Improving the Interfacial Microstructure and Properties of Al/Mg Bimetal by a Novel Mo Coating Combined with Ultrasonic Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
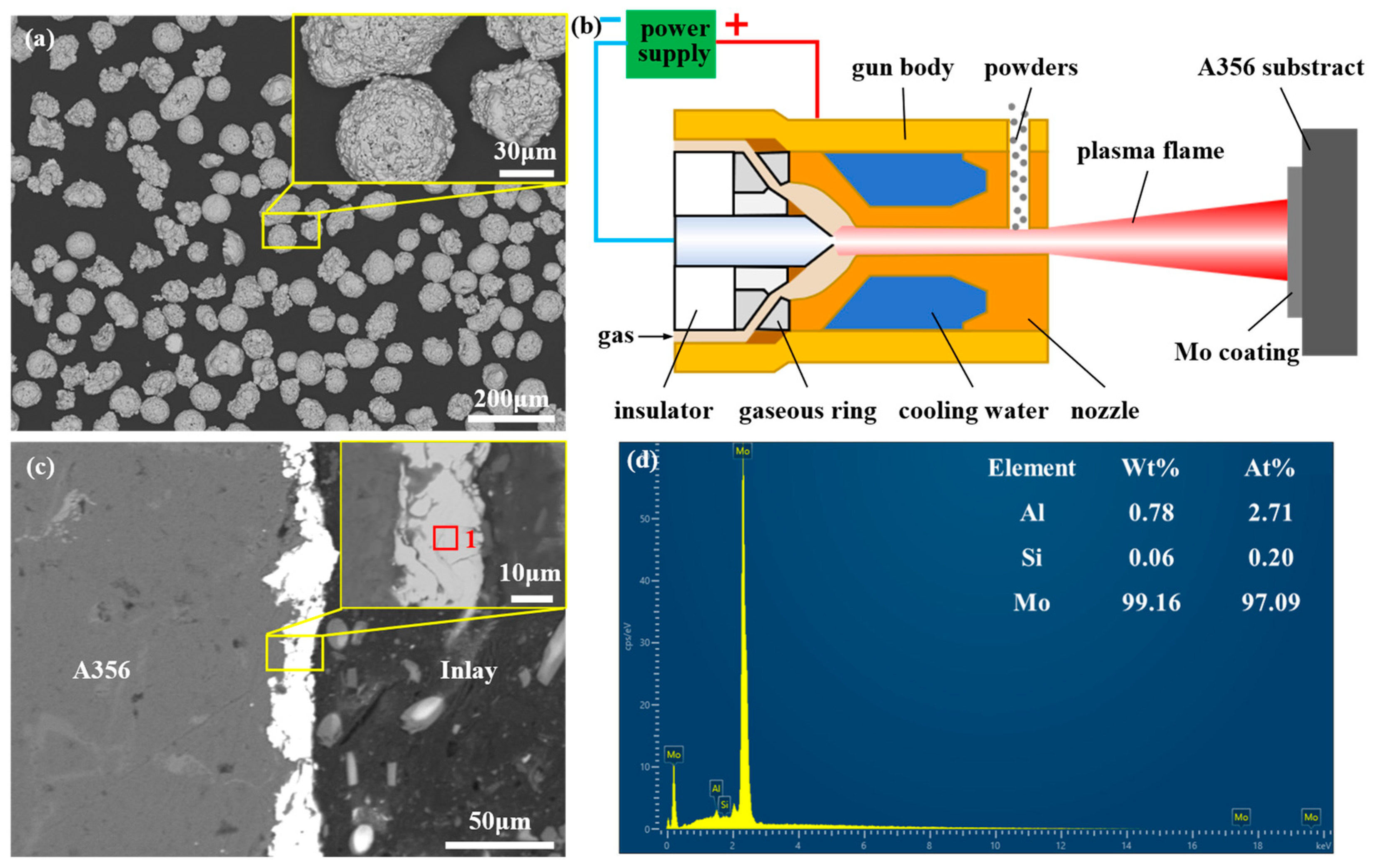
2.2. Preparation Process of Mo Coating
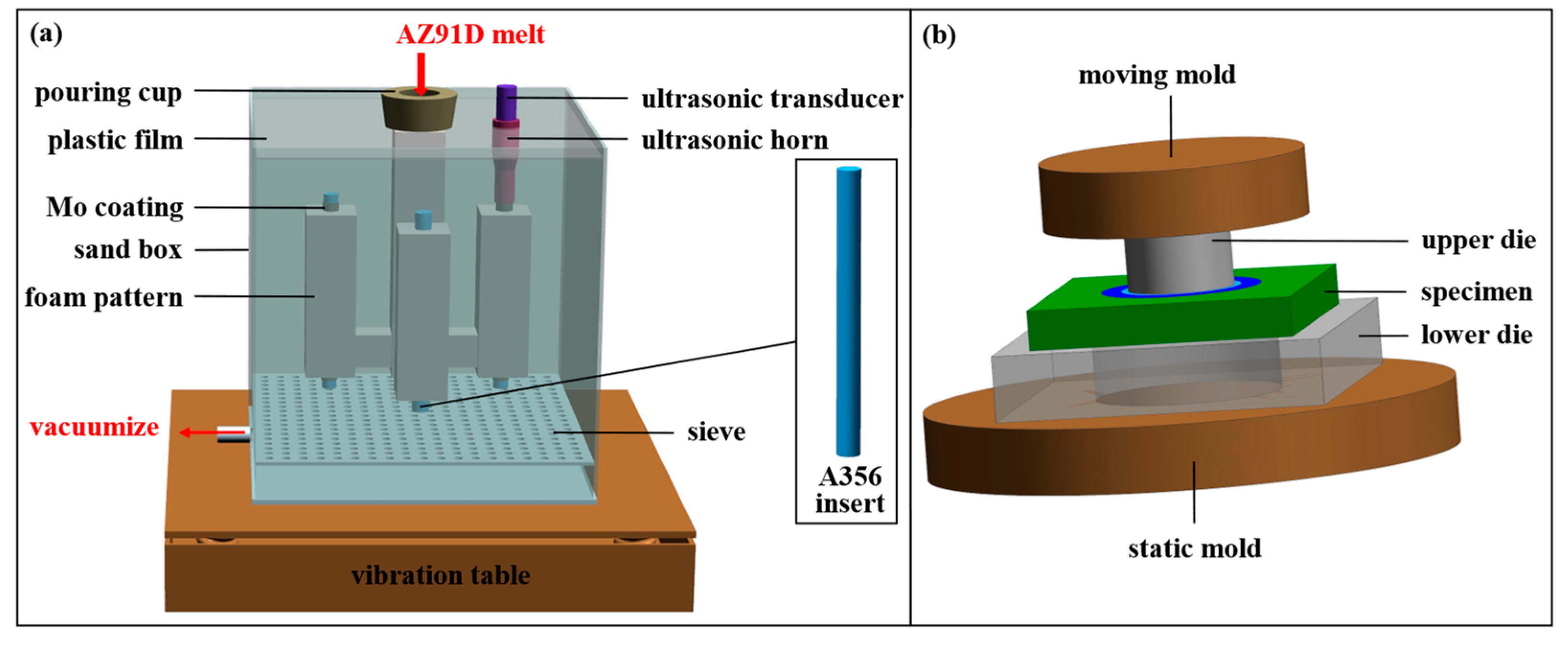
2.3. Compound Casting Process
2.4. Characterization
3. Results
3.1. Microstructure
3.2. Mechanical Properties
4. Discussion
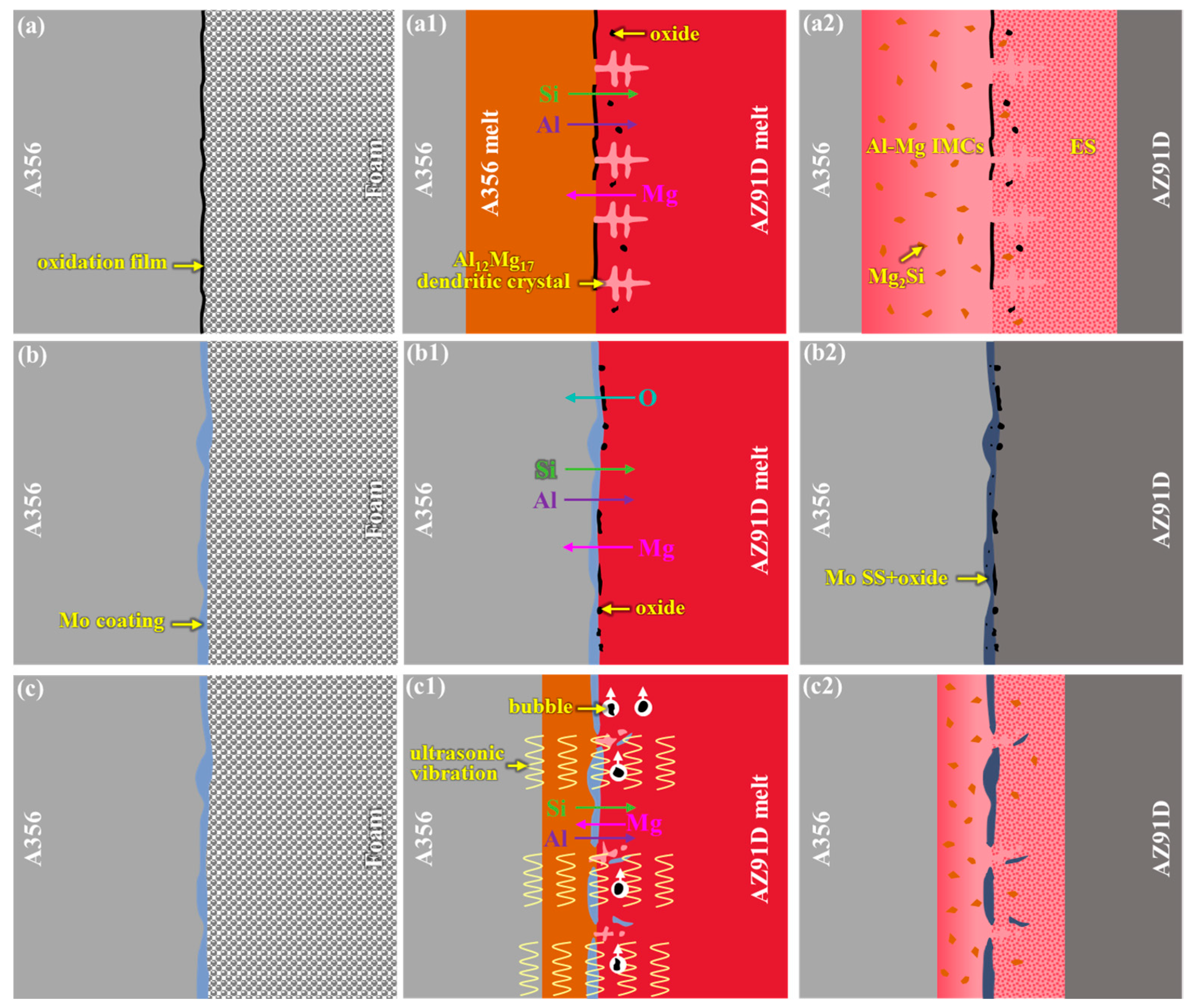
4.1. Interfacial Formation Mechanism
4.2. Interfacial Enhancement Mechanism
5. Conclusions
- (1)
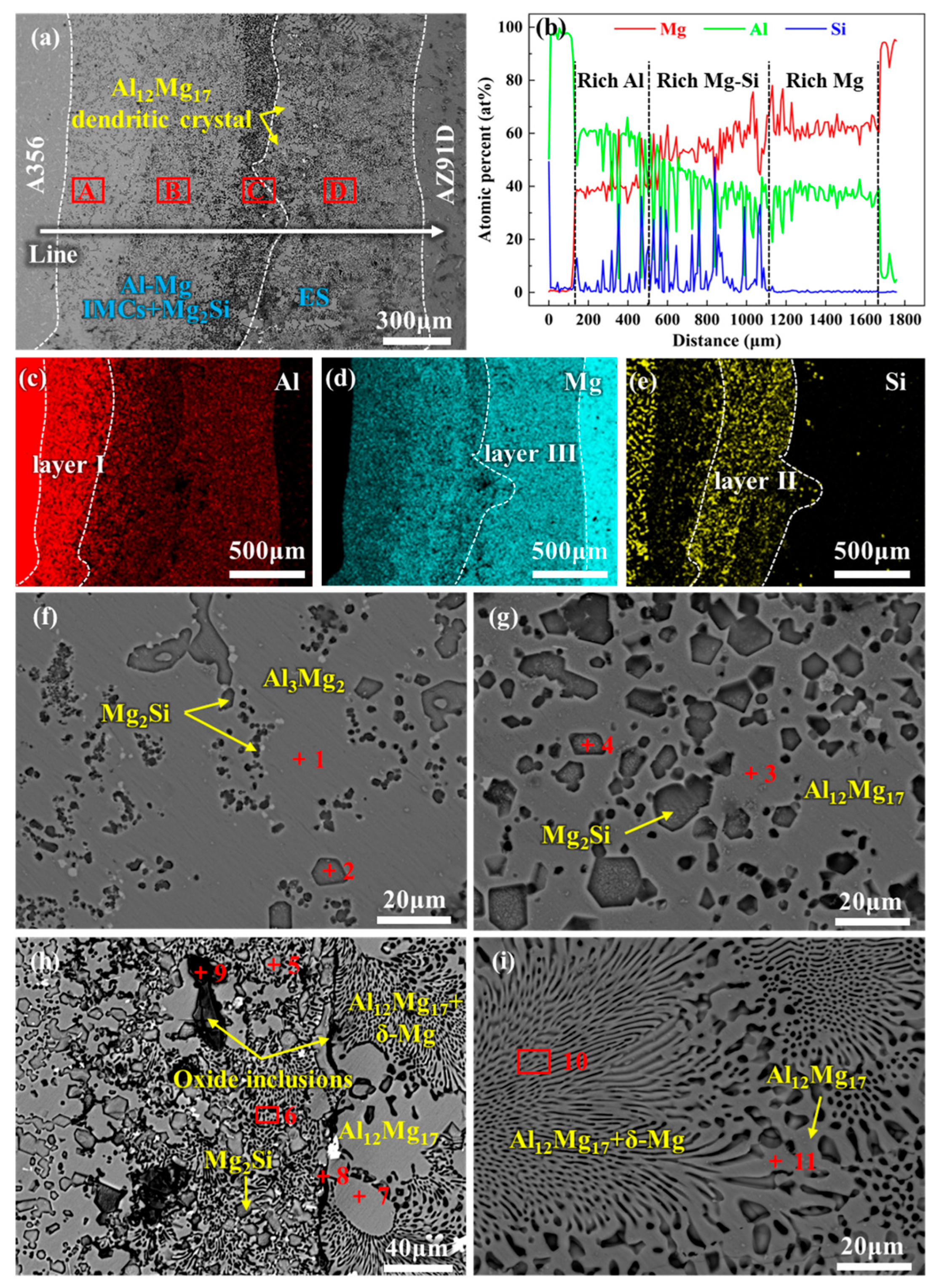
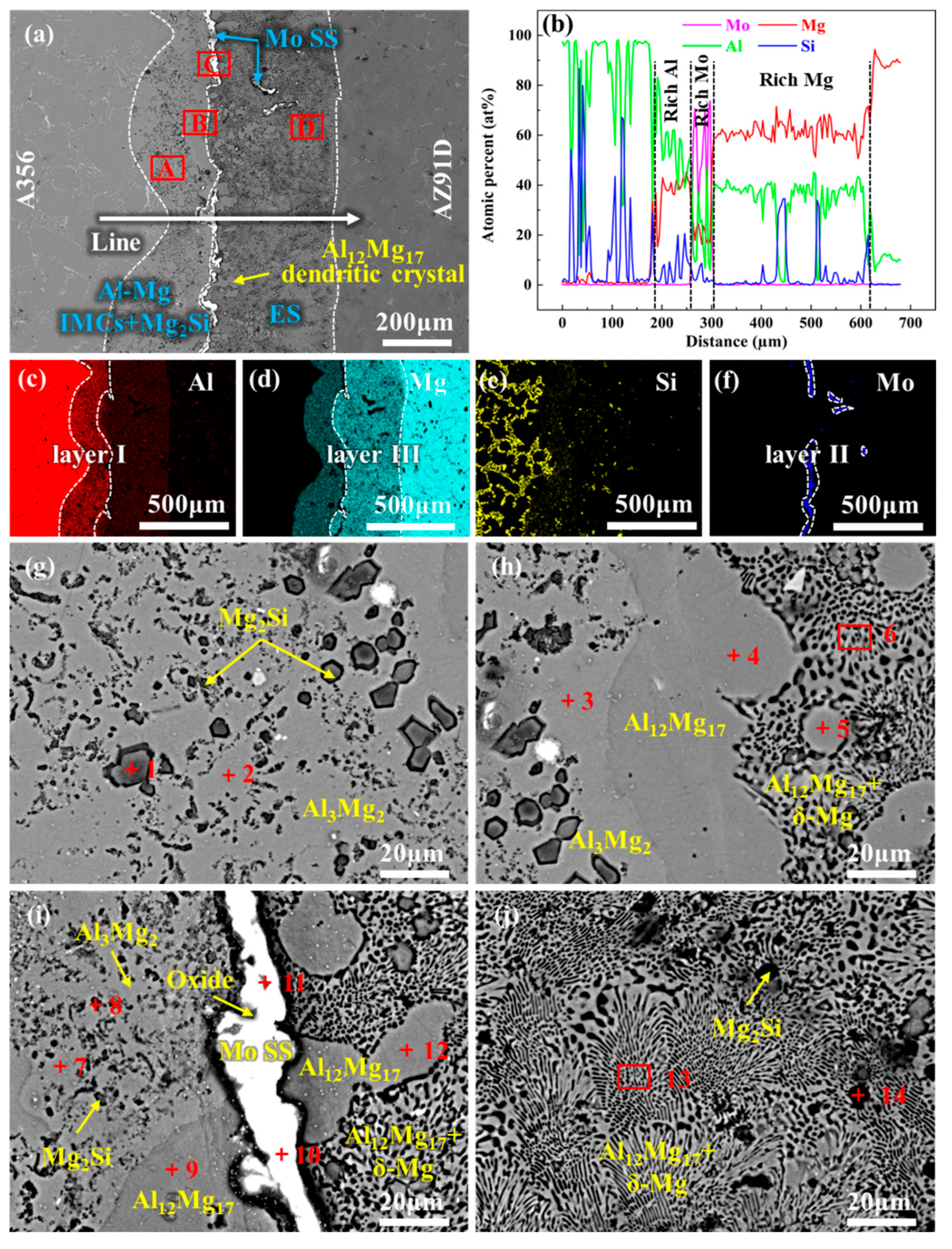
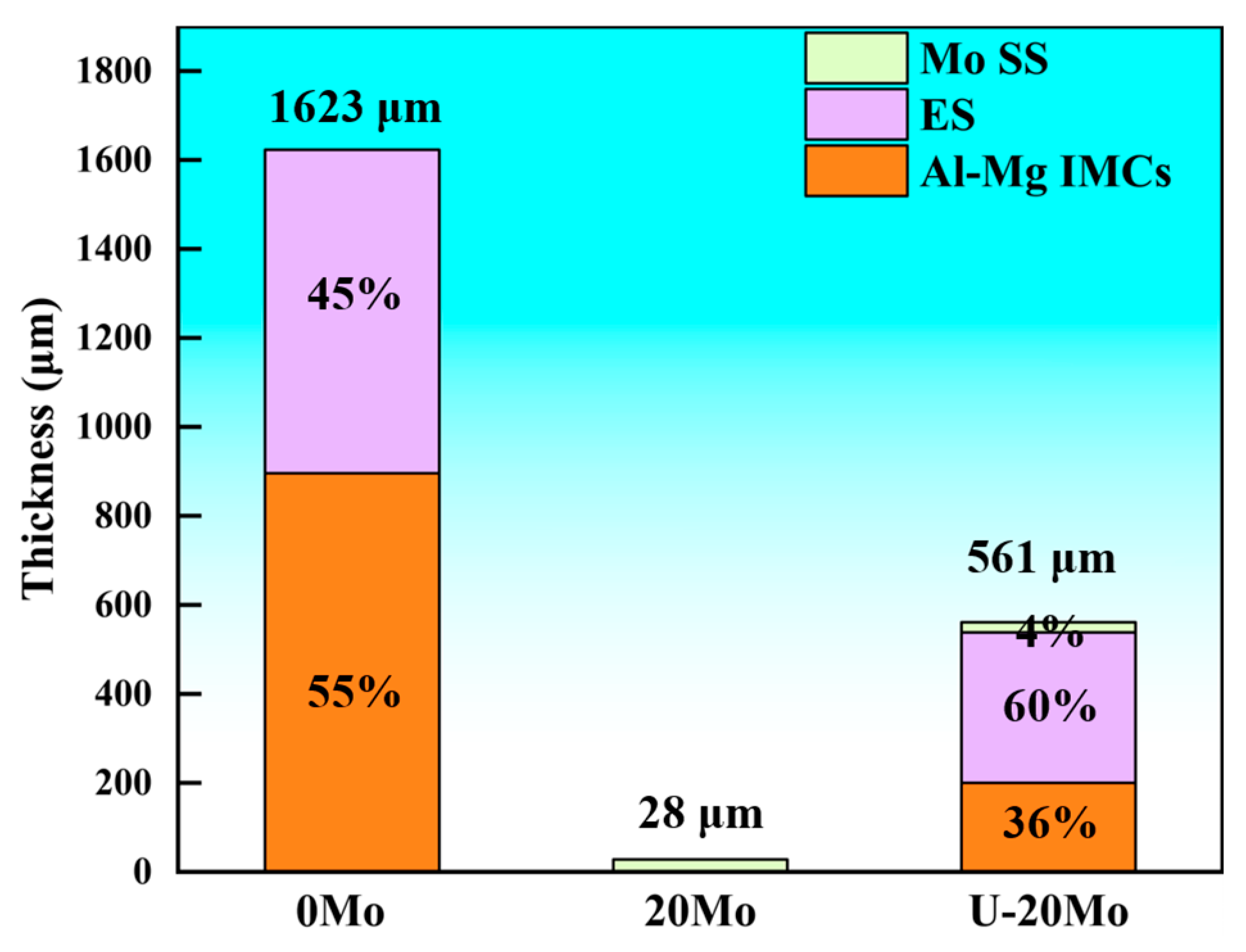
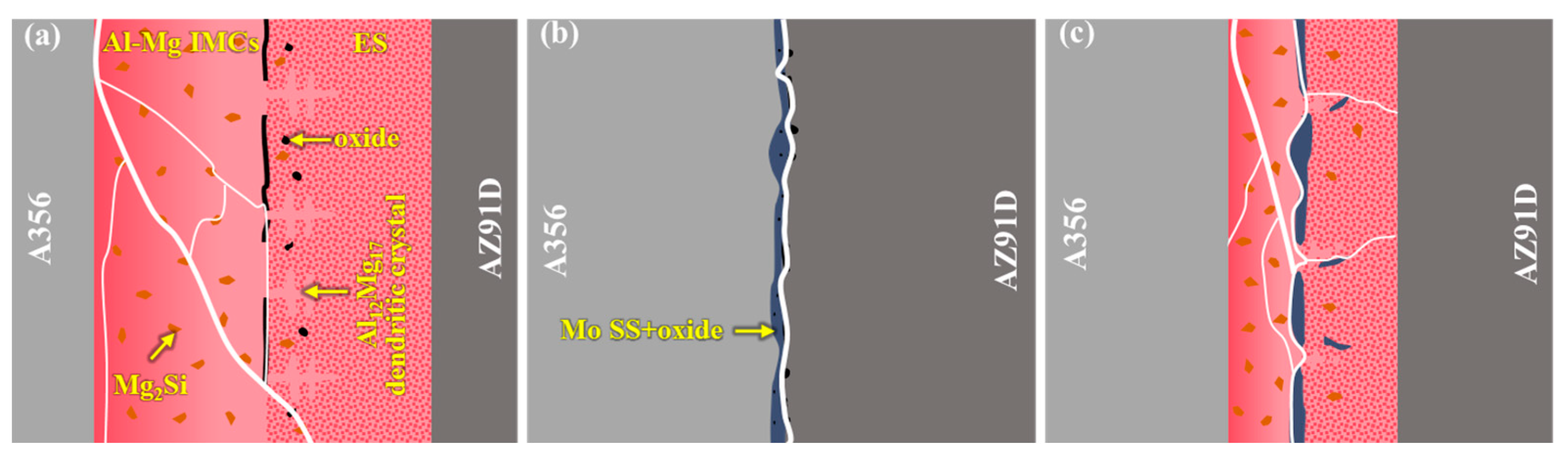
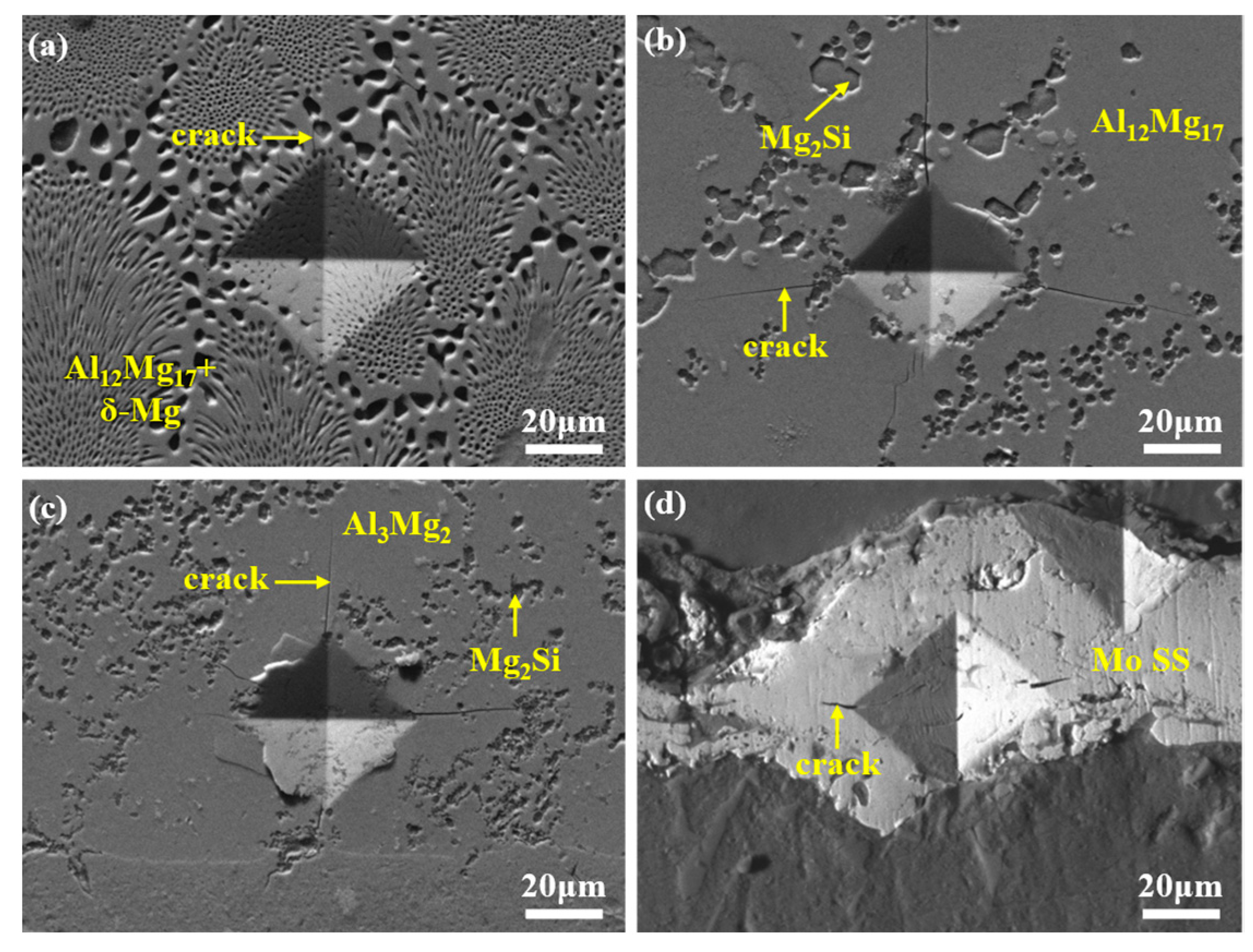
- In the absence of a Mo coating, the interfacial layer between Al and Mg has a thickness of approximately 1623 μm, predominantly composed of Al-Mg IMCs, Mg2Si, and ES. With the introduction of a 20 μm Mo coating, the high melting point of the Mo coating inhibits the Al-Mg interfacial reaction, leading to the complete elimination of Al-Mg phases and Mg2Si in the interfacial layer. Instead, the interfacial layer is dominated by Mo SS and oxide inclusions, with its thickness reduced to 28 μm. Under ultrasonic field, the Mo coating is partially fractured at localized regions, causing phases identical to those in the 0Mo specimen with a Mo SS layer. Consequently, the interfacial layer thickness decreases to 561 μm, accompanied by a reduced proportion of the Al-Mg IMC layer.
- (2)
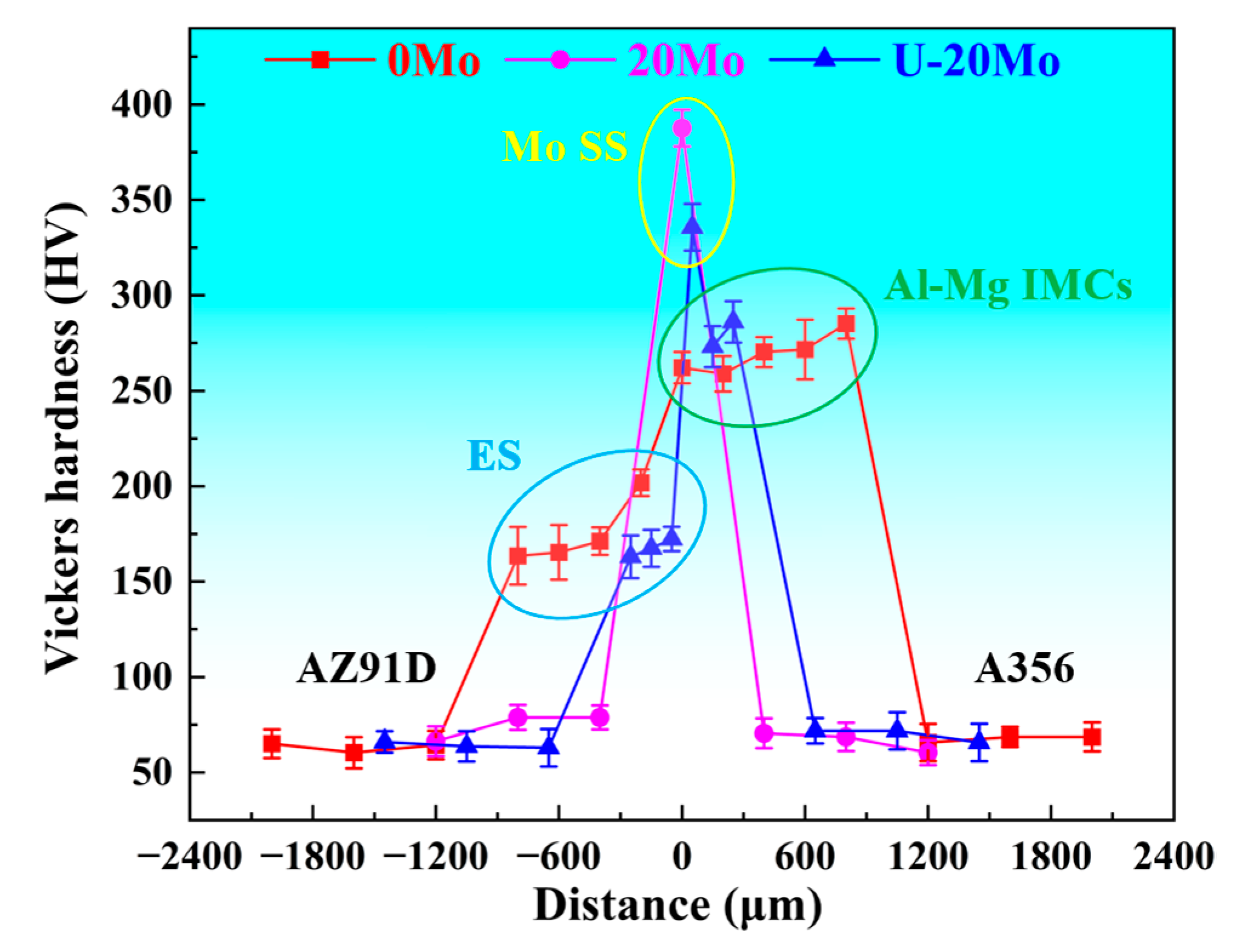
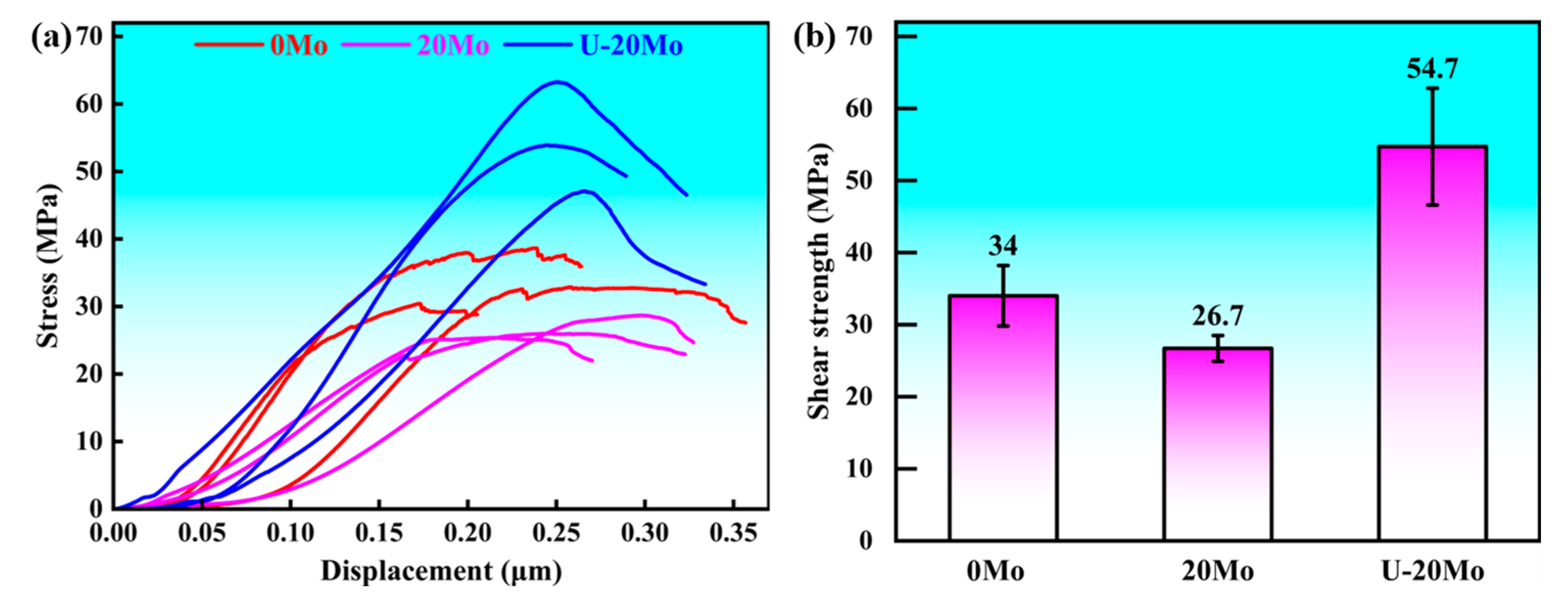
- The microhardness of the interfacial layer is significantly higher than that of the matrix, with slight variations among different reaction sublayers. Specifically, the Mo SS layer exhibits the highest hardness (330–390 HV), while the eutectic layer shows the lowest hardness (160–170 HV). Shear strength measurements indicate that the 20Mo specimen has a lower shear strength than the 0Mo specimen. Under ultrasonic treatment, the shear strength increases significantly to 54.7 MPa, representing a 60.9% improvement compared to the 0Mo specimen. Fracture analysis reveals that all three specimens exhibit brittle fracture characteristics.
- (3)
- The interfacial formation and fracture mechanisms vary among different specimens. For the 0Mo specimen, the interfacial formation mechanism involves melting and diffusion. In the presence of a high-melting-point Mo coating, interface formation is dominated by elemental diffusion. Under ultrasonic treatment, due to the ultrasonic vibration-induced disruption of the Mo coating’s integrity, its interface formation mechanism reverts to melting and diffusion. The enhanced shear strength of the U-20Mo specimen compared to the 0Mo specimen is attributed to the elimination of oxide inclusion defects and a reduced proportion of Al-Mg brittle phases. Thus, the combination of the Mo coatings and ultrasound field is required to achieve the strengthening effect on Al/Mg bimetals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.X.; Xiong, S.M. Research Progress on Thermal Conductivity of High-Pressure Die-Cast Aluminum Alloys. Metals 2024, 14, 370. [Google Scholar] [CrossRef]
- Liu, K.; Wang, H.; Li, J.; Geng, S.; Chen, Z.; Okulov, A. A Review on Factors Influencing Solidification Cracking of Magnesium Alloys During Welding. Met. Mater. Int. 2024, 30, 1723–1742. [Google Scholar] [CrossRef]
- Zhi, P.; Liu, L.; Chang, J.; Liu, C.; Zhang, Q.; Zhou, J.; Liu, Z.; Fan, Y. Advances in the Study of Magnesium Alloys and Their Use in Bone Implant Material. Metals 2022, 12, 1500. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloys 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Li, S.S.; Yue, X.; Li, Q.Y.; Peng, H.L.; Dong, B.X.; Liu, T.S.; Yang, H.Y.; Fan, J.; Shu, S.L.; Qiu, F.; et al. Development and applications of aluminum alloys for aerospace industry. J. Mater. Res. Technol. 2023, 27, 944–983. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G.; Liu, H.; Wang, Y.; Li, J. Grain Refinement Mechanism and Research Progress of Magnesium Alloy Incorporating Zr. Acta Met. Sin. 2024, 60, 129–142. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhang, Z.; Wang, J.; Yu, Y.; Fan, Z. Preparation, interfacial regulation and strengthening of Mg/Al bimetal fabricated by compound casting: A review. J. Magnes. Alloys 2023, 11, 3059–3098. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, W.; Xu, Y.; Yu, L.; Peng, Z.; Li, G.; Fan, Z. Achieving high strength of Al/Mg bimetal interface with multiple strengthening mechanisms via a novel combination treatment of La addition and ultrasonic vibration. J. Alloys Compd. 2025, 1018, 179312. [Google Scholar] [CrossRef]
- Li, G.; Yang, W.; Jiang, W.; Guan, F.; Jiang, H.; Wu, Y.; Fan, Z. The role of vacuum degree in the bonding of Al/Mg bimetal prepared by a compound casting process. J. Mater. Process. Technol. 2019, 265, 112–121. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Fan, Z.; Jiang, Z.; Liu, X.; Liu, F. Effects of pouring temperature on microstructure, mechanical properties, and fracture behavior of Al/Mg bimetallic composites produced by lost foam casting process. Int. J. Adv. Manuf. Technol. 2017, 91, 1355–1368. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, G.; Yang, L.; Liu, X. Effects of Melt-to-Solid Insert Volume Ratio on the Microstructures and Mechanical Properties of Al/Mg Bimetallic Castings Produced by Lost Foam Casting. Metall. Mater. Trans. A 2016, 47, 6487–6497. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Luo, A.A. Improved Interfacial Bonding in Magnesium/Aluminum Overcasting Systems by Aluminum Surface Treatments. Metall. Mater. Trans. B 2014, 45, 2495–2503. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Luo, A.A. A novel aluminum surface treatment for improved bonding in magnesium/aluminum bimetallic castings. Scr. Mater. 2014, 86, 52–55. [Google Scholar] [CrossRef]
- Xu, G.; Luo, A.A.; Chen, Y.; Sachdev, A.K. Interfacial phenomena in magnesium/aluminum bi-metallic castings. Mater. Sci. Eng. A 2014, 595, 154–158. [Google Scholar] [CrossRef]
- Yan, H.; Xia, Y.; Luan, T.; Leng, X.; Yan, J. Evolution of interfacial IMCs and mechanical performance of joints of Mg/Al dissimilar metals soldered by using Ga-coating protection. Mater. Sci. Eng. A 2022, 836, 142687. [Google Scholar] [CrossRef]
- Paidar, M.; Mehrez, S.; Babaei, B.; Memon, S.; Ojo, O.O.; Lankarani, H.M. Dissimilar welding of AA5083 to AZ31 Mg alloys using modified friction stir clinching brazing. Mater. Lett. 2021, 301, 129764. [Google Scholar] [CrossRef]
- Zhao, H.; Gong, P.; Ji, S.; Gong, X. Enhancing the Strength of Al/Mg Dissimilar Friction Stir Lap Welded Joint by Zn Filler Addition Combined with an Appropriate Heat Input. Arch. Met. Mater. 2022, 67, 137–146. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Z.; Chang, L.; Guo, D.; Yu, J.; Cui, M. A two-step bonding process for preparing 6061/AZ31 bimetal assisted with liquid molten zinc interlayer: The process and microstructure. J. Adhes. Sci. Technol. 2022, 36, 2093–2115. [Google Scholar] [CrossRef]
- Ji, S.; Niu, S.; Liu, J. Dissimilar Al/Mg alloys friction stir lap welding with Zn foil assisted by ultrasonic. J. Mater. Sci. Technol. 2019, 35, 1712–1718. [Google Scholar] [CrossRef]
- Guo, Y.; Quan, G.; Ren, L.; Liu, B.; Al-Ezzi, S.; Pan, H. Effect of Zn interlayer thickness on the microstructure and mechanical properties of two-step diffusion bonded joint of ZK60Mg and 5083Al. Vacuum 2019, 161, 353–360. [Google Scholar] [CrossRef]
- Ji, S.; Niu, S.; Liu, J.; Meng, X. Friction stir lap welding of Al to Mg assisted by ultrasound and a Zn interlayer. J. Mater. Process. Technol. 2019, 267, 141–151. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Xu, Z.; Chen, S.; Peng, L.; Yan, J. Joint formation and mechanical properties of ultrasonic-assisted transient liquid bonding of dissimilar Al/Mg with a Zn interlayer in air. J. Manuf. Process. 2022, 77, 632–641. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, H.; Wang, B.; Ji, A.; Liu, J.; Feng, J. Improving weld strength of arc-assisted ultrasonic seam welded Mg/Al joint with Sn interlayer. Mater. Des. 2016, 98, 262–271. [Google Scholar] [CrossRef]
- Patel, V.K.; Bhole, S.D.; Chen, D.L. Improving weld strength of magnesium to aluminium dissimilar joints via tin interlayer during ultrasonic spot welding. Sci. Technol. Weld. Join. 2012, 17, 342–347. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Shah, L.H.; Gerlich, A.P. Dissimilar laser welding of an AA6022-AZ31 lap-joint by using Ni-interlayer: Novel beam-wobbling technique, processing parameters, and metallurgical characterization. Opt. Laser Technol. 2019, 112, 349–362. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Yu, Y.; Fan, Z. Effect of different Ni interlayers on interfacial microstructure and bonding properties of Al/Mg bimetal using a novel compound casting. J. Manuf. Process. 2020, 50, 614–628. [Google Scholar] [CrossRef]
- Yin, F.; Liu, C.; Zhang, Y.; Qin, Y.; Liu, N. Effect of Ni interlayer on characteristics of diffusion bonded Mg/Al joints. Mater. Sci. Technol. 2018, 34, 1104–1111. [Google Scholar] [CrossRef]
- Dong, S.; Lin, S.; Zhu, H.; Wang, C.; Cao, Z. Effect of Ni interlayer on microstructure and mechanical properties of Al/Mg dissimilar friction stir welding joints. Sci. Technol. Weld. Join. 2022, 27, 103–113. [Google Scholar] [CrossRef]
- Kumar, S.; Wu, C. Eliminating intermetallic compounds via Ni interlayer during friction stir welding of dissimilar Mg/Al alloys. J. Mater. Res. Technol. 2021, 15, 4353–4369. [Google Scholar] [CrossRef]
- Liu, N.; Liu, C.; Liang, C.; Zhang, Y. Influence of Ni Interlayer on Microstructure and Mechanical Properties of Mg/Al Bimetallic Castings. Metall. Mater. Trans. A 2018, 49, 3556–3564. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Yu, Y.; Fan, Z. Improving mechanical properties of AZ91D magnesium/A356 aluminum bimetal prepared by compound casting via a high velocity oxygen fuel sprayed Ni coating. J. Magnes. Alloys 2022, 10, 1075–1085. [Google Scholar] [CrossRef]
- Qi, X.; Liu, L. Fusion welding of Fe-added lap joints between AZ31B magnesium alloy and 6061 aluminum alloy by hybrid laser–tungsten inert gas welding technique. Mater. Des. 2012, 33, 436–443. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, Y.; Li, Z.; Zhao, S.; Gao, M. Effects of beam oscillation on interface layer and mechanical properties of laser-arc hybrid lap welded Al/Mg dissimilar metals. Intermetallics 2021, 133, 107175. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, G.; Li, L.; Shen, Q.; Zhang, L. Formation of intermetallic compounds in Mg–Ag–Al joints during diffusion bonding. J. Mater. Sci. 2014, 49, 7298–7308. [Google Scholar] [CrossRef]
- Peng, H.; Chen, D.L.; Bai, X.F.; Wang, P.Q.; Li, D.Y.; Jiang, X.Q. Microstructure and mechanical properties of Mg-to-Al dissimilar welded joints with an Ag interlayer using ultrasonic spot welding. J. Magnes. Alloys 2020, 8, 552–563. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X.; Liu, S. Microstructure of laser-TIG hybrid welds of dissimilar Mg alloy and Al alloy with Ce as interlayer. Scr. Mater. 2006, 55, 383–386. [Google Scholar] [CrossRef]
- Papis, K.J.M.; Loeffler, J.F.; Uggowitzer, P.J. Light metal compound casting. Sci. China Ser. E Technol. Sci. 2009, 52, 46–51. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, X.; Ma, Y.; Liu, S.; Zang, L.; Chen, Y. Microstructure and Corrosion Behavior of Friction Stir-Welded 6061 Al/AZ31 Mg Joints with a Zr Interlayer. Materials 2019, 12, 1115. [Google Scholar] [CrossRef]
- Li, X.; Liang, W.; Zhao, X.; Zhang, Y.; Fu, X.; Liu, F. Bonding of Mg and Al with Mg–Al eutectic alloy and its application in aluminum coating on magnesium. J. Alloys Compd. 2009, 471, 408–411. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, G.; Wang, Y.; Xiao, Y.; Shen, Q.; Zhang, L. Effect of Al thin film and Ni foil interlayer on diffusion bonded Mg–Al dissimilar joints. J. Alloys Compd. 2013, 556, 139–142. [Google Scholar] [CrossRef]
- Yang, T.; Wang, K.; Zhang, D.; Huang, J. Contact-reaction brazing of an AZ31 magnesium/3003 aluminum alloy using a silver-copper-zinc interlayer. J. Mater. Process. Technol. 2017, 249, 531–537. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, M.; Li, L.; Luo, G.; Shen, Q.; Zhang, L. Accelerated Bonding of Magnesium and Aluminum with a CuNiAgCuNi Sandwich Interlayer by Plasma-Activated Sintering. Metall. Mater. Trans. A 2016, 47A, 631–636. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Zhang, Z.; Fan, Z. Microstructure, mechanical properties and corrosion resistance of A356 aluminum/AZ91D magnesium bimetal prepared by a compound casting combined with a novel Ni-Cu composite interlayer. J. Mater. Process. Technol. 2021, 288, 116874. [Google Scholar] [CrossRef]
- Peng, Z.; Zhou, T.; Li, Y.; Cui, Z.; Wu, Z.; Yan, J. Microstructure and mechanical performance of AZ31/2024 dissimilar alloy joints using a multi-interlayer of Ni/Al/Zn via ultrasonic-assisted transient liquid phase bonding. Mater. Des. 2021, 197, 109218. [Google Scholar] [CrossRef]
- Penner, P.; Liu, L.; Gerlich, A.; Zhou, Y. Feasibility study of resistance spot welding of dissimilar Al/Mg combinations with Ni based interlayers. Sci. Technol. Weld. Join. 2013, 18, 541–550. [Google Scholar] [CrossRef]
- Abdollahzadeh, A.; Shokuhfar, A.; Cabrera, J.M.; Zhilyaev, A.P.; Omidvar, H. In-situ nanocomposite in friction stir welding of 6061-T6 aluminum alloy to AZ31 magnesium alloy. J. Mater. Process. Technol. 2019, 263, 296–307. [Google Scholar] [CrossRef]
- Wang, Y. Exploring the effectiveness of integrating augmented reality-based materials to support writing activities. Comput. Educ. 2017, 113, 162–176. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Jiang, W.; Xu, Y.; Li, Q.; Liu, W.; Yao, S.; Yao, P.; Fan, Z. Microstructure characteristic, formation mechanism and mechanical properties of AZ91D/A356 bimetallic interface with a novel Ni-Cr composite coating. J. Alloys Compd. 2024, 1005, 176121. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, W.; Li, Q.; Niu, Y.; Yu, L.; Li, G.; Fan, Z. Significantly improved bonding strength of Al/Mg bimetallic interface by compound casting via FeCoNiCrCu high entropy alloy coating. J. Mater. Res. Technol. 2024, 30, 6870–6876. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, W.; Li, Q.; Yu, L.; Yu, X.; Fan, Z. Combined effects of ultrasonic vibration and FeCoNiCrCu coating on interfacial microstructure and mechanical properties of Al/Mg bimetal by compound casting. China Foundry 2024, 21, 588–598. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, W.; Li, Q.; Yu, L.; Yu, X.; Peng, Z.; Fan, Z. Superior bonding of Al/Mg interface by introducing nanocrystals via high-entropy alloy coating and ultrasonic vibration. Mater. Res. Lett. 2025, 13, 131–139. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, C.; Zhou, Z.; Long, H.; Jia, J.; He, L.; Long, Y. Dissimilar laser lap welding of Mg and Al alloys using a CoCrFeNi medium-entropy alloy interlayer. Opt. Laser Technol. 2023, 157, 108639. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Yu, Y.; Fan, Z. Microstructure evolution, mechanical properties and fracture behavior of Al-xSi/AZ91D bimetallic composites prepared by a compound casting. J. Magnes. Alloys 2024, 12, 1944–1964. [Google Scholar] [CrossRef]
- Guan, F.; Jiang, W.; Zhang, Z.; Li, G.; Wang, J.; Fan, Z. Significantly Enhanced Mg/Al Bimetallic Interface by Compound Casting via Combination of Gd Addition and Vibration. Metall. Mater. Trans. A 2023, 54, 3389–3399. [Google Scholar] [CrossRef]
- Guan, F.; Jiang, W.; Zhang, Z.; Wang, J.; Li, G.; Fan, Z. Interfacial microstructure, mechanical properties and strengthening mechanism of Mg/Al bimetallic composites produced by a novel compound casting with the addition of Gd. Mater. Charact. 2023, 200, 112898. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, W.; Li, G.; Wang, J.; Guan, F.; Jie, G.; Fan, Z. Effect of La on microstructure, mechanical properties and fracture behavior of Al/Mg bimetallic interface manufactured by compound casting. J. Mater. Sci. Technol. 2022, 105, 214–225. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, W.; Guan, F.; Wang, J.; Li, G.; Fan, Z. Understanding the microstructural evolution and strengthening mechanism of Al/Mg bimetallic interface via the introduction of Y. Mater. Sci. Eng. A 2022, 840, 142974. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, W.; Guan, F.; Li, G.; Fan, Z. Interface formation and strengthening mechanisms of Al/Mg bimetallic composite via compound casting with rare earth Ce introduction. Mater. Sci. Eng. A 2022, 854, 143830. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, W.; Li, G.; Wang, J.; Guan, F.; Jie, G.; Fan, Z. Improved interface bonding of Al/Mg bimetal fabricated by compound casting with Nd addition. Mater. Sci. Eng. A 2021, 826, 141998. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, R.; Klarner, A.D.; Luo, A.A.; Chen, Y. Predicting and controlling interfacial microstructure of magnesium/aluminum bimetallic structures for improved interfacial bonding. J. Magnes. Alloys 2020, 8, 578–586. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, W.; Xu, Y.; Yu, L.; Niu, Y.; Fan, Z. Development of prominent bonding strength in Al/Mg bimetal composites prepared by ultrasonic vibration-assisted compound casting: Effects of ultrasonic powers. J. Mater. Sci. Technol. 2024, 197, 78–93. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, W.; Xu, Y.; Yu, L.; Yu, X.; Fan, Z. New insights into the influencing mechanism of ultrasonic vibration on interface of Al/Mg bimetal composites by compound casting using simulation calculation and experimental verification. Compos. Part B 2024, 284, 111726. [Google Scholar] [CrossRef]
- Yu, L.; Ma, Y.; Li, Q.; Xu, Y.; Yu, X.; Peng, Z.; Jiang, W. Effect of electromagnetic stirring on interfacial microstructure and mechanical properties of Al/Mg bimetal produced by lost foam compound casting. J. Mater. Res. Technol. 2024, 33, 2101–2111. [Google Scholar] [CrossRef]
- Qie, X.; Zhang, Z.; Li, Q.; Guan, F.; Fan, Z.; Jiang, W. Strengthening of compound casting Al/Mg bimetallic interface with Ni interlayer by vibration assisted treatment. J. Mater. Res. Technol. 2023, 26, 1736–1742. [Google Scholar] [CrossRef]
- Li, Q.; Guan, F.; Xu, Y.; Zhang, Z.; Fan, Z.; Jiang, W. Development of Al/Mg Bimetal Processed by Ultrasonic Vibration-Assisted Compound Casting: Effects of Ultrasonic Vibration Treatment Duration Time. Materials 2023, 16, 5009. [Google Scholar] [CrossRef]
- Li, G.; Guan, F.; Jiang, W.; Xu, Y.; Zhang, Z.; Fan, Z. Effects of mechanical vibration on filling and solidification behavior, microstructure and performance of Al/Mg bimetal by lost foam compound casting. China Foundry 2023, 20, 469–479. [Google Scholar] [CrossRef]
- Guan, F.; Fan, S.; Wang, J.; Li, G.; Zhang, Z.; Jiang, W. Effect of Vibration Acceleration on Interface Microstructure and Bonding Strength of Mg–Al Bimetal Produced by Compound Casting. Metals 2022, 12, 766. [Google Scholar] [CrossRef]
- Guan, F.; Jiang, W.; Li, G.; Zhu, J.; Wang, J.; Jie, G.; Fan, Z. Effect of vibration on interfacial microstructure and mechanical properties of Mg/Al bimetal prepared by a novel compound casting. J. Magnes. Alloys 2022, 10, 2296–2309. [Google Scholar] [CrossRef]
- Guan, F.; Jiang, W.; Wang, J.; Li, G.; Zhang, Z.; Fan, Z. Development of high strength Mg/Al bimetal by a novel ultrasonic vibration aided compound casting process. J. Mater. Process. Technol. 2022, 300, 117441. [Google Scholar] [CrossRef]
- Wang, J.; Guan, F.; Jiang, W.; Li, G.; Zhang, Z.; Fan, Z. The role of vibration time in interfacial microstructure and mechanical properties of Al/Mg bimetallic composites produced by a novel compound casting. J. Mater. Res. Technol. 2021, 15, 3867–3879. [Google Scholar] [CrossRef]
- Mola, R.; Bucki, T. Characterization of the Bonding Zone in AZ91/AlSi12 Bimetals Fabricated by Liquid-Solid Compound Casting Using Unmodified and Thermally Modified AlSi12 Alloy. J. Mech. Eng. 2020, 66, 439–448. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhang, Z.; Wang, J.; Fan, Z. Interfacial characteristics, mechanical properties and fracture behaviour of Al/Mg bimetallic composites by compound casting with different morphologies of Al insert. Int. J. Cast Met. Res. 2022, 35, 84–101. [Google Scholar] [CrossRef]
- Kalyankar, V.; Chudasama, G. On the metallurgical challenges of intermetallic compound in steel/Al dissimilar resistance spot welding: Significance, growth and controlling mechanisms. Adv. Mater. Process. Technol. 2024, 10, 2062–2094. [Google Scholar] [CrossRef]
- Shang, S.; Sun, H.; Pan, B.; Wang, Y.; Krajewski, A.M.; Banu, M.; Li, J.; Liu, Z. Forming mechanism of equilibrium and non-equilibrium metallurgical phases in dissimilar aluminum/steel (Al–Fe) joints. Sci. Rep. 2021, 11, 24251. [Google Scholar] [CrossRef] [PubMed]
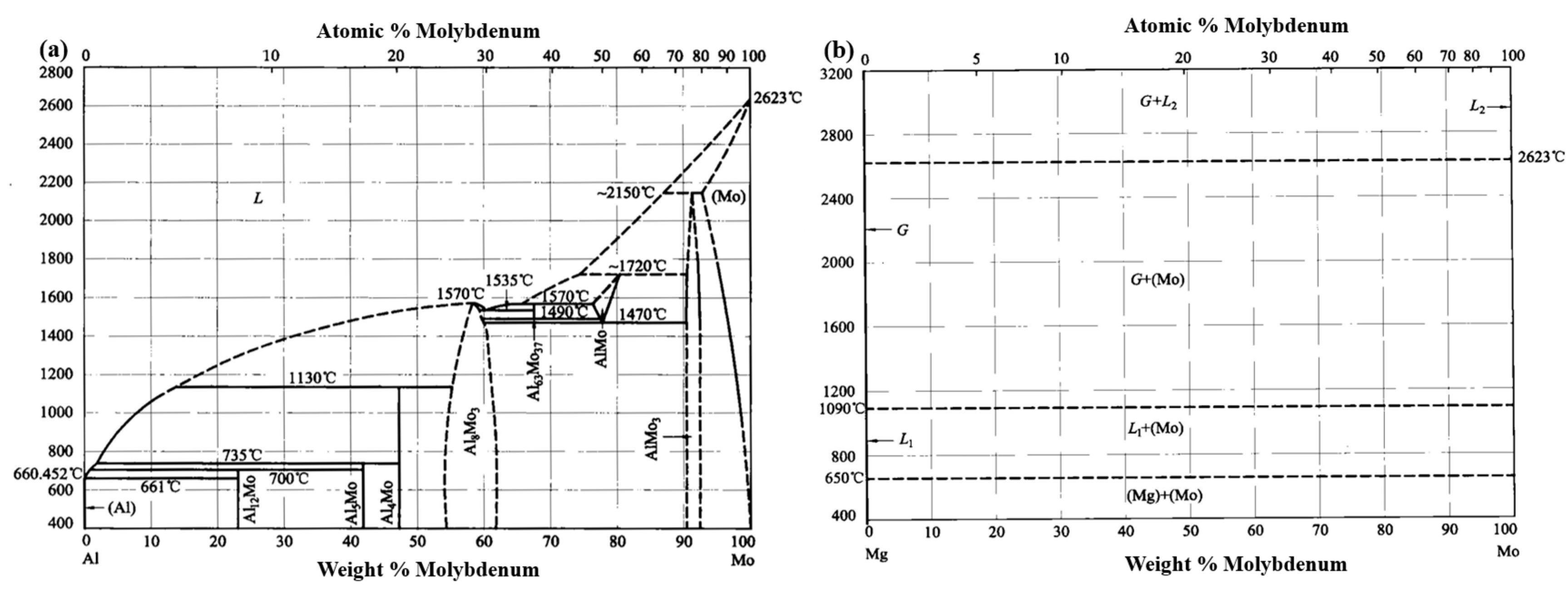
- Saunders, N.; Thermotech, L. The Al-Mo system (aluminum-molybdenum). J. Phase Equilib. 1997, 18, 370–378. [Google Scholar] [CrossRef]
- Villars, P. (Ed.) Mg-Mo Binary Phase Diagram 0-100 at.% Mo; Springer Materials (Online Database): Cham, Switzerland, 2011; Dataset ID: c_0901564; Available online: https://materials.springer.com/isp/phase-diagram/docs/c_0901564 (accessed on 12 August 2025).
- Guo, C.; Li, C.; Masset, P.J.; Du, Z. A thermodynamic description of the Al-Mo-Si system. Calphad 2012, 36, 100–109. [Google Scholar] [CrossRef]
- Kiejna, A.; Nieminen, R.M. Density-functional study of oxygen adsorption on Mo(112). J. Chem. Phys. 2005, 122, 44712. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Jiang, H.; Fan, Z. Effect of insert materials on microstructure and mechanical properties of Al/Mg bimetal produced by a novel solid-liquid compound process. J. Manuf. Process. 2019, 47, 62–73. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Yang, W.; Jiang, Z.; Guan, F.; Jiang, H.; Fan, Z. New Insights into the Characterization and Formation of the Interface of A356/AZ91D Bimetallic Composites Fabricated by Compound Casting. Metall. Mater. Trans. A 2019, 50, 1076–1090. [Google Scholar] [CrossRef]
- Jiang, W.M.; Fan, Z.T.; Li, G.Y. Characteristics and Formation Mechanism of the Interface of Mg/Al Bimetallic Composites Prepared by Lost Foam Casting. Mater. Sci. Forum 2018, 941, 2054–2059. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, C.; Zhu, C.; Li, Y.; Luo, T.; Liu, C.; Xu, S.; Yang, Y. Effect of Mg/Si mass ratio on microstructure and mechanical properties of Al-Mg-Si cast aluminum alloy. China Foundry 2025, 22, 163–172. [Google Scholar] [CrossRef]


| Element | Mass Fraction (%) | ||||||
|---|---|---|---|---|---|---|---|
| Mn | Zn | Si | Ti | Fe | Mg | Al | |
| A356 | - | - | 6.81 | 0.017 | 0.205 | 0.439 | Bal. |
| AZ91D | 0.62 | 0.23 | - | - | - | Bal. | 9.08 |
| Position | Element (at%) | Phase | |||
|---|---|---|---|---|---|
| Al | Mg | Si | O | ||
| 1 | 63.56 | 36.44 | - | - | Al3Mg2 |
| 2 | - | 58.48 | 41.52 | - | Mg2Si |
| 3 | 42.20 | 57.80 | - | - | Al12Mg17 |
| 4 | - | 62.08 | 37.92 | - | Mg2Si |
| 5 | - | 57.15 | 42.85 | - | Mg2Si |
| 6 | 10.28 | 89.72 | - | - | ES |
| 7 | 40.38 | 59.62 | - | - | Al12Mg17 |
| 8 | 12.71 | 23.15 | - | 64.14 | Oxide |
| 9 | 11.24 | 33.22 | 8.76 | 46.78 | Oxide |
| 10 | 11.35 | 88.65 | - | - | ES |
| 11 | 37.29 | 62.71 | - | - | Al12Mg17 |
| Position | Element (at%) | Phase | ||||
|---|---|---|---|---|---|---|
| Al | Mg | Si | Mo | O | ||
| 1 | 0.78 | 3.62 | - | 95.60 | - | Mo SS |
| 2 | 2.84 | 14.57 | - | 15.18 | 67.4 | Oxide |
| 3 | 3.71 | 32.06 | - | 15.71 | 48.51 | Oxide |
| 4 | 44.72 | 55.28 | - | - | - | Al12Mg17 |
| 5 | 1.98 | 8.39 | - | 17.70 | 71.92 | Oxide |
| 6 | 0.78 | 4.31 | - | 94.91 | - | Mo SS |
| 7 | 4.01 | 13.01 | - | 21.56 | 61.42 | Oxide |
| 8 | 2.44 | 23.45 | - | 8.95 | 65.16 | Oxide |
| Position | Element (at%) | Phase | ||||
|---|---|---|---|---|---|---|
| Al | Mg | Si | Mo | O | ||
| 1 | - | 35.71 | 64.29 | - | - | Mg2Si |
| 2 | 38.01 | 61.99 | - | - | - | Al3Mg2 |
| 3 | 38.95 | 61.05 | - | - | - | Al3Mg2 |
| 4 | 47.85 | 52.15 | - | - | - | Al12Mg17 |
| 5 | 40.13 | 59.87 | - | - | - | Al12Mg17 |
| 6 | 14.99 | 85.01 | - | - | - | ES |
| 7 | 59.59 | 40.41 | - | - | - | Al3Mg2 |
| 8 | - | 36.66 | 63.34 | - | - | Mg2Si |
| 9 | 48.40 | 51.60 | - | - | - | Al12Mg17 |
| 10 | 4.38 | 24.46 | 1.23 | 69.92 | Mo SS | |
| 11 | 11.40 | 12.28 | - | 13.53 | 62.79 | Oxide |
| 12 | 38.98 | 61.02 | - | - | - | Al12Mg17 |
| 13 | 11.29 | 88.71 | - | - | - | ES |
| 14 | - | 33.87 | 66.13 | - | - | Mg2Si |
| Specimen | Thickness (μm) | |||
|---|---|---|---|---|
| Al-Mg IMCs | ES | Mo SS | Overall Interface Layer | |
| 0Mo | 896 | 727 | 0 | 1623 |
| 20Mo | 0 | 0 | 28 | 28 |
| U-20Mo | 200 | 338 | 23 | 561 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Fan, X.; Du, H.; Li, G.; Wang, X.; Kang, X.; Zeng, Q. Improving the Interfacial Microstructure and Properties of Al/Mg Bimetal by a Novel Mo Coating Combined with Ultrasonic Field. Materials 2025, 18, 4005. https://doi.org/10.3390/ma18174005
Hu J, Fan X, Du H, Li G, Wang X, Kang X, Zeng Q. Improving the Interfacial Microstructure and Properties of Al/Mg Bimetal by a Novel Mo Coating Combined with Ultrasonic Field. Materials. 2025; 18(17):4005. https://doi.org/10.3390/ma18174005
Chicago/Turabian StyleHu, Jiaze, Xiuru Fan, Haoheng Du, Guangyu Li, Xiaoqiong Wang, Xing Kang, and Qiantong Zeng. 2025. "Improving the Interfacial Microstructure and Properties of Al/Mg Bimetal by a Novel Mo Coating Combined with Ultrasonic Field" Materials 18, no. 17: 4005. https://doi.org/10.3390/ma18174005
APA StyleHu, J., Fan, X., Du, H., Li, G., Wang, X., Kang, X., & Zeng, Q. (2025). Improving the Interfacial Microstructure and Properties of Al/Mg Bimetal by a Novel Mo Coating Combined with Ultrasonic Field. Materials, 18(17), 4005. https://doi.org/10.3390/ma18174005







