Nanostructured Metal Oxide from Metallic Glass for Water Splitting: Effect of Hydrothermal Duration on Structure and Performance
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Telford, M. The case for bulk metallic glass. Mater. Today 2004, 7, 36–43. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.; Koshiba, H.; Kato, H.; Yavari, A.R. Cobalt-based bulk glassy alloy with ultrahigh strength and soft magnetic properties. Nat. Mater. 2003, 2, 661–663. [Google Scholar] [CrossRef]
- Tian, L.; Cheng, Y.-Q.; Shan, Z.-W.; Li, J.; Wang, C.-C.; Han, X.-D.; Sun, J.; Ma, E. Approaching the ideal elastic limit of metallic glasses. Nat. Commun. 2012, 3, 609. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Park, E.S.; Chang, H.J.; Kim, D.H. Mg-rich Mg–Ni–Gd ternary bulk metallic glasses with high compressive specific strength and ductility. J. Mater. Res. 2007, 22, 334–338. [Google Scholar] [CrossRef]
- Schroers, J.; Johnson, W.L. Ductile bulk metallic glass. Phys. Rev. Lett. 2004, 93, 255506. [Google Scholar] [CrossRef]
- Chen, M. Mechanical behavior of metallic glasses: Microscopic understanding of strength and ductility. Annu. Rev. Mater. Res. 2008, 38, 445–469. [Google Scholar] [CrossRef]
- Hao, G.J.; Zhang, Y.; Lin, J.P.; Wang, Y.L.; Lin, Z.; Chen, G.L. Bulk metallic glass formation of Ti-based alloys from low purity elements. Mater. Lett. 2006, 60, 1256–1260. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, B. The correlation of damping capacity with grain-boundary precipitates in Fe–Cr-based damping alloys annealed at high temperature. Mater. Sci. Eng. A 2004, 366, 45–49. [Google Scholar] [CrossRef]
- Carmo, M.; Sekol, R.C.; Ding, S.; Kumar, G.; Schroers, J.; Taylor, A.D. Bulk metallic glass nanowire architecture for electrochemical applications. ACS Nano 2011, 5, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- An, B.W.; Gwak, E.J.; Kim, K.; Kim, Y.C.; Jang, J.; Kim, J.Y.; Park, J.U. Stretchable, Transparent Electrodes as Wearable Heaters Using Nanotrough Networks of Metallic Glasses with Superior Mechanical Properties and Thermal Stability. Nano Lett. 2016, 16, 471–478. [Google Scholar] [CrossRef]
- Doubek, G.; Sekol, R.C.; Li, J.; Ryu, W.; Gittleson, F.S.; Nejati, S.; Moy, E.; Reid, C.; Carmo, M.; Linardi, M. Guided evolution of bulk metallic glass nanostructures: A platform for designing 3D electrocatalytic surfaces. Adv. Mater. 2016, 28, 1940–1949. [Google Scholar] [CrossRef]
- Tiwari, K.; Douest, Y.; Giraldo-Osorno, P.M.; Galipaud, J.; Douillard, T.; Blanchard, N.; Palmquist, A.; Courtois, N.; Fabrègue, D.; Chevalier, J. Nanotopographical design and corrosion resistance improvement of Ti40Zr10Cu36Pd14 glassy alloy using alkaline chemical treatment. J. Alloys Compd. 2025, 1024, 180150. [Google Scholar] [CrossRef]
- Moghbeli, B.; Khanouki, M.T.A.; Ehteshamzadeh, M. Corrosion assessment of ZrCuAgAl bulk metallic glass in NaCl solutions with different pH values. J. Alloys Compd. 2025, 1036, 181793. [Google Scholar] [CrossRef]
- Guo, C.; Liu, G.; Zhang, S.; Wang, T. Controlling the static quenching temperature to improve the corrosion resistance performance of Zr-based bulk metallic glass. J. Non-Cryst. Solids 2025, 660, 123540. [Google Scholar] [CrossRef]
- Yang, Y.; Zhuang, C.; Liu, X.; Chen, L.; Zhu, Y.; Zhang, X.; Gao, M.; Li, H.; Shi, Z.; Liang, S. Study on the corrosion properties of Ti41.4Zr28.52Cu6.44Nb8.0Be15.64 metallic glass-based composites in binary acid-salt solutions. J. Alloys Compd. 2025, 1017, 179133. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, H.J.; Kim, T.K.; Hong, S.H.; Wang, W.M.; Choi, T.J.; Kim, K.B. Formation of photo-reactive heterostructure from a multicomponent amorphous alloy with atomically random distribution. J. Mater. Sci. Technol. 2022, 109, 245–253. [Google Scholar] [CrossRef]
- Bates, S.; Zografi, G.; Engers, D.; Morris, K.; Crowley, K.; Newman, A. Analysis of amorphous and nanocrystalline solids from their X-ray diffraction patterns. Pharm. Res. 2006, 23, 2333–2349. [Google Scholar] [CrossRef]
- Inoue, A.; Nishiyama, N.; Amiya, K.; Zhang, T.; Masumoto, T. Ti-based amorphous alloys with a wide supercooled liquid region. Mater. Lett. 1994, 19, 131–135. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Kim, W.T.; Kim, D.-H. Glass Forming Ability and Crystallization Behavior in Amorphous Ti50Cu32−xNi15Sn3Bex (x = 0, 1, 3, 7) Alloys. Mater. Trans. 2002, 43, 1243–1247. [Google Scholar] [CrossRef][Green Version]
- Dhawan, A.; Raetzke, K.; Faupel, F.; Sharma, S.K. Air oxidation of Zr65Cu17.5Ni10Al7.5 in its amorphous and supercooled liquid states, studied by thermogravimetric analysis. Phys. Status Solidi 2003, 199, 431–438. [Google Scholar] [CrossRef]
- Sharma, S.K.; Strunskus, T.; Ladebusch, H.; Faupel, F. Surface oxidation of amorphous Zr65Cu17.5Ni10Al7.5 and Zr46.75Ti8.25Cu7.5Ni10Be27.5. Mater. Sci. Eng. A 2001, 304, 747–752. [Google Scholar] [CrossRef]
- Premkumar, H.; Vadivel, S.; Al-Lohedan, H.; Syed, S.R.M. Synthesis and Characterization of Ternary ZnO/CuO/NiO Nanocomposite for Pseudocapacitive Energy Storage application. Vacuum 2025, 241, 114650. [Google Scholar] [CrossRef]
- Oleksak, R.P.; Hostetler, E.B.; Flynn, B.T.; McGlone, J.M.; Landau, N.P.; Wager, J.F.; Stickle, W.F.; Herman, G.S. Thermal oxidation of Zr–Cu–Al–Ni amorphous metal thin films. Thin Solid. Films 2015, 595, 209–213. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Salgueiriño-Maceira, V.; Liz-Marzán, L.M. Deposition of silver nanoparticles on silica spheres by pretreatment steps in electroless plating. Chem. Mater. 2001, 13, 1630–1633. [Google Scholar] [CrossRef]
- González-Angeles, A.; Mendoza-Suárez, G.; Grusková, A.; Dosoudil, R.; Ortega-Zempoalteca, R. Magnetic studies of Sn2+–Sn4+-substituted barium hexaferrites synthesized by mechanical alloying. Mater. Lett. 2004, 58, 2906–2910. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Lu, M.; Kuo, C.; Chen, L. Direct Conversion of Single-Layer SnO Nanoplates to Multi-Layer SnO2 Nanoplates with Enhanced Ethanol Sensing Properties. Adv. Funct. Mater. 2009, 19, 2453–2456. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Tu, Y.; Chen, S.; Li, X.; Gorbaciova, J.; Gillin, W.P.; Krause, S.; Briscoe, J. Control of oxygen vacancies in ZnO nanorods by annealing and their influence on ZnO/PEDOT: PSS diode behaviour. J. Mater. Chem. C 2018, 6, 1815–1821. [Google Scholar] [CrossRef]
- Lackner, P.; Zou, Z.; Mayr, S.; Diebold, U.; Schmid, M. Using photoelectron spectroscopy to observe oxygen spillover to zirconia. Phys. Chem. Chem. Phys. 2019, 21, 17613–17620. [Google Scholar] [CrossRef]
- Rosendal, V.; Pryds, N.; Petersen, D.H.; Brandbyge, M. Electron-vacancy scattering in SrNbO3 and SrTiO3: A density functional theory study with nonequilibrium Green’s functions. Phys. Rev. B 2024, 109, 205129. [Google Scholar] [CrossRef]
- Md Saad, S.K.; Ali Umar, A.; Ali Umar, M.I.; Tomitori, M.; Rahman, M.Y.A.; Mat Salleh, M.; Oyama, M. Two-Dimensional, Hierarchical Ag-Doped TiO2 Nanocatalysts: Effect of the Metal Oxidation State on the Photocatalytic Properties. ACS Omega 2018, 3, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen Doped Titanium Dioxide (N-TiO2): Synopsis of Synthesis Methodologies, Doping Mechanisms, Property Evaluation and Visible Light Photocatalytic Applications. Photochem 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Sharma, S.K.; Strunskus, T.; Ladebusch, H.; Zaporojtchenko, V.; Faupel, F. XPS study of the initial oxidation of the bulk metallic glass Zr46.75Ti8.25Cu7.5Ni10Be27.5. J. Mater. Sci. 2008, 43, 5495–5503. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014; ISBN 1482208687. [Google Scholar]
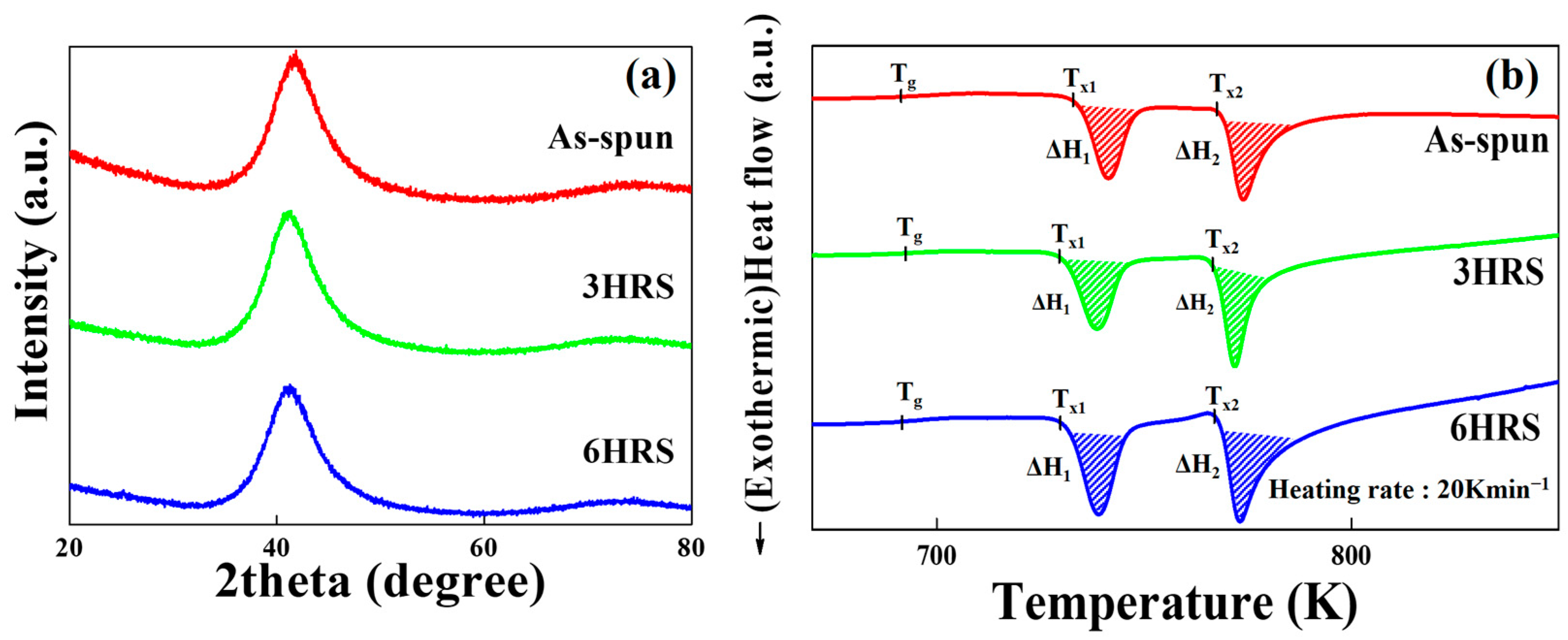

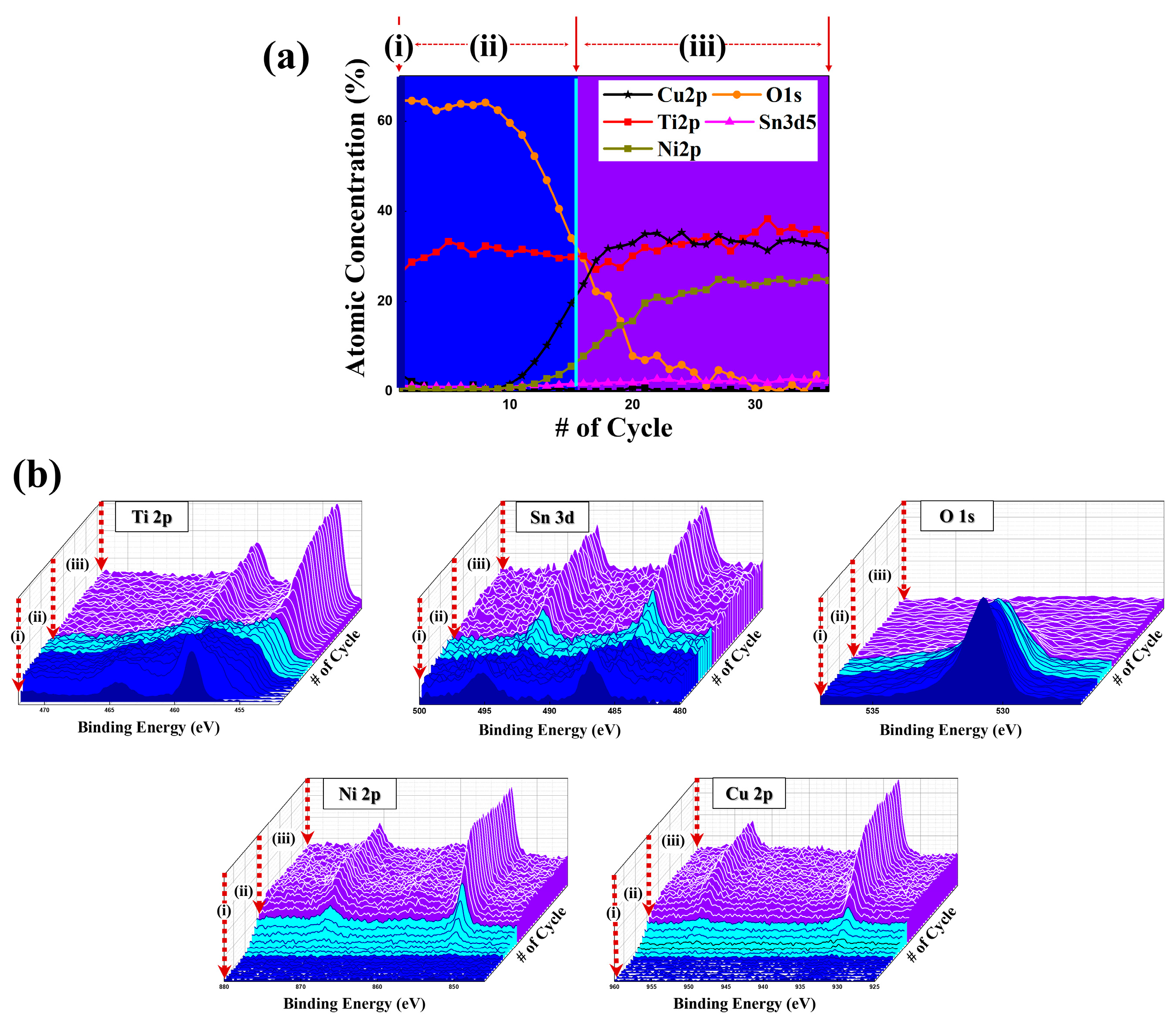
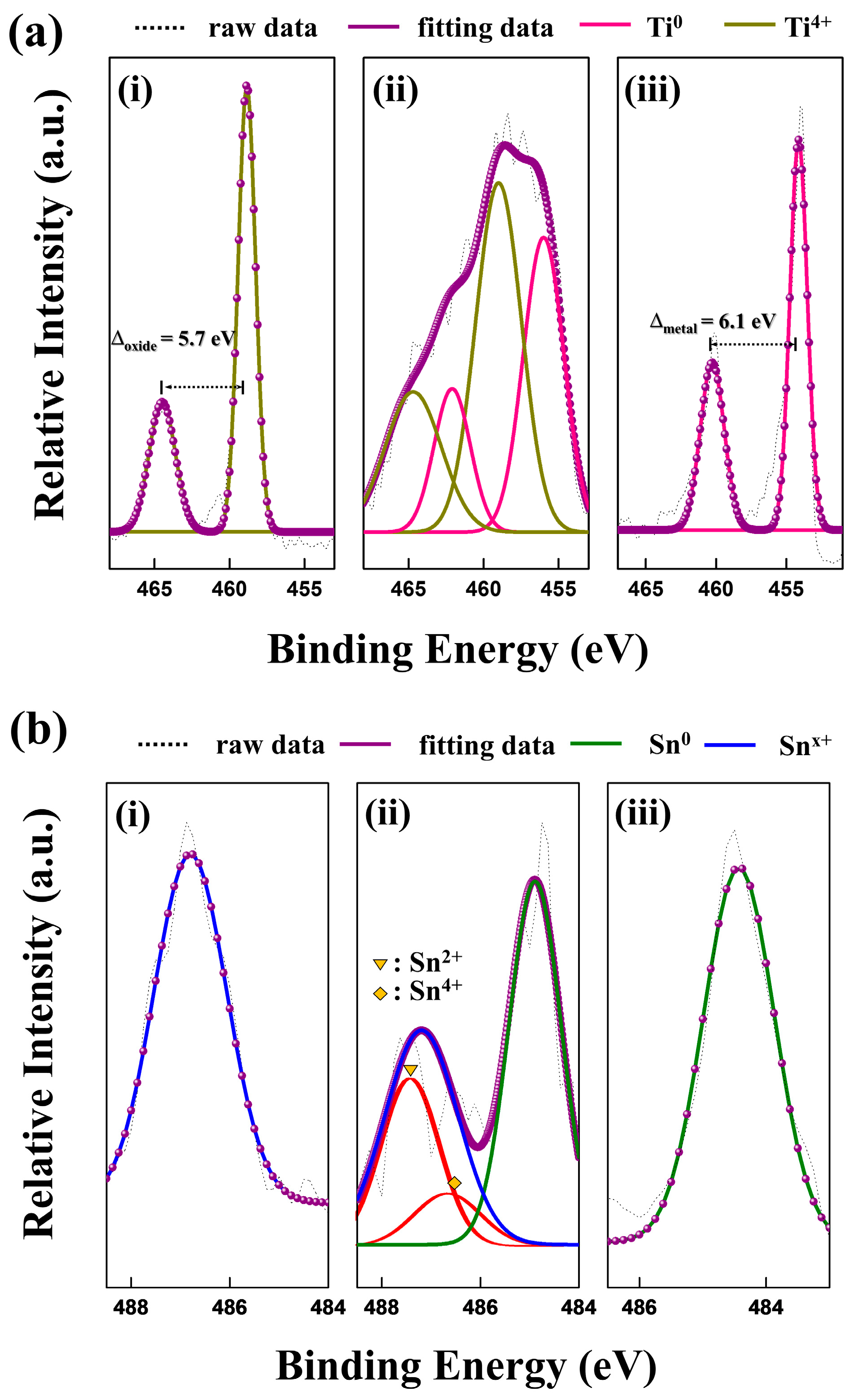
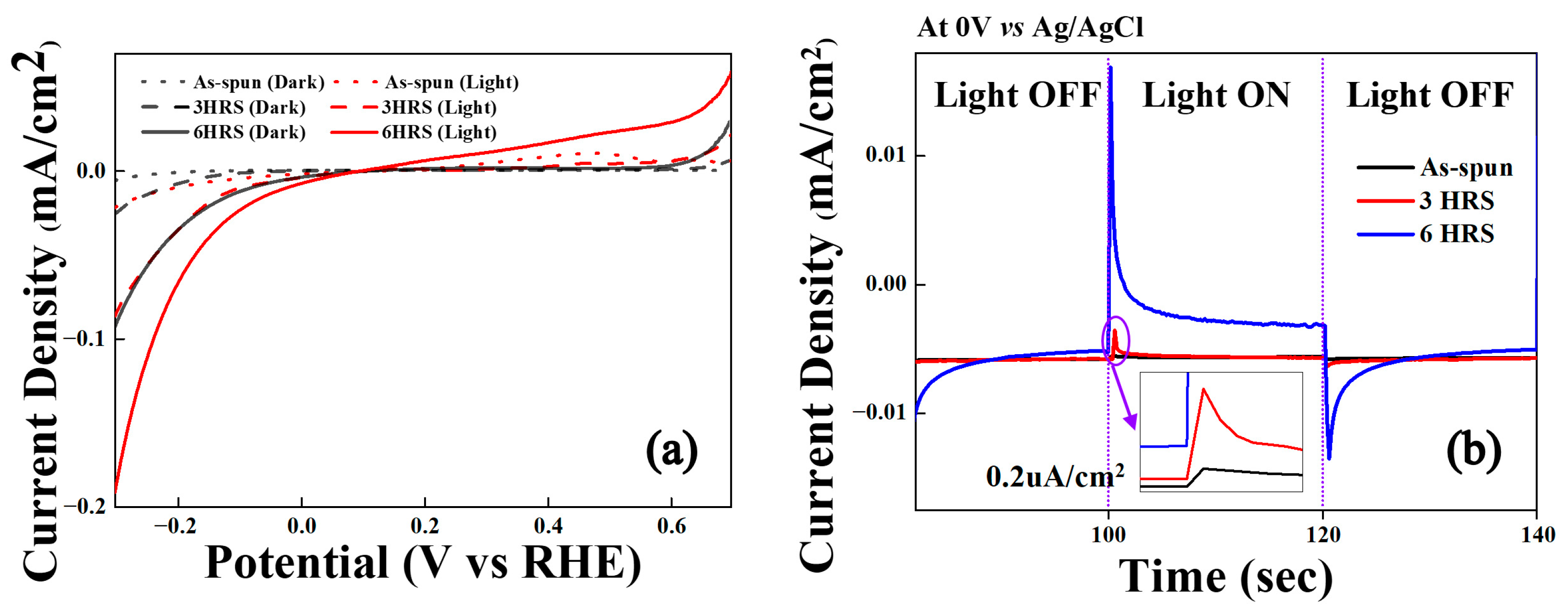
| Sample | Tg (K) | Tx1 (K) | ΔH1 (J/g) | Tx2 (K) | ΔH2 (J/g) |
|---|---|---|---|---|---|
| As-spun | 691.4 | 732.8 | –36.7 | 767.7 | –49.5 |
| 3HRS | 692.5 | 729.5 | –39.4 | 766.5 | –53.6 |
| 6HRS | 691.6 | 729.8 | –37.5 | 766.9 | –53.5 |
| 6HRS | Binding Energy (eV) | |||||
|---|---|---|---|---|---|---|
| Ti 2p | Sn 3d5/2 | |||||
| Layer | Surface | Oxide | Metal | Surface | Oxide | Metal |
| Ti4+ | Ti4+ | Snx+ | Snx+ | |||
| 464.5 | 464.7 | 486.8 | 487.2 | |||
| 458.8 | 459.0 | |||||
| Ti0 | Ti0 | Sn0 | Sn0 | |||
| 462.1 | 461.4 | 484.9 | 484.4 | |||
| 456.0 | 455.3 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.J.; Kim, T.K.; Eckert, J.; Hong, S.H.; Kim, K.B. Nanostructured Metal Oxide from Metallic Glass for Water Splitting: Effect of Hydrothermal Duration on Structure and Performance. Materials 2025, 18, 4082. https://doi.org/10.3390/ma18174082
Park HJ, Kim TK, Eckert J, Hong SH, Kim KB. Nanostructured Metal Oxide from Metallic Glass for Water Splitting: Effect of Hydrothermal Duration on Structure and Performance. Materials. 2025; 18(17):4082. https://doi.org/10.3390/ma18174082
Chicago/Turabian StylePark, Hae Jin, Tae Kyung Kim, Jürgen Eckert, Sung Hwan Hong, and Ki Buem Kim. 2025. "Nanostructured Metal Oxide from Metallic Glass for Water Splitting: Effect of Hydrothermal Duration on Structure and Performance" Materials 18, no. 17: 4082. https://doi.org/10.3390/ma18174082
APA StylePark, H. J., Kim, T. K., Eckert, J., Hong, S. H., & Kim, K. B. (2025). Nanostructured Metal Oxide from Metallic Glass for Water Splitting: Effect of Hydrothermal Duration on Structure and Performance. Materials, 18(17), 4082. https://doi.org/10.3390/ma18174082







