Mitigating Strength Loss in Geopolymers in Low-Temperature Environments by Sodium Nitrite Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design Proportions
2.3. Setting Time Test
2.4. Compressive Test Methods
2.5. XRD Analysis
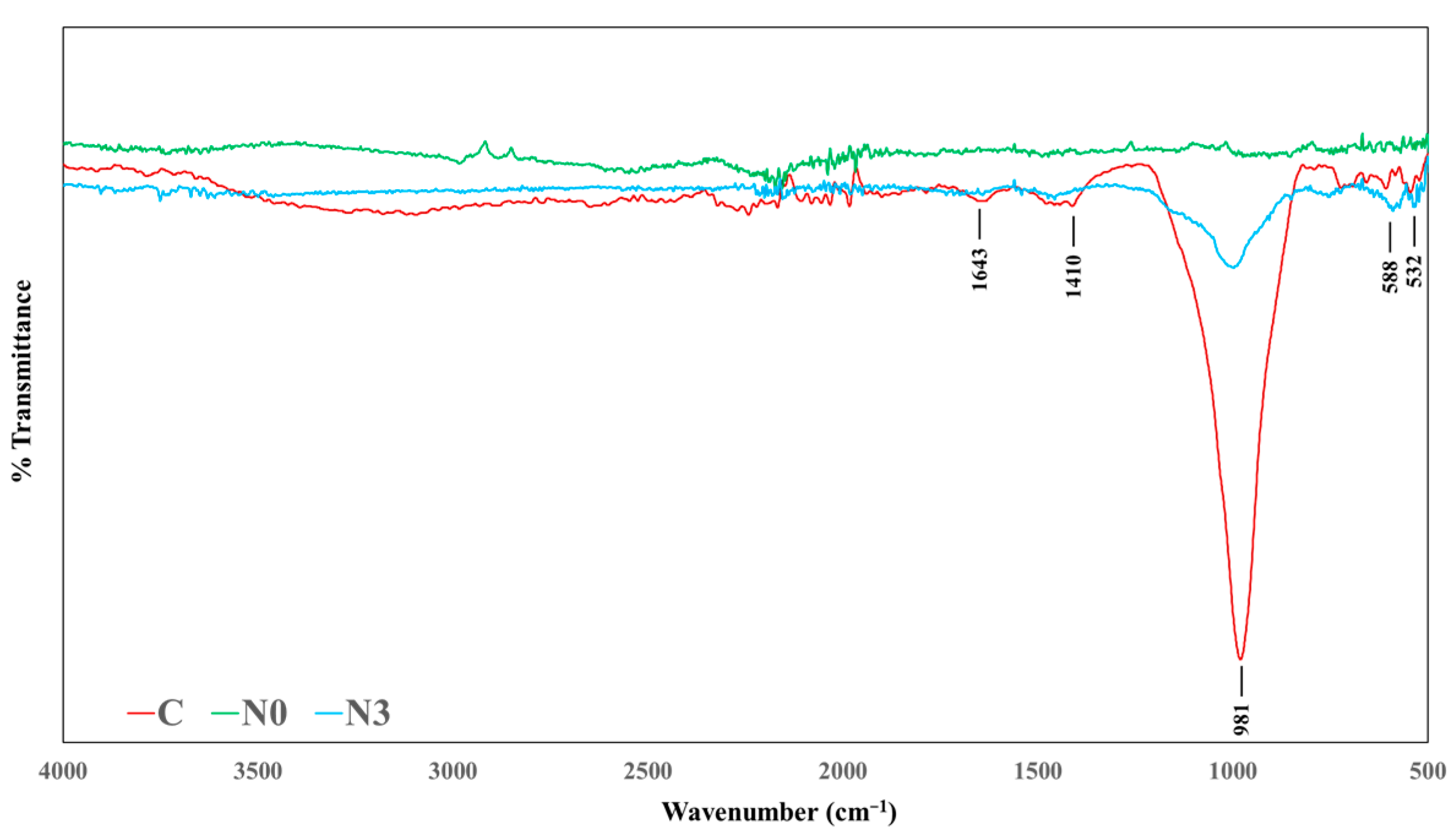
2.6. FTIR Characterization
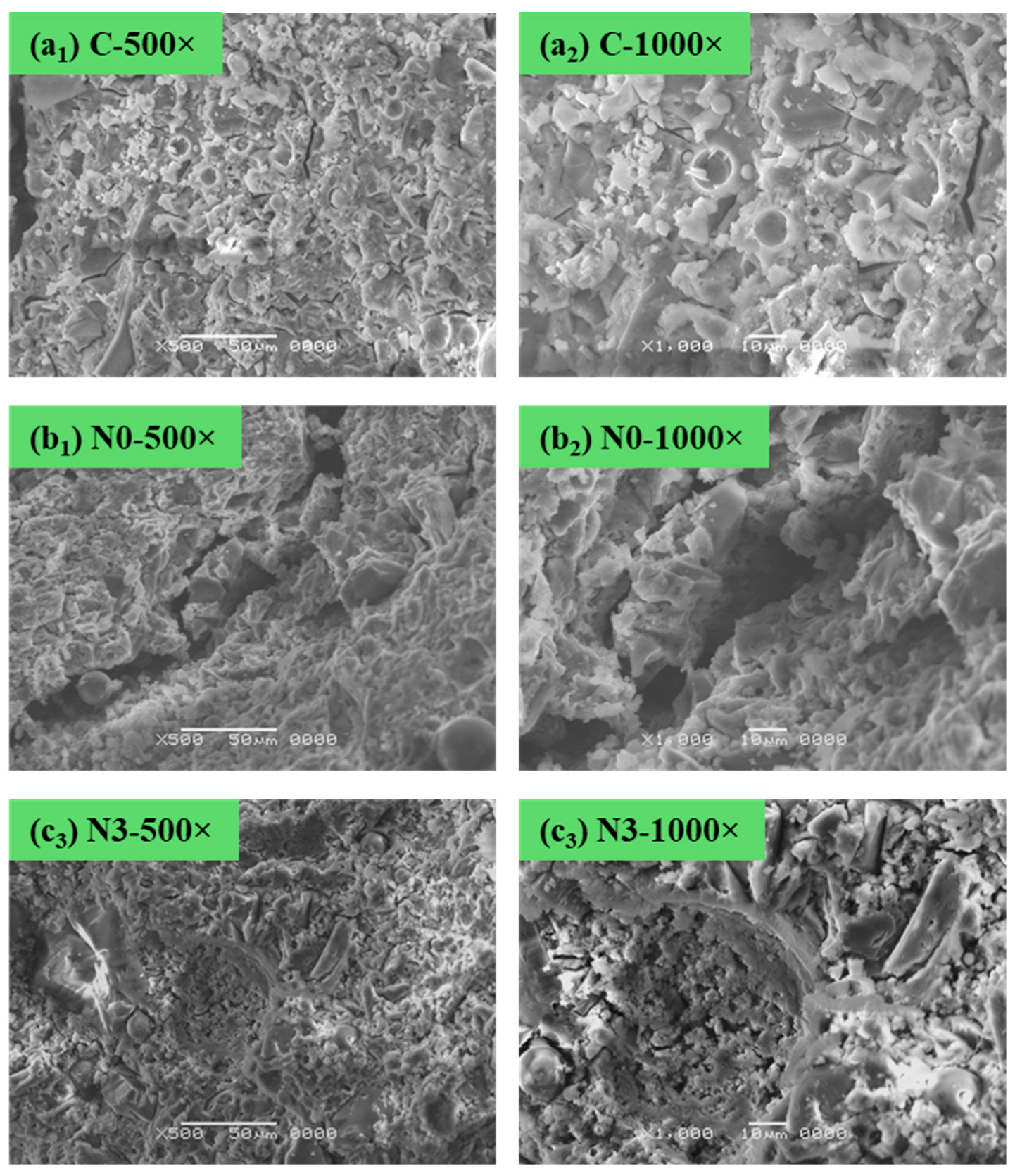
2.7. Morphological Analysis
3. Results and Discussion
3.1. Setting Time
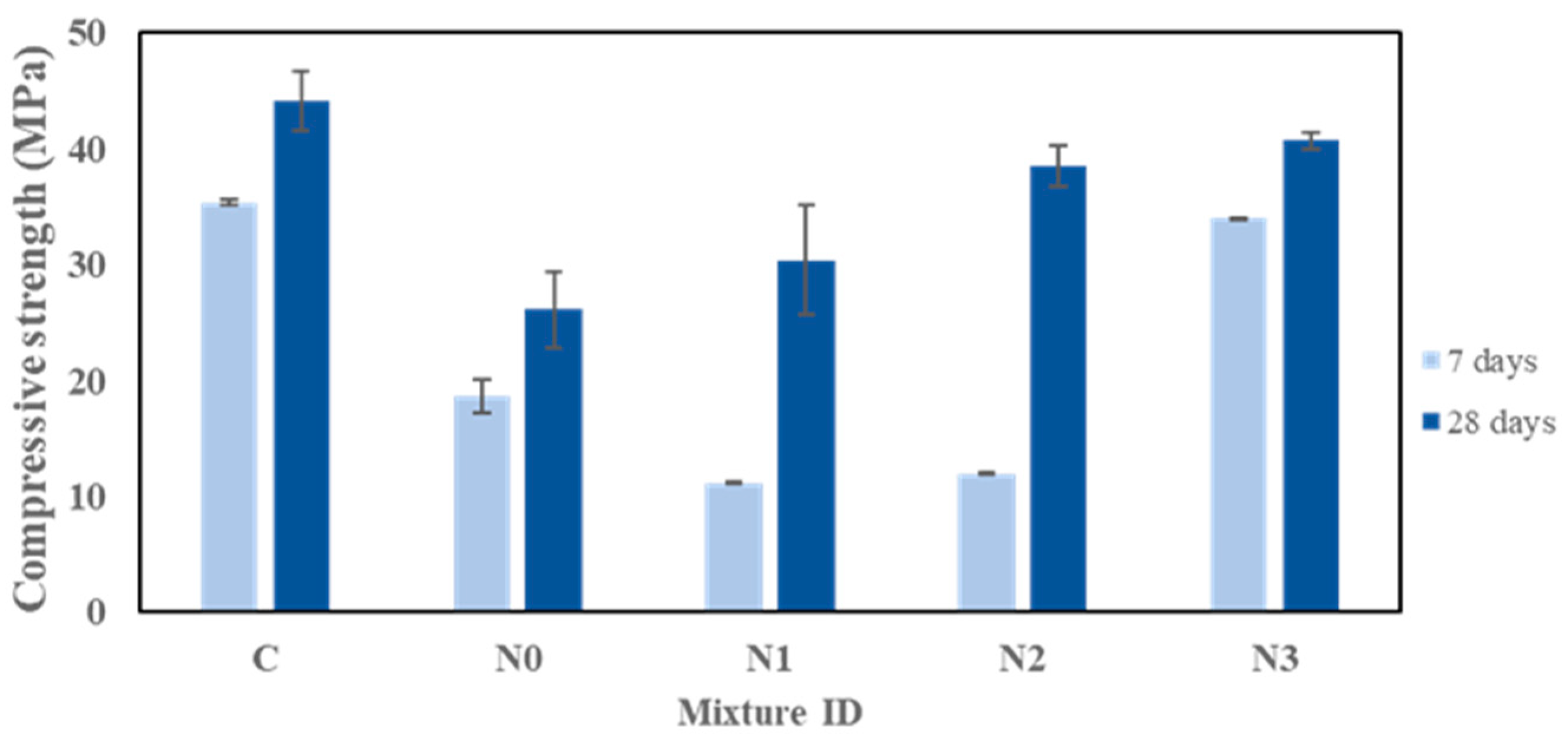
3.2. Compressive Strength
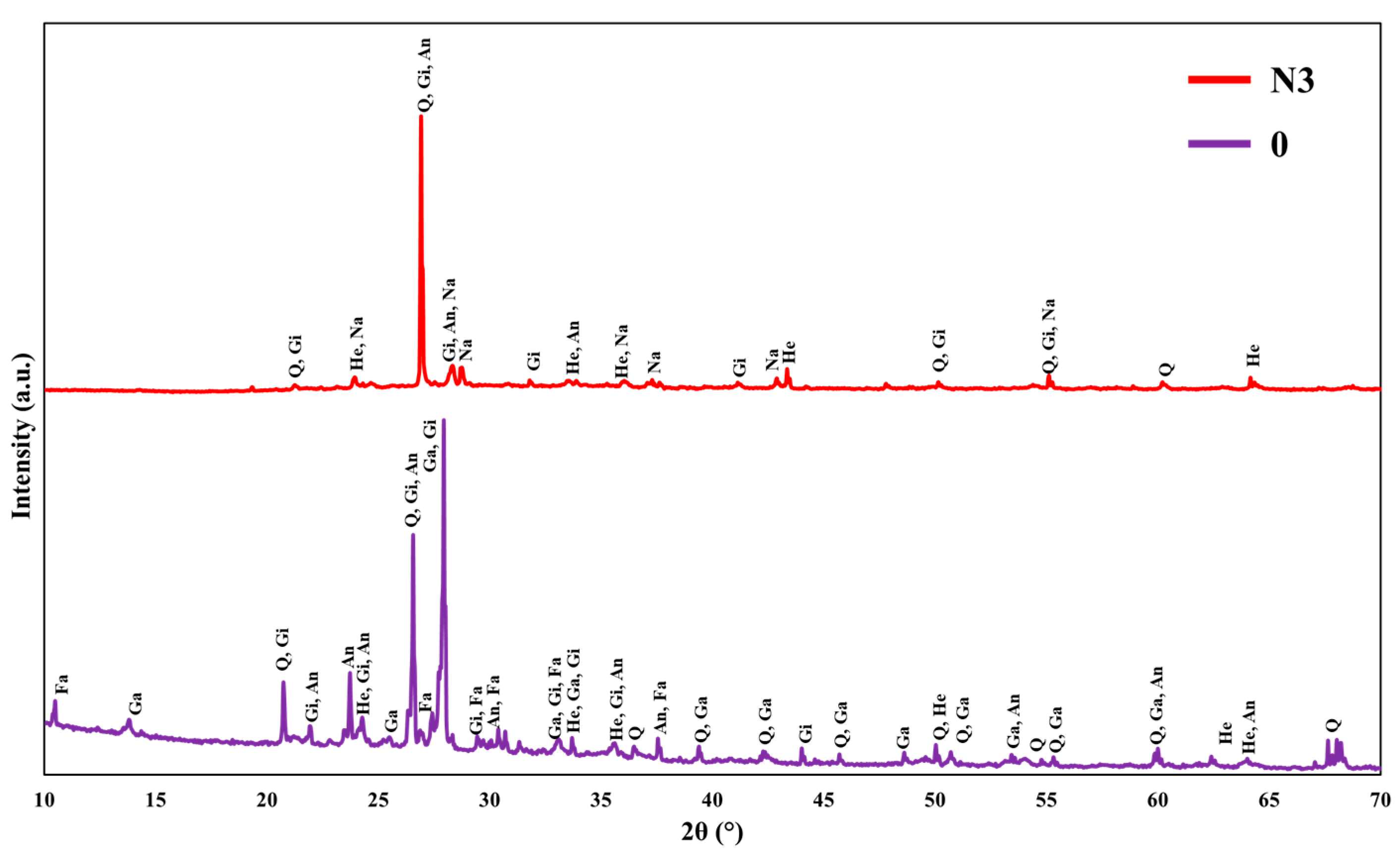
3.3. XRD Characterization
3.4. FTIR Analysis
3.5. Morphological Analysis with SEM Characterization
4. Conclusions
- The addition of 3 wt% NaNO2 significantly improves the compressive strength retention of geopolymers during low-temperature curing (40.7 MPa at 28 days), which is comparable to 44.2 MPa for the control samples.
- Geopolymers without NaNO2 show the severe degradation of strength when cured under subzero conditions due to incomplete geopolymerization, supported by the absence of Si–O–T bonds in FTIR spectral data and the identifiable cracking in SEM images.
- The XRD results indicate that the NaNO2 addition retained the stable geopolymer gel structure in gismondine, and the absence of the faujasite phase indicates the increased degree of geopolymerization. Furthermore, the formation of the new sodium aluminum amide phase could be responsible for stabilizing the performance of the geopolymer in low-temperature environments.
- SEM observations reveal less cracks in the geopolymer containing NaNO2, which suggests a better microstructural integrity and resistance to fracture propagation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, D.; Feng, Y.; Xia, Q.; Tian, J.; Li, X. Research on mechanical properties and durability of early frozen concrete: A review. Constr. Build. Mater. 2024, 425, 135988. [Google Scholar] [CrossRef]
- Matalkah, F.; Soroushian, P. Freeze thaw and deicer salt scaling resistance of concrete prepared with alkali aluminosilicate cement. Constr. Build. Mater. 2018, 163, 200–213. [Google Scholar] [CrossRef]
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J.G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- Martínez, A.; Miller, S.A. A review of drivers for implementing geopolymers in construction: Codes and constructability. Resour. Conserv. Recycl. 2023, 199, 107238. [Google Scholar] [CrossRef]
- Harmaji, A.; Jafari, R.; Simard, G. Valorization of Residue from Aluminum Industries: A Review. Materials 2024, 17, 5152. [Google Scholar] [CrossRef]
- Harmaji, A.; Kirana, M.C.; Jafari, R. Machine Learning to Predict Workability and Compressive Strength of Low- and High-Calcium Fly Ash–Based Geopolymers. Crystals 2024, 14, 830. [Google Scholar] [CrossRef]
- Pang, S.; Zhang, X.; Lei, B.; Fan, H.; Liu, J.; Ju, P.; Gao, Y. Effect of freeze-thaw cycle on deterioration of mechanical properties of fibre-reinforced geopolymer cemented aeolian sand. Constr. Build. Mater. 2024, 452, 138943. [Google Scholar] [CrossRef]
- Sun, P.; Wu, H.-C. Chemical and freeze–thaw resistance of fly ash-based inorganic mortars. Fuel 2013, 111, 740–745. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Wang, Q.; Zhang, T.; Yang, F.; Xu, Y.; Zhan, J. Experimental Analysis and Establishment of Strength Attenuation Model of POM Fiber Reinforced Geopolymeric Recycled Concrete under Freeze-Thaw Cycles. Materials 2023, 16, 1699. [Google Scholar] [CrossRef]
- Min, Y.; Wu, J.; Li, B.; Zhang, M.; Zhang, J. Experimental study of freeze–thaw resistance of a one-part geopolymer paste. Case Stud. Constr. Mater. 2022, 17, e01269. [Google Scholar] [CrossRef]
- Wei, X.; Ming, F.; Li, D.; Chen, L.; Liu, Y. Influence of Water Content on Mechanical Strength and Microstructure of Alkali-Activated Fly Ash/GGBFS Mortars Cured at Cold and Polar Regions. Materials 2020, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; Habermehl-Cwirzen, K.; Hedlund, H.; Cwirzen, A. A Review of the Mechanical Properties and Durability of Ecological Concretes in a Cold Climate in Comparison to Standard Ordinary Portland Cement-Based Concrete. Materials 2020, 13, 3467. [Google Scholar] [CrossRef] [PubMed]
- Kumar Das, J.; Pradhan, B. Effect of sodium nitrite on chloride-induced corrosion of steel in concrete. Mater. Today: Proc. 2022, 65, 636–643. [Google Scholar] [CrossRef]
- Teklegiorgis, N.S.; Pradhan, B.; Prusty, J.K.; Das, J.K. Effect of Sodium Nitrite as Corrosion Inhibitor against Chloride-Induced Corrosion of Steel Rebar in Geopolymer Concrete Containing Fly Ash and GGBS. J. Mater. Civ. Eng. 2022, 34, 04022007. [Google Scholar] [CrossRef]
- Simatupang, P.H.; Hanafi, R.; Purwasasmita, B.S.; Imran, I.; Pane, I. The Study of Red Mud Addition Influences in Metakaolinite-Based Geopolymer Characteristics. Adv. Mater. Res. 2012, 450-451, 281–285. [Google Scholar] [CrossRef]
- Harmaji, A.; Sunendar, B. Utilization of Fly Ash, Red Mud, and Electric Arc Furnace Dust Slag for Geopolymer. Mater. Sci. Forum 2016, 841, 157–161. [Google Scholar] [CrossRef]
- Khouchaf, L.; Boulahya, K.; Das, P.P.; Nicolopoulos, S.; Kis, V.K.; Lábár, J.L. Study of the Microstructure of Amorphous Silica Nanostructures Using High-Resolution Electron Microscopy, Electron Energy Loss Spectroscopy, X-ray Powder Diffraction, and Electron Pair Distribution Function. Materials 2020, 13, 4393. [Google Scholar] [CrossRef]
- Walkley, B.; Ke, X.; Hussein, O.; Provis, J.L. Thermodynamic properties of sodium aluminosilicate hydrate (N–A–S–H). Dalton Trans. 2021, 50, 13968–13984. [Google Scholar] [CrossRef]
- Srinivasan, C.B.; Narasimhan, N.L.; Ilango, S.V. Development of rapid-set high-strength cement using statistical experimental design. Cem. Concr. Res. 2003, 33, 1287–1292. [Google Scholar] [CrossRef]
- Su, Y.; Luo, B.; Luo, Z.; Huang, H.; Li, J.; Wang, D. Effect of Accelerators on the Workability, Strength, and Microstructure of Ultra-High-Performance Concrete. Materials 2021, 15, 159. [Google Scholar] [CrossRef]
- Al-musawi, H.; Huang, H.; Di Benedetti, M.; Guadagnini, M.; Pilakoutas, K. Effect of shrinkage on rapid hardening plain and recycled steel fibre concrete overlays. Cem. Concr. Compos. 2022, 125, 104246. [Google Scholar] [CrossRef]
- Xu, Y.; He, T.; Ma, X. The influence of calcium nitrate/sodium nitrate on the hydration process of cement paste mixed with alkali free liquid accelerator. Constr. Build. Mater. 2022, 347, 128555. [Google Scholar] [CrossRef]
- Perera, D.S.; Uchida, O.; Vance, E.R.; Finnie, K.S. Influence of curing schedule on the integrity of geopolymers. J. Mater. Sci. 2007, 42, 3099–3106. [Google Scholar] [CrossRef]
- Huseien, G.F.; Khamehchi, M.; Kubba, Z.; Benjeddou, O.; Mahmoodi, M.J. Freeze-thaw cycle and abrasion resistance of alkali-activated FA and POFA-based mortars: Role of high volume GBFS incorporation. Heliyon 2023, 9, e17672. [Google Scholar] [CrossRef]
- Ye, W.; Feng, T.; Sun, W.; Gómez-Zamorano, L.Y. Enhancing steel slag cement mortar performance under low-temperature curing through alkali activation: Mechanisms and implications. Front. Mater. 2025, 12, 1576078. [Google Scholar] [CrossRef]
- Paswan, R.; Das, S. Elucidating the evolution of pore structure, microstructural damage, and micromechanical response in cement pastes containing microencapsulated phase change materials under freeze-thaw conditions. Cem. Concr. Compos. 2024, 154, 105743. [Google Scholar] [CrossRef]
- Ma, X.; Hu, S.; Sun, H.; Zhang, C.; Yang, Y.; Huo, Y. Exploring the influence of sodium nitrite on the early-age freeze resistance of low-carbon sulphoaluminate cement (SAC). J. Build. Eng. 2024, 84, 108489. [Google Scholar] [CrossRef]
- Siyal, A.A.; Mohamed, R.M.S.R.; Shamsuddin, R.; Ridzuan, M.B. A comprehensive review of synthesis kinetics and formation mechanism of geopolymers. RSC Adv. 2024, 14, 446–462. [Google Scholar] [CrossRef]
- Wang, Z.; Rehemituli, R.; Zhang, X. Study on the Compressive Strength of Alkali Activated Fly Ash and Slag under the Different Silicate Structure. Materials 2021, 14, 2227. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, L.; Gbozee, M. Thermal stability of geopolymers used as supporting materials for TiO2 film coating through sol-gel process: Feasibility and improvement. Constr. Build. Mater. 2016, 125, 1114–1126. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Thu, T.T.; Tran, A.T.H.; Le, O.T.K.; Sagadevan, S.; Kaus, N.H.M. Effect of Red Mud and Rice Husk Ash-Based Geopolymer Composites on the Adsorption of Methylene Blue Dye in Aqueous Solution for Wastewater Treatment. ACS Omega 2023, 8, 41258–41272. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, K.; Sun, W.; Zeng, Y.; Zou, Z. Mechanics and microstructure analysis of geopolymer utilizing ilmenite tailing and metakaolin powder as alkali-activated materials. Case Stud. Constr. Mater. 2024, 21, e03567. [Google Scholar] [CrossRef]
- Iftikhar, S.; Rashid, K.; Haq, E.U.; Zafar, I.; Alqahtani, F.K.; Khan, M.I. Synthesis and characterization of sustainable geopolymer green clay bricks: An alternative to burnt clay brick. Constr. Build. Mater. 2020, 259, 119659. [Google Scholar] [CrossRef]
- Ionescu, B.A.; Barbu, A.-M.; Lăzărescu, A.-V.; Rada, S.; Gabor, T.; Florean, C. The Influence of Substitution of Fly Ash with Marble Dust or Blast Furnace Slag on the Properties of the Alkali-Activated Geopolymer Paste. Coatings 2023, 13, 403. [Google Scholar] [CrossRef]
- Erfanimanesh, A.; Sharbatdar, M.K. Mechanical and microstructural characteristics of geopolymer paste, mortar, and concrete containing local zeolite and slag activated by sodium carbonate. J. Build. Eng. 2020, 32, 101781. [Google Scholar] [CrossRef]
- Agista, M.N.; Khalifeh, M.; Braga, R.; Freitas, J. Low-density granite-based geopolymer for well cementing: The role of burnt lime in enhancing early strength. Geoenergy Sci. Eng. 2024, 242, 213249. [Google Scholar] [CrossRef]
- Özen, S.; Uzal, B. Effect of characteristics of natural zeolites on their geopolymerization. Case Stud. Constr. Mater. 2021, 15, e00715. [Google Scholar] [CrossRef]
- Nikolov, A.; Rostovsky, I.; Nugteren, H. Geopolymer materials based on natural zeolite. Case Stud. Constr. Mater. 2017, 6, 198–205. [Google Scholar] [CrossRef]
- Wu, K.; Han, H.; Xu, L.; Gao, Y.; Yang, Z.; Jiang, Z.; De Schutter, G. The improvement of freezing–thawing resistance of concrete by cellulose/polyvinyl alcohol hydrogel. Constr. Build. Mater. 2021, 291, 123274. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S.J. The effect of silica availability on the mechanism of geopolymerisation. Cem. Concr. Res. 2011, 41, 210–216. [Google Scholar] [CrossRef]
- Yuniati, M.D.; Hirajima, T.; Miki, H.; Sasaki, K. Silicate Covering Layer on Pyrite Surface in the Presence of Silicon–Catechol Complex for Acid Mine Drainage Prevention. Mater. Trans. 2015, 56, 1733–1741. [Google Scholar]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Arima, T.; Villacorte-Tabelin, M. Utilization of Palm Oil Fuel Ash (POFA) as an Admixture for the Synthesis of a Gold Mine Tailings-Based Geopolymer Composite. Minerals 2023, 13, 232. [Google Scholar] [CrossRef]
- Harmaji, A.; Jafari, R.; Simard, G. Durable bauxite-residue geopolymers: Enhancing scaling resistance with fly ash and waste glass powder. Constr. Build. Mater. 2025, 491, 142661. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Shahriari, E. Heterogeneous Photodecolorization of Methyl Green Catalyzed by Fe(II)-o-Phenanthroline/Zeolite Y Nanocluster. Int. J. Photoenergy 2011, 2011, 518153. [Google Scholar] [CrossRef][Green Version]
- Firdous, R.; Stephan, D.; Djobo, J.N.Y. Natural pozzolan based geopolymers: A review on mechanical, microstructural and durability characteristics. Constr. Build. Mater. 2018, 190, 1251–1263. [Google Scholar] [CrossRef]
- Merabtene, M.; Kacimi, L.; Clastres, P. Elaboration of geopolymer binders from poor kaolin and dam sludge waste. Heliyon 2019, 5, e01938. [Google Scholar] [CrossRef]
- Valencia-Saavedra, W.; Robayo-Salazar, R.; de Gutiérrez, R.M. Alkali-Activated Hybrid Cements Based on Fly Ash and Construction and Demolition Wastes Using Sodium Sulfate and Sodium Carbonate. Molecules 2021, 26, 7572. [Google Scholar] [CrossRef] [PubMed]
- Husem, M.; Gozutok, S. The effects of low temperature curing on the compressive strength of ordinary and high performance concrete. Constr. Build. Mater. 2005, 19, 49–53. [Google Scholar] [CrossRef]
- Hasholt, M.T.; Christensen, K.U.; Pade, C. Frost resistance of concrete with high contents of fly ash-A study on how hollow fly ash particles distort the air void analysis. Cem. Concr. Res. 2019, 119, 102–112. [Google Scholar] [CrossRef]
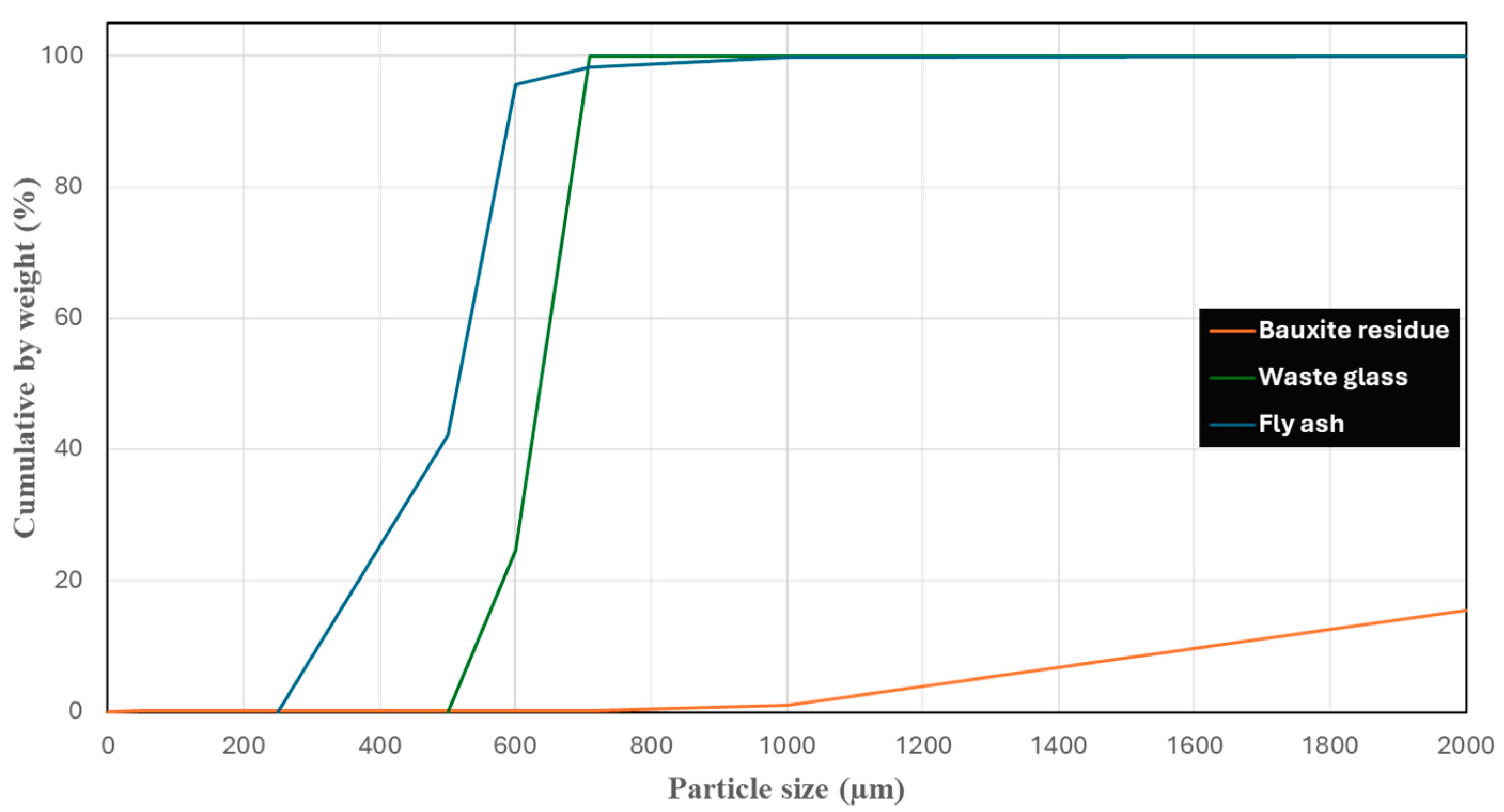
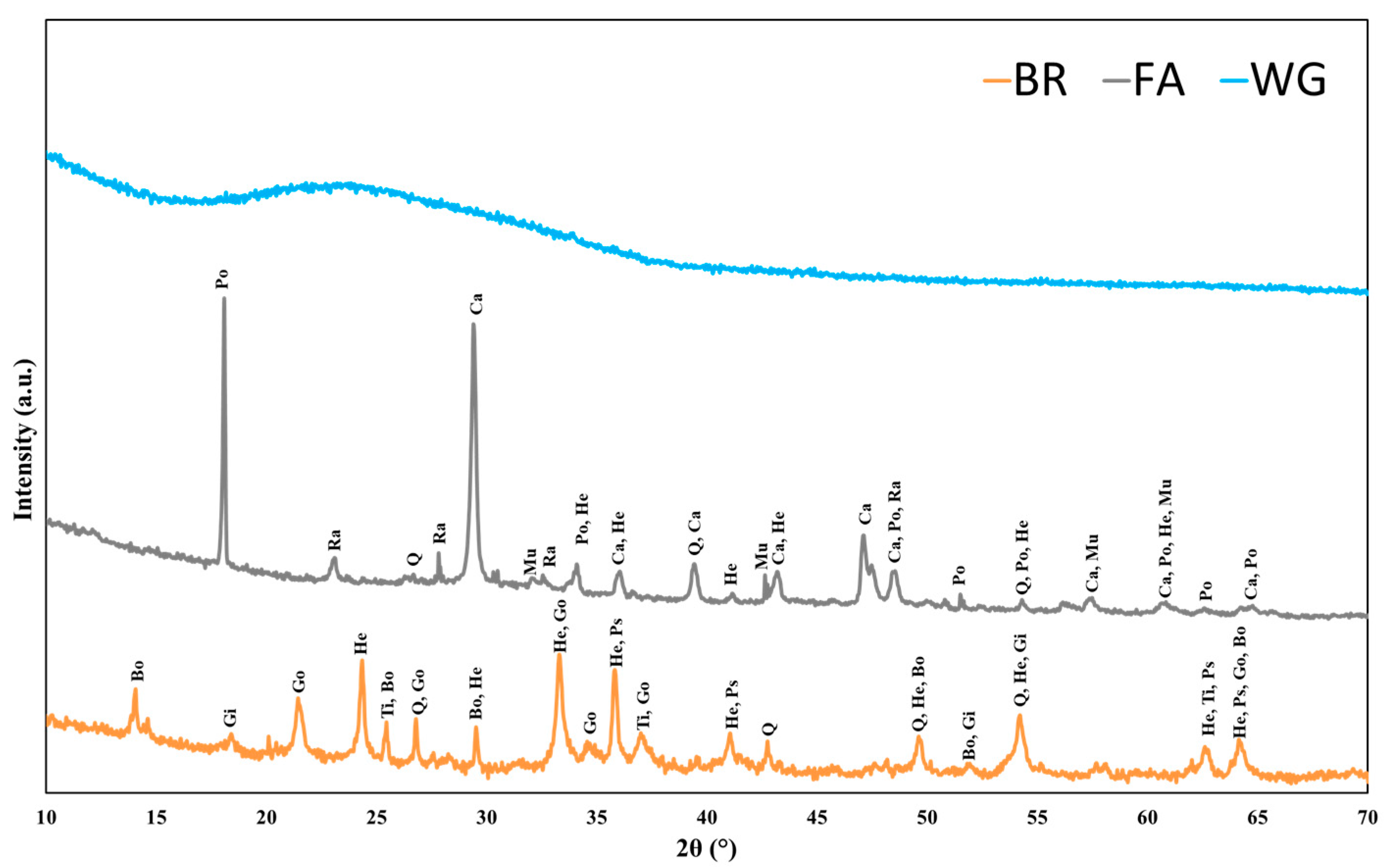

| Oxide Mass (%) | Bauxite Residue | Fly Ash | Waste Glass |
|---|---|---|---|
| SiO2 | 11.1 | 43.5 | 72.2 |
| Al2O3 | 17.2 | 21.1 | 1.6 |
| Fe2O3 | 44.4 | 26 | 0.4 |
| MgO | 0.05 | 0.7 | 1.1 |
| CaO | 2.6 | 3.8 | 11.2 |
| Na2O | 6.4 | 0.5 | 12.9 |
| K2O | 0.06 | 1.7 | 0.5 |
| LOI | 11.9 | 1.9 | - |
| Name | Bauxite Residue (kg/m3) | Fly Ash (kg/m3) | Waste Glass Powder (kg/m3) | Fine Aggregate (kg/m3) | NaOH (kg/m3) | Na2SiO3 (kg/m3) | NaNO2 (kg/m3) | Curing Method |
|---|---|---|---|---|---|---|---|---|
| C | 302.8 | 113.5 | 340.7 | 1514 | 101 | 202 | - | ambient |
| N0 | 302.8 | 113.5 | 340.7 | 1514 | 101 | 202 | - | subzero |
| N1 | 302.8 | 113.5 | 340.7 | 1514 | 101 | 202 | 7.6 | subzero |
| N2 | 302.8 | 113.5 | 340.7 | 1514 | 101 | 202 | 15.2 | subzero |
| N3 | 302.8 | 113.5 | 340.7 | 1514 | 101 | 202 | 22.8 | subzero |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmaji, A.; Jafari, R. Mitigating Strength Loss in Geopolymers in Low-Temperature Environments by Sodium Nitrite Addition. Materials 2025, 18, 3987. https://doi.org/10.3390/ma18173987
Harmaji A, Jafari R. Mitigating Strength Loss in Geopolymers in Low-Temperature Environments by Sodium Nitrite Addition. Materials. 2025; 18(17):3987. https://doi.org/10.3390/ma18173987
Chicago/Turabian StyleHarmaji, Andrie, and Reza Jafari. 2025. "Mitigating Strength Loss in Geopolymers in Low-Temperature Environments by Sodium Nitrite Addition" Materials 18, no. 17: 3987. https://doi.org/10.3390/ma18173987
APA StyleHarmaji, A., & Jafari, R. (2025). Mitigating Strength Loss in Geopolymers in Low-Temperature Environments by Sodium Nitrite Addition. Materials, 18(17), 3987. https://doi.org/10.3390/ma18173987








