Advances of Welding Technology of Glass for Electrical Applications
Abstract
1. Introduction
2. A Review of Glass Welding
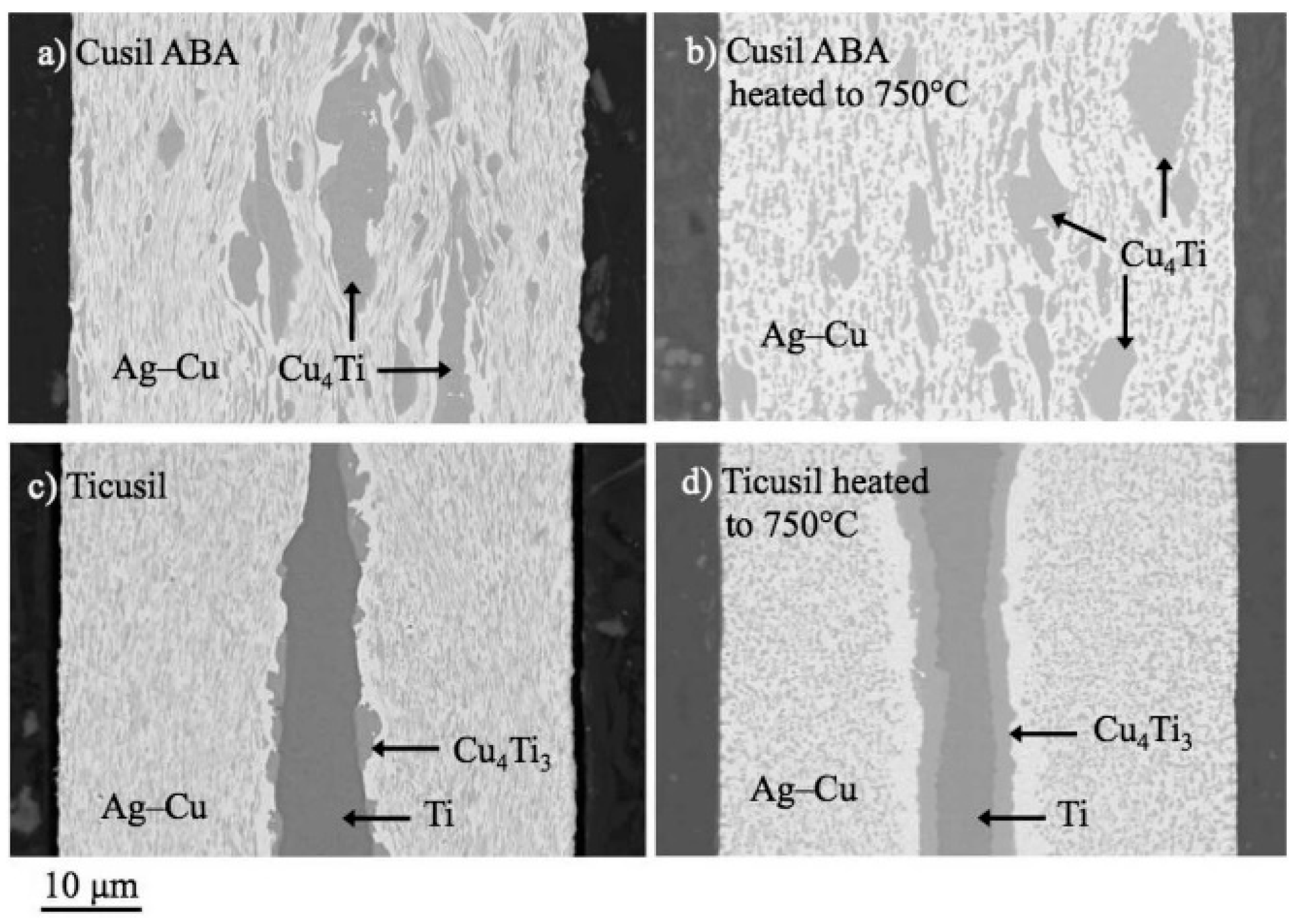
2.1. Brazing of Glass
2.2. Ultrasonic Welding
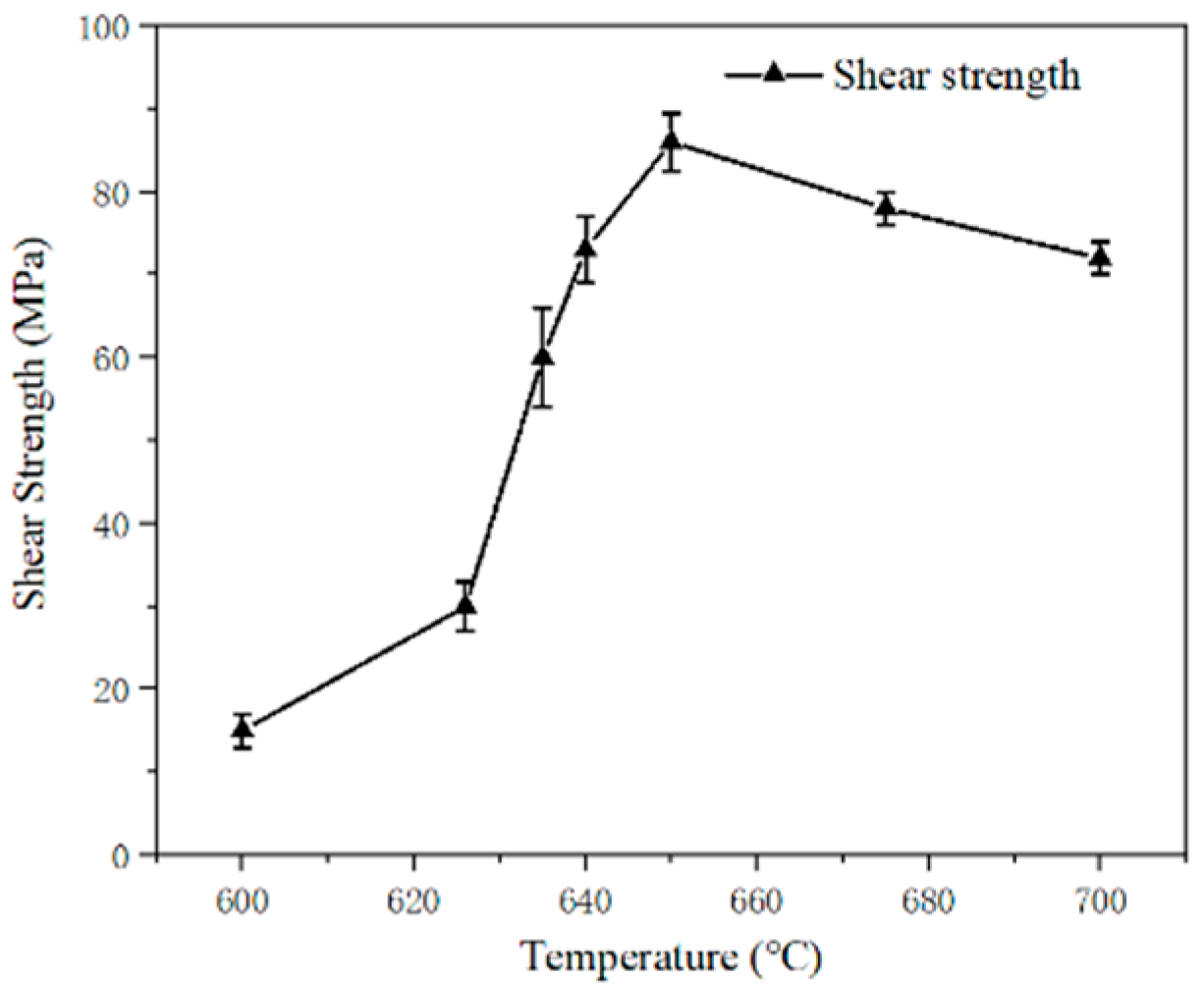
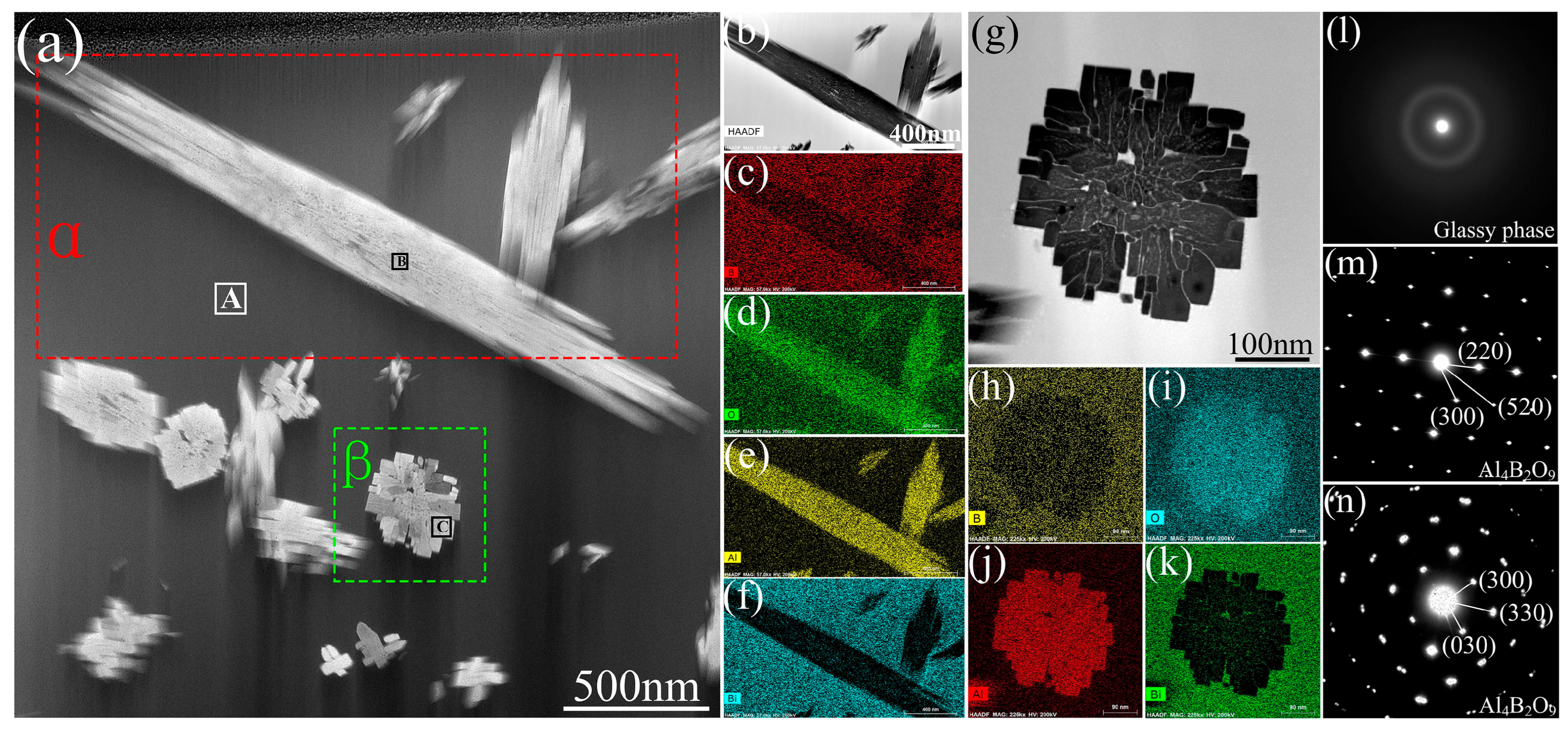
2.3. Ultrasonic-Assisted Brazing
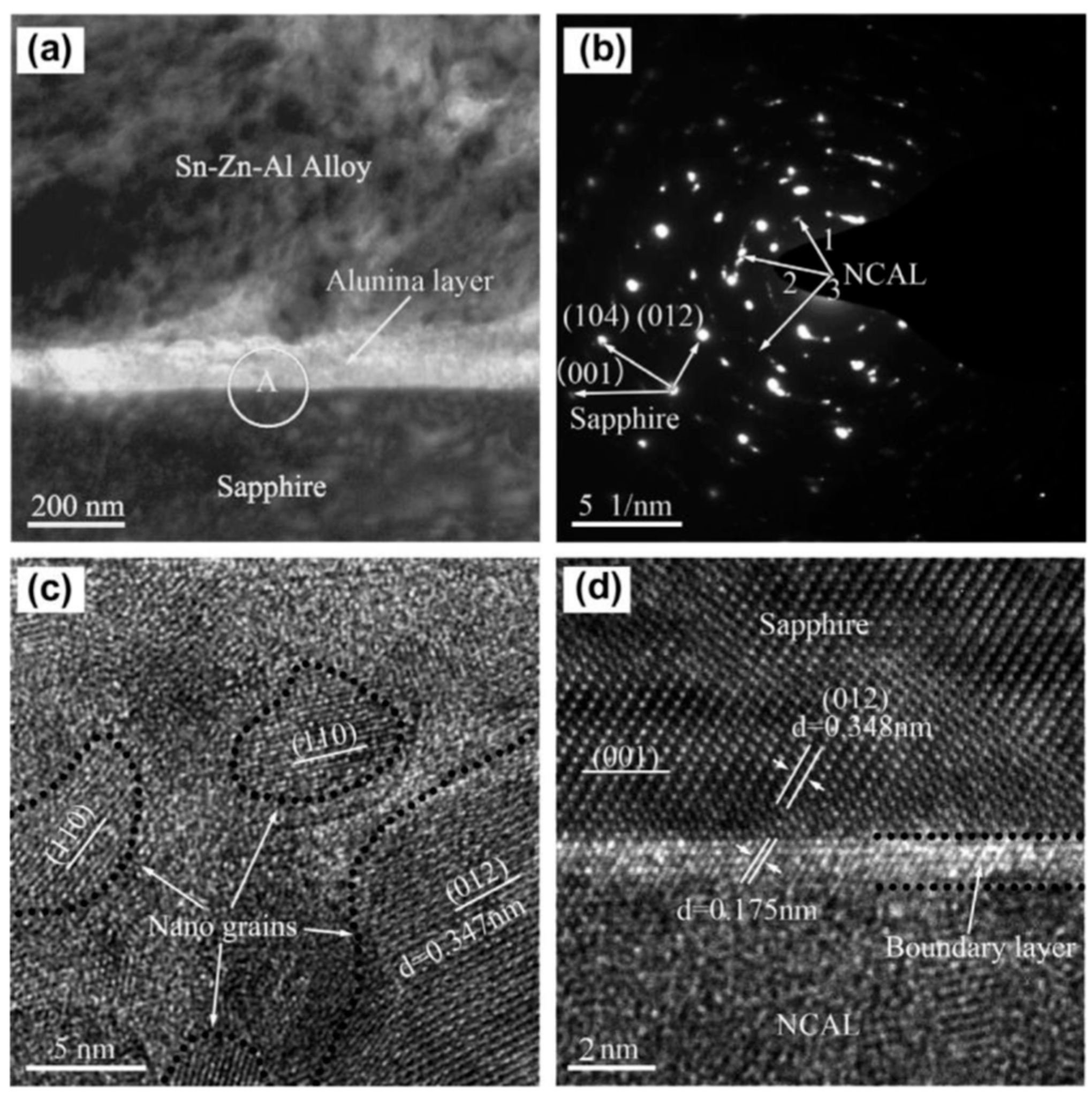
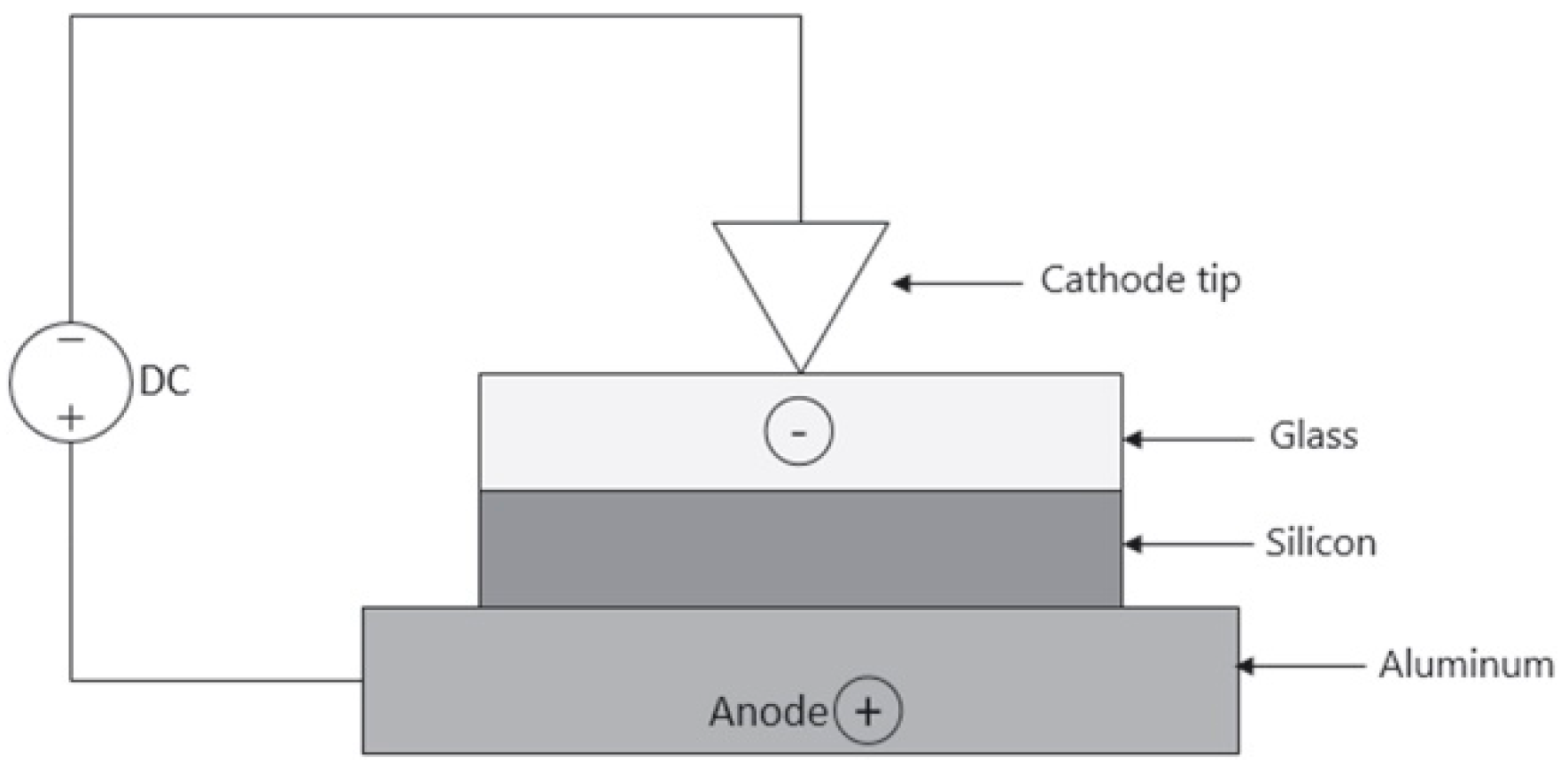
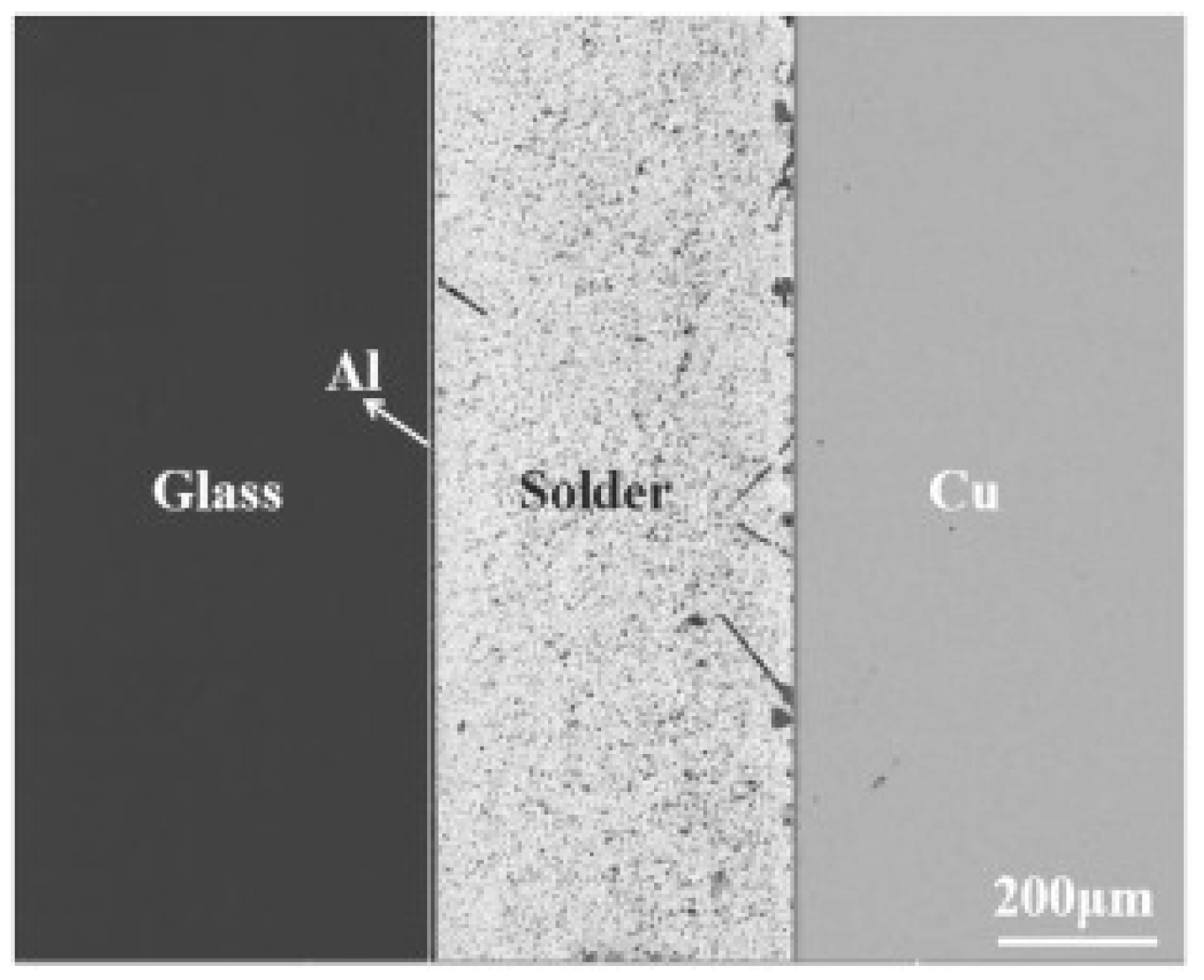
2.4. Anodic Bonding
2.4.1. Direct Bonding
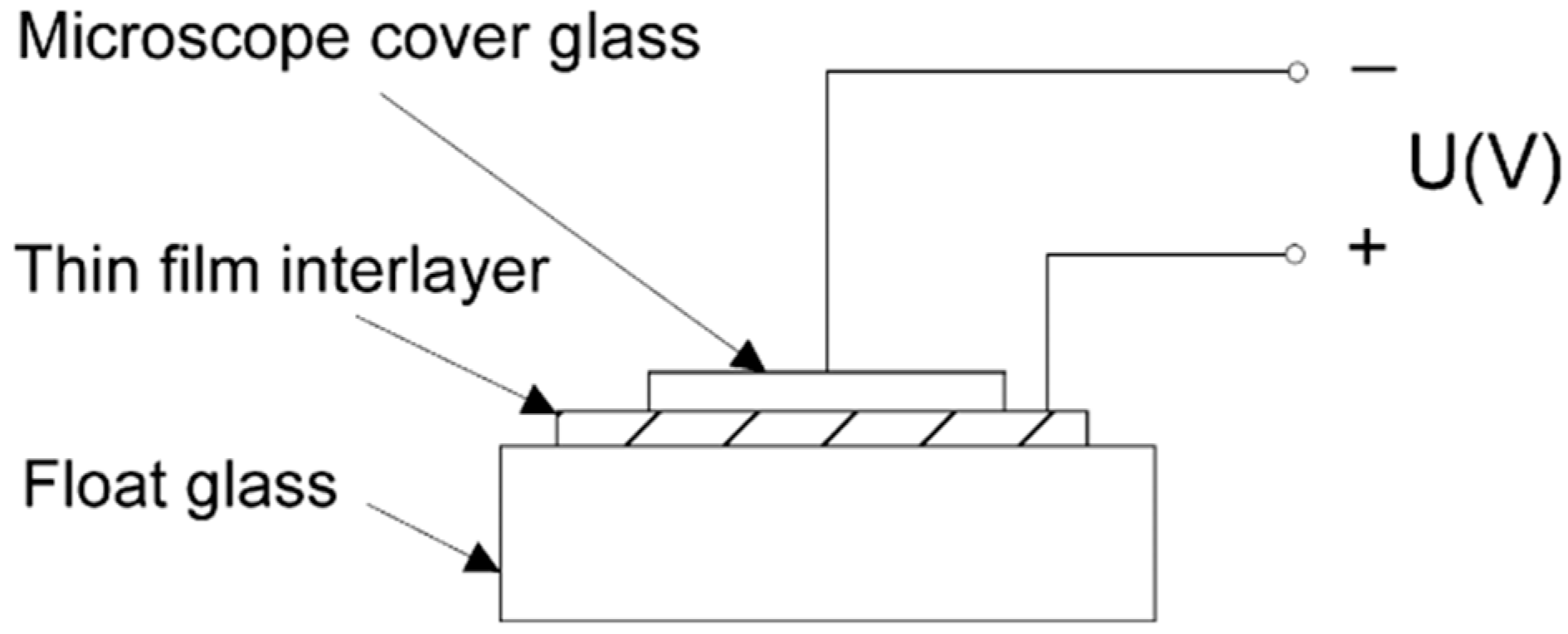
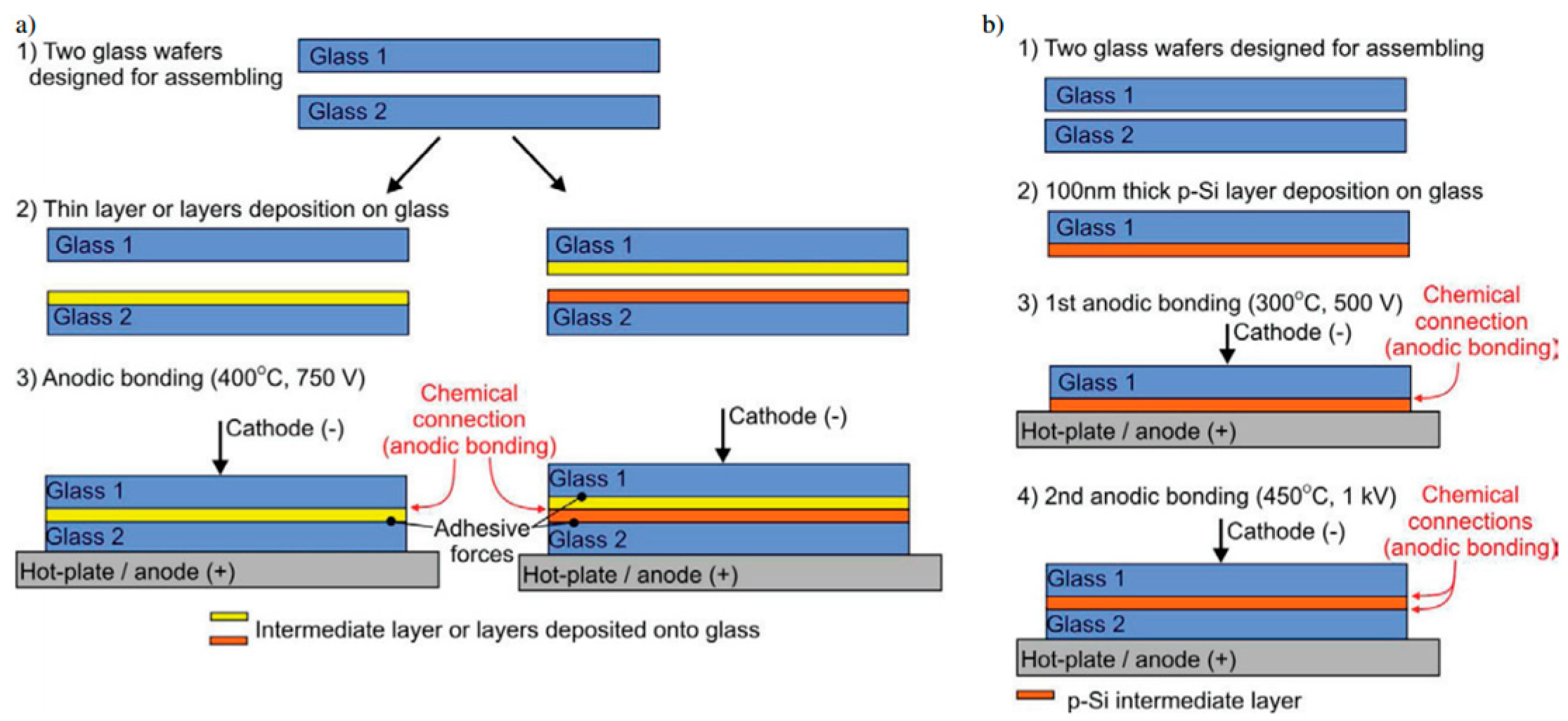
2.4.2. Indirect Bonding
2.5. Application of Lasers in Glass Welding
2.5.1. Glass–Glass Welding by NS Laser
2.5.2. Glass–Glass Welding by CO2 Laser
2.5.3. Glass–Glass Welding by USP Laser
3. Future Trends in Glass Welding
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, F.; Rettenmayr, M. Joining of SiO2 glass and 316L stainless steel using Bi–Ag-based active solders. J. Mater. Sci. 2021, 56, 3444–3454. [Google Scholar] [CrossRef]
- Moore, L.A.; Smith, C.M. Fused silica as an optical material. Opt. Mater. Express 2022, 12, 3043–3059. [Google Scholar] [CrossRef]
- Schroeter, A.; Nawrodt, R.; Schnabel, R.; Reid, S.; Martin, I.; Rowan, S.; Schwarz, C.; Koettig, T.; Neubert, R.; Thürk, M.; et al. On the mechanical quality factors of cryogenic test masses from fused silica and crystalline quartz. arXiv 2007, arXiv:0709.4359. [Google Scholar] [CrossRef]
- Sinev, L.; Petrov, I. Ceramics Linear thermal expansion coefficient (at temperatures from 130 to 800 k) of borosilicate glasses suitable for silicon compounds in microelectronics. Glass Ceram. 2016, 73, 32–35. [Google Scholar] [CrossRef]
- Min, P.; Tong, X.; Yuan, Y.; Li, H.; Cheng, Y.; Liu, H.; Song, P.; Wang, H. Silicon-to-silicon high strength joining with borosilicate glass interlayer using tape casting method. Ceram. Int. 2025, 51, 35405–35413. [Google Scholar] [CrossRef]
- Kahr, M.; Domke, M.; Steiner, H.; Hortschitz, W.; Stifter, M. Borosilicate Glass MEMS Lorentz Force Magnetometer. Proceedings 2018, 2, 788. [Google Scholar] [CrossRef]
- Li, S.L.; Chen, Q. Mechanism and Research Progress of Ultrashort Pulse Laser Welding of Glass Materials. MW Met. Formin 2024, 22, 1–10. (In Chinese) [Google Scholar]
- Brandon, D.; Kaplan, W.D. Joining Processes: An introduction; John Wiley & Sons: Hoboken, NJ, USA, 1997; p. 5. [Google Scholar]
- Hélie, D.; Lacroix, F.; Vallée, R. Bonding of optical materials by femtosecond laser welding for aerospace and high power laser applications. In Proceedings of the Photonics North 2012, Montréal, QC, Canada, 6–8 June 2012. [Google Scholar]
- Banse, H. Laser Beam Soldering—Technology for the Setup of Optical Systems. Ph.D Thesis, University Jena, Jena, Germany, 2005. (In German). [Google Scholar]
- Wu, Q.; Lorenz, N.; Cannon, K.M.; Hand, D.P. Glass frit as a hermetic joining layer in laser based joining of miniature device. IEEE Trans. Compon. Packag. Technol. 2010, 33, 470–477. [Google Scholar] [CrossRef]
- Kazakov, N.F. Diffusion Bonding of Materials; Elsevier: Amsterdam, The Netherlands, 2013; pp. 17–48. [Google Scholar]
- Banse, H.; Eberhardt, R.; Beckert, R. Laserstrahllöten-Technologie zum Aufbau optischer Systeme. In Proceedings of the Internationales Wissenschaftliches Kolloquium, Thuringia, Germany, 19–23 September 2005. [Google Scholar]
- Dachev, D.; Kazilas, M.; Alfano, G.; Omairey, S. Towards Reliable Adhesive Bonding: A Comprehensive Review of Mechanisms, Defects, and Design Considerations. Materials 2025, 18, 2724. [Google Scholar] [CrossRef]
- Guo, W.; Wang, T.; Lin, T.; Guo, S.; He, P. Bismuth borate zinc glass braze for bonding sapphire in air. Mater. Charact. 2018, 137, 67–76. [Google Scholar] [CrossRef]
- Kuckert, H.; Born, C.; Wagner, G.; Eifler, D. Helium-tight Sealing of Glass with Metal by Ultrasonic Welding. Adv. Eng. Mater. 2001, 3, 903–905. [Google Scholar] [CrossRef]
- Cui, W.; Li, S.; Yan, J.; He, J.; Liu, Y. Ultrasonic-assisted brazing of sapphire with high strength Al–4.5 Cu–1.5 Mg alloy. Ceram. Int. 2015, 41, 8014–8022. [Google Scholar] [CrossRef]
- Howlader, M.; Suehara, S.; Suga, T. Room temperature wafer level glass/glass bonding. Sens. Actuators A Phys. 2006, 127, 31–36. [Google Scholar] [CrossRef]
- Wang, W. Laser Welding of Fiber and Quartz Glass Ferrule. Micromachines 2023, 14, 939. [Google Scholar] [CrossRef]
- Gao, Z.; He, J.; Jia, X.; Yi, Z.; Li, C.; Zhang, S.; Wang, C.; Duan, J. High-strength welding of silica glass using double-pulse femtosecond laser under non-optical contact conditions. Photonics 2024, 11, 945. [Google Scholar] [CrossRef]
- Guo, W.; Fu, L.; He, P.; Lin, T.; Shen, Z.; Liu, X.C.; Wang, T.; Wang, C. Low-temperature brazing of alumina ceramics with bismuth-borate glass in air. Mater. Charact. 2019, 149, 158–164. [Google Scholar] [CrossRef]
- Schmid, F.; Khattak, C.P.; Rogers, H.H.; Felt, D.M.; Askinazi, J.; Wientzen, R.V. Current status of very large sapphire crystal growth for optical applications. In Proceedings of the Window and Dome Technologies and Materials VI, Orlando, FL, USA, 26 July 1999. [Google Scholar]
- He, L.; Chen, C.; Li, H.; Li, Y.; Yi, R. Research advances in joining processes of sapphire. Int. J. Adv. Manuf. Technol. 2022, 121, 59–81. [Google Scholar] [CrossRef]
- Dalton, R.H. Solder glass sealing. J. Am. Ceram. Soc. 1956, 39, 109–112. [Google Scholar] [CrossRef]
- Guo, W.; Wang, T.; Lin, T.; He, P. Bonding sapphire in air by using Bi2O3–B2O3 glass braze. Mater. Lett. 2018, 210, 117–120. [Google Scholar] [CrossRef]
- Ali, M.; Knowles, K.M.; Mallinson, P.M.; Fernie, J.A. Interfacial reactions between sapphire and Ag–Cu–Ti-based active braze alloys. Acta Mater. 2016, 103, 859–869. [Google Scholar] [CrossRef]
- Ali, M.; Knowles, K.M.; Mallinson, P.M.; Fernie, J.A. Microstructural evolution and characterisation of interfacial phases in Al2O3/Ag–Cu–Ti/Al2O3 braze joints. Acta Mater. 2015, 96, 143–158. [Google Scholar] [CrossRef]
- Yi, R.; Chen, C.; Li, Y.; Peng, H.; Zhang, H.; Ren, X. The bonding between glass and metal. Int. J. Adv. Manuf. Technol. 2020, 111, 963–983. [Google Scholar] [CrossRef]
- Shemer, H.F. Thermal expansion of binary alkaline-earth borate glasses. J. Res. Natl. Bur. Stand. 1956, 56, 73–79. [Google Scholar] [CrossRef]
- Kim, J. Weld ability of Cu54Zr22Ti18Ni6 bulk metallic glass by ultrasonic welding processing. Mater. Lett. 2014, 130, 160–163. [Google Scholar] [CrossRef]
- Shakil, M.; Tariq, N.; Ahmad, M.; Choudhary, M.; Akhter, J.; Babu, S.S. Effect of ultrasonic welding parameters on microstructure and mechanical properties of dissimilar joints. Mater. Des. 2014, 55, 263–273. [Google Scholar] [CrossRef]
- Wagner, G.; Walther, F.; Nebe, T.; Eifle, D. Glass/glass joints by ultrasonic welding. Glass Technol. 2003, 44, 152–155. [Google Scholar]
- Silva, J.J.; Da Silva, R.G.; Morelli, C.L.; López, E.A.; Santos, T.F. Trends and Future Projections in Ultrasonic Welding Research for Hybrid Materials. Polymers 2025, 17, 1124. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhai, W.; Wei, B.; Hu, H. Acoustic field and convection pattern within liquid material during ultrasonic processing. Wuli Xuebao 2017, 66, 194303. [Google Scholar]
- Fang, Y.; Yamamoto, T.; Komarov, S. Cavitation and acoustic streaming generated by different sonotrode tips. Ultrason. Sonochemistry 2018, 48, 79–87. [Google Scholar] [CrossRef]
- Wu, X.W. Research on Study on the Technology and Mechanism of Ultrasonic Assisted Low Temperature Active Soldering of SiO2 Glass; Harbin Institute of Technology: Harbin, China, 2022. (In Chinese) [Google Scholar]
- Muhrat, A.; Barbosa, J. Ultrasonic-assisted brazing of titanium joints using Al-Si based fillers: Numerical and experimental process design. Metal 2021, 11, 1686. [Google Scholar] [CrossRef]
- Zhao, D.; Li, D.; Xiao, Y.; Li, M.; Chen, W. Acoustic cavitation-induced microstructure evolution in ultrasonically brazed Al/Cu joints using Zn-Al alloy fillers. Ultrason. Sonochemistry 2024, 109, 107001. [Google Scholar] [CrossRef]
- Cui, W.; Yan, J.; Dai, Y.; Li, D. Building a nano-crystalline α-alumina layer at a liquid metal/sapphire interface by ultrasound. Ultrason. Sonochemistry 2015, 22, 108–112. [Google Scholar] [CrossRef]
- Brown, J.S.; Wilson, C.; Bohlen, C.; Choi, H.; Thompson, L.; Bostwick, J.B. Failure modes and bonding strength of ultrasonically-soldered glass joints. J. Mater. Process. Technol. 2022, 299, 117385. [Google Scholar] [CrossRef]
- Wallis, G.; Pomerantz, D.I. Field assisted glass-metal sealing. J. Appl. Phys. 1969, 40, 3946–3949. [Google Scholar] [CrossRef]
- Tan, J.; Cai, C.; Ming, X.; Yu, X.; Zhao, S.; Tu, S.T.; Liu, H. Morphology and stress at silicon-glass interface in anodic bonding. Appl. Surf. Sci. 2016, 387, 139–148. [Google Scholar] [CrossRef]
- Han, W.H.; Yu, J.Z. Flatness conditions required for room-temperature bonding of silicon wafers. J. Semicond. 2001, 12, 1516–1518. (In Chinese) [Google Scholar]
- Gerlach, A.; Maas, D.; Seidel, D.; Bartuch, H.; Schundau, S.; Kaschlik, K. Low-temperature anodic bonding of silicon to silicon wafers by means of intermediate glass layers. Microsyst. Technol. 1999, 5, 144–149. [Google Scholar] [CrossRef]
- Esashi, M.; Nakano, A.; Shoji, S.; Hebiguchi, H. Low-temperature silicon-to-silicon anodic bonding with intermediate low melting point glan. Sens. Actuators A Phys. 1990, 23, 931–934. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Y.; Lina, K.; Zhang, L.; Wang, B.; Sasangka, W.A.; Lee, K.E.K.; Chua, S.J.; Michel, J.; Fitzgerald, E. A review of silicon-based wafer bonding processes, an approach to realize the monolithic integration of Si-CMOS and III–V-on-Si wafers. J. Semicond. 2021, 42, 023106. [Google Scholar] [CrossRef]
- Du, C.; Zhao, Y.; Yong, L. Effect of surface cleaning process on the wafer bonding of silicon and pyrex glass. J. Inorg. Organomet. Polym. Mater. 2023, 33, 673–679. [Google Scholar] [CrossRef]
- Liu, C.R.; Meng, Q.S.; Hu, L.F.; Hu, M.Y. Microstructure and Formation Mechanism of Anodic Bonding Interface between Pyrex Glass and Kovar Alloy. Trans. China Weld. Inst. 2008, 2, 73–76. (In Chinese) [Google Scholar]
- Pang, Z.M.; Hu, L.F.; Jia, X.; Han, W.T.; Li, Z.H. Glass to Kovar Alloy Bonding Based on Anodic Bonding. Hot Work. Technol. 2023, 52, 119–122. (In Chinese) [Google Scholar]
- Howlader, M.; Kibria, M.G.; Zhang, F. Hybrid plasma bonding of germanium and glass wafers at low temperature. Mater. Lett. 2010, 64, 1532–1535. [Google Scholar] [CrossRef]
- Chen, D.M.; Hu, L.F.; Shi, F.R.; Meng, Q.S. Mechanism and Mechanical Properties of Silicon-Glass-Silicon Anodic Bonding. Trans. China Weld. Inst. 2019, 40, 123–127+166. (In Chinese) [Google Scholar]
- Sundaresh, S.; Deshpande, M.G.; Sundaram, K.B. New inexpensive simple experimental setup for anodic bonding of silicon-glass. ECS J. Solid State Sci. Technol. 2021, 10, 054002. [Google Scholar] [CrossRef]
- Jia, X. Study on the Interface Behavior and Mechanical Properties of Glass-Metal Joining. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2022. (In Chinese). [Google Scholar]
- Berthold, A.; Nicola, L.; Sarro, P.M.; Vellekoop, M.J. Glass-to-glass anodic bonding with standard IC technology thin films as intermediate layers. Sens. Actuators A Phys. 2000, 82, 224–228. [Google Scholar] [CrossRef]
- Unno, K.; Shibata, T.; Makino, E. Micromachining of diamond probes for atomic force microscopy applications. Sens. Actuators A Phys. 2001, 88, 247–255. [Google Scholar] [CrossRef]
- Mrozek, P. Anodic bonding of glasses with interlayers for fully transparent device applications. Sens. Actuators A Phys. 2009, 151, 77–80. [Google Scholar] [CrossRef]
- Mrozek, P. Glass-to-glass anodic bonding using TiNx interlayers for fully transparent device applications. Sens. Actuators A Phys. 2012, 174, 139–143. [Google Scholar] [CrossRef]
- Knapkiewicz, P.; Cichy, B.; Posadowski, W.; Tadaszak, K.; Sniadek, P.; Dziuban, J. Anodic bonding of glass-to-glass through magnetron spattered nanometric silicon layer. Procedia Eng. 2011, 25, 1629–1632. [Google Scholar] [CrossRef]
- Lin, Z.X.; Wang, S.G.; Liu, Q.; Zeng, Y.B.; Guo, H. Anodic Bonding of Glass-Silicon-Glass Three-Layer Structure with Si3N4 Thin Film. Transducer Microsyst. Technol. 2013, 32, 63–66+70. (In Chinese) [Google Scholar]
- Tang, W.; Jiao, J.; Wang, X.; Zhang, H. A novel method of anodic bonding. In Proceedings of the 2009 4th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Shenzhen, China, 5–9 January 2009. [Google Scholar]
- Elrefaey, A.; Janczak-Rusch, J.; Koebel, M.M. Direct glass-to-metal joining by simultaneous anodic bonding and soldering with activated liquid tin solder. J. Mater. Process. Technol. 2014, 214, 2716–2722. [Google Scholar] [CrossRef]
- Hu, L.; Xue, Y.; Wang, H. Glass-Cu joining by anodic bonding and soldering with eutectic Sn-9Zn solder. J. Alloys Compd. 2019, 789, 558–566. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, X.; Shi, Q.; He, T.; Sun, Z.; Guo, X.; Liu, W.; Sulaiman, O.B.; Dong, B.; Lee, C. Development trends and perspectives of future sensors and MEMS/NEMS. Micromachines 2019, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yang, F.; Zhang, D. Modeling of anodic bonding with SiO2 dielectric as interlayer. J. Micromechanics Microengineering 2020, 30, 105003. [Google Scholar] [CrossRef]
- Karlen, S.; Haesler, J.; Overstolz, T.; Bergonzi, G.; Lecomte, S. Sealing of MEMS atomic vapor cells using Cu-Cu thermocompression bonding. J. Microelectromechanical Syst. 2019, 29, 95–99. [Google Scholar] [CrossRef]
- Lin, Y.; Nie, J.; Bai, Y.; Li, S.; Xu, L.; Wang, F.; Ding, Y.; Tian, J.; Li, Y.; Chen, X.; et al. Anodic bonding driven by the pulse current signal of triboelectric nanogenerator. Nano Energy 2020, 73, 104759. [Google Scholar] [CrossRef]
- Yu, P.; Pan, C.; Xue, J. The anodic bonding between K4 glass and Si. Mater. Lett. 2005, 59, 2492–2495. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, L.; Liu, W.; Chou, Z.; Jia, W.; Mu, W. Study on the mechanism of glass-SiC-glass anodic bonding process. J. Micromechanics Microengineering 2024, 34, 045010. [Google Scholar] [CrossRef]
- Bhudolia, S.K.; Gohel, G.; Leong, K.F.; Barsotti Jr, R.J. Investigation on ultrasonic welding attributes of novel carbon/elium®® composites. Materials 2020, 13, 1117. [Google Scholar] [CrossRef]
- Lapadatu, A.C.; Jakobsen, H. Handbook of Silicon Based MEMS Materials and Technologies, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 599–610. [Google Scholar]
- Miyamoto, I.; Cvecek, K.; Schmidt, M. Advances of Laser Welding Technology of Glass-Science and Technology. J. Laser Micro/Nanoeng. 2020, 15, 63–76. [Google Scholar]
- Sleiman, K.; Legutko, M.; Rettschlag, K.; Jäschke, P.; Overmeyer, L.; Kaierle, S. CO2 laser based welding of borosilicate glass by Laser Glass Deposition. Procedia CIRP 2022, 111, 466–469. [Google Scholar] [CrossRef]
- Sun, G. Research on the Forming of Laser Welding for Vacuum Glass. Master’s Thesis, Yangzhou University, Yangzhou, China, 2012. (In Chinese). [Google Scholar]
- Jia, X.; Luo, J.; Li, K.; Wang, C.; Li, Z.; Wang, M.; Jiang, Z.; Veiko, V.P.; Duan, J. Ultrafast laser welding of transparent materials: From principles to applications. Int. J. Extrem. Manuf. 2025, 7, 032001. [Google Scholar] [CrossRef]
- Hong, K.M.; Shin, Y.C. Prospects of laser welding technology in the automotive industry: A review. J. Mater. Process. Technol. 2017, 245, 46–69. [Google Scholar] [CrossRef]
- Gu, B. The Latest Developments and Future Trends of Laser Processing Technology in Industrial Manufacturing. MW Met. Form. 2025, 3, 1–6+16. (In Chinese) [Google Scholar]
- Sysoev, V.K.; Planida, B.N.; Verlan, A.A.; Vyatlev, P.A.; Pomerantsev, M.A.; Aliev, T.A.; Papchenko, B.P. Laser welding of quartz glass workpieces. Glass Ceram. 2012, 68, 389–392. [Google Scholar] [CrossRef]
- Yoon, S.H.; Hwang, J.R.; Na, S.J. A study on the plasma-augmented laser welding for small-diameter STS tubes. Int. J. Adv. Manuf. Technol. 2007, 32, 1134–1143. [Google Scholar] [CrossRef]
- De, P.A.; Höche, T. Laser welding of glasses using a nanosecond pulsed Nd:YAG laser. Opt. Lasers Eng. 2017, 90, 1–9. [Google Scholar] [CrossRef]
- He, Q. Numerical Simulation and Experimental Study on Laser Sealing of Vacuum Flat Glass. Master’s Thesis, Yangzhou University, Yangzhou, China, 2019. (In Chinese). [Google Scholar]
- De Pablos-Martin, A.; Benndorf, G.; Tismer, S.; Mittag, M.; Cismak, A.; Lorenz, M.; Grundmann, M.; Höche, T. Laser-welded fused silica substrates using a luminescent fresnoite-based sealant. Opt. Laser Technol. 2016, 80, 176–185. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, L.; Zhang, Q.; Li, J.; Zhao, D.; Wang, H.; Lue, Q. Investigation of the reaction mechanism and optical transparency in nanosecond laser welding of glasses assisted with titanium film. Appl. Opt. 2020, 59, 940–947. [Google Scholar] [CrossRef]
- Bin Mohd Zawawi, M.Z.; Kim, T.; Jung, M.; Im, J.; Kang, S. Engineering Sustainable fabrication of glass nanostructures using infrared transparent mold assisted by CO2 laser scanning irradiation. J. Manuf. Sci. Eng. 2018, 140, 121005. [Google Scholar] [CrossRef]
- Katayama, S. Handbook of Laser Welding Technologies; Elsevier: Amsterdam, The Netherlands, 2013; pp. 19–21. [Google Scholar]
- Nam, G.J.; Seo, M.H.; Ryu, K.H.; Moon, S.W.; Hong, Y.S.; Ko, D.S. Laser cutting and sealing methods of capillary glass tube. In Proceedings of the Fifth International Symposium on Laser Precision Microfabrication, Nara, Japan, 11–14 May 2004. [Google Scholar]
- Tang, W.L. The Research of Sealing Glass Ampoule by CO2 Laser; Huazhong University of Science and Technology: Wuhan, China, 2008. (In Chinese) [Google Scholar]
- Levesque, M.; Labranche, B.; Forest, R.; Savard, É.; Deshaies, S.; Cournoyer, A. Welding of glass pieces. Phys. Procedia 2010, 5, 139–144. [Google Scholar] [CrossRef]
- Wang, W.H.; Yu, Q.X.; Jiang, X.S. CO2 Laser Hot-Melt Welding Process for Ultrathin Fused Quartz Glass. Chin. J. Lasers 2012, 39, 53–59. (In Chinese) [Google Scholar]
- Cai, Z.C.; Hu, Z.Y.; Cheng, G.L.; Zhang, Z.Y.; Wang, Z.H.; Wang, W. Research on Laser Welding Process of Fused Quartz Glass Based on ANSYS. Equip. Manuf. Technol. 2014, 5, 71–73. (In Chinese) [Google Scholar]
- Pohl, L.; Witzendorff, P.; Suttmann, O.; Overmeyer, L. Automated laser-based glass fusing with powder additive. In Proceedings of the ICALEO 2014: 33nd International Congress on Laser Materials Processing, Laser Microprocessing and Nanomanufacturing, San Diego, CA, USA, 19–23 October 2014. [Google Scholar]
- Pohl, l.; Witzendorff, P.; Chatzizyrli, E.; Suttmann, O.; Overmeyer, L. CO2 laser welding of glass: Numerical simulation and experimental study. Int. J. Adv. Manuf. Technol. 2017, 90, 397–403. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sui, G.H. Development of High-Temperature Fiber Optic Pressure Sensor Based on CO2 Laser Welding. Metrol. Meas. Technol. 2021, 41, 30–33. (In Chinese) [Google Scholar]
- Alexeev, I.; Cvecek, K.; Schmidt, C.; Miyamoto, I.; Frick, T.; Schmidt, M. Characterization of Shear Strength and Bonding Energy of Laser Produced Welding Seams in Glass. J. Laser Micro/Nanoeng. 2012, 7, 279–283. [Google Scholar] [CrossRef]
- Weber, M.J. Handbook of Optical Material, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 5–156. [Google Scholar]
- Topcu, T.; Robicheaux, F. Dichotomy between tunneling and multiphoton ionization in atomic photoionization: Keldysh parameter γ versus scaled frequency Ω. Physical Review A—At. Mol. Opt. Physic 2012, 86, 053407. [Google Scholar] [CrossRef]
- Tamaki, T.; Watanabe, W.; Nishii, J.; Itoh, K. Welding of transparent materials using femtosecond laser pulses. Jpn. J. Appl. Phys. 2005, 44, L687–L689. [Google Scholar] [CrossRef]
- Watanabe, W.; Onda, S.; Tamaki, T.; Itoh, K.; Nishii, J. Space-selective laser joining of dissimilar transparent materials using femtosecond laser pulses. Appl. Phys. Lett. 2006, 89, 021106. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J.; Lee, Y.; Kim, T.; Kim, D.; Jang, W.; Lim, K.S.; Sohn, I.B.; Lee, J. Femtosecond laser bonding of glasses and ion migration in the interface. Appl. Phys. A 2010, 101, 147–152. [Google Scholar] [CrossRef]
- Itoh, K.; Ozeki, Y. Novel processes in laser microfabrication and microjoining. In Proceedings of the International Congress on Applications of Lasers & Electro-Optics, Anaheim, CA, USA, 26–30 September 2010. [Google Scholar]
- Ji, C.; Yang, C.; Wang, C.; Yu, Z.; Wu, J.; Yuan, L.; Long, Y. A review of the progress and challenges in ultrafast laser micro-welding of brittle optical materials. Opt. Laser Technol. 2025, 192, 113570. [Google Scholar] [CrossRef]
- Miyamoto, I.; Cvecek, K.; Schmidt, C. Evaluation of nonlinear absorptivity in internal modification of bulk glass by ultrashort laser pulses. Opt. Express 2011, 19, 10714–10727. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, I.; Cvecek, K.; Schmidt, M. Crack-free conditions in welding of glass by ultrashort laser pulse. Opt. Express 2013, 21, 14291–14302. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Döring, S.; Tünnermann, A.; Nolte, S. Bonding of glass with femtosecond laser pulses at high repetition rates. Appl. Phys. A 2011, 103, 257–261. [Google Scholar] [CrossRef]
- Cvecek, K.; Miyamoto, I.; Johannes, S.; Viet, B.; Sonja, S. Strength of joining seams in glass welded by ultra-fast lasers depending on focus height. J. Laser Micro Nanoeng. 2012, 7, 68. [Google Scholar] [CrossRef]
- Huang, S.; Chen, R.; Zhang, H.; Ye, J.; Yang, X.; Sheng, J. A study of welding process in connecting borosilicate glass by picosecond laser pulses based on response surface methodology. Opt. Laser Technol. 2020, 131, 106427. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, Y.; Liu, Y.; Fu, X. Technology ANSYS Workbench simulation of glass welding by femtosecond laser pulses. Infrared Phys. Technol. 2019, 98, 334–340. [Google Scholar] [CrossRef]
- Kaiser, E. Laser Welding of Glass Replaces Glueing Procedure: Glass welding with a femtosecond laser brings economic advantages and new design options. Laser Tech. J. 2016, 13, 22–25. [Google Scholar] [CrossRef]
- Tamaki, T.; Watanabe, W.; Itoh, K. Laser micro-welding of transparent materials by a localized heat accumulation effect using a femtosecond fiber laser at 1558 nm. Opt. Express 2006, 14, 10460–10468. [Google Scholar] [CrossRef]
- Cvecek, K.; Dehmel, S.; Miyamoto, I.; Schmidt, M. A review on glass welding by ultra-short laser pulses. Int. J. Extrem. Manuf. 2019, 1, 042001. [Google Scholar] [CrossRef]
- Wu, S.Z. Nvestigation on the Control of the Surface and Interfaceby Laser Micro/Nanofabrication Technologies. Ph.D. Thesis, Jilin University, Jilin, China, 2014. (In Chinese). [Google Scholar]
- Richter, S.; Zimmermann, F.; Sutter, D.; Budnicki, A.; Tünnermann, A.; Nolte, S. Ultrashort pulse laser welding of glasses without optical contacting. In Proceedings of the Ultrafast Optics: Biomedical, Scientific, and Industrial Applications XVII, San Francisco, CA, USA, 6–10 August 2017. [Google Scholar]
- Miao, Y.; Ting, H.; Rongshi, X. Long focal length green femtosecond laser welding of glass. Chin. J. Lasers 2020, 47, 090200510. [Google Scholar] [CrossRef]
- Chen, J.; Carter, R.M.; Thomson, R.R.; Hand, D.P. Avoiding the requirement for pre-existing optical contact during picosecond laser glass-to-glass welding. Opt. Express 2015, 23, 18645–18657. [Google Scholar] [CrossRef]
- Shanshool, H.M.; Naser, H.; Hadi, N.M.; Flaih, H.A.; Abbas, F.M.; Hussin, M.J.; Hindal, S. Parameters affecting the welding of transparent materials using femtosecond laser pulses. Lasers Manuf. Mater. Process. 2020, 7, 59–73. [Google Scholar] [CrossRef]
- Jiang, N.; Qian, R.; Yin, H.; Ni, C.; Liu, Y.; Qiao, H.; Wang, C. Mechanistic optimization of wide-gap ultrafast laser quartz glass welding with plasma dynamics. J. Mater. Process. Technol. 2025, 340, 118840. [Google Scholar] [CrossRef]
- Richter, S.; Zimmermann, F.; Eberhardt, R.; Tünnermann, A.; Nolte, S. Toward laser welding of glasses without optical contacting. Appl. Phys. A 2015, 121, 1–9. [Google Scholar] [CrossRef]
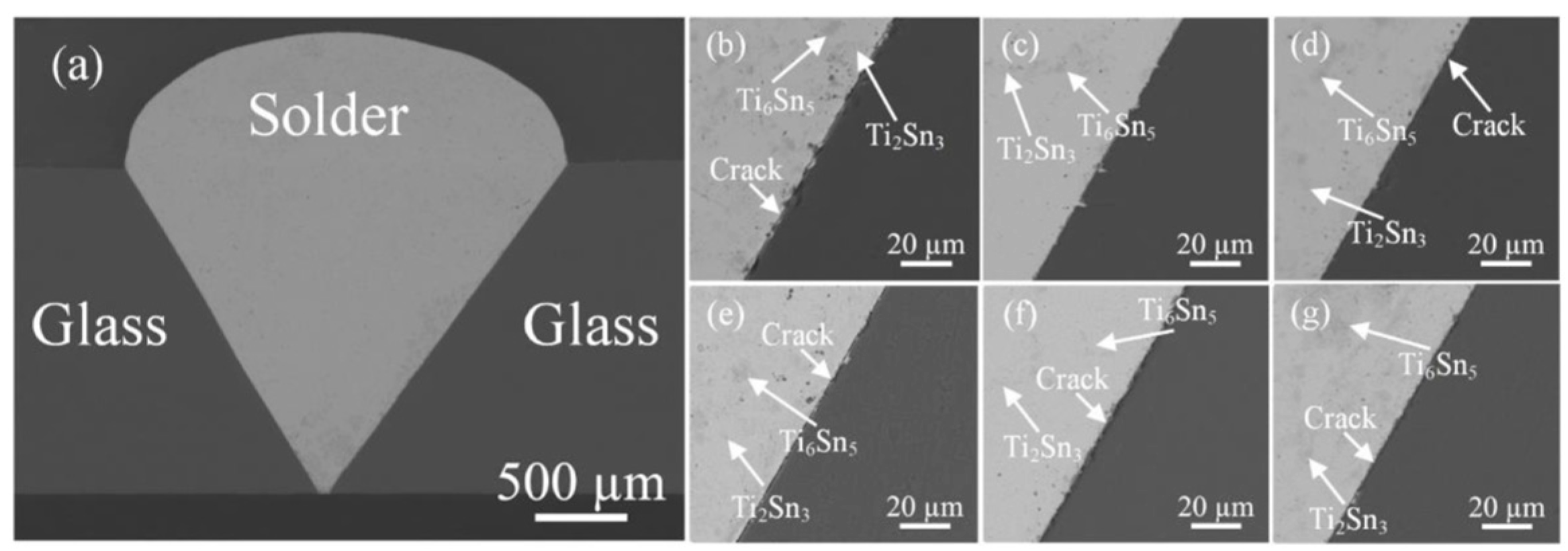
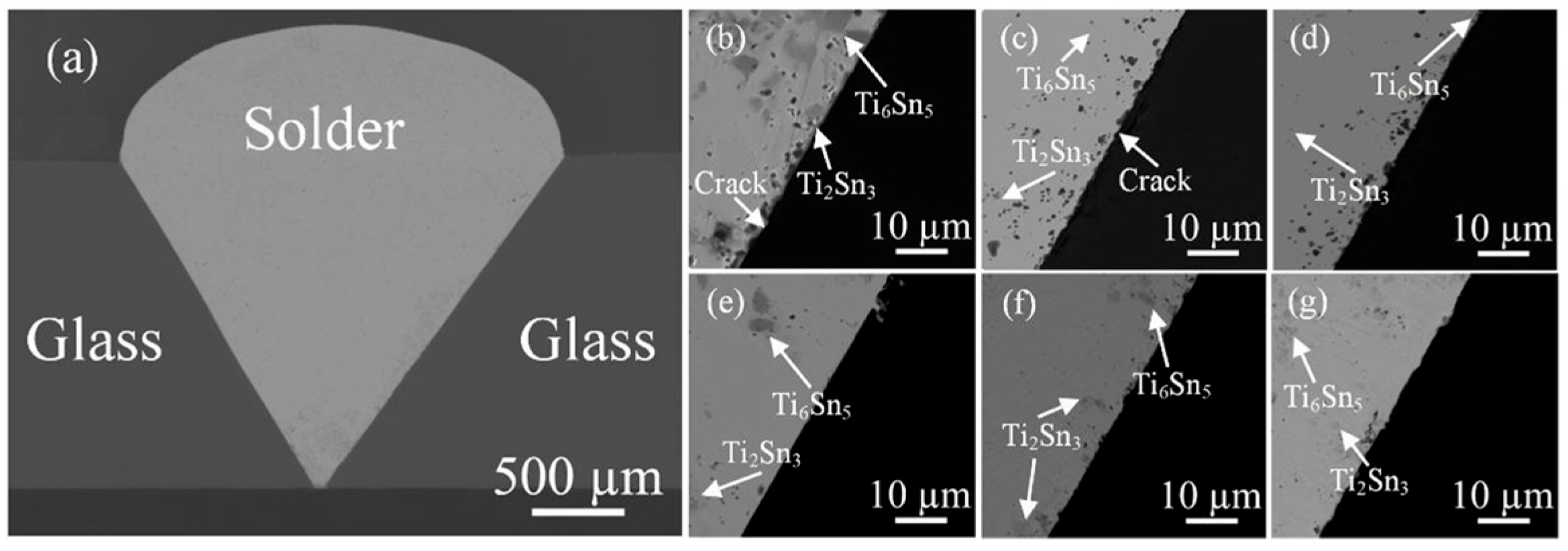
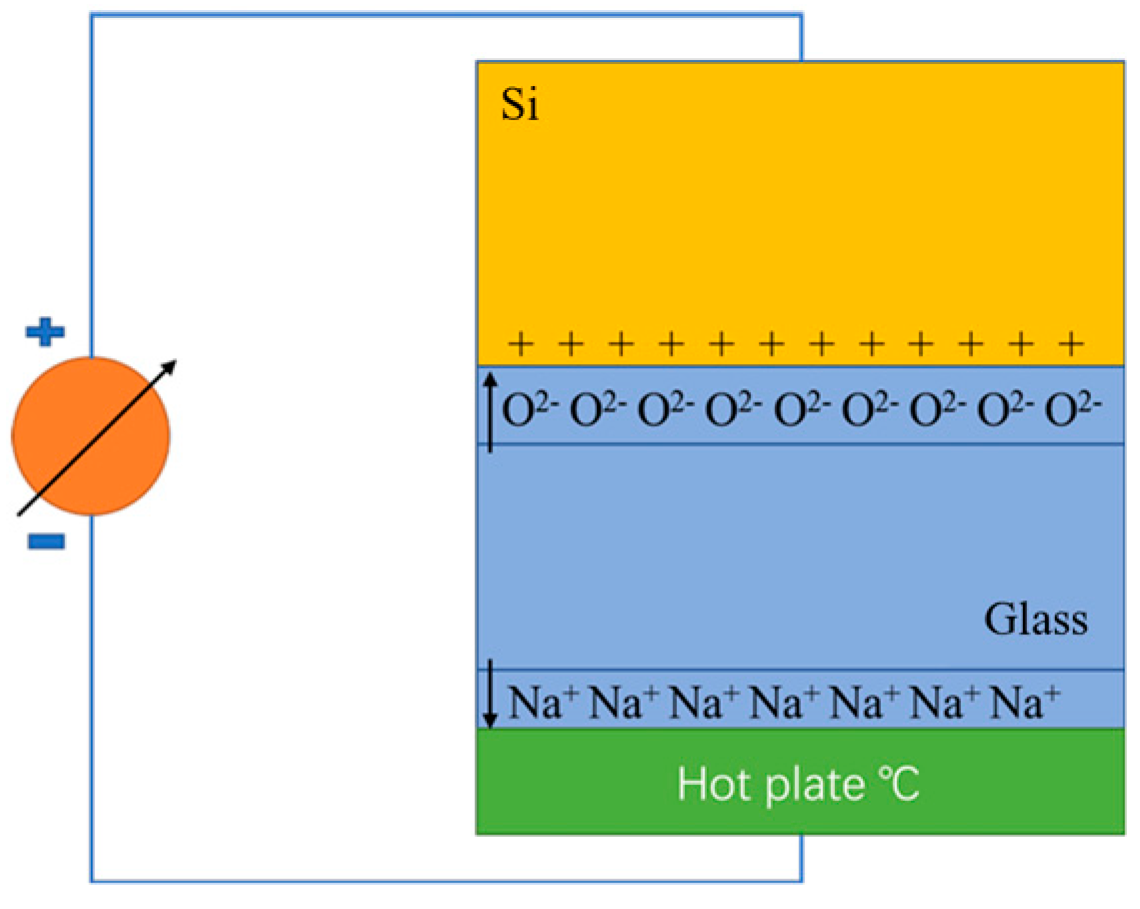
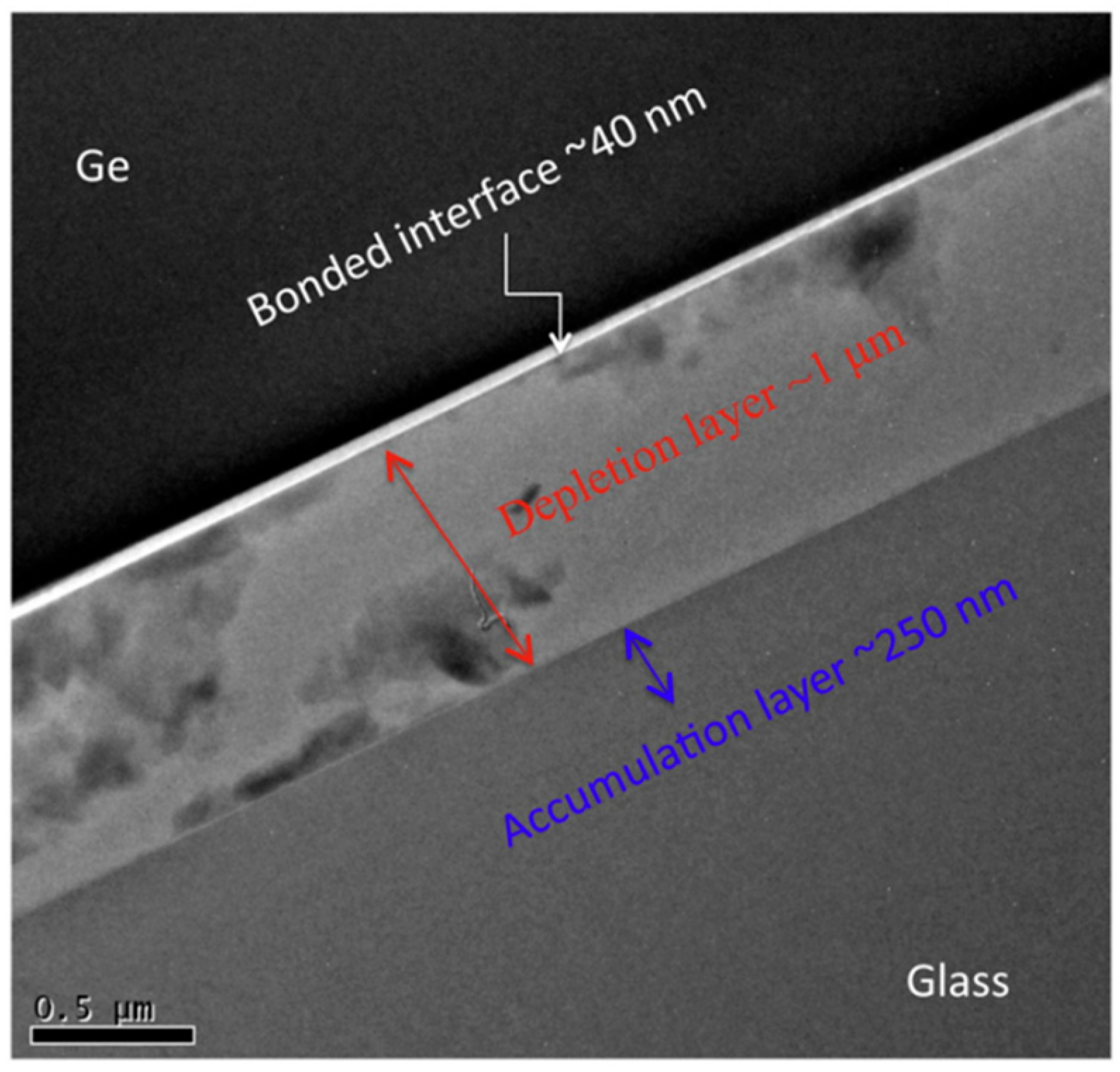
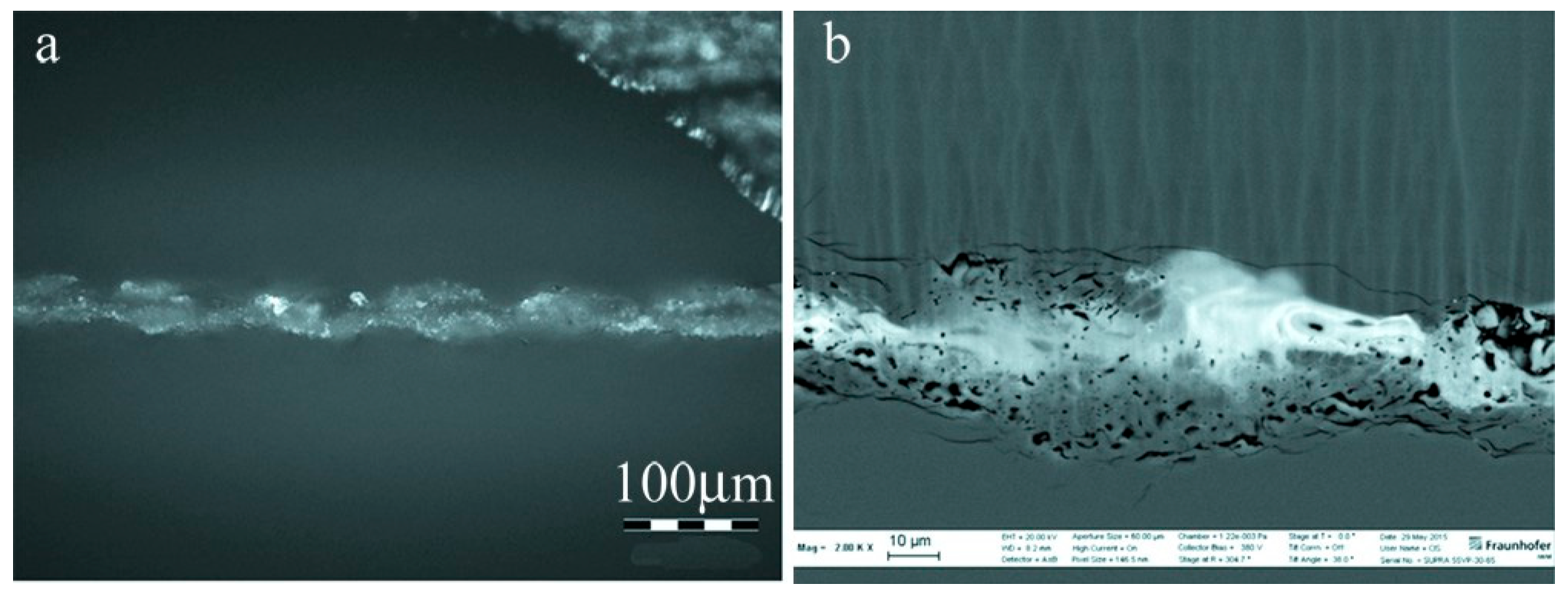
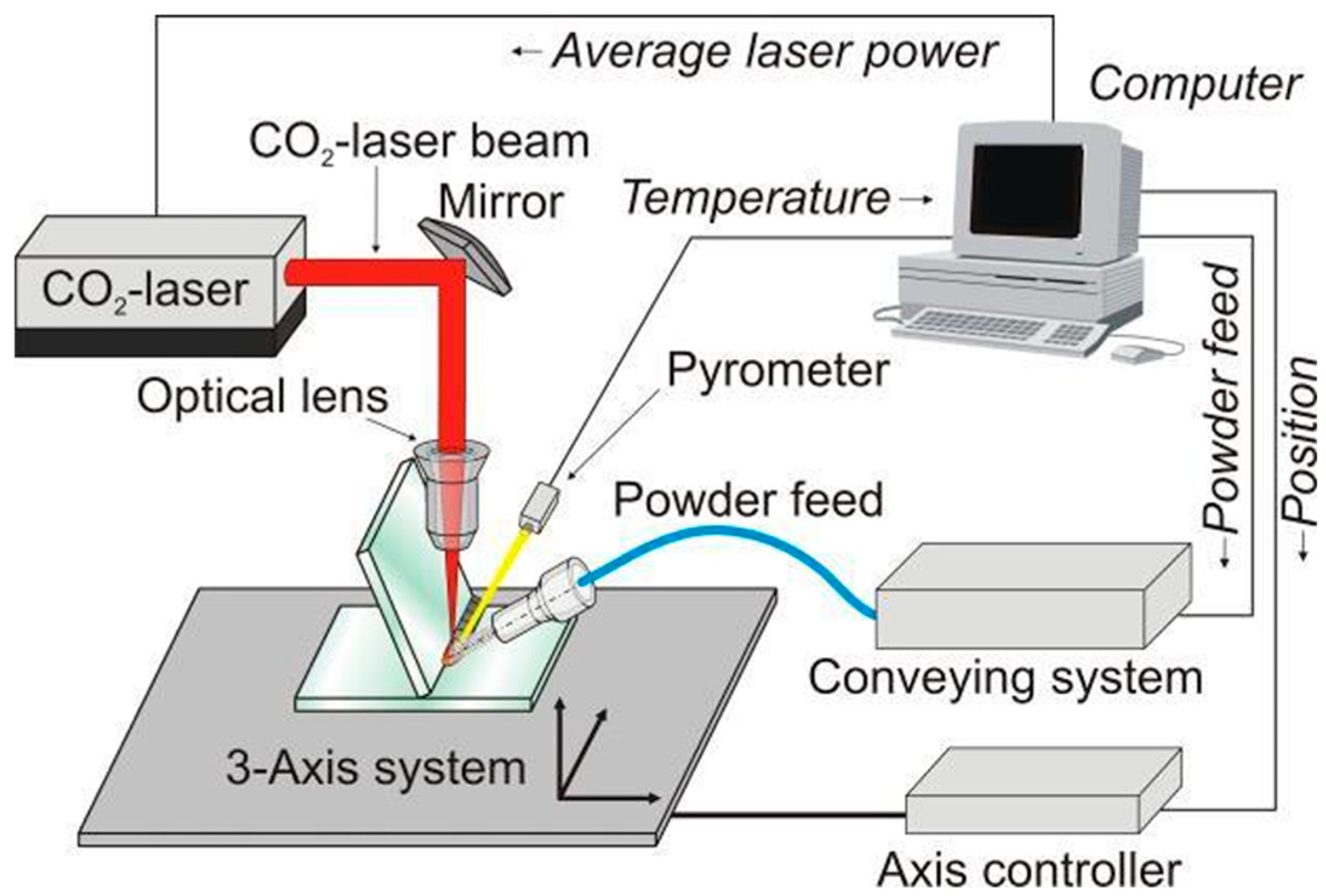
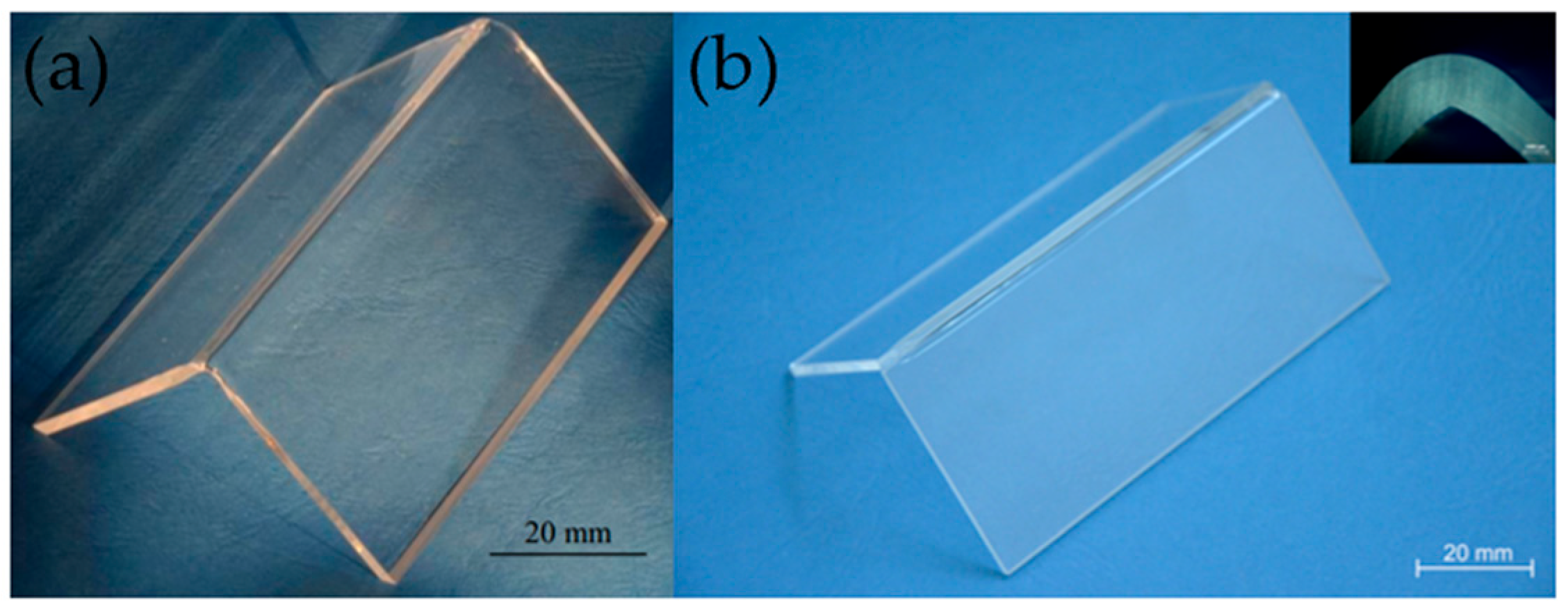
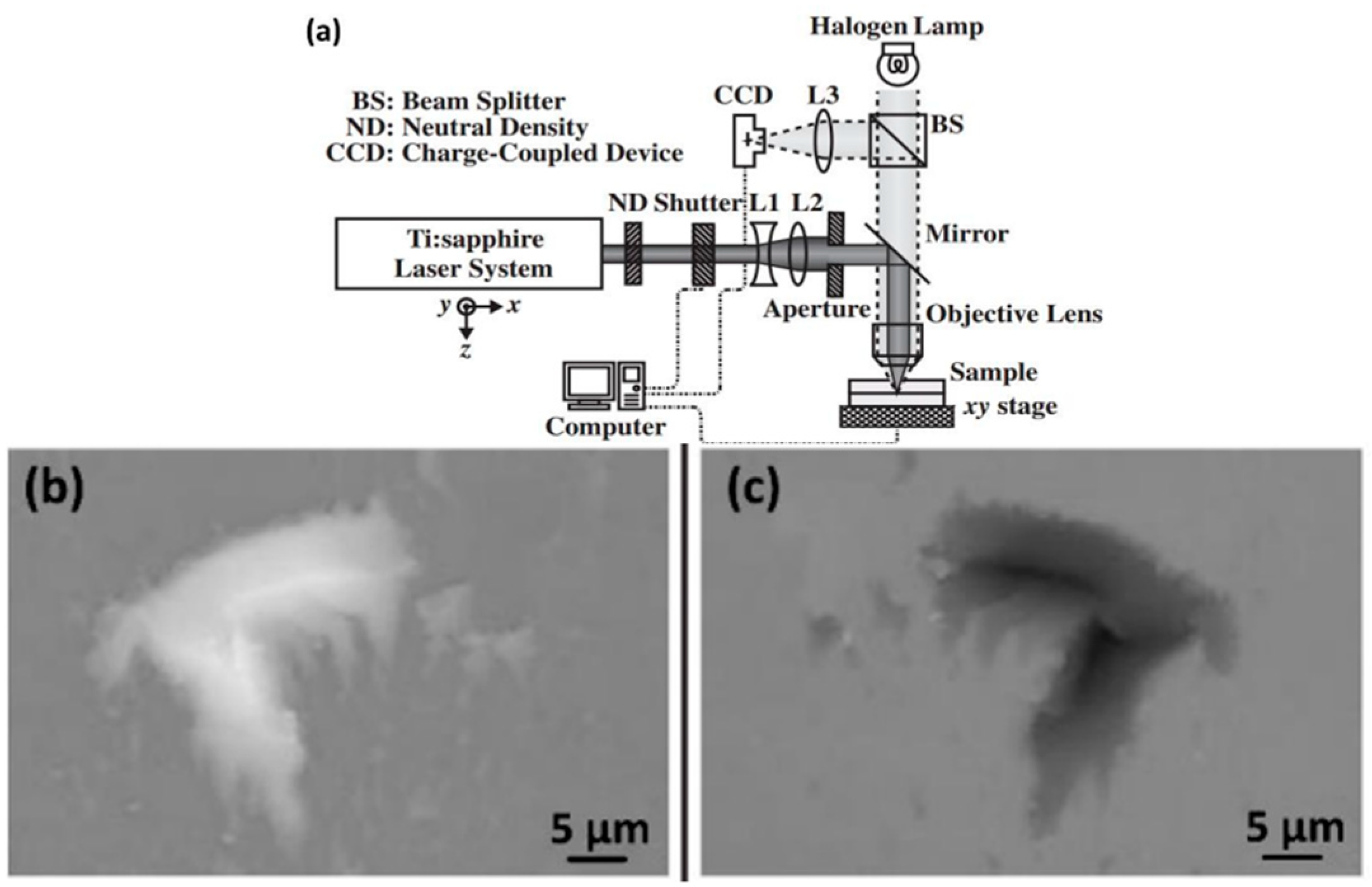
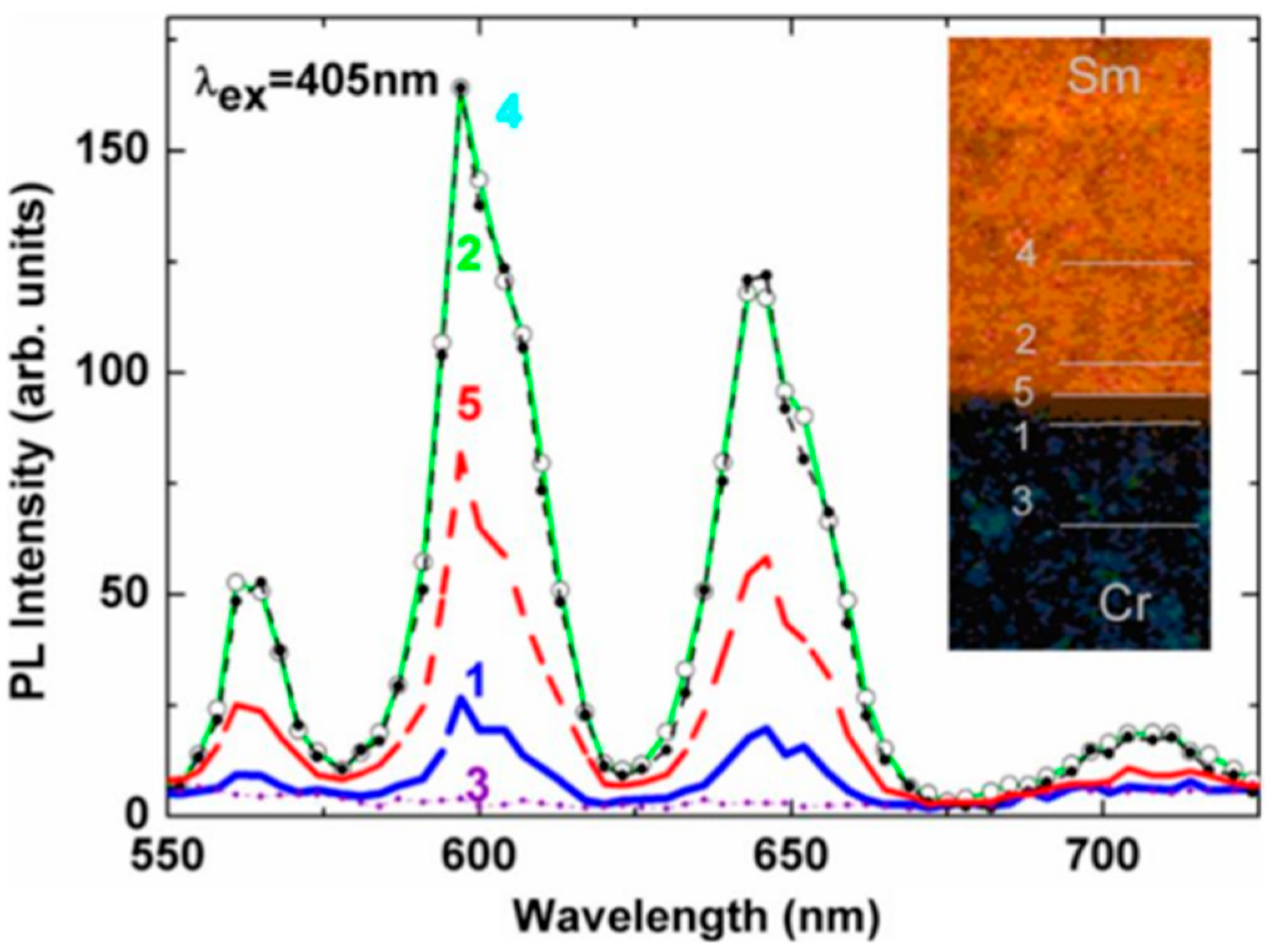
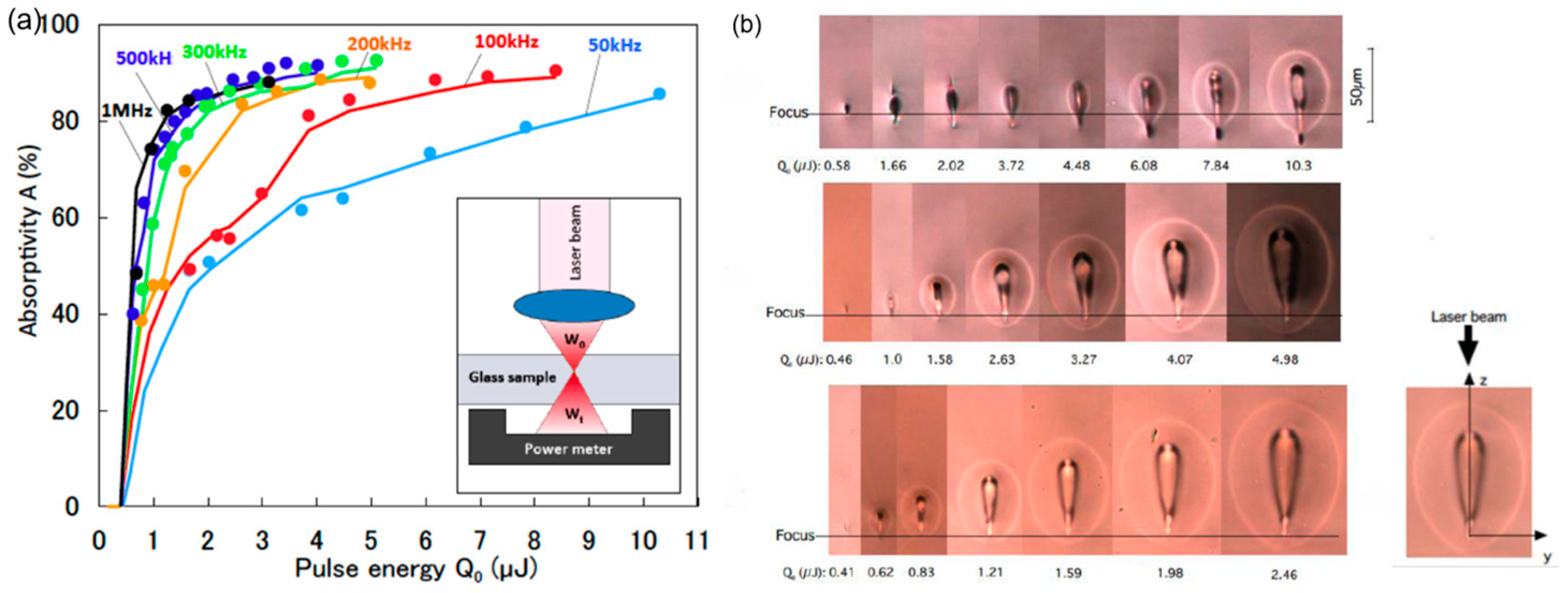
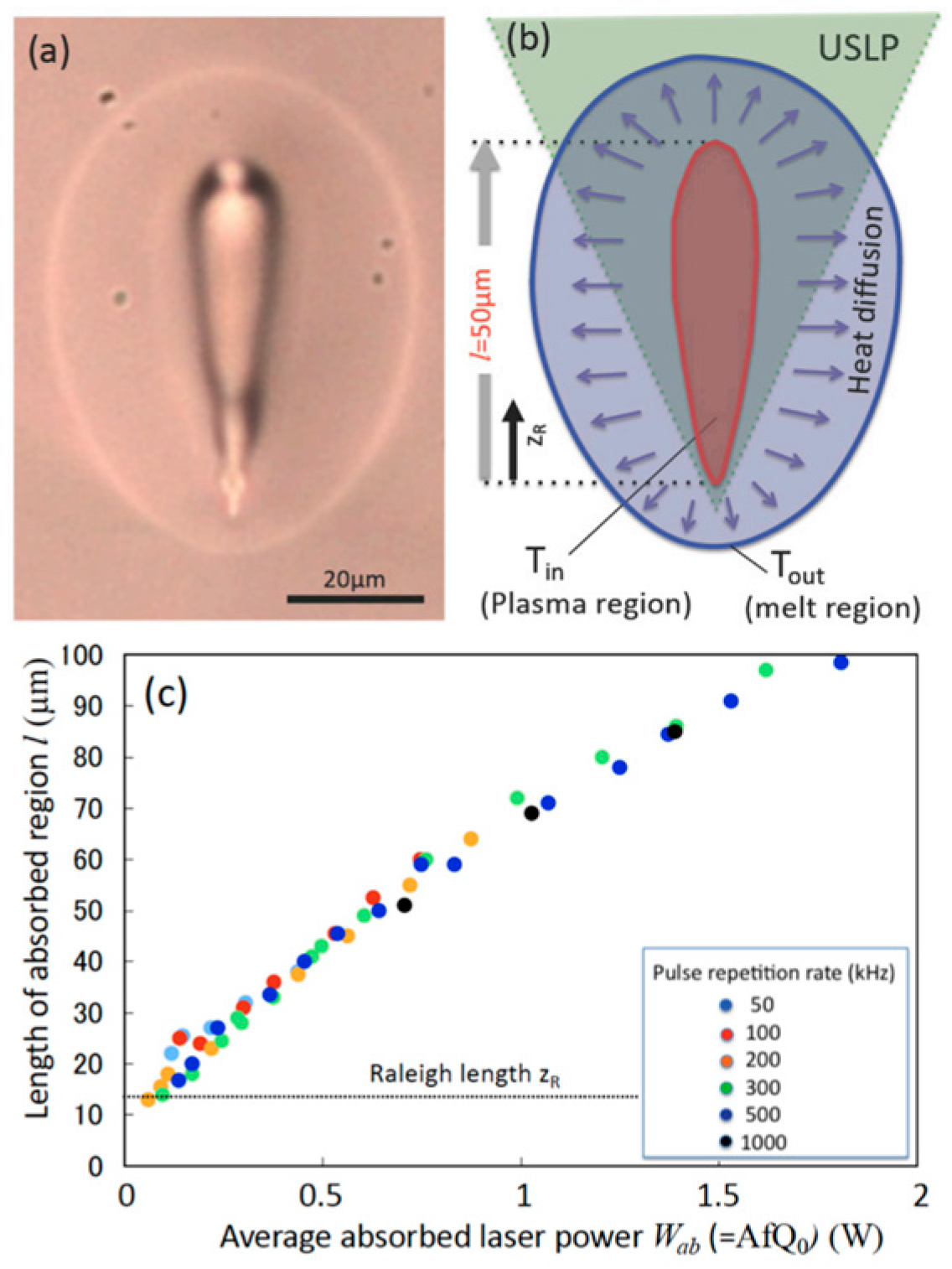
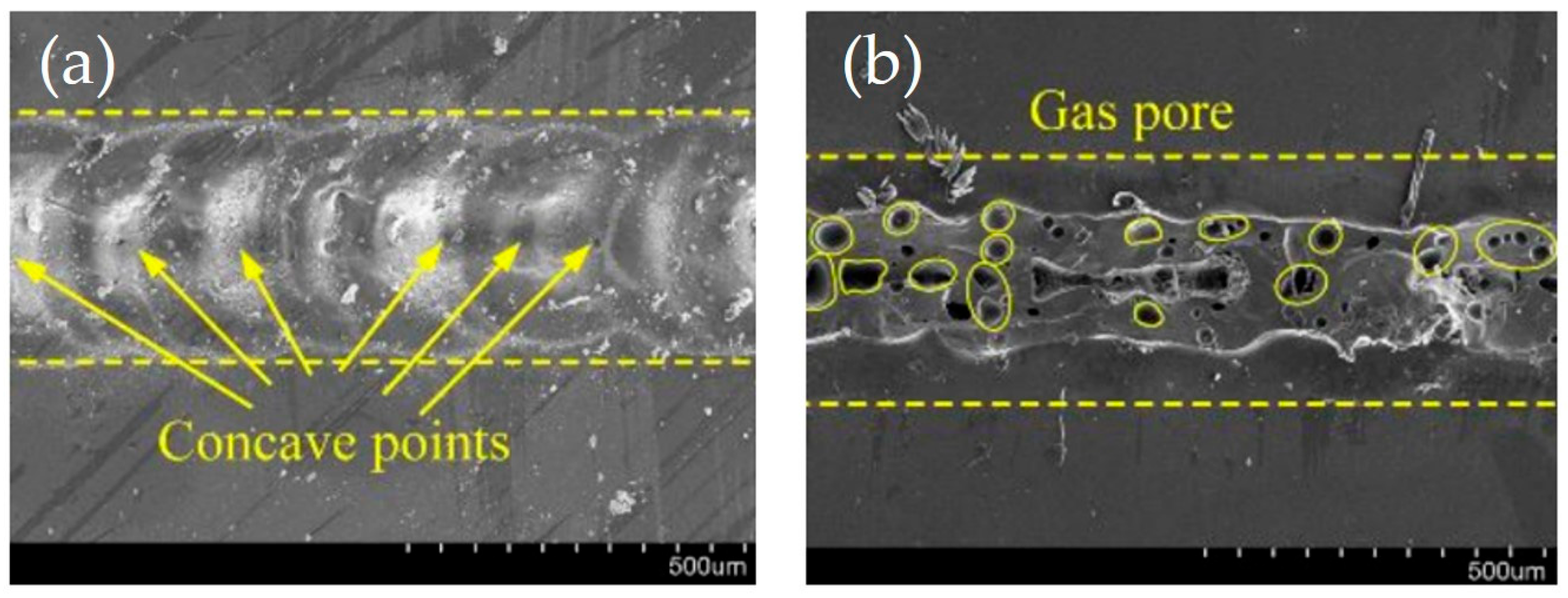
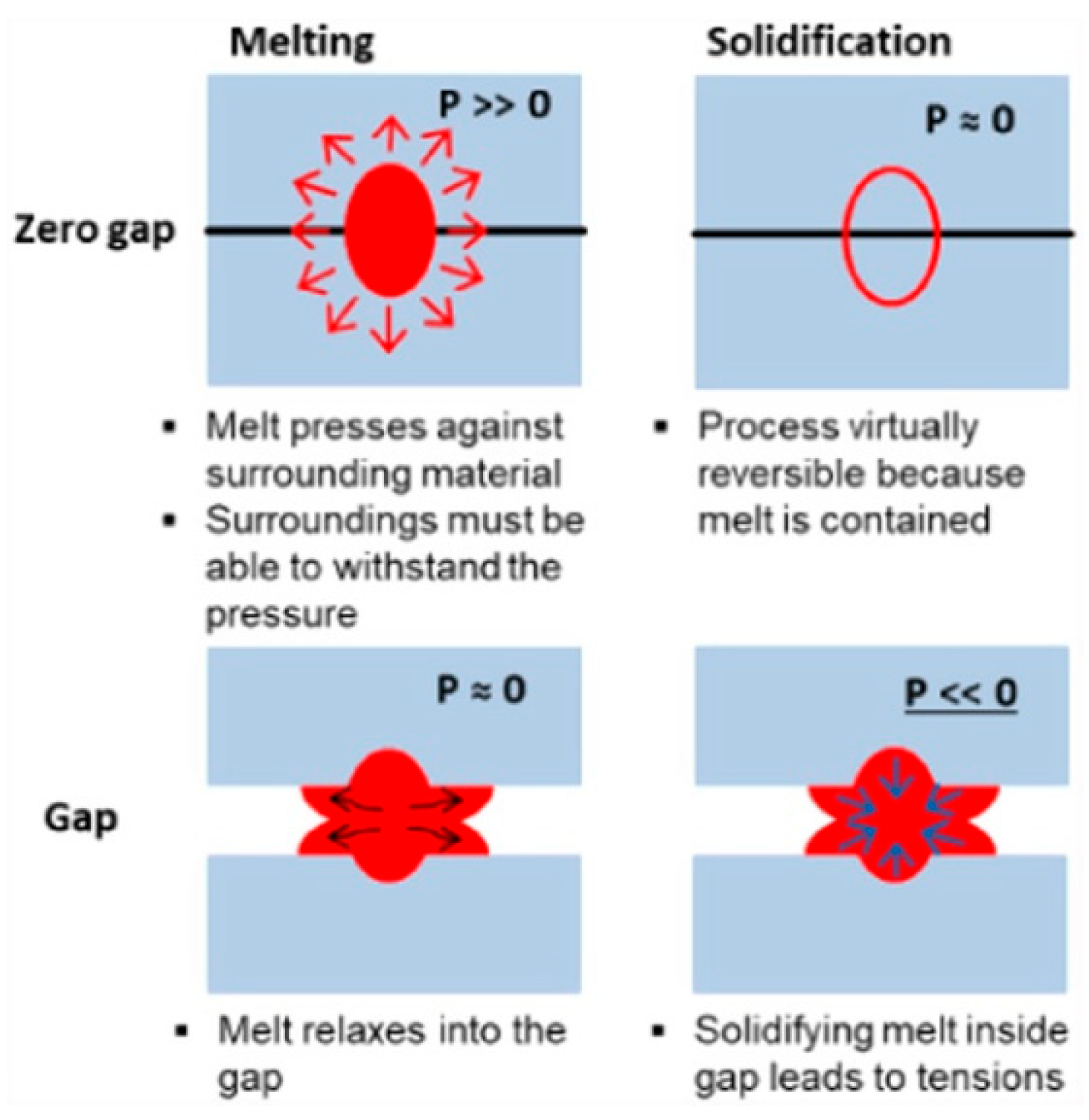
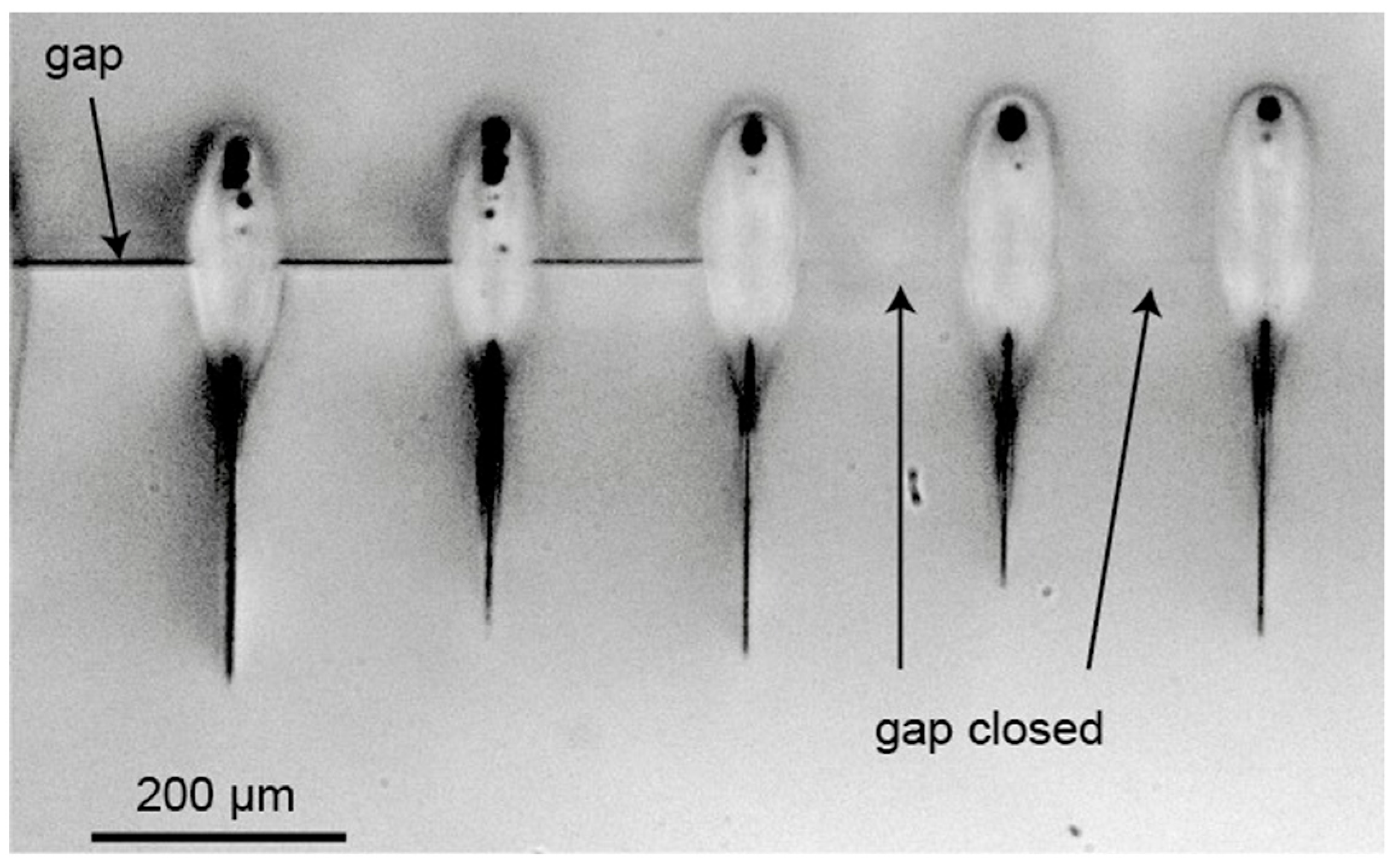
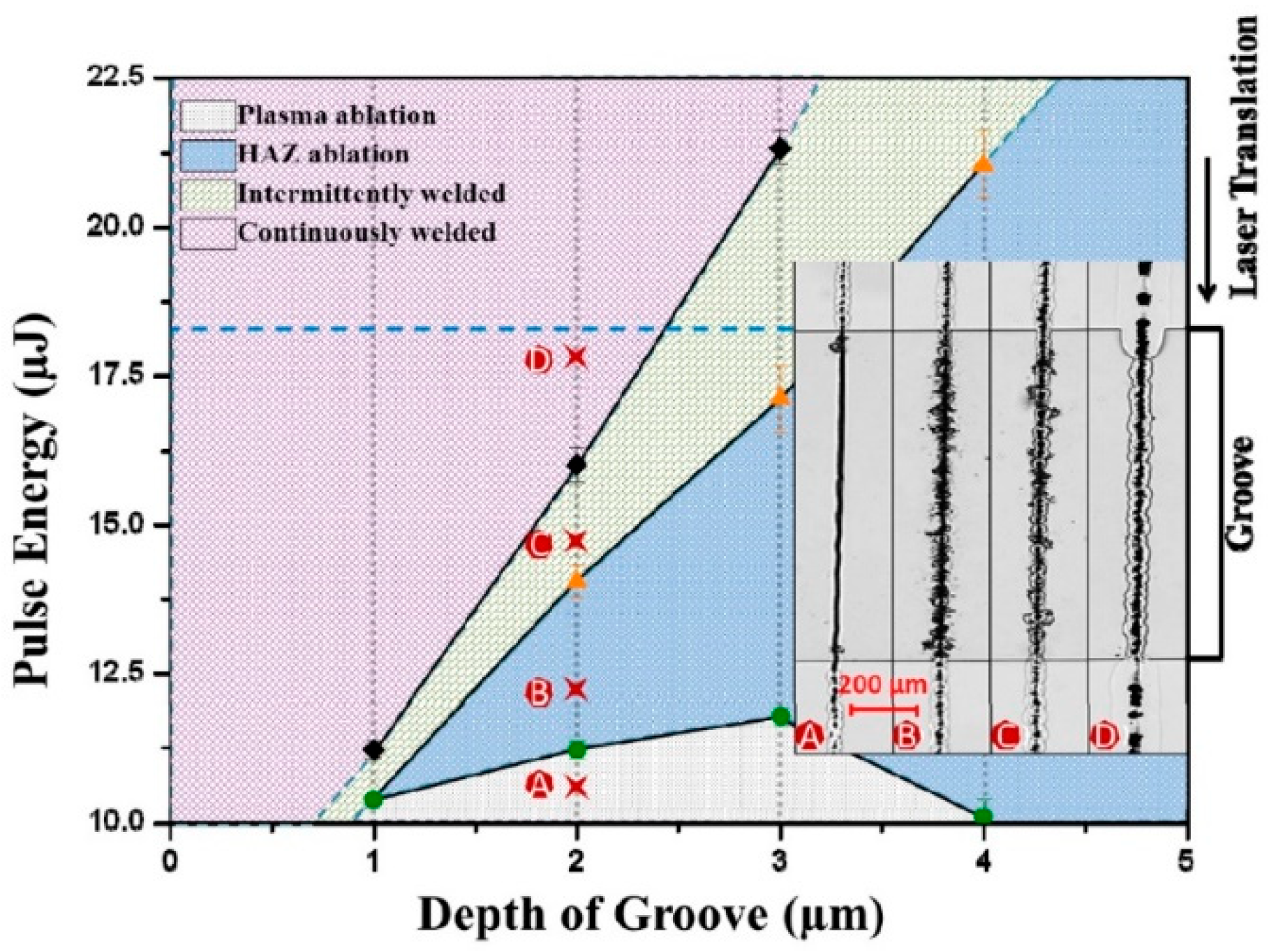
| Method | Advantages | Limitations | Typical Applications | Industrial Status |
|---|---|---|---|---|
| Brazing of Glass | High strength; good hermeticity | Residual stress from thermal mismatch; sensitive to glass composition | Micro-mechanical devices; vacuum equipment | Widely used in aerospace and optical assemblies |
| Ultrasonic Welding | Small heat-affected zone | Limited strength; requires smooth surfaces; rarely used directly on glass | - | - |
| Ultrasonic-assisted Brazing | Minimizes thermal damage; suitable for heat-sensitive glass | Risk of porosity at interface; oxidation if prolonged | Microfluidic devices; MEMS components | Experimental, emerging in precision applications |
| Anodic Bonding | High quality; exhibits good long-term stability | Requires smooth surface and matching CTE | MEMS sensors; microelectronic packaging | Widely adopted in MEMS and microsensors |
| Laser Welding | High precision; non-contact | High cost; needs good optical contact | Optical devices; MEMS sensors | Partially industrialized, mainly in optics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, D.; Ma, L.; Lu, J.; Wang, D.; Li, X. Advances of Welding Technology of Glass for Electrical Applications. Materials 2025, 18, 4096. https://doi.org/10.3390/ma18174096
Yan D, Ma L, Lu J, Wang D, Li X. Advances of Welding Technology of Glass for Electrical Applications. Materials. 2025; 18(17):4096. https://doi.org/10.3390/ma18174096
Chicago/Turabian StyleYan, Dejun, Lili Ma, Jiaqi Lu, Dasen Wang, and Xiaopeng Li. 2025. "Advances of Welding Technology of Glass for Electrical Applications" Materials 18, no. 17: 4096. https://doi.org/10.3390/ma18174096
APA StyleYan, D., Ma, L., Lu, J., Wang, D., & Li, X. (2025). Advances of Welding Technology of Glass for Electrical Applications. Materials, 18(17), 4096. https://doi.org/10.3390/ma18174096




