Effect of Temperature and Pyrolysis Atmosphere on Pore Structure of Sintered Coal Gangue Ceramsites
Abstract
1. Introduction
2. Materials and Methods
2.1. Coal Gangue (CG)
2.2. Preparation of Coal Gangue Ceramsites (CGCs)
2.3. Characterization Methods
3. Results and Discussion
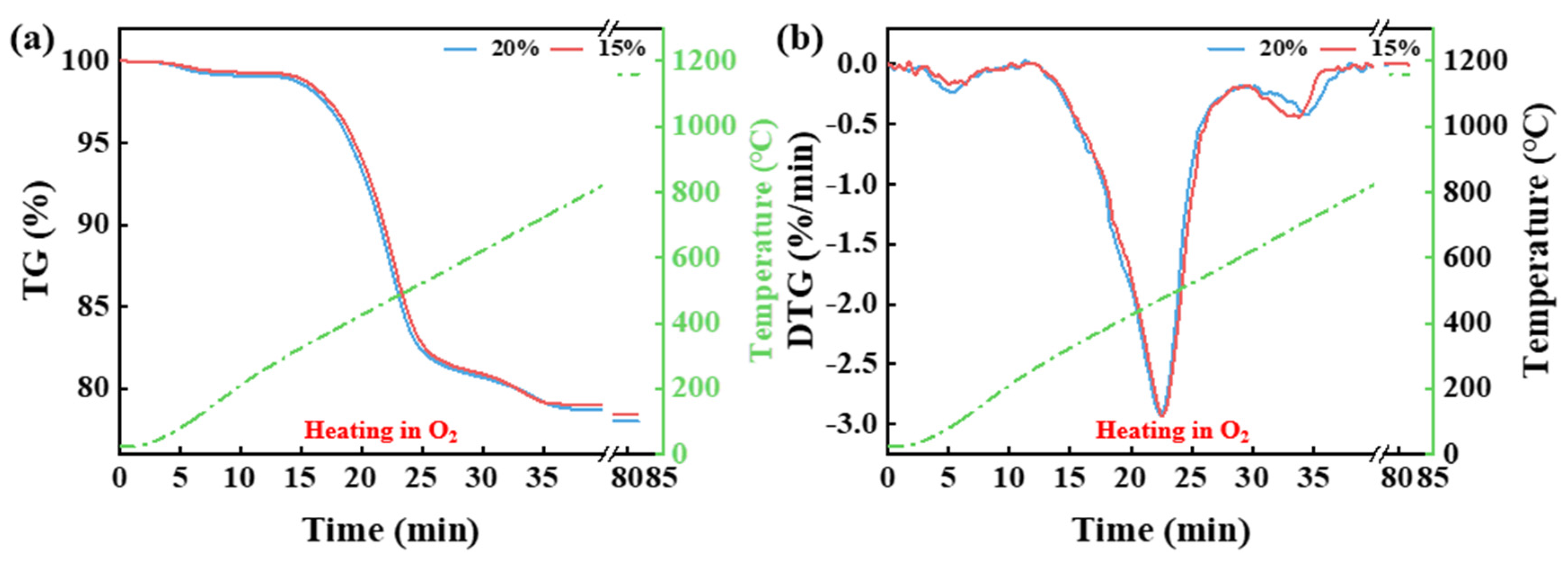
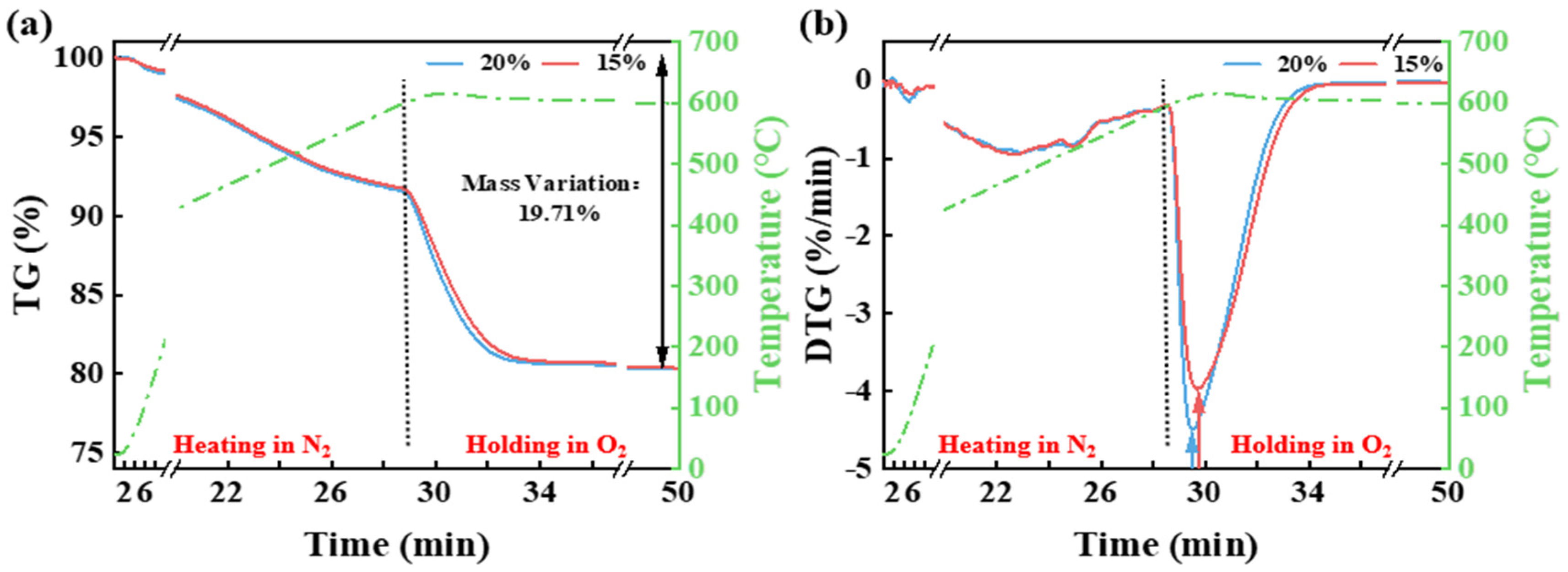
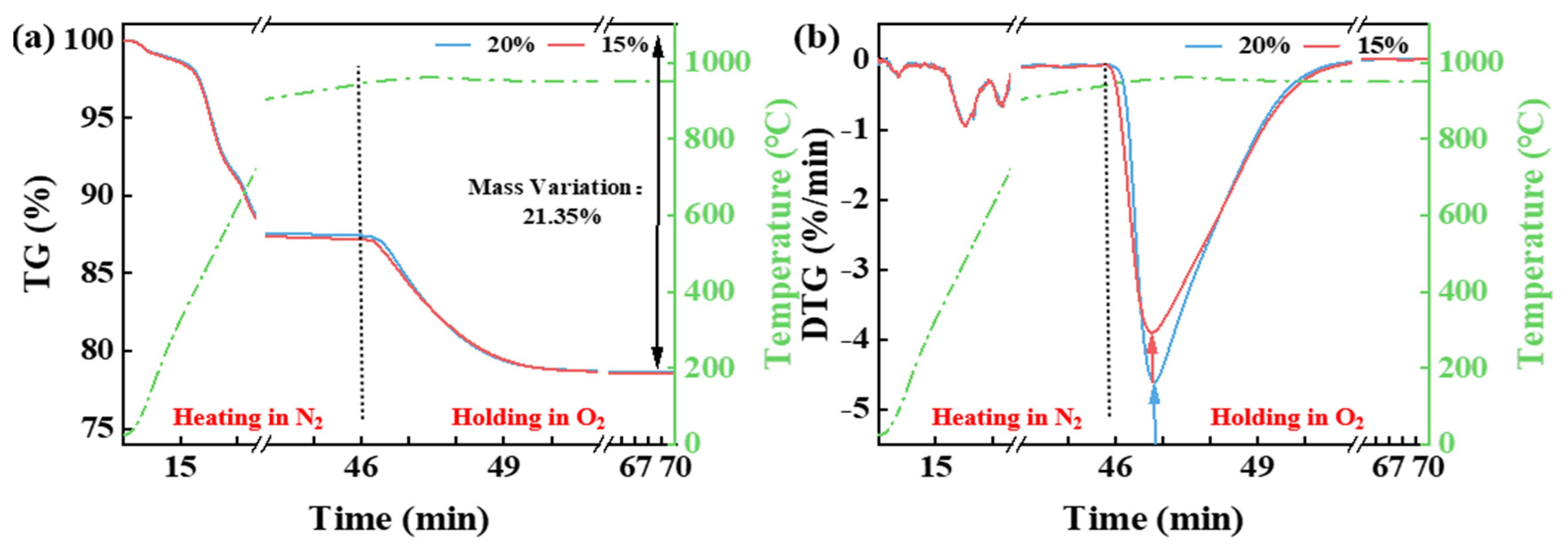
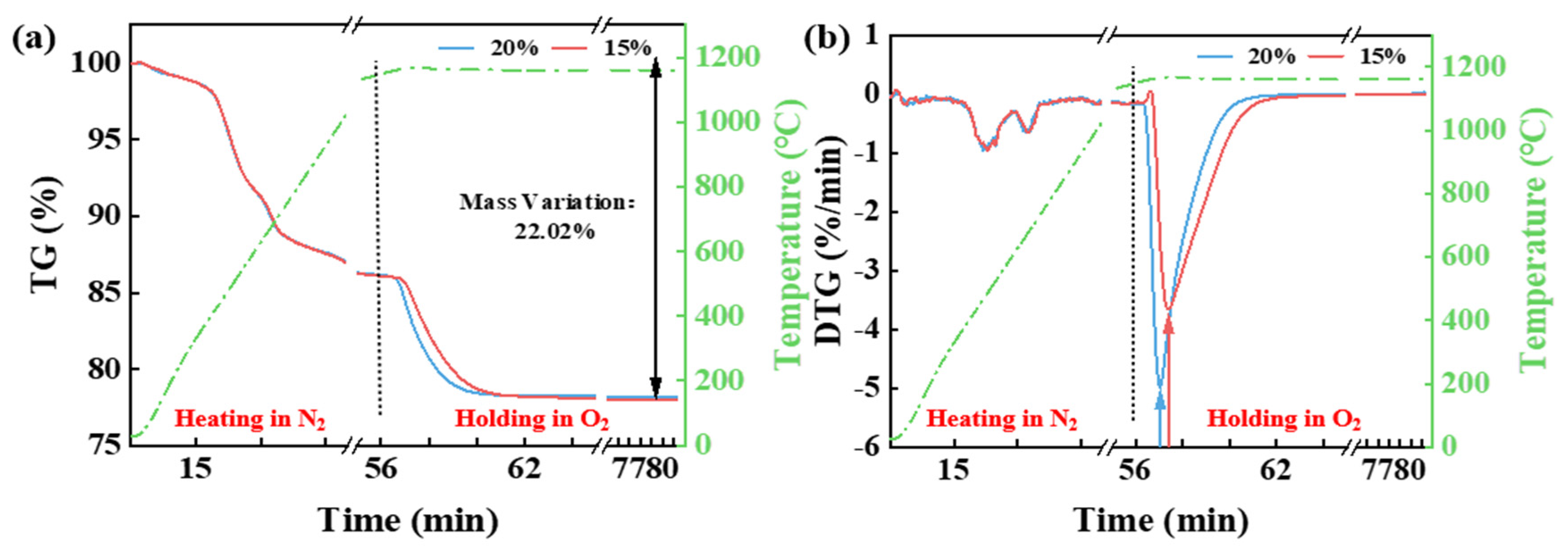
3.1. Effect of Temperature and Pyrolysis Atmosphere on the Thermal Release of CG
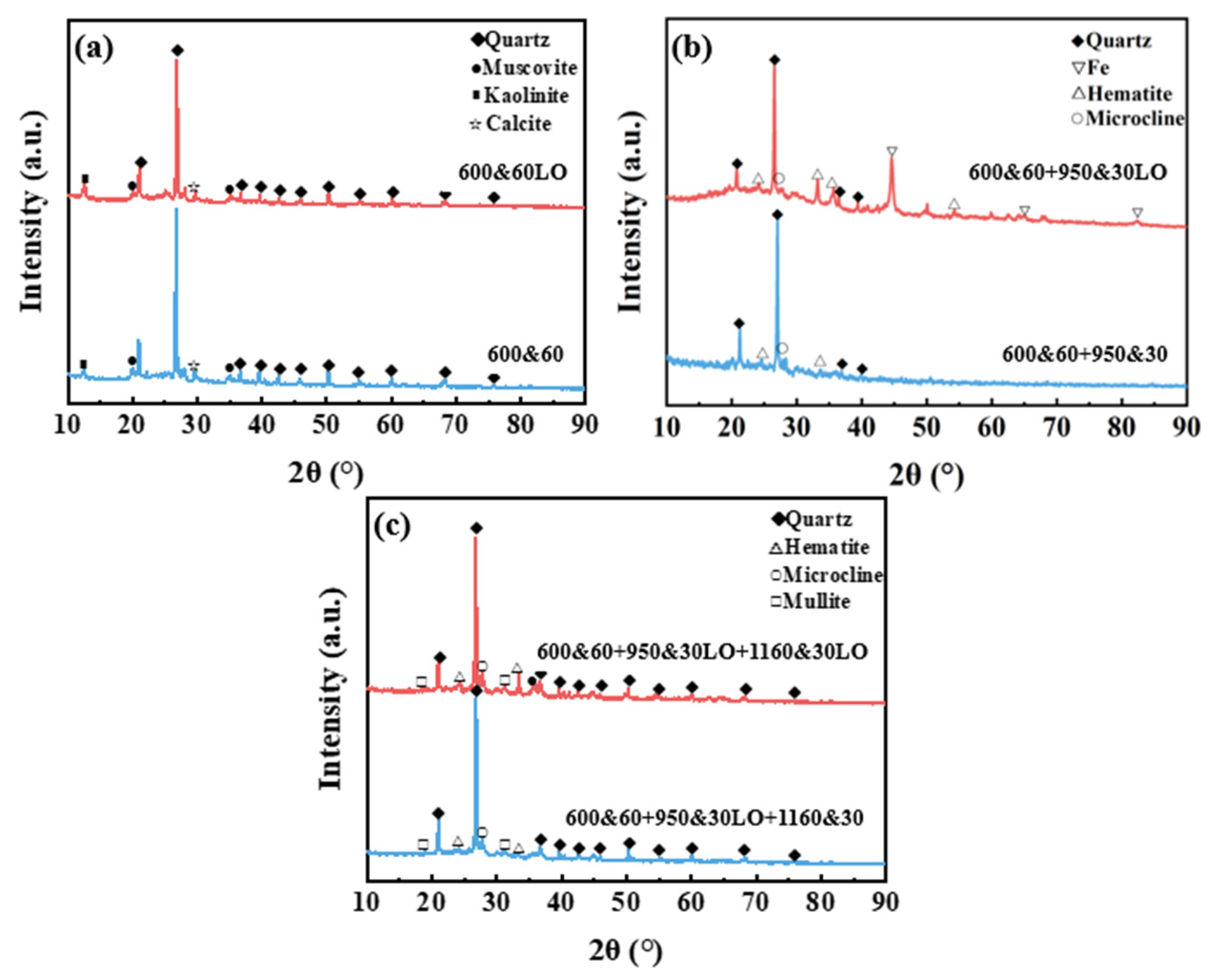
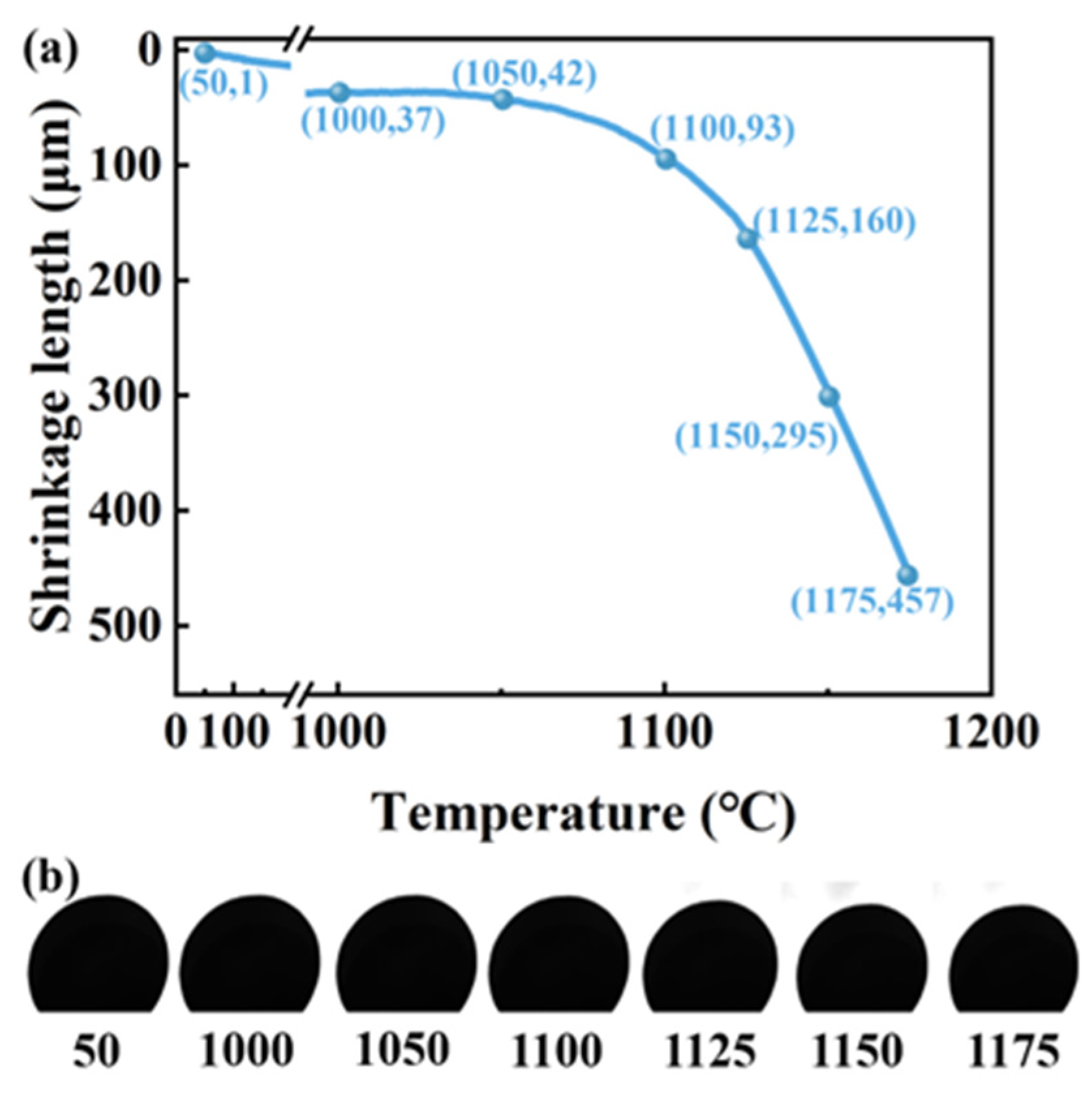
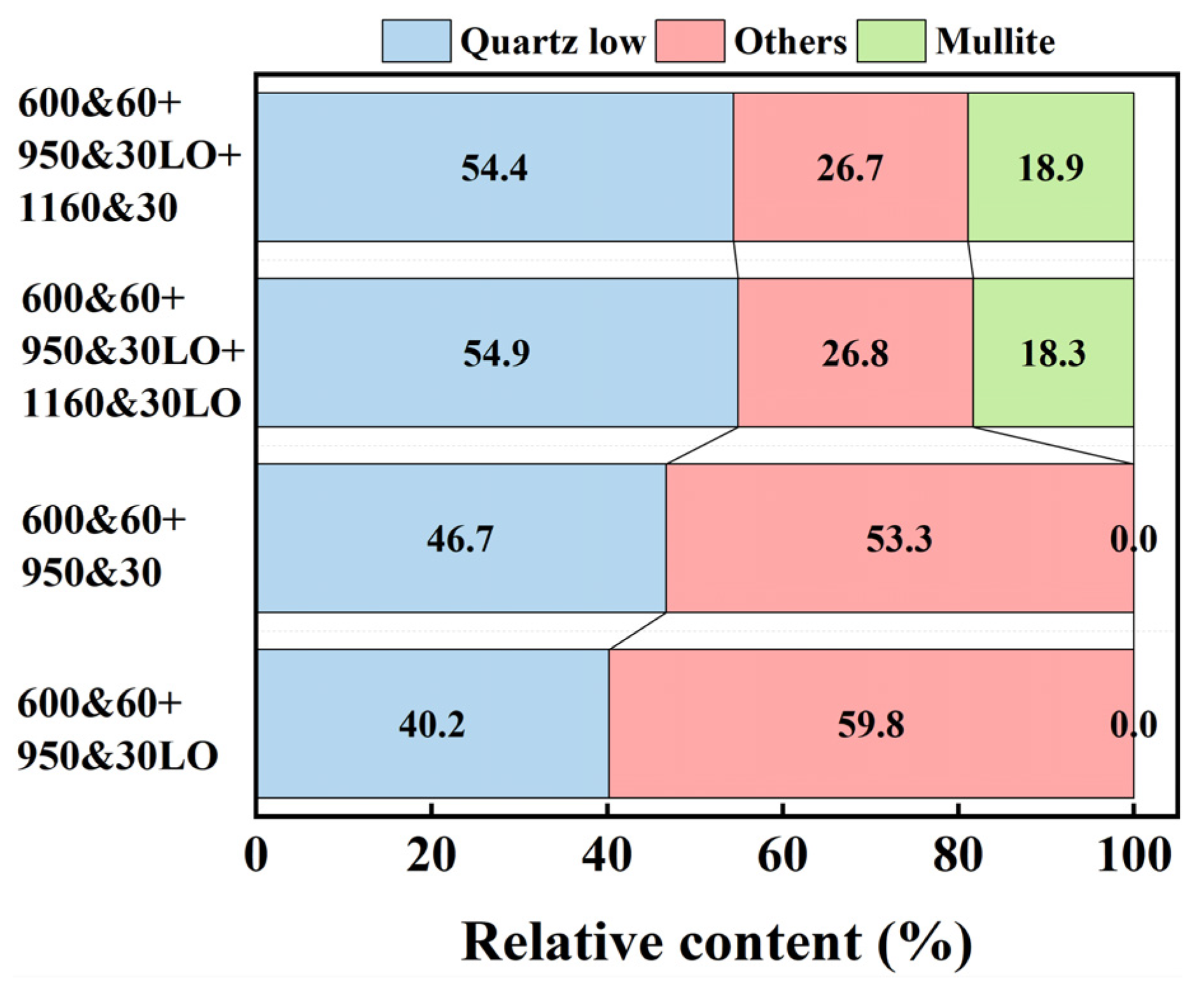
3.2. Phase Transformation and Shrinkage During the Sintering Process of CGCs
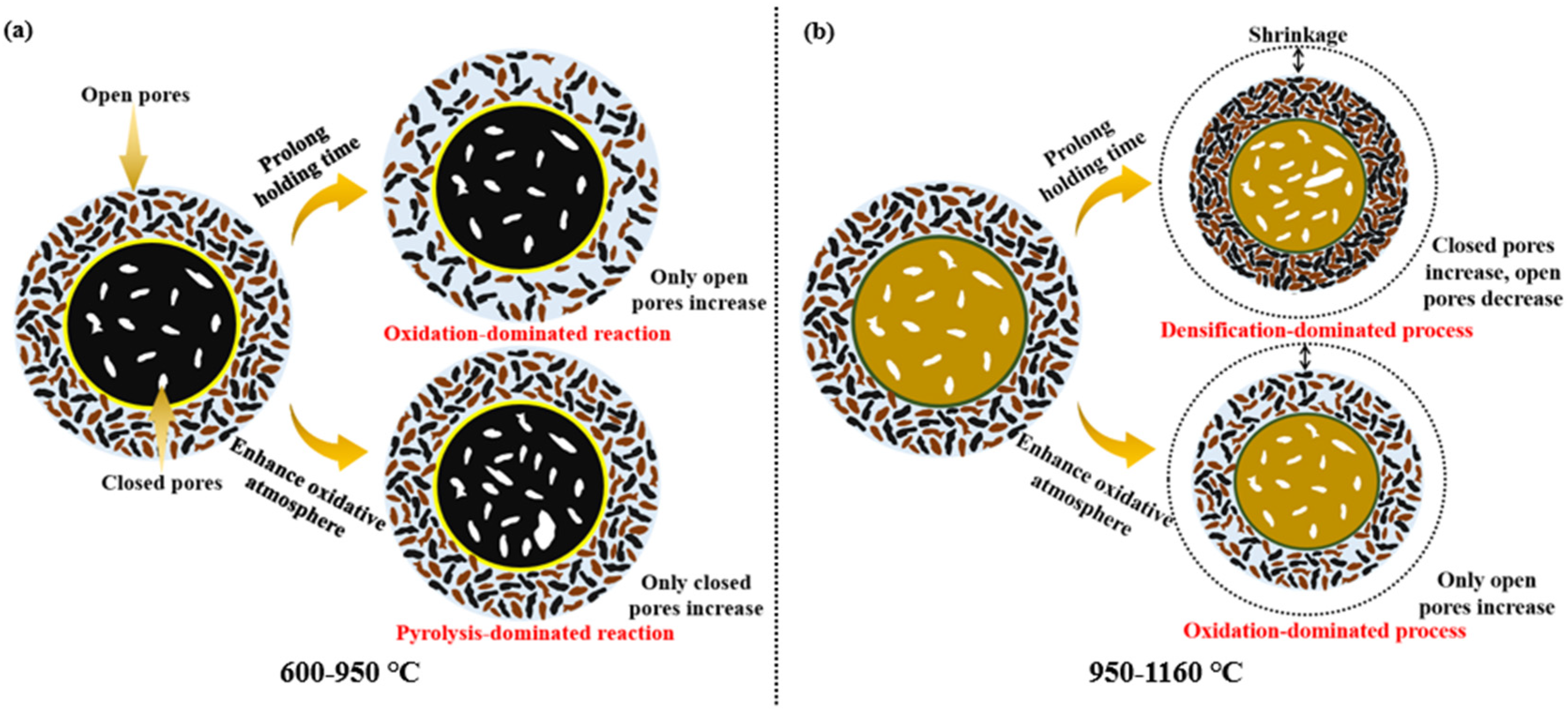
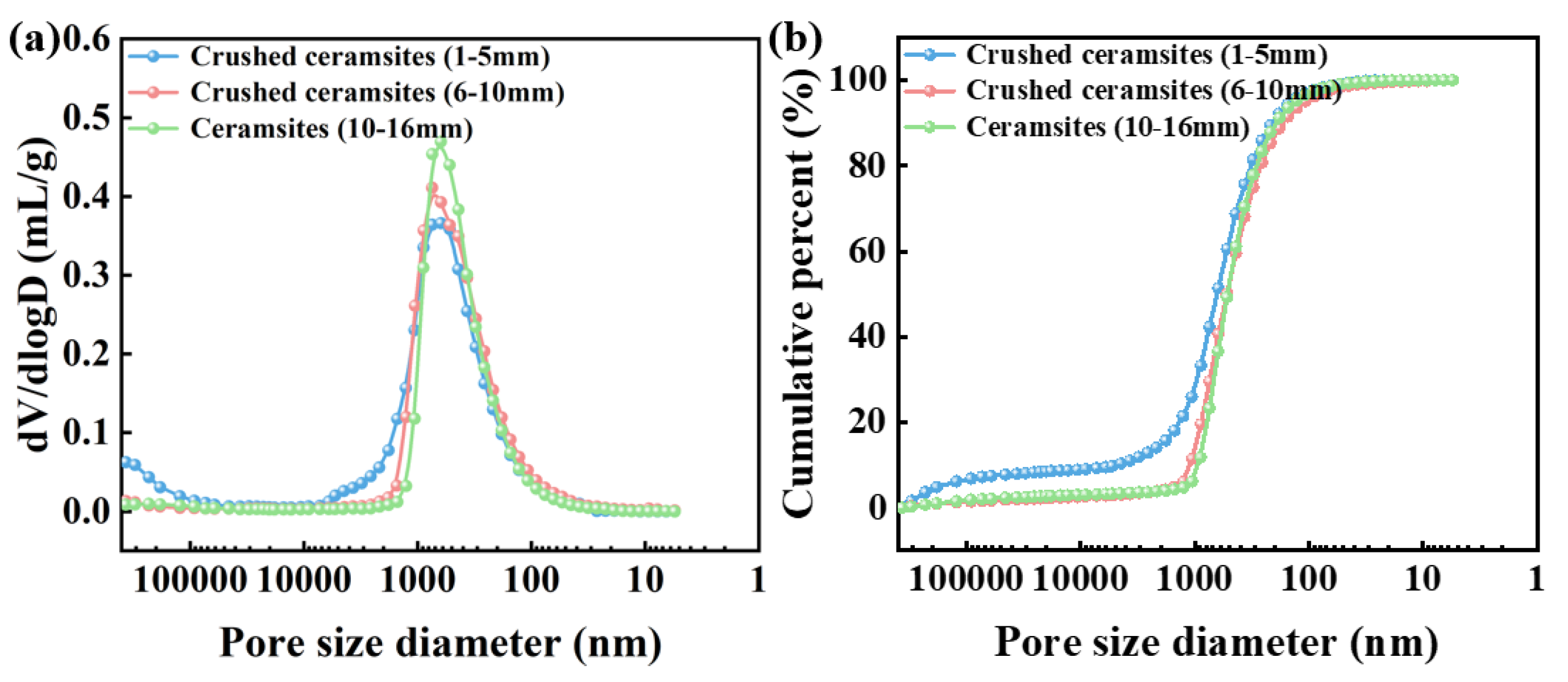
3.3. Effect of Temperature Regime and Pyrolysis Atmosphere on the Pore Structure of CGCs
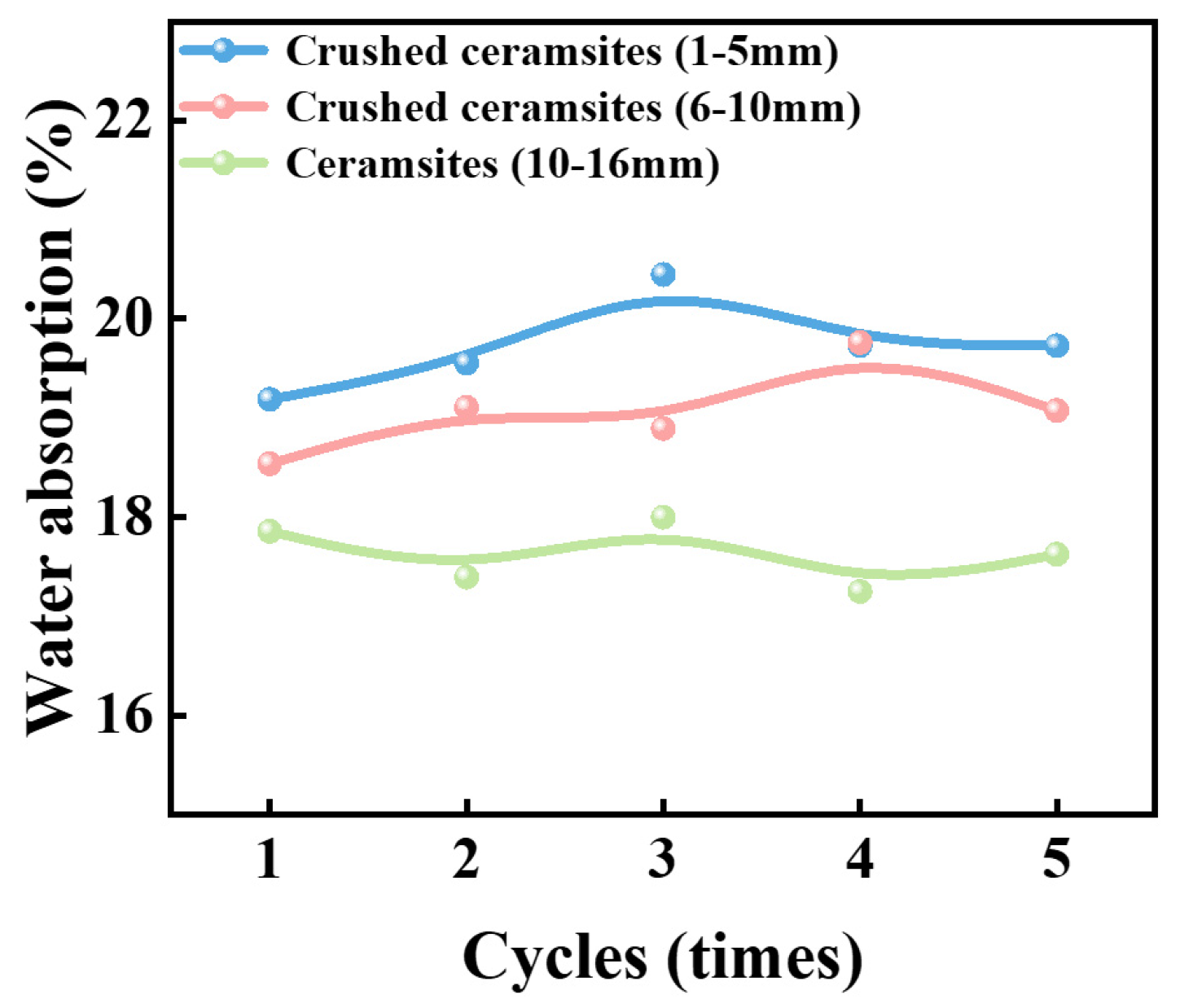
3.4. Moisture Retention and Water Storage Characteristics of Porous Ceramsites with Different Sizes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.J.; Hu, R.S. Research Environmental Pollution and Management of Coal Gangue in Pingdingshan. Adv. Mater. Res. 2013, 2695, 750–755. [Google Scholar] [CrossRef]
- Wu, H.; Chen, C.; Song, W.; Hou, W. High-capacity utilization of coal gangue as supplementary cementitious material, geopolymer, and aggregate: A review. Constr. Build. Mater. 2024, 435, 136857. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.M. Progress and trend of bulk utilization technology of metallurgical solid wastes in China. Chin. J. Eng. 2021, 43, 1713–1724. [Google Scholar]
- Guanhua, J.; Yanlin, W. Preparation of CFB fly ash/sewage sludge ceramsite and the morphological transformation and release properties of sulfur. Constr. Build. Mater. 2023, 373, 130864. [Google Scholar] [CrossRef]
- Du, T.; Wang, D. Optimizing the formulation of coal gangue planting substrate using wastes: The sustainability of coal mine ecological restoration. Ecol. Eng. 2020, 143, 105669. [Google Scholar] [CrossRef]
- Nanyan, H.; Yafei, L. Preparation and performance of porous ceramsite for Ag+ removal in sewage treatment with total phosphorus tailings. J. Clean. Prod. 2023, 413, 137515. [Google Scholar]
- Yi, H.; Jie, X.D. Preparation of gangue ceramsite by sintering pot test and potential analysis of waste heat recovery from flue gas. J. Iron Steel Res. Int. 2023, 30, 1401–1410. [Google Scholar] [CrossRef]
- Abzalov, V.M.; Bragin, V.V.; Klein, V.I.; Evstyugin, S.N.; Solodukhin, A.A. Thermal systems of conveyer roasting machines. Steel Transl. 2010, 40, 813–815. [Google Scholar] [CrossRef]
- Pan, X.; Liu, J.; Qi, Y.; Zheng, H.; Pei, J.; Yu, H. Preparation and formation mechanism of ultra-lightweight ceramsite from high-calcium red mud. Constr. Build. Mater. 2025, 490, 142484. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Zhang, X. Progress of lightweight and robust ceramsite from solid waste: Preparation, properties and applications. Constr. Build. Mater. 2025, 482, 141601. [Google Scholar] [CrossRef]
- El Hafidi, E.M.; Mortadi, A.; Lizoul, B.; Hairch, Y.; Mghaiouini, R.; Sabor, A.; Mnaouer, K.; Chahid, E.G.; Jebbari, S.; El Moznine, R.; et al. Assessment of sand and hearth ash filtration for wastewater treatment and novel monitoring via complex conductivity. EMJE 2025, 10, 1137–1148. [Google Scholar] [CrossRef]
- Duan, X.; Huang, Y.; Li, Y.; Zhang, W.; Huang, Z. Evolution mechanism of pore structure in sintered coal gangue ceramsites. Ceram. Int. 2023, 49, 31385–31395. [Google Scholar] [CrossRef]
- Gu, H.L.; Yu, M.G. Analysis on the zero-g period and characteristic temperature of coal gangue during adiabatic oxidation process. J. China Coal Soc. 2011, 36, 648–653. [Google Scholar]
- Wiles, D.B.; Young, R.A. A new computer program for Rietveld analysis of X-ray powder diffraction patterns. J. Appl. Crystallogr. 1981, 14, 149–151. [Google Scholar] [CrossRef]
- Bish, D.L.; Howard, S.A. Quantitative phase analysis using the Rietveld method. J. Appl. Crystallogr. 1988, 21, 86–91. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, N.Y. A novel route for the fabrication of melilite-spinel porous ceramics with ultralow thermal conductivity and sufficient strength. Ceram. Int. 2022, 24, 37488–37491. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Su, Y.; Luo, Y. Characteristics of pine wood oxidative pyrolysis: Degradation behavior, carbon oxide production and heat properties. J. Anal. Appl. Pyrolysis 2012, 98, 137–143. [Google Scholar] [CrossRef]
- Daouk, E.; Van de Steene, L. Thick wood particle pyrolysis in an oxidative atmosphere. Chem. Eng. Sci. 2015, 126, 608–615. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Effect of Oxygen Concentration on Pinewood Pyrolysis Behavior. Proc. CSEE 2011, 31, 117–123. [Google Scholar]
- Zhang, C.; Shan, B. Comparison of cadmium and lead sorption by Phyllostachys pubescens biochar produced under a low-oxygen pyrolysis atmosphere. Bioresource Technol. 2017, 238, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Hu, J. Experimental study on the effect of temperature and low oxygen conditions on pore structure of molding biochar. Chem. Ind. Eng. Prog. 2018, 37, 3100–3106. [Google Scholar]
- Fu, P.; Hu, S. Evaluation of the porous structure development of chars from pyrolysis of rice straw: Effects of pyrolysis temperature and heating rate. J. Anal. Appl. Pyrolysis 2012, 98, 177–183. [Google Scholar] [CrossRef]
- Fu, P.; Yi, W. Effect of temperature on gas composition and char structural features of pyrolyzed agricultural residues. Bioresource Technol. 2011, 102, 8211–8219. [Google Scholar] [CrossRef] [PubMed]



| Temp. | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | S | K2O | TiO2 | Na2O | LOI | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 °C | 55.14 | 20.10 | 5.26 | 0.76 | 1.59 | 0.40 | 2.57 | 0.79 | 1.24 | 11.76 | 99.65 |
| 1150 °C | 59.67 | 20.78 | 6.72 | 2.00 | 1.50 | 0.43 | 2.90 | 0.82 | 1.58 | 2.91 | 99.33 |
| Proximate Analysis (wt.%) | Qnet,ar (Kcal/kg) | |||||
|---|---|---|---|---|---|---|
| Mt | Mad | Aad | Vad | FCad | 573 | |
| 2.7 | 0.66 | 84.86 | 9.57 | 4.91 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Duan, X.; Li, Y. Effect of Temperature and Pyrolysis Atmosphere on Pore Structure of Sintered Coal Gangue Ceramsites. Materials 2025, 18, 3386. https://doi.org/10.3390/ma18143386
Zhao B, Duan X, Li Y. Effect of Temperature and Pyrolysis Atmosphere on Pore Structure of Sintered Coal Gangue Ceramsites. Materials. 2025; 18(14):3386. https://doi.org/10.3390/ma18143386
Chicago/Turabian StyleZhao, Baoqiang, Xiangjie Duan, and Yu Li. 2025. "Effect of Temperature and Pyrolysis Atmosphere on Pore Structure of Sintered Coal Gangue Ceramsites" Materials 18, no. 14: 3386. https://doi.org/10.3390/ma18143386
APA StyleZhao, B., Duan, X., & Li, Y. (2025). Effect of Temperature and Pyrolysis Atmosphere on Pore Structure of Sintered Coal Gangue Ceramsites. Materials, 18(14), 3386. https://doi.org/10.3390/ma18143386





