Physical–Chemical Assessment and Antimicrobial Activity of Chlortetracycline-Loaded Collagen Sponges
Abstract
1. Introduction
2. Materials and Methods
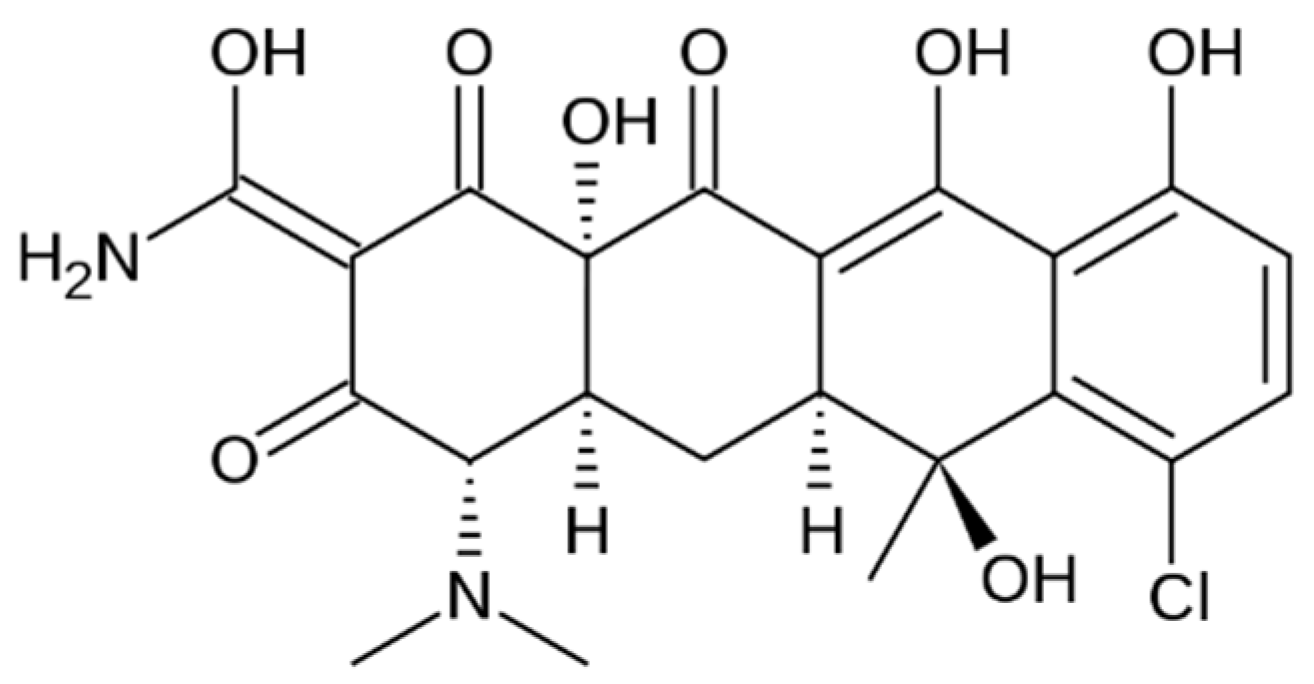
2.1. Materials
2.2. Preparation of Chlortetracycline-Loaded Collagen Sponges
2.3. Experimental Techniques
3. Results and Discussion
3.1. FT-IR Characterization of Chlortetracycline–Collagen Interactions
3.2. Optical Characterization by UV–VIS–NIR Spectroscopy
3.3. Assessment of Hydrophilic Properties via Swelling Test
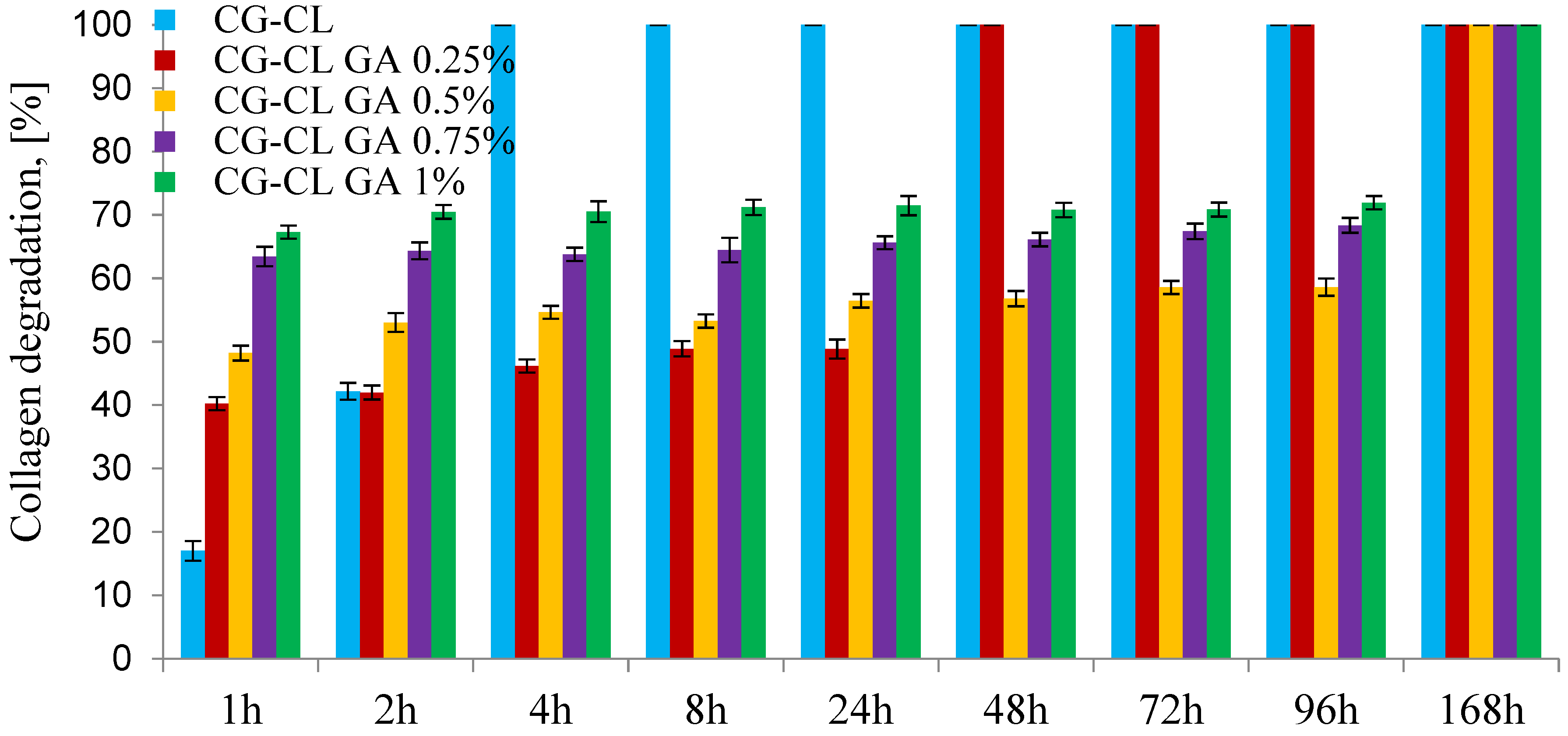
3.4. In Vitro Enzymatic Degradation Test
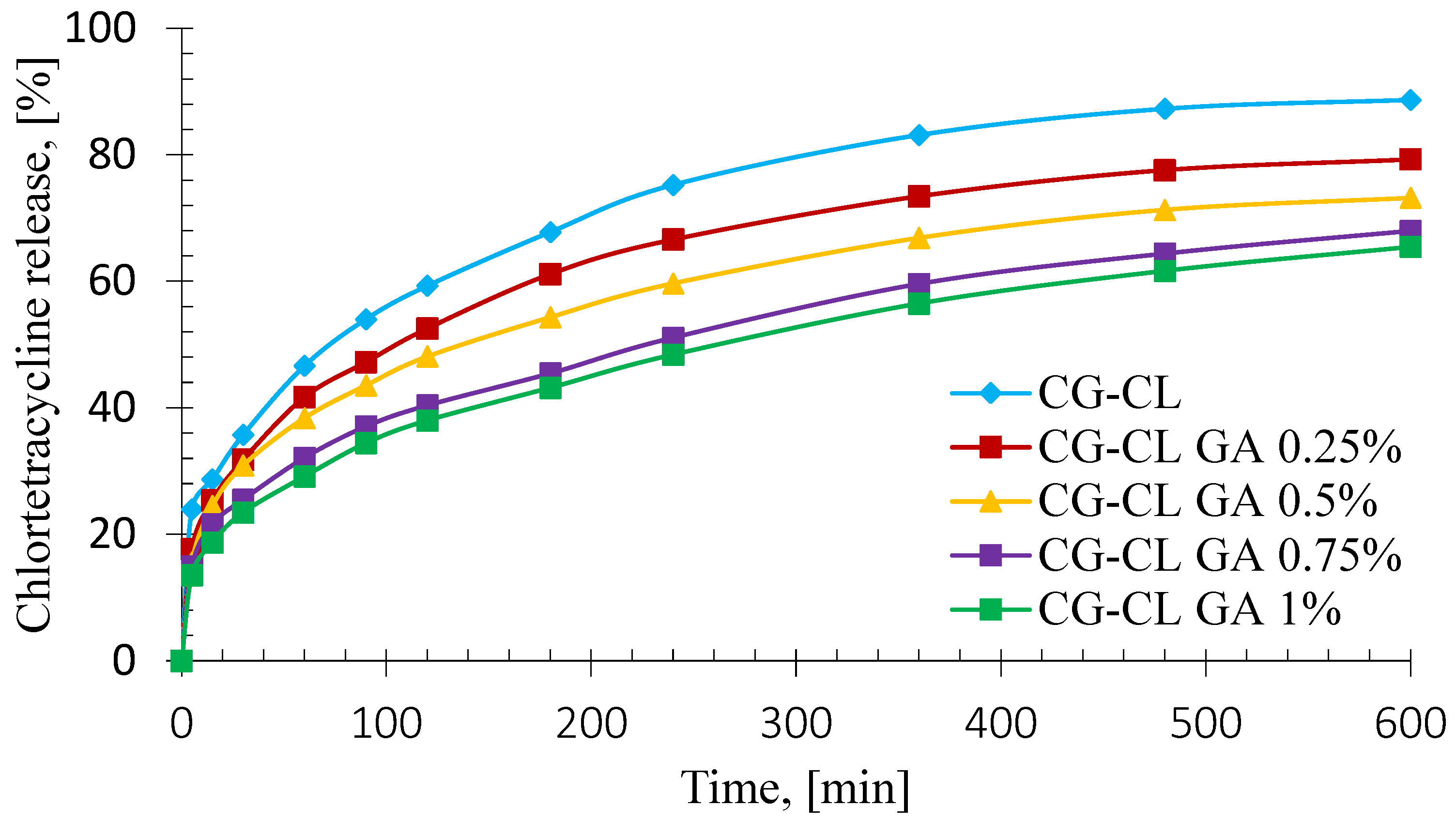
3.5. In Vitro Kinetics Release of Chlortetracycline
3.6. Evaluation of Antibacterial Activity
- (a)
- Drug incorporation and release
- (b)
- Interaction with Gram-Positive bacteria (e.g., S. aureus, E. faecalis)
- (c)
- Interaction with Gram-Negative bacteria (e.g., E. coli)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| E. coli | Escherichia coli |
| E. faecalis | Enterococcus faecalis |
| S. aureus | Staphylococcus aureus |
| GA | Glutaraldehyde |
| CG | Collagen Gel |
| CL | Chlortetracycline HCL |
| NaOH | Sodium Hydroxide |
| ATR | Attenuated Total Reflection |
| LB | Luria Bertani |
| MIC | Minimum inhibitory Concentration |
| OD | Optical density |
| SD | Standard Deviation |
| OTC | Oxytetracycline Hydrochloride |
| CP | Chloramphenicol |
| DXC | Doxycycline Hydrochloride |
| IB | Ibuprofen |
| R | Correlation Coefficient |
| AIC | Akaike Information Criterion |
References
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart dental materials for antimicrobial applications. Bioact. Mater. 2023, 24, 1–19. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, Z.; Jiang, Y.; Xu, L.; Qian, H.; Wang, Y.; Wang, W. Recent advances on nanomaterials for antibacterial treatment of oral diseases. Mater. Today Bio 2023, 20, 100635. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Mikulewicz, M. Multifunctional cellulose-based biomaterials for dental applications: A sustainable approach to oral health and regeneration. Ind. Crops Prod. 2023, 203, 117142. [Google Scholar] [CrossRef]
- Marin, E. History of dental biomaterials: Biocompatibility, durability and still open challenges. Herit. Sci. 2023, 11, 207. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Kulawik, M.; Kwiatek, J.; Bikiaris, D.; Cielecka-Piontek, J. Novel Applications of Natural Biomaterials in Dentistry—Properties, Uses, and Development Perspectives. Materials 2025, 18, 2124. [Google Scholar] [CrossRef]
- Sheehy, E.J.; Cunniffe, G.M.; O’Brien, F.J. 5-Collagen-based biomaterials for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 127–150. [Google Scholar]
- Ersanli, C.; Tzora, A.; Skoufos, I.; Voidarou, C.; Zeugolis, D.I. Recent Advances in Collagen Antimicrobial Biomaterials for Tissue Engineering Applications: A Review. Int. J. Mol. Sci. 2023, 24, 7808. [Google Scholar] [CrossRef]
- Tihan, G.T.; Rău, I.; Zgârian, R.G.; Ghica, M.V. Collagen-based biomaterials for ibuprofen delivery. Comptes Rendus. Chim. 2016, 19, 390–394. [Google Scholar] [CrossRef]
- Wosicka-Frąckowiak, H.; Poniedziałek, K.; Woźny, S.; Kuprianowicz, M.; Nyga, M.; Jadach, B.; Milanowski, B. Collagen and Its Derivatives Serving Biomedical Purposes: A Review. Polymers 2024, 16, 2668. [Google Scholar] [CrossRef]
- Tihan, G.T.; Rău, I.; Zgârian, R.G.; Ungureanu, C.; Barbaresso, R.C.; Kaya, M.G.A.; Dinu-Pîrvu, C.; Ghica, M.V. Oxytetracycline versus Doxycycline Collagen Sponges Designed as Potential Carrier Supports in Biomedical Applications. Pharmaceutics 2019, 11, 363. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Dental materials with antibiofilm properties. Dent. Mater. 2014, 30, e1–e16. [Google Scholar] [CrossRef]
- Jiao, Y.; Tay, F.R.; Niu, L.-n.; Chen, J.-h. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Protin, A.; Cameli, C.; Sérandour, A.-L.; Hamon, J.; Chaux, A.-G.; Guillemin, M.; Thibaut, F. Application of a topical collagen agent after tooth extraction to control hemostasis should be immediate and not delayed: A comparative randomized trial. J. Oral Med. Oral Surg. 2023, 29, 34. [Google Scholar] [CrossRef]
- Kim, J.-W.; Seong, T.-W.; Cho, S.; Kim, S.-J. Randomized controlled trial on the effectiveness of absorbable collagen sponge after extraction of impacted mandibular third molar: Split-mouth design. BMC Oral Health 2020, 20, 77. [Google Scholar] [CrossRef]
- Stępień, A.; Stępień, P.; Kornatowska, J. Dexmedetomidine as an additive to local anesthesia in dentistry. Prospect. Pharm. Sci. 2024, 22, 58–61. [Google Scholar] [CrossRef]
- Rajasekhar, R.; Soman, S.; Sebastian, V.M. Antimicrobial efficacy of calcium hypochlorite in endodontics: A systematic review of in vitro studies. Turk. Endod. J. 2022, 7, 28–42. [Google Scholar] [CrossRef]
- Zou, X.; Zheng, X.; Liang, Y.; Zhang, C.; Fan, B.; Liang, J.; Ling, J.; Bian, Z.; Yu, Q.; Hou, B.; et al. Expert consensus on irrigation and intracanal medication in root canal therapy. Int. J. Oral Sci. 2024, 16, 23. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Perinpanayagam, H.; Oh, S.; Kim, A.-R.; Han, S.-H.; Kum, K.-Y. Endodontic biofilms: Contemporary and future treatment options. Restor. Dent. Endod. 2019, 44, e7. [Google Scholar] [CrossRef] [PubMed]
- Warguła, J. Not only foils and packaging. Part 1: About the applications of polymers in ophthalmology, dentistry and medical equipment. Prospect. Pharm. Sci. 2024, 22, 135–145. [Google Scholar] [CrossRef]
- Qiu, W.; Zhou, Y.; Li, Z.; Huang, T.; Xiao, Y.; Cheng, L.; Peng, X.; Zhang, L.; Ren, B. Application of Antibiotics/Antimicrobial Agents on Dental Caries. BioMed Res. Int. 2020, 2020, 5658212. [Google Scholar] [CrossRef]
- Makaoui, N.; Moghni, N.; Boutemak, K.; Akkache, L.; Imoudache, H.; Zafour, A.H.-Z. Synergistic effect of synthesized ZnO nanoflowers coupled with various antibiotics against pathogenic microbes: Characterization, antibacterial and antifungal activity assessment. Nano-Struct. Nano-Objects 2023, 36, 101047. [Google Scholar] [CrossRef]
- Panwar, M.; Gupta, S.H. Local Drug Delivery with Tetracycline Fiber: An Alternative to Surgical Periodontal Therapy. Med. J. Armed Forces India 2009, 65, 244–246. [Google Scholar] [CrossRef]
- Braz, V.S.; Melchior, K.; Moreira, C.G. Escherichia coli as a Multifaceted Pathogenic and Versatile Bacterium. Front. Cell. Infect. Microbiol. 2020, 10, 548492. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Rehani, U.; Agarwal, A.; Kaushik, M.; Adlakha, V. Antimicrobial Efficacy of Endodontic Irrigants Against Enterococcus Faecalis and Escherichia coli: An in vitro study. Int. J. Clin. Pediatr. Dent. 2013, 6, 178–182. [Google Scholar] [CrossRef]
- Seng, M.L.; Estrada, R.S.E. Escherichia coli in root canal. Investig. Medicoquir. 2012, 4, 214–220. [Google Scholar]
- Hage, W.; Sarkis, D.K.; Kallassy, M.; Mallah, M.; Zogheib, C. In vitro evaluation of Enterococcus faecalis growth in different conditions on dentinal substrate. Biomater. Invest. Dent. 2023, 10, 2287668. [Google Scholar] [CrossRef]
- Chivatxaranukul, P.; Dashper, S.G.; Messer, H.H. Dentinal tubule invasion and adherence by Enterococcus faecalis. Int. Endod. J. 2008, 41, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, F.; Shakir, M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef]
- Castillo-Villagomez, P.; Madla-Cruz, E.; Lopez-Martinez, F.; Rodriguez-Delgado, I.; Flores-Treviño, J.J.; Malagon-Santiago, G.I.; de La Garza-Ramos, M.A. Antimicrobial effectiveness of root canal sealers against Enterococcus faecalis. Biomater. Investig. Dent. 2022, 9, 47–51. [Google Scholar] [CrossRef]
- BiblioMed Directory for Scholarly Articles. Available online: https://www.bibliomed.org/ (accessed on 30 June 2025).
- Tuon, F.F.; Suss, P.H.; Telles, J.P.; Dantas, L.R.; Borges, N.H.; Ribeiro, V.S.T. Antimicrobial Treatment of Staphylococcus aureus Biofilms. Antibiotics 2023, 12, 87. [Google Scholar] [CrossRef]
- Touaitia, R.; Mairi, A.; Ibrahim, N.A.; Basher, N.S.; Idres, T.; Touati, A. Staphylococcus aureus: A Review of the Pathogenesis and Virulence Mechanisms. Antibiotics 2025, 14, 470. [Google Scholar] [CrossRef]
- Ul Haq, I.; Khan, T.A.; Krukiewicz, K. Etiology, pathology, and host-impaired immunity in medical implant-associated infections. J. Infect. Public Health 2024, 17, 189–203. [Google Scholar] [CrossRef]
- Patianna, G.; Valente, N.A. The adjunctive use of locally delivered tetracyclines in periodontal therapy: A narrative review of the recent literature. J. Int. Dent. Med. Res. 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Sousa, F.F.O.; Luzardo-Álvarez, A.; Pérez-Estévéz, A.; Seoane-Prado, R.; Blanco-Méndez, J. Sponges containing tetracycline loaded-PLGA-zein microparticles as a periodontal controlled release device. J. Drug Deliv. Sci. Technol. 2020, 59, 101858. [Google Scholar] [CrossRef]
- Manuel, R.P.; Tania, G.G.; Rafael, S.P.; Antonio, P.E.; José, B.M.; Asteria, L.; Pablo, C.B.; Alejandro, L.P.; Pablo, Á.N.; Benjamín, M.B. In Vitro Development of a New Sponge-Based Delivery System for Intracanal Antimicrobial Administration in Endodontic Treatment. J. Clin. Med. 2021, 10, 2725. [Google Scholar] [CrossRef]
- Petrescu, N.; Crisan, B.; Aghiorghiesei, O.; Sarosi, C.; Mirica, I.C.; Lucaciu, O.; Iușan, S.A.L.; Dirzu, N.; Apostu, D. Gradual Drug Release Membranes and Films Used for the Treatment of Periodontal Disease. Membranes 2022, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Tihan, G.T.; Ungureanu, C.; Barbaresso, R.C.; Zgârian, R.G.; Rău, I.; Meghea, A.; Albu, M.G.; Ghica, M.V. Chloramphenicol collagen sponges for local drug delivery in dentistry. Comptes Rendus. Chim. 2015, 18, 986–992. [Google Scholar] [CrossRef]
- Barbaresso, R.; Rău, I.; Zgârian, R.G.; Meghea, A.; Ghica, M.V. Niflumic acid-collagen delivery systems used as anti-inflammatory drugs and analgesics in dentistry. Comptes Rendus. Chim. 2014, 17, 12–17. [Google Scholar] [CrossRef]
- Available online: https://www.shimclinic.com/singapore/meds/chlortralim-ophthalmic-ointment-1/ (accessed on 30 June 2025).
- AdvaCare Pharma USA. Chlortetracycline Eye Ointment. Available online: https://www.advacarepharma.com/en/pharmaceuticals/chlortetracycline-eye-ointment (accessed on 30 June 2025).
- Veterinary Medicines. Ophtocycline 10 mg/g Eye Ointment for Horses, Dogs and Cats. Available online: https://medicines.health.europa.eu/veterinary/ro/600000034271 (accessed on 30 June 2025).
- FRILAB Laboratories Pharmaceutiques. Aureomycine® 3%. Available online: https://frilab.ch/en/product/aureomycine-3/ (accessed on 30 June 2025).
- Medikamio. Aureocort 1 mg/g + 30 mg/g Salbe. Available online: https://medikamio.com/de-at/medikamente/aureocort-1-mgg-30-mgg-salbe/pil (accessed on 30 June 2025).
- Ravindra, D.; Huang, G.; Hallett, K.; Burgner, D.P.; Gwee, A.; Silva, M.J. Antibiotic Exposure and Dental Health: A Systematic Review. Pediatrics 2023, 152, e2023061350. [Google Scholar] [CrossRef]
- Akota, I.; Alvsaker, B.; Bjùrnland, T. The effect of locally applied gauze drain impregnated with chlortetracycline ointment in mandibular third-molar surgery. Acta Odontol. Scand. 1998, 56, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Heydariyan, Z.; Soofivand, F.; Dawi, E.A.; Al-Kahdum, S.A.A.; Hameed, N.M.; Salavati-Niasari, M. A comprehensive review: Different approaches for encountering of bacterial infection of dental implants and improving their properties. J. Drug Deliv. Sci. Technol. 2023, 84, 104401. [Google Scholar] [CrossRef]
- Kundabala, M.; Jagadish, S.; Ramya, S. Efficacy of Ledermix as a root canal medicament in symptomatic teeth: A clinical study. J. Interdiscip. Dent. 2014, 4, 85–88. [Google Scholar] [CrossRef]
- Albu, M.G. Collagen Gels and Matrices for Biomedical Applications; Lambert Academic Publishing: Saarbrücken, Germany, 2011; pp. 23–24. [Google Scholar]
- Arpornmaeklong, P.; Pripatnanont, P.; Suwatwirote, N. Properties of chitosan-collagen sponges and osteogenic differentiation of rat-bone-marrow stromal cells. Int. J. Oral Surg. 2008, 37, 357–366. [Google Scholar] [CrossRef]
- Sorca, B.V.; Kaya, D.A.; Kaya, M.G.A.; Enachescu, M.; Ghetu, D.-M.; Enache, L.-B.; Boerasu, I.; Coman, A.E.; Rusu, L.C.; Constantinescu, R.; et al. Bone Fillers with Balance Between Biocompatibility and Antimicrobial Properties. Biomimetics 2025, 10, 100. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Pearson, J.C.; Gillett, E.; Gadri, N.D.; Dionne, B. Tetracyclines, the old and the new: A narrative review. CMI Commun. 2025, 2, 105059. [Google Scholar] [CrossRef]
- Setiawati, E.M. The effectiveness of 0.5–0.7% tetracycline gel to reduced subgingival plaque bacteria. Dent. J. 2008, 41, 114–117. [Google Scholar] [CrossRef][Green Version]
- Seymour, R.A.; Heasman, P.A. Tetracyclines in the management of periodontal diseases. A review. J. Clin. Periodontol. 1995, 22, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Zgârian, R.; Tihan, G.; Barbaresso, R.C.; Rau, I. Spectral characterization of some collagen based composite for dental application. U.P.B. Sci. Bull. Ser. B 2016, 78, 99–110. [Google Scholar]
- Albu, M.G.; Ghica, M.V.; Giurginca, M.; Trandafir, V.; Popa, L.; Cotrut, C. Spectral characteristics and antioxidant properties of tannic acid immobilized on collagen drug-delivery systems. Rev. Chim. 2009, 60, 666–672. [Google Scholar]
- David, G.; Simionescu, B.; Maier, S.; Balhui, C. Micro-/Nanostructured polymeric materials: Poly(ε-caprolactone) crosslinked collagen sponges. Dig. J. Nanomater. Biostruct. 2011, 6, 1575–1585. [Google Scholar]
- Joshi, P.; Mallepogu, P.; Kaur, H.; Singh, R.; Sodhi, I.; Samal, S.K.; Jena, K.C.; Sangamwar, A.T. Explicating the molecular level drug-polymer interactions at the interface of supersaturated solution of the model drug: Albendazole. Eur. J. Pharm. Sci. 2021, 167, 106014. [Google Scholar] [CrossRef] [PubMed]
- Ioan, D.-C.; Rău, I.; Albu Kaya, M.G.; Radu, N.; Bostan, M.; Zgârian, R.G.; Tihan, G.T.; Dinu-Pîrvu, C.-E.; Lupuliasa, A.; Ghica, M.V. Ciprofloxacin-Collagen-Based Materials with Potential Oral Surgical Applications. Polymers 2020, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.G.A.; Simonca, A.G.; Rau, I.; Coman, A.E.; Marin, M.M.; Popa, L.; Trusca, R.; Dinu-Pirvu, C.-E.; Ghica, M.V. Topical Biocomposites Based on Collagen, Hyaluronic Acid and Metronidazole as Periodontitis Treatment. Pharmaceuticals 2024, 17, 1336. [Google Scholar] [CrossRef]
- Romero, A.I.; Villegas, M.; Cid, A.G.; Parentis, M.L.; Gonzo, E.E.; Bermúdez, J.M. Validation of kinetic modeling of progesterone release from polymeric membranes. Asian J. Pharm. Sci. 2018, 13, 54–62. [Google Scholar] [CrossRef]
- Cavanaugh, J.E.; Neath, A.A. The Akaike information criterion: Background, derivation, properties, application, interpretation, and refinements. Wiley Interdiscip. Rev. Comput. Stat. 2019, 11, e1460. [Google Scholar] [CrossRef]
- Berteanu, E.; Tihan, G.T.; Zgarian, R.G.; Tatia, R.; Totea, G.; Ionita, D.; Tcacenco, L.; Iordachel, C.; Demetrescu, I. Biopolymeric Membranes with Immobilized Alkaline Phosphatase and Metallic Zn Ion for Biomedical Applications. RO134094-A2, 21 November 2018. [Google Scholar]
- Mullally, C.A.; Fahriani, M.; Mowlaboccus, S.; Coombs, G.W. Non-faecium non-faecalis enterococci: A review of clinical manifestations, virulence factors, and antimicrobial resistance. Clin. Microbiol. Rev. 2024, 37, e0012123. [Google Scholar] [CrossRef]
- Aun, E.; Kisand, V.; Laht, M.; Telling, K.; Kalmus, P.; Väli, Ü.; Brauer, A.; Remm, M.; Tenson, T. Molecular Characterization of Enterococcus Isolates from Different Sources in Estonia Reveals Potential Transmission of Resistance Genes Among Different Reservoirs. Front. Microbiol. 2021, 12, 601490. [Google Scholar] [CrossRef]
- Nikolic, P.; Mudgil, P. The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- ul Islam, S. 5-Antibacterial drugs. In Infectious Diseases; ul Islam, S., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 119–148. [Google Scholar]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef]
- Winterhalter, M. Antibiotic uptake through porins located in the outer membrane of Gram-negative bacteria. Expert Opin. Drug Deliv. 2021, 18, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Pacific BioLabs. Why You Should Be Using the MTT to Test Cytotoxicity? Available online: https://pacificbiolabs.com/why-you-should-be-using-the-mtt-to-test-cytot/ (accessed on 12 July 2025).
- Balat, A.; Gürel, H.G.; Ordueri, N.E. In vitro evaluation of cytotoxicity of fixed functional appliances. BMC Oral Health 2025, 25, 375. [Google Scholar] [CrossRef]
- Wiertelak-Makała, K.; Szymczak-Pajor, I.; Bociong, K.; Śliwińska, A. Considerations About Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 152. [Google Scholar] [CrossRef]
- Sjögren, G.; Sletten, G.; Dahl, J.E. Cytotoxicity of dental alloys, metals, and ceramics assessed by Millipore filter, agar overlay, and MTT tests. J. Prosthet. Dent. 2000, 84, 229–236. [Google Scholar] [CrossRef]
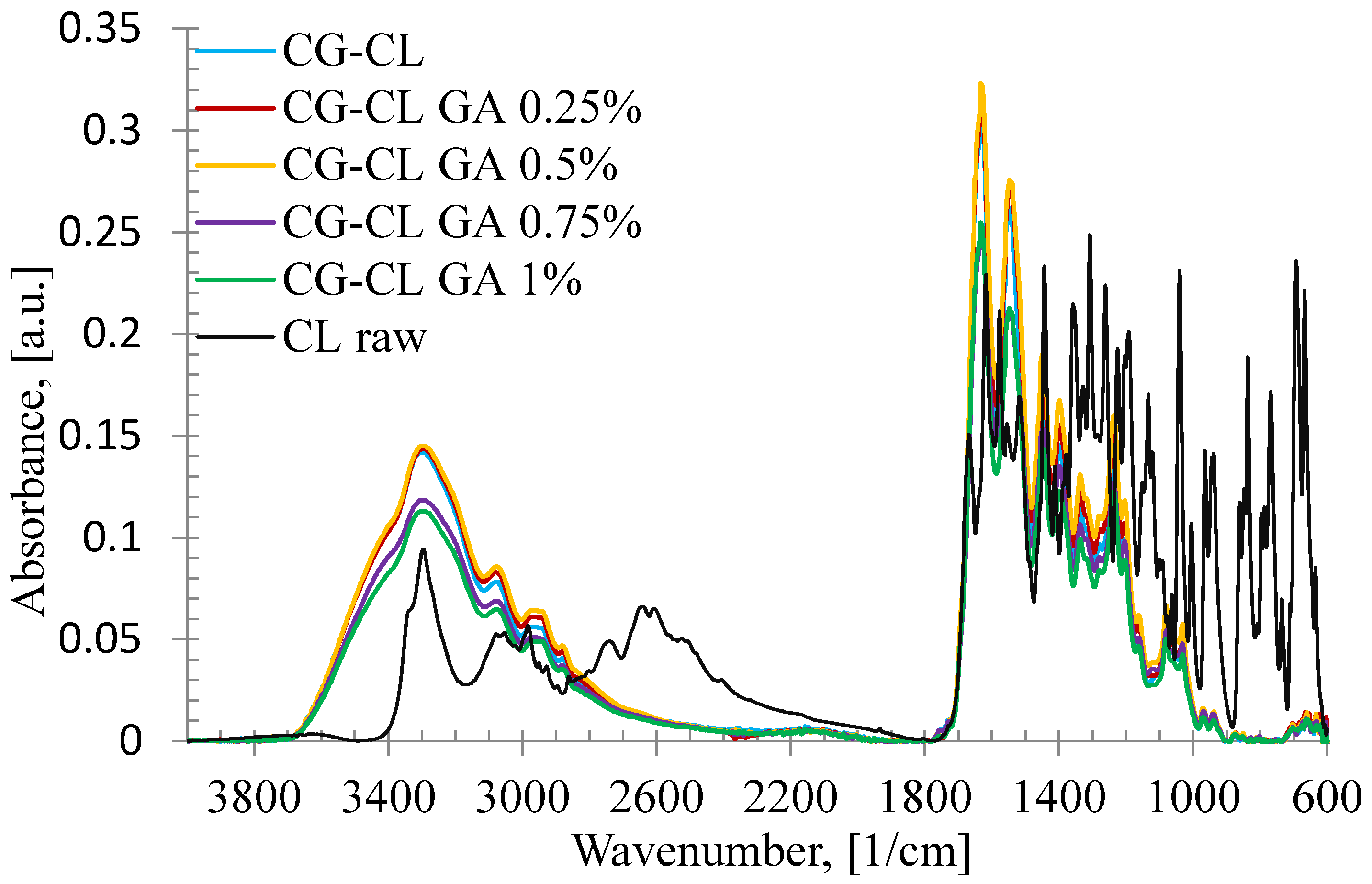
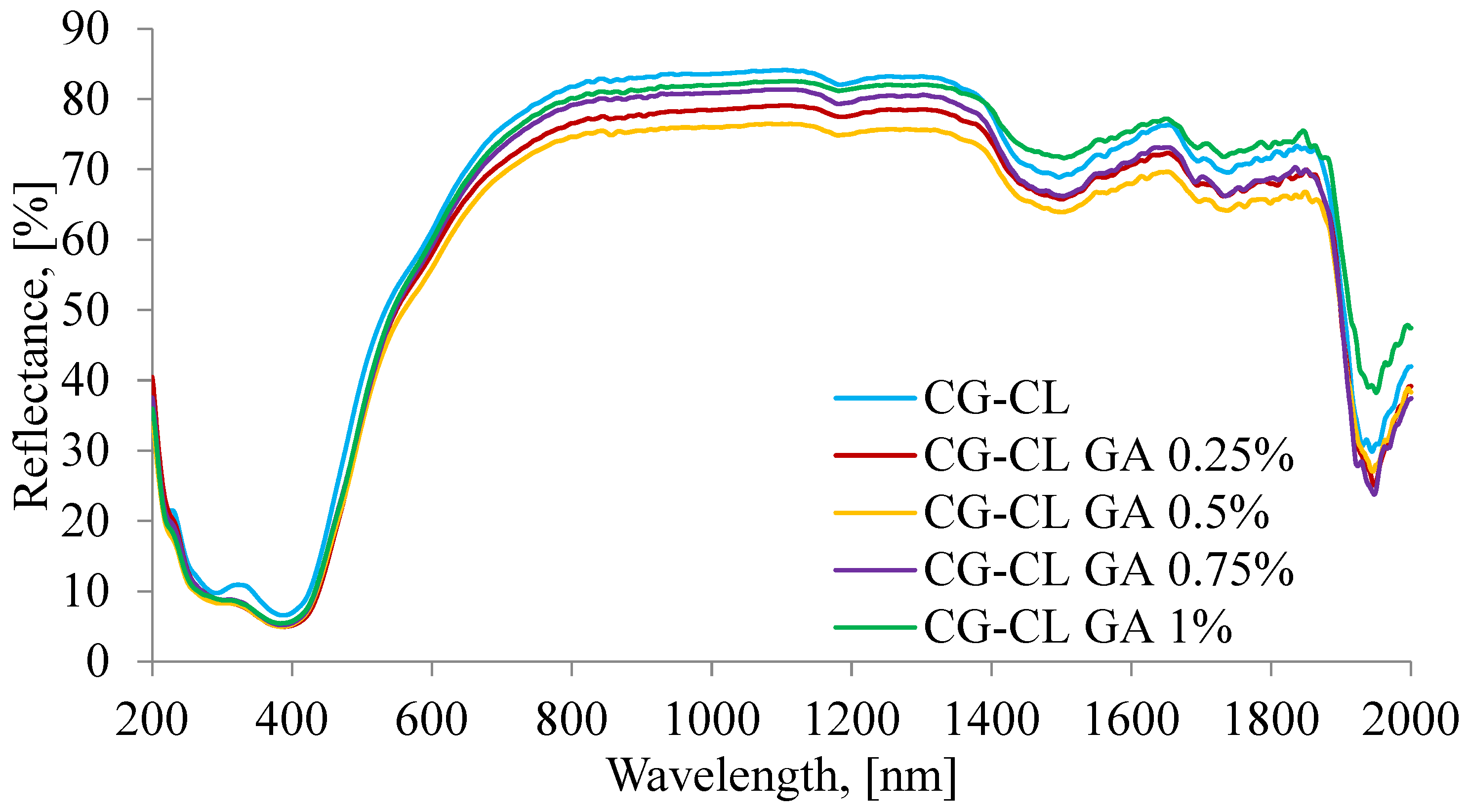
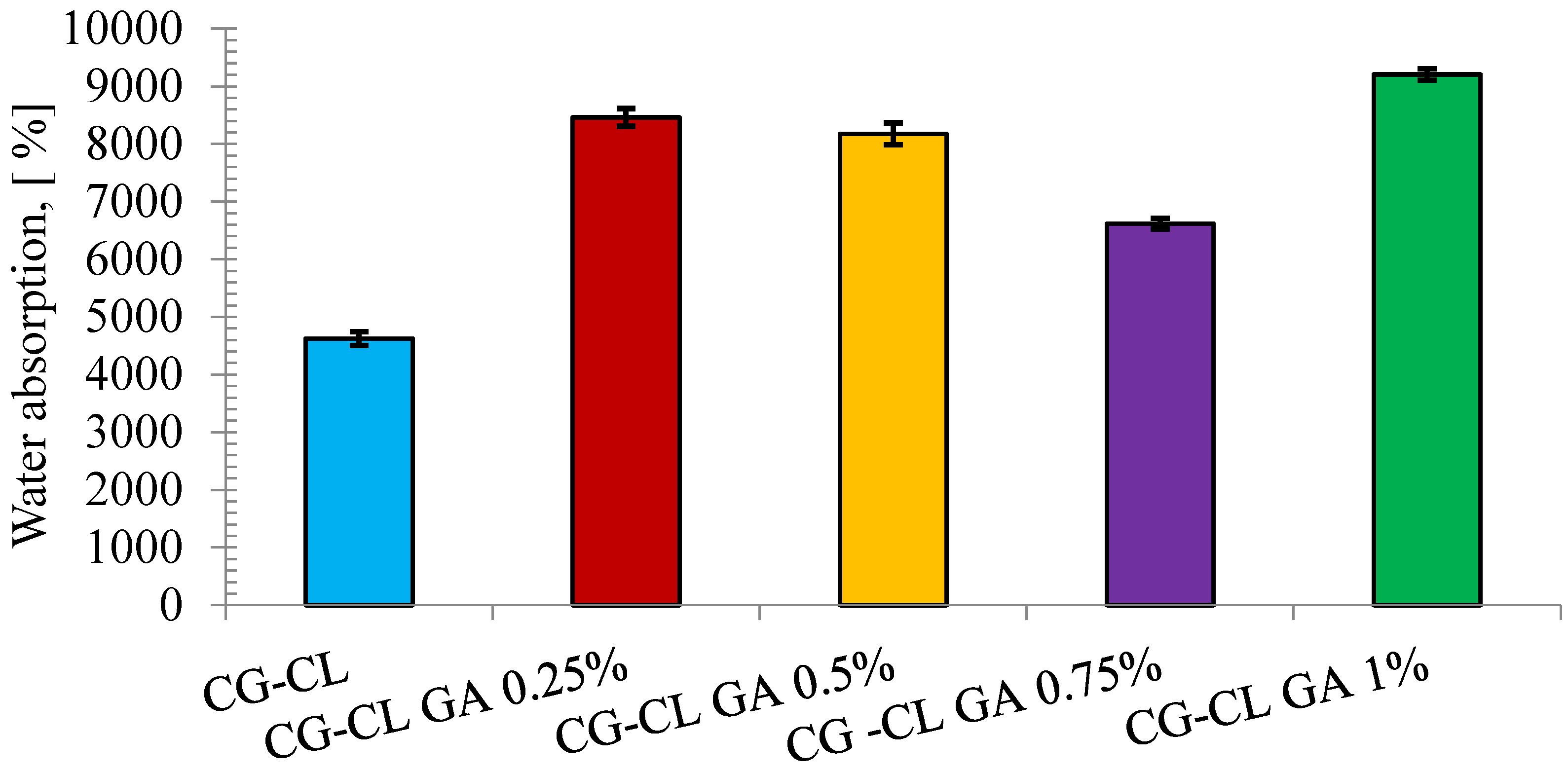
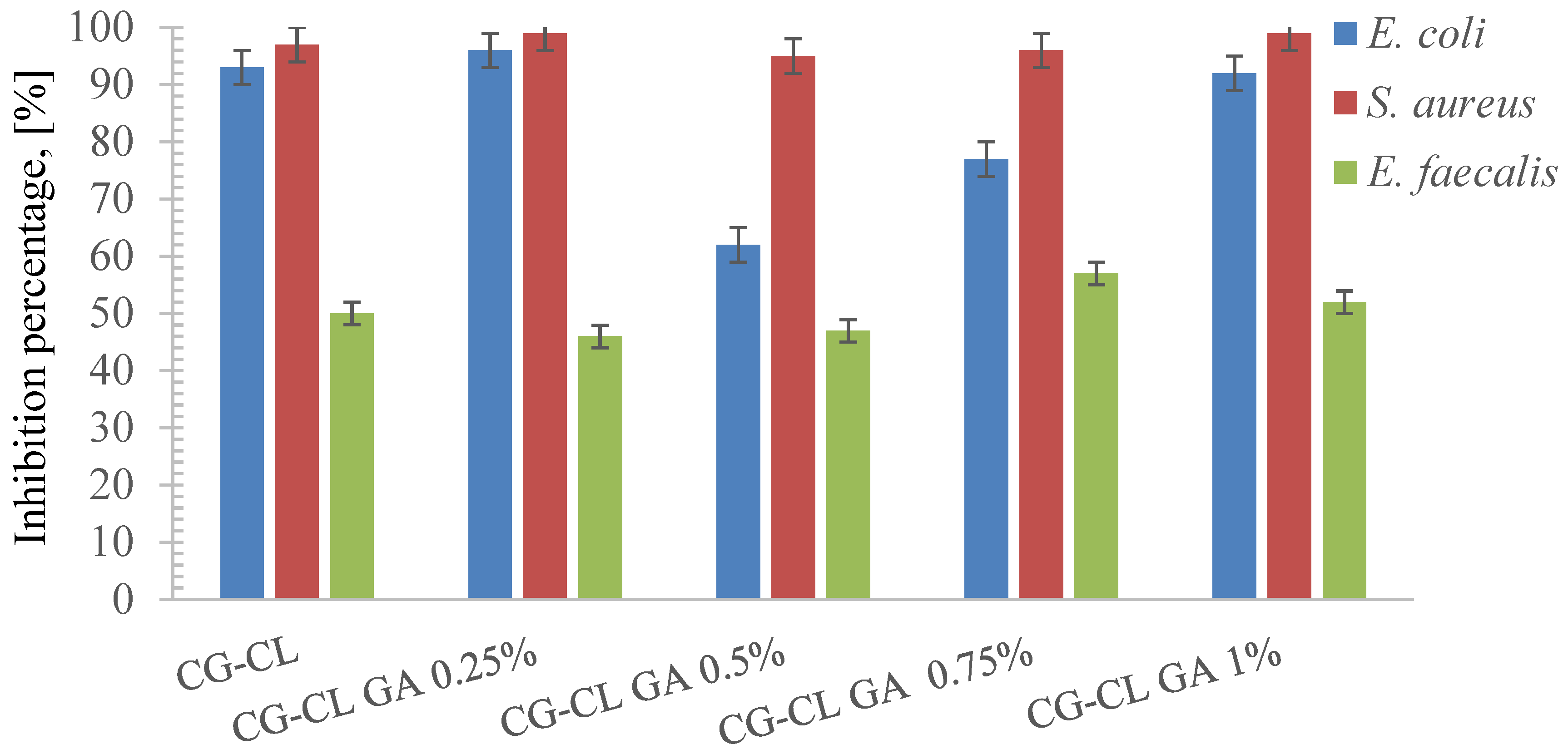
| Sponge | AOH/AI | AI/AOH | Δυ |
|---|---|---|---|
| CG-CL | 0.4557 | 2.1942 | 85 |
| CG-CL GA 0.25% | 0.4516 | 2.2142 | 82 |
| CG-CL GA 0.5% | 0.4489 | 2.2274 | 85 |
| CG-CL GA 0.75% | 0.4719 | 2.1191 | 85 |
| CG-CL GA 1% | 0.4441 | 2.2518 | 86 |
| Band | λ, [nm] | ||||
|---|---|---|---|---|---|
| CG-CL | CG-CL GA 0.25% | CG-CL GA 0.5% | CG-CL GA 0.75% | CG-CL GA 1% | |
| -CO-NH- | 293 | 304 | 291 | 296 | 307 |
| νCH2 | 1184 | 1185 | 1180 | 1181 | 1181 |
| νOH ass | 1497 | 1499 | 1502 | 1502 | 1503 |
| δO-H | 1944 | 1948 | 1946 | 1948 | 1950 |
| Sponge | Higuchi Model | Power Law Model | Kinetic Constant, k (1/minn) | Release Exponent, n | CL Released (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| R | Adj R2 | AICc | R | Adj R2 | AICc | ||||
| CG-CL | 0.9675 | 0.9297 | −55.52 | 0.9958 | 0.9910 | −80.19 | 0.142 | 0.29 | 88.69 |
| CG-CL GA 0.25% | 0.9688 | 0.9326 | −58.46 | 0.9960 | 0.9914 | −83.12 | 0.120 | 0.30 | 79.31 |
| CG-CL GA 0.5% | 0.9702 | 0.9355 | −61.35 | 0.9978 | 0.9954 | −93.01 | 0.112 | 0.30 | 73.22 |
| CG-CL GA 0.75% | 0.9838 | 0.9647 | −71.05 | 0.9994 | 0.9988 | −111.7 | 0.085 | 0.32 | 67.99 |
| CG-CL GA 1% | 0.9879 | 0.9736 | −75.24 | 0.9992 | 0.9992 | −117.3 | 0.073 | 0.34 | 65.48 |
| Microorganism | MIC (µg/mL) |
|---|---|
| Escherichia coli | (100, 200] |
| Staphylococcus aureus | (50, 100] |
| Enterococcus faecalis | (6.25, 12.5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tihan, G.T.; Ungureanu, C.; Rău, I.; Zgârian, R.G.; Barbaresso, R.C.; Albu Kaya, M.G.; Dinu-Pîrvu, C.-E.; Ghica, M.V. Physical–Chemical Assessment and Antimicrobial Activity of Chlortetracycline-Loaded Collagen Sponges. Materials 2025, 18, 4029. https://doi.org/10.3390/ma18174029
Tihan GT, Ungureanu C, Rău I, Zgârian RG, Barbaresso RC, Albu Kaya MG, Dinu-Pîrvu C-E, Ghica MV. Physical–Chemical Assessment and Antimicrobial Activity of Chlortetracycline-Loaded Collagen Sponges. Materials. 2025; 18(17):4029. https://doi.org/10.3390/ma18174029
Chicago/Turabian StyleTihan, Graţiela Teodora, Camelia Ungureanu, Ileana Rău, Roxana Gabriela Zgârian, Răzvan Constantin Barbaresso, Mădălina Georgiana Albu Kaya, Cristina-Elena Dinu-Pîrvu, and Mihaela Violeta Ghica. 2025. "Physical–Chemical Assessment and Antimicrobial Activity of Chlortetracycline-Loaded Collagen Sponges" Materials 18, no. 17: 4029. https://doi.org/10.3390/ma18174029
APA StyleTihan, G. T., Ungureanu, C., Rău, I., Zgârian, R. G., Barbaresso, R. C., Albu Kaya, M. G., Dinu-Pîrvu, C.-E., & Ghica, M. V. (2025). Physical–Chemical Assessment and Antimicrobial Activity of Chlortetracycline-Loaded Collagen Sponges. Materials, 18(17), 4029. https://doi.org/10.3390/ma18174029











