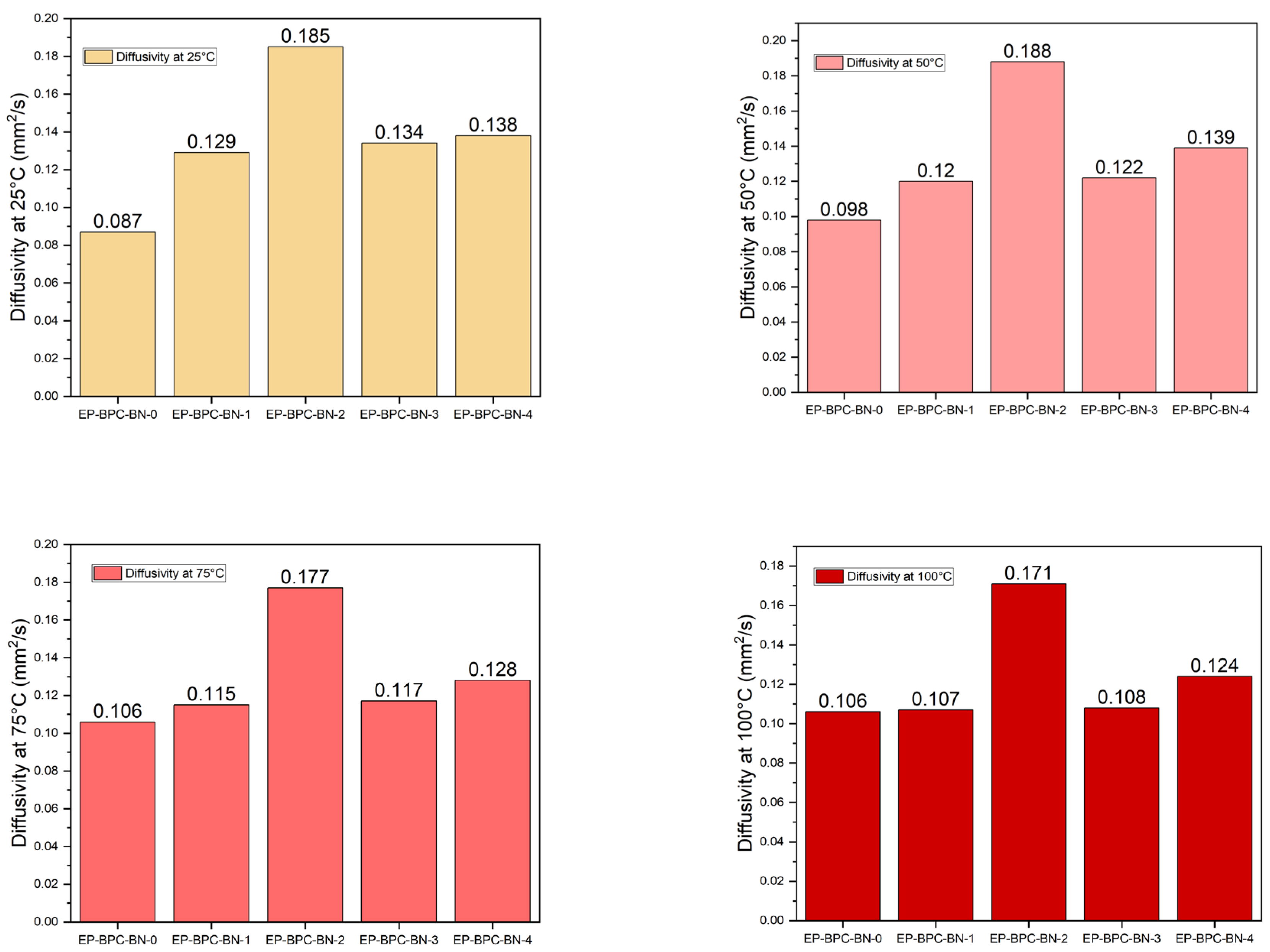
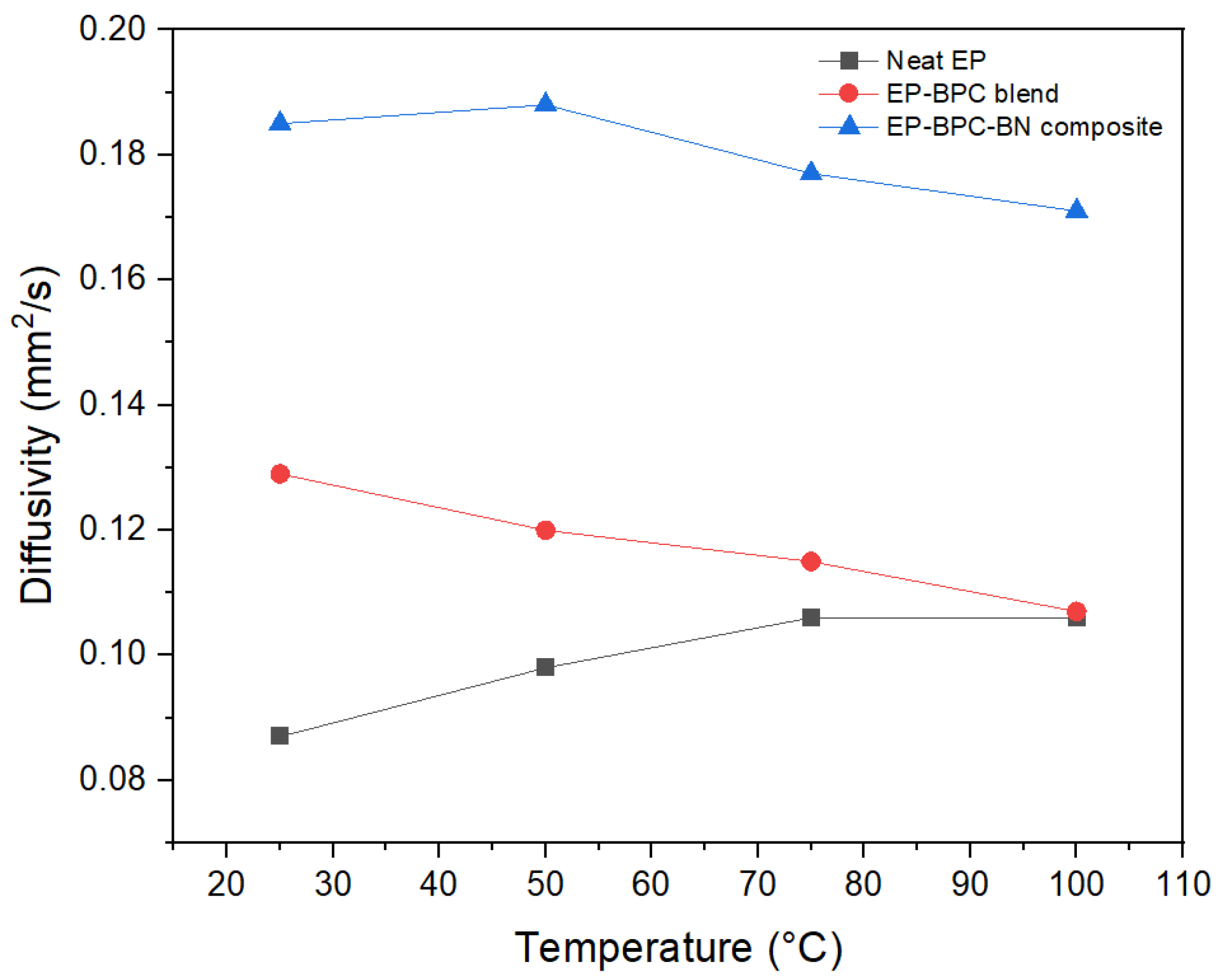
3.6. Thermal Properties Analysis
3.7. Microstructure Analysis
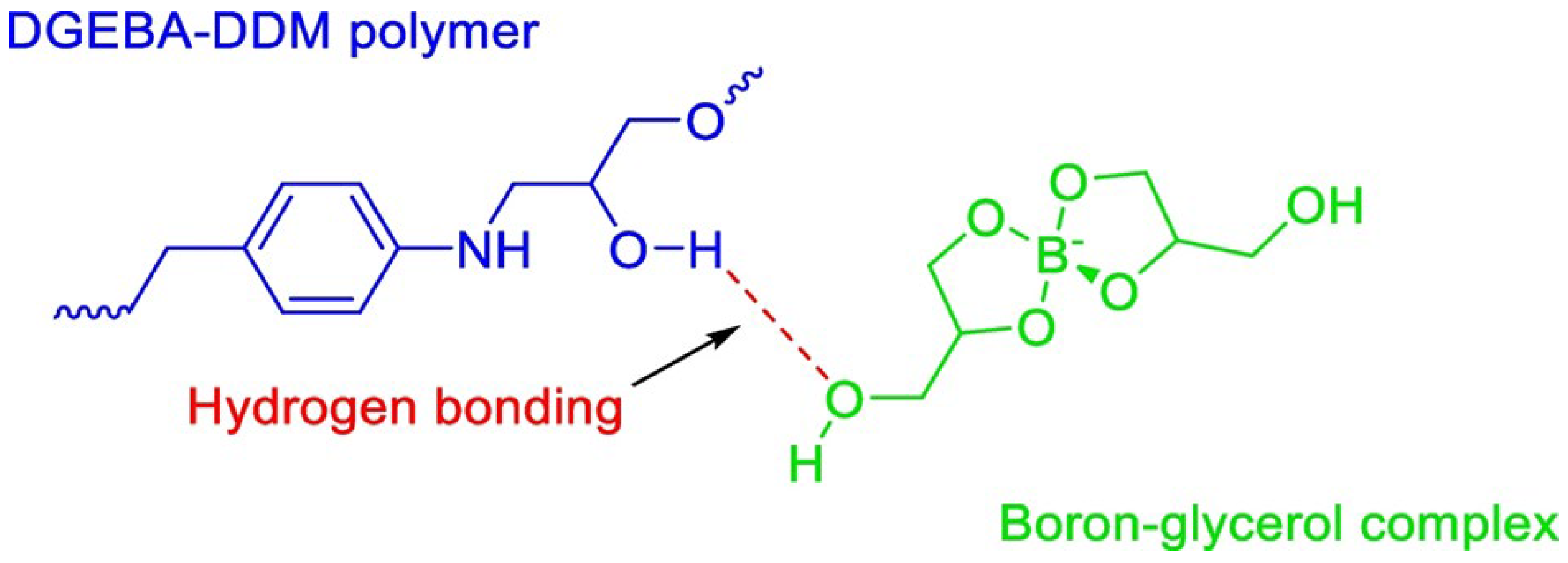
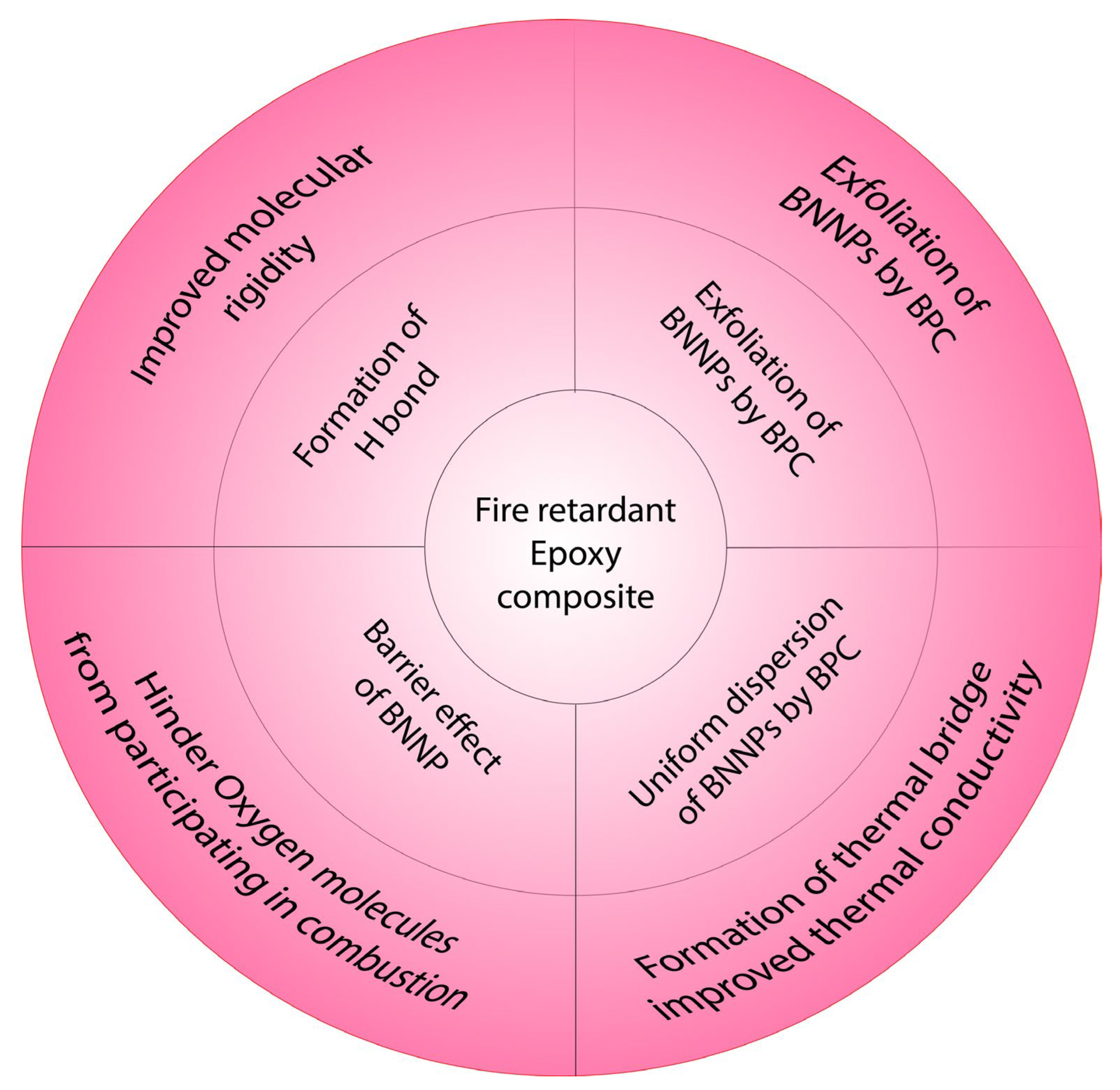
4. Flame-Retardant Mechanism
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, K.; Tang, G.; Gao, R.; Jiang, S. In situ growth of 0D silica nanospheres on 2D molybdenum disulfide nanosheets: Towards reducing fire hazards of epoxy resin. J. Hazard. Mater. 2018, 344, 1078–1089. [Google Scholar] [CrossRef]
- Kong, Q.; Sun, Y.; Zhang, C.; Guan, H.; Zhang, J.; Wang, D.-Y.; Zhang, F. Ultrathin iron phenyl phosphonate nanosheets with appropriate thermal stability for improving fire safety in epoxy. Compos. Sci. Technol. 2019, 182, 107748. [Google Scholar] [CrossRef]
- Jiang, J.; Cheng, Y.; Liu, Y.; Wang, Q.; He, Y.; Wang, B. Intergrowth charring for flame-retardant glass fabric-reinforced epoxy resin composites. J. Mater. Chem. A 2015, 3, 4284–4290. [Google Scholar] [CrossRef]
- Li, P.; Zheng, Y.; Shi, T.; Wang, Y.; Li, M.; Chen, C.; Zhang, J. A solvent-free graphene oxide nanoribbon colloid as filler phase for epoxy-matrix composites with enhanced mechanical, thermal and tribological performance. Carbon 2016, 96, 40–48. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.; Liu, Q.; He, Y. A comprehensive review of reactive flame-retardant epoxy resin: Fundamentals, recent developments, and perspectives. Polym. Degrad. Stab. 2022, 201, 109976. [Google Scholar] [CrossRef]
- Mathews, L.D.; Capricho, J.C.; Peerzada, M.; Salim, N.V.; Parameswaranpillai, J.; Hameed, N. Recent progress and multifunctional applications of fire-retardant epoxy resins. Mater. Today Commun. 2022, 33, 104702. [Google Scholar] [CrossRef]
- Wang, J. Effect of a Novel Polysilicone on the Flame Retardancy and Thermal Degradation of Epoxy Resin. J. Polym. Mater. 2018, 35, 33–44. [Google Scholar] [CrossRef]
- Chen, S.; Ai, L.; Zhang, T.; Liu, P.; Liu, W.; Pan, Y.; Liu, D. Synthesis and application of a triazine derivative containing boron as flame retardant in epoxy resins. Arab. J. Chem. 2020, 13, 2982–2994. [Google Scholar] [CrossRef]
- Zhou, S.; Tao, R.; Dai, P.; Luo, Z.; He, M. Two-step fabrication of lignin-based flame retardant for enhancing the thermal and fire retardancy properties of epoxy resin composites. Polym. Compos. 2020, 41, 2025–2035. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhang, L.; Molleja, J.G.; Wang, D.-Y. Bimetallic metal-organic frameworks and graphene oxide nano-hybrids for enhanced fire retardant epoxy composites: A novel carbonization mechanism. Carbon 2019, 153, 407–416. [Google Scholar] [CrossRef]
- Szeluga, U.; Pusz, S.; Kumanek, B.; Olszowska, K.; Kobyliukh, A.; Trzebicka, B. Effect of graphene filler structure on electrical, thermal, mechanical, and fire retardant properties of epoxy-graphene nanocomposites-a review. Crit. Rev. Solid State Mater. Sci. 2021, 46, 152–187. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, Q.T.; Bach, T.P. Mechanical properties and flame retardancy of epoxy resin/nanoclay/multiwalled carbon nanotube nanocomposites. J. Chem. 2019, 2019, 3105205. [Google Scholar] [CrossRef]
- Wang, Q.; Su, D.S.; Wang, D.-Y. Carbon nanotube/epoxy composites for improved fire safety. ACS Appl. Nano Mater. 2020, 3, 4253–4264. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Li, Y.; Liang, C.; Zhang, J. Novel Ti3C2Tx MXene/epoxy intumescent fire-retardant coatings for ancient wooden architectures. J. Appl. Polym. Sci. 2021, 138, 50649. [Google Scholar] [CrossRef]
- Gong, K.; Yin, L.; Pan, H.; Mao, S.; Liu, L.; Zhou, K. Novel exploration of the flame retardant potential of bimetallic MXene in epoxy composites. Compos. Part B Eng. 2022, 237, 109862. [Google Scholar] [CrossRef]
- Espinosa, M.; Galia, M.; Cadiz, V. Novel phosphorilated flame retardant thermosets: Epoxy–benzoxazine–novolac systems. Polymer 2004, 45, 6103–6109. [Google Scholar] [CrossRef]
- Rajaei, M.; Kim, N.; Bickerton, S.; Bhattacharyya, D. A comparative study on effects of natural and synthesised nano-clays on the fire and mechanical properties of epoxy composites. Compos. Part B Eng. 2019, 165, 65–74. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, W.-J.; Zhou, Q.-K.; Gao, T.-Y.; Xu, G.-M.; Tai, Q.-L.; Zhu, S.-E.; Lu, H.-D.; Yuen, R.K.K.; Yang, W.; et al. Highly thermo-stable resveratrol-based flame retardant for enhancing mechanical and fire safety properties of epoxy resins. Chem. Eng. J. 2022, 450, 138475. [Google Scholar] [CrossRef]
- Terekhov, I.V.; Chistyakov, E.M.; Filatov, S.N.; Deev, I.S.; Kurshev, E.V.; Lonskii, S.L. Factors Influencing the Fire-Resistance of Epoxy Compositions Modified with Epoxy-Containing Phosphazenes. Inorg. Mater. Appl. Res. 2019, 10, 1429–1435. [Google Scholar] [CrossRef]
- Terekhov, I.V.; Filatov, S.N.; Chistyakov, E.M.; Borisov, R.S.; Kireev, V.V. Halogenated hydroxy-aryloxy phosphazenes and epoxy oligomers based on them. Russ. J. Appl. Chem. 2013, 86, 1600–1604. [Google Scholar] [CrossRef]
- Sun, X.; Li, Z.; Das, O.; Hedenqvist, M.S. Superior flame retardancy and smoke suppression of epoxy resins with zinc ferrite@polyphosphazene nanocomposites. Compos. Part A Appl. Sci. Manuf. 2023, 167, 107417. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Wang, C.; Huang, W.; Tian, Q.; Fu, Q.; Yan, W. Flame-Retardant Performance of Epoxy Resin Composites with SiO2 Nanoparticles and Phenethyl-Bridged DOPO Derivative. ACS Omega 2021, 6, 666–674. [Google Scholar] [CrossRef]
- Bifulco, A.; Parida, D.; Salmeia, K.A.; Lehner, S.; Stämpfli, R.; Markus, H.; Malucelli, G.; Branda, F.; Gaan, S. Improving flame retardancy of in-situ silica-epoxy nanocomposites cured with aliphatic hardener: Combined effect of DOPO-based flame-retardant and melamine. Compos. Part C Open Access 2020, 2, 100022. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Chen, C.; Ye, Y.; Zeng, H.; Qu, H.; Liu, J.; Zhou, X.; Long, S.; Xie, X. Highly thermally conductive flame retardant epoxy nanocomposites with multifunctional ionic liquid flame retardant-functionalized boron nitride nanosheets. J. Mater. Chem. A 2018, 6, 20500–20512. [Google Scholar] [CrossRef]
- Shahari, S.; Fathullah, M.; Abdullah, M.M.A.B.; Shayfull, Z.; Mia, M.; Budi Darmawan, V.E. Recent developments in fire retardant glass fibre reinforced epoxy composite and geopolymer as a potential fire-retardant material: A review. Constr. Build. Mater. 2021, 277, 122246. [Google Scholar] [CrossRef]
- Mathews, L.D.; Capricho, J.C.; Salim, N.; Parameswaranpillai, J.; Moinuddin, K.; Hameed, N. Intrinsically modified self-extinguishing fire-retardant epoxy resin using boron-polyol complex. J. Polym. Res. 2023, 30, 271. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Gu, J. Chapter 6—Thermally conductive polymer composites. In Thermally Conductive Polymer Composites; Gu, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 149–196. [Google Scholar]
- Wang, Z.; Wu, Z.; Weng, L.; Ge, S.; Jiang, D.; Huang, M.; Mulvihill, D.M.; Chen, Q.; Guo, Z.; Jazzar, A.; et al. A Roadmap Review of Thermally Conductive Polymer Composites: Critical Factors, Progress, and Prospects. Adv. Funct. Mater. 2023, 33, 2301549. [Google Scholar] [CrossRef]
- Harris, R.H.; Douglas, J.F.; Winey, K.I.; Kashiwagi, T.; Du, F.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat Mater 2005, 4, 928–933. [Google Scholar]
- Kwon, O.-S.; Lee, D.; Lee, S.P.; Kang, Y.G.; Kim, N.C.; Song, S.H. Enhancing the mechanical and thermal properties of boron nitride nanoplatelets/elastomer nanocomposites by latex mixing. RSC Adv. 2016, 6, 59970–59975. [Google Scholar] [CrossRef]
- He, Y.-M.; Wang, Q.-Q.; Liu, W.; Liu, Y.-S. Functionalization of boron nitride nanoparticles and their utilization in epoxy composites with enhanced thermal conductivity. Phys. Status Solidi (A) 2014, 211, 677–684. [Google Scholar] [CrossRef]
- Aghajani, A.; Ehsani, M.; Khajavi, R.; Kalaee, M.; Zaarei, D. Thermally conductive and mechanically strengthened bio-epoxy/boron nitride nanocomposites: The effects of particle size, shape, and combination. Polym. Compos. 2022, 43, 9027–9039. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Tang, G.; Chen, J.; Huang, Z.-Q.; Hu, Y. Biobased polyelectrolyte multilayer-coated hollow mesoporous silica as a green flame retardant for epoxy resin. J. Hazard. Mater. 2018, 342, 689. [Google Scholar] [CrossRef]
- AS NZS 3837:1998; Method of Test for Heat and Smoke Release Rates for Materials and Products Using an Oxygen Consumption Calorimeter. Standards Australia Limited/Standards New Zealand: Sydney, Australia, 1998.
- White, R.P.; Lipson, J.E.G. Polymer Free Volume and Its Connection to the Glass Transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar] [CrossRef]
- Zang, L.; Wagner, S.; Ciesielski, M.; Mueller, P.; Doering, M. Novel Star-shaped and Hyperbranched Phosphorus-containing Flame Retardants in Epoxy Resins. Polym. Adv. Technol. 2011, 22, 1182. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, H. A novel nitrogen, phosphorus, and boron ionic pair compound toward fire safety and mechanical enhancement effect for epoxy resin. Polym. Adv. Technol. 2020, 31, 885–897. [Google Scholar] [CrossRef]
- Filyanov, Y.M. Effect of a filler on the glass transition temperature of an epoxy resin and its relation to the filled polymer properties. Polym. Sci. USSR 1978, 20, 2074–2078. [Google Scholar] [CrossRef]
- Khare, K.S.; Khare, R. Effect of carbon nanotube dispersion on glass transition in cross-linked epoxy–carbon nanotube nanocomposites: Role of interfacial interactions. J. Phys. Chem. B 2013, 117, 7444–7454. [Google Scholar] [CrossRef]
- Moradi, S.; Calventus, Y.; Román, F.; Hutchinson, J.M. Achieving High Thermal Conductivity in Epoxy Composites: Effect of Boron Nitride Particle Size and Matrix-Filler Interface. Polymers 2019, 11, 1156. [Google Scholar] [CrossRef]
- Chiguma, J.; Johnson, E.; Shah, P.; Gornopolskaya, N.; Jones, W.E., Jr. Thermal diffusivity and thermal conductivity of epoxy-based nanocomposites by the laser flash and differential scanning calorimetry techniques. Open J. Compos. Mater. 2013, 3, 34468. [Google Scholar] [CrossRef]
- Sun, G.; Bi, J. Scalable production of boron nitride nanosheets in ionic liquids by shear-assisted thermal treatment. Ceram. Int. 2021, 47, 7776–7782. [Google Scholar] [CrossRef]
- Shi, Y.; Hamsen, C.; Jia, X.; Kim, K.K.; Reina, A.; Hofmann, M.; Hsu, A.L.; Zhang, K.; Li, H.; Juang, Z.-Y. Synthesis of few-layer hexagonal boron nitride thin film by chemical vapor deposition. Nano Lett. 2010, 10, 4134–4139. [Google Scholar] [CrossRef]
- Lee, D.; Lee, B.; Park, K.H.; Ryu, H.J.; Jeon, S.; Hong, S.H. Scalable Exfoliation Process for Highly Soluble Boron Nitride Nanoplatelets by Hydroxide-Assisted Ball Milling. Nano Lett. 2015, 15, 1238–1244. [Google Scholar] [CrossRef]
- Moon, O.M.; Kang, B.C.; Lee, S.B.; Boo, J.H. Temperature effect on structural properties of boron oxide thin films deposited by MOCVD method. Thin Solid Film. 2004, 464–465, 164–169. [Google Scholar] [CrossRef]
- Hameed, N.; Salim, N.V.; Walsh, T.R.; Wiggins, J.S.; Ajayan, P.M.; Fox, B.L. Ductile thermoset polymers via controlling network flexibility. Chem. Commun. 2015, 51, 9903–9906. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Peng, S.; Hameed, N.; Guo, Q.; Mai, Y.-W. A new route to nanostructured thermosets with block ionomer complexes. Soft Matter 2012, 8, 688–698. [Google Scholar] [CrossRef]
- Zheng, G.; Wei, X.J.; Wang, Q.; Ai, J.J. The Synthesis of High Yield Diglycerin Borate. Appl. Mech. Mater. 2011, 55–57, 312–316. [Google Scholar] [CrossRef]
- Peng, H.; Wang, D.; Zhang, L.; Li, M.; Liu, M.; Wang, C.; Fu, S. Amorphous cobalt borate nanosheets grown on MoS2 nanosheet for simultaneously improving the flame retardancy and mechanical properties of polyacrylonitrile composite fiber. Compos. Part B Eng. 2020, 201, 108298. [Google Scholar] [CrossRef]
- Gorbachev, R.V.; Riaz, I.; Nair, R.R.; Jalil, R.; Britnell, L.; Belle, B.D.; Hill, E.W.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Hunting for Monolayer Boron Nitride: Optical and Raman Signatures. Small 2011, 7, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.S.; Deshmukh, A.; Suárez-Villagrán, M.Y.; Das, R.; Pal, V.; Dey, S.; Miller, J.H.; Machado, L.D.; Kumbhakar, P.; Tiwary, C.S. 2D Hexagonal Boron Nitride-Coated Cotton Fabric with Self-Extinguishing Property. ACS Appl. Mater. Interfaces 2020, 12, 45274–45280. [Google Scholar] [CrossRef]
- Mehra, N.; Li, Y.; Zhu, J. Small organic linkers with hybrid terminal groups drive efficient phonon transport in polymers. J. Phys. Chem. C 2018, 122, 10327–10333. [Google Scholar] [CrossRef]
- Kim, G.-H.; Lee, D.; Shanker, A.; Shao, L.; Kwon, M.S.; Gidley, D.; Kim, J.; Pipe, K.P. High thermal conductivity in amorphous polymer blends by engineered interchain interactions. Nat. Mater. 2015, 14, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Feng, Y.; Feng, W. Three-dimensional interconnected networks for thermally conductive polymer composites: Design, preparation, properties, and mechanisms. Mater. Sci. Eng. R: Rep. 2020, 142, 100580. [Google Scholar] [CrossRef]
- Guan, F.-L.; Gui, C.-X.; Zhang, H.-B.; Jiang, Z.-G.; Jiang, Y.; Yu, Z.-Z. Enhanced thermal conductivity and satisfactory flame retardancy of epoxy/alumina composites by combination with graphene nanoplatelets and magnesium hydroxide. Compos. Part B Eng. 2016, 98, 134–140. [Google Scholar] [CrossRef]



| Sample ID | DGEBA Resin (g) | 25% BPC (g) | DDM (g) | BNNP (g) | BN (wt%) | BPC (wt%) | BPC–BN (wt%) |
|---|---|---|---|---|---|---|---|
| EP-BPC-BN-0 | 10 | 0 | 3 | 0 | 0 | 0 | 0 |
| EP-BPC-BN-1 | 10 | 2 | 3 | 0 | 0.00 | 13.33 | 13.33 |
| EP-BPC-BN-2 | 10 | 2 | 3 | 0.03 | 0.20 | 13.33 | 13.51 |
| EP-BPC-BN-3 | 10 | 2 | 3 | 0.06 | 0.41 | 13.33 | 13.68 |
| EP-BPC-BN-4 | 10 | 2 | 3 | 0.09 | 0.60 | 13.33 | 13.86 |
| EP-BPC-BN-5 | 10 | 2 | 3 | 0.12 | 0.80 | 13.33 | 14.03 |
| Sample | UL-94 |
|---|---|
| EP-BPC-BN-0 | No rating (NR) |
| EP-BPC-BN-1 | V-1 |
| EP-BPC-BN-2 | V-0 |
| EP-BPC-BN-3 | V-0 |
| Wave Number (cm−1) | Intensity | Caused by | Effect | |||
|---|---|---|---|---|---|---|
| EP-BPC-BN-0 | EP-BPC-BN-1 | EP-BPC-BN-2 | ||||
| 1370 | None | None | Low | B-N tensile vibration | Non-combustible BNNPs in the composite | |
| 811 | None | None | Low | B-N-B bending vibrations | Non-combustible BNNPs in the composite | |
| 1290 | None | None | Low | B-O deformation vibration | Functionalisation of BNNPs with OH | |
| 3100–3590 | Low | Medium | High | O-H tensile vibrations | Functionalisation of BNNPs with OH | |
| 658 | None | Medium | Medium | Boron spiro structure vibration | Presence of BPC complex in the composite | |
| 2931 | Low | High | Medium | C-H stretching vibrations | Presence of BPC complex in the composite | |
| 2850 | Low | High | Medium | C-H stretching vibrations | Presence of BPC complex in the composite | |
| Composites | Time to PHRR (s) | PHRR (Kw/m2) | PHRR% Increase (%) | Average HRR (Kw/m2) | TSR (m2/m2) | TSR % Increase (%) | TTI (s) |
|---|---|---|---|---|---|---|---|
| Neat plywood | 55 | 317 | - | 112 | 68 | - | 22 |
| Neat EP-coated plywood | 80 | 358 | 13 | 111 | 113 | 66 | 23 |
| FREP-coated plywood | 60 | 335 | 6 | 109 | 110 | 62 | 23 |
| Carbon fibre-reinforced plywood composite | |||||||
| Neat CFEP | 80 | 236 | - | 98 | 145 | - | 26 |
| CFFREP | 90 | 285 | 21 | 113 | 207 | 43 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathews, L.D.; Mateti, S.; Parameswaranpillai, J.; Hameed, N.; Salim, N.V. Enhanced Fire Retardancy of Epoxy Resins upon Addition of Boron Nitride Nanoparticles Using Boron Polyol Complex. Materials 2025, 18, 4101. https://doi.org/10.3390/ma18174101
Mathews LD, Mateti S, Parameswaranpillai J, Hameed N, Salim NV. Enhanced Fire Retardancy of Epoxy Resins upon Addition of Boron Nitride Nanoparticles Using Boron Polyol Complex. Materials. 2025; 18(17):4101. https://doi.org/10.3390/ma18174101
Chicago/Turabian StyleMathews, Lalson D., Srikanth Mateti, Jyotishkumar Parameswaranpillai, Nishar Hameed, and Nisa V. Salim. 2025. "Enhanced Fire Retardancy of Epoxy Resins upon Addition of Boron Nitride Nanoparticles Using Boron Polyol Complex" Materials 18, no. 17: 4101. https://doi.org/10.3390/ma18174101
APA StyleMathews, L. D., Mateti, S., Parameswaranpillai, J., Hameed, N., & Salim, N. V. (2025). Enhanced Fire Retardancy of Epoxy Resins upon Addition of Boron Nitride Nanoparticles Using Boron Polyol Complex. Materials, 18(17), 4101. https://doi.org/10.3390/ma18174101







