Lithium Adsorption Using Graphene Oxide: Modeling, Regeneration, and Mechanistic Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Measurement
3. Results and Discussion
3.1. GO Characterization
3.2. Adsorption Studies
3.2.1. Effect of GO Dosage
3.2.2. Effect of pH
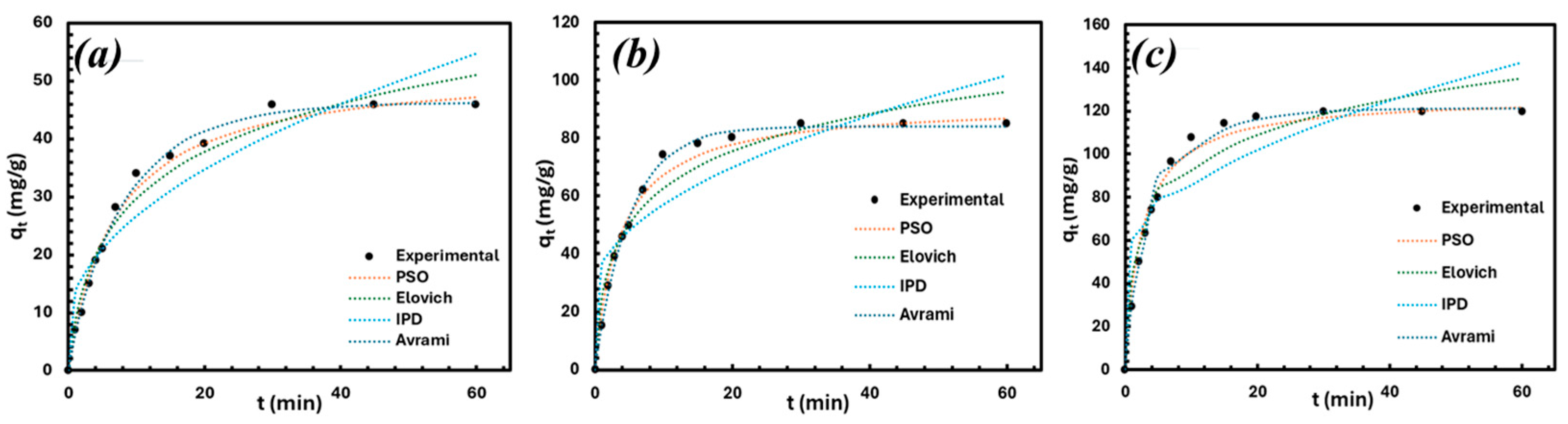
3.3. Kinetic Modeling
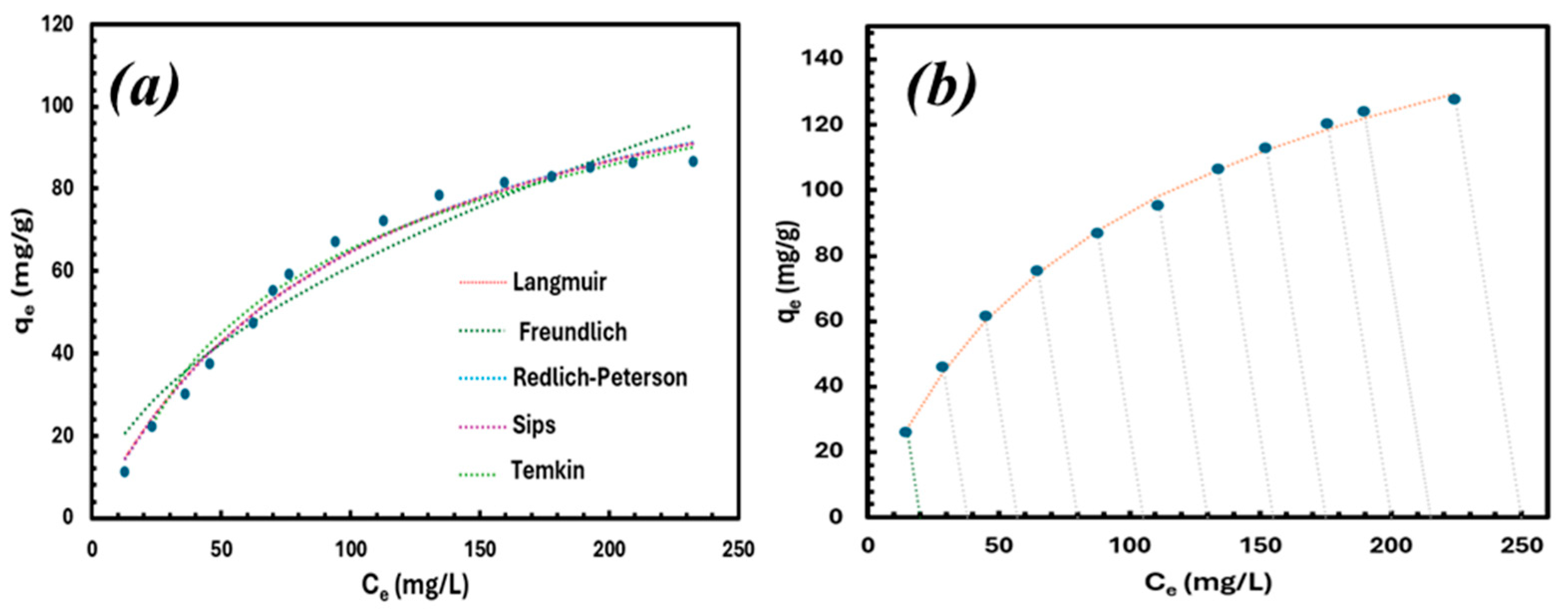
3.4. Adsorption Isotherm
3.5. Effect of Salinity
3.6. Comparison of Adsorption Capacities
3.7. Regeneration Studies
3.8. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Li | Lithium |
| C0 | Initial lithium adsorbate concentration |
| Ce | Equilibrium lithium adsorbate concentration |
| GO | Graphene Oxide |
| PFO | Pseudo-first order kinetic model |
| PSO | Pseudo-second order kinetic model |
| Qe | Capacity at a specific concentration |
| qmax | Maximum adsorption capacity |
| Qt | Capacity at time t |
| R2 | Coefficient of determination error analysis |
| SSE | Sum of the square of the errors |
References
- Kumar, A.; Fukuda, H.; Hatton, T.A.; Lienhard, J.H. Lithium recovery from oil and gas produced water: A need for a growing energy industry. ACS Energy Lett. 2019, 4, 1471–1474. [Google Scholar] [CrossRef]
- Padan, E.; Landau, M. Sodium-Proton (Na+/H+) Antiporters: Properties and Roles in Health and Disease; Springer: Cham, Switzerland, 2016; Volume 16. [Google Scholar]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and lithium ion batteries for applications in microelectronic devices: A review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Emsley, J. Nature’s Building Blocks: An A–Z Guide to the Elements, 2nd ed.; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Tarascon, J.M. Is lithium the new gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hao, H.; Zhao, F.; Liu, Z. Global Lithium Flow. 1994–2015: Implications for improving resource efficiency and security. Environ. Sci. Technol. 2018, 52, 2827–2834. [Google Scholar] [CrossRef]
- Riley, J.P.; Tongudai, M. The lithium content of sea water. Deep. Sea Res. Oceanogr. Abstr. 1964, 11, 563–568. [Google Scholar] [CrossRef]
- Zhu, X.; Yue, H.; Sun, W.; Zhang, L.; Cui, Q.; Wang, H. Study on adsorption extraction process of lithium ion from West Taijinar brine by shaped titanium-based lithium ion sieves. Sep. Purif. Technol. 2021, 274, 119099. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Sun, S.; Yu, J. Adsorption of lithium ions on lithium-aluminum hydroxides: Equilibrium and kinetics. Can. J. Chem. Eng. 2020, 98, 544–555. [Google Scholar] [CrossRef]
- Luo, X.; Guo, B.; Luo, J.; Deng, F.; Zhang, S.; Luo, S.; Crittenden, J. Recovery of lithium from wastewater using development of li ion-imprinted polymers. ACS Sustain. Chem. Eng. 2015, 3, 460–467. [Google Scholar] [CrossRef]
- Liu, M.; Wu, D.; Qin, D.; Yang, G. Spray-drying assisted layer-structured H2TiO3 ion sieve synthesis and lithium adsorption performance. Chin. J. Chem. Eng. 2022, 45, 258–267. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, J.; Liu, Y.; Guo, Y.; Deng, T.; Yu, X. Synthesis of granulated H4Mn5O12/chitosan with improved stability by a novel cross-linking strategy for lithium adsorption from aqueous solutions. Chem. Eng. J. 2021, 426, 131689. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Sun, S. Performance of a synthetic resin for lithium adsorption in waste liquid of extracting aluminum from fly-ash. Chin. J. Chem. Eng. 2022, 44, 115–123. [Google Scholar] [CrossRef]
- Gao, J.M.; Du, Z.; Zhao, Q.; Guo, Y.; Cheng, F. Enhanced Li+ adsorption by magnetically recyclable iron-doped lithium manganese oxide ion-sieve: Synthesis, characterization, adsorption kinetics and isotherm. J. Mater. Res. Technol. 2021, 13, 228–240. [Google Scholar] [CrossRef]
- Kamran, U.; Heo, Y.J.; Lee, J.W.; Park, S.J. Chemically modified activated carbon decorated with MnO2 nanocomposites for improving lithium adsorption and recovery from aqueous media. J. Alloys Compd. 2019, 794, 425–434. [Google Scholar] [CrossRef]
- Park, H.J.; Singhal, N.; Jho, E.H. Lithium sorption properties of HMnO in seawater and wastewater. Water Res. 2015, 87, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Wahib, S.A.; Da’na, D.A.; Zaouri, N.; Hijji, Y.M.; Al-Ghouti, M.A. Adsorption and recovery of lithium ions from groundwater using date pits impregnated with cellulose nanocrystals and ionic liquid. J. Hazard. Mater. 2022, 421, 126657. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-based technologies for lithium recovery from water lithium resources: A review. J. Memb. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Dimakis, N.; Salas, I.; Gonzalez, L.; Vadodaria, O.; Ruiz, K.; Bhatti, M.I. Li and Na Adsorption on Graphene and Graphene Oxide Examined by Density Functional Theory, Quantum Theory of Atoms in Molecules, and Electron Localization Function. Molecules 2019, 24, 754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barabanova, L.; Buldum, A. A First Principles Study of Lithium Adsorption in Nanoporous Graphene. Nanomaterials 2024, 14, 1528. [Google Scholar] [CrossRef]
- Arnold, B.C.; da Silva Machado, E.; Martins, J.B.L.; Paterno, L.G.; dos Santos Politi, J.R. Exploring the Electronic Structure of Graphene and Graphene Ultrathin Films with Adsorbed Lithium. J. Phys. Chem. C 2025, 129, 7879–7893. [Google Scholar] [CrossRef]
- Nour, S.A.; Hong, S.; Choi, D.; Arafat, H.; AlMarzooqi, F. Interfacial Adsorption and Recovery of Lithium Ions Using Sulfonated Graphene Oxide and Ti3C2Tx Mxene Nanocomposite Hydrogels. Desalination 2025, 606, 118766. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Abu-Nada, A.; Abdala, A.; McKay, G. Isotherm and Kinetic Modeling of Strontium Adsorption on Graphene Oxide. Nanomaterials 2021, 11, 2780. [Google Scholar] [CrossRef] [PubMed]
- Kaliva, M.; Vamvakaki, M. Nanomaterials characterization. In Polymer Science and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 401–433. [Google Scholar]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef]
- Lam, K.F.; Yeung, K.L.; McKay, G. A rational approach in the design of selective mesoporous adsorbents. Langmuir 2006, 22, 9632–9641. [Google Scholar] [CrossRef]
- Palchoudhury, S.; Baalousha, M.; Lead, J.R. Methods for measuring concentration (mass, surface area, and number) of nanomaterials. In Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2015; Volume 8, pp. 153–181. [Google Scholar]
- Ren, X.; Li, J.; Tan, X.; Wang, X. Comparative study of graphene oxide, activated carbon and carbon nanotubes as adsorbents for copper decontamination. Dalton Trans. 2013, 42, 5266–5274. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelo¨ster stoffe. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Lopes, E.C.; dos Anjos, F.S.; Vieira, E.F.; Cestari, A.R. An alternative Avrami equation to evaluate kinetic parameters of the interaction of Hg (II) with thin chitosan membranes. J. Colloid Interface Sci. 2003, 263, 542–547. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- McKay, G. The adsorption of dyestuffs from aqueous solutions using activated carbon. III. Intraparticle diffusion processes. J. Chem. Technol. Biotechnol. 1983, 33, 196–204. [Google Scholar] [CrossRef]
- Langmuir, I. A new adsorption isotherm. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Colloid and Capillary Chemistry; Methuen: London, UK, 1926; pp. 6–8. [Google Scholar]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Temkin, M.I. Kinetics of heterogeneous catalysis. J. Phys. Chem. 1940, 14, 1153–1158. [Google Scholar]
- Zhong, J.; Lin, S.; Yu, J. Li+ adsorption performance and mechanism using lithium/aluminum layered double hydroxides in low grade brines. Desalination 2021, 505, 114983. [Google Scholar] [CrossRef]
- Widyabuningsih, D.; Hielwana, H.; Pauti, E.; Ridwan, I.; Rispiandi, R.; Andrijanto, E. Lithium recovery using hydrogen manganese oxide adsorbent derived from spinel lithium manganese oxide. Rekayasa Bahan Alam Dan Energi Berkelanjutan 2023, 7, 30–34. [Google Scholar] [CrossRef]
- Liu, J.; Fu, L.; Sun, W.; Cui, Q.; Zhu, X.; Yue, H.; Wang, H. Design and adsorption performance of hollow structure nano-metatitanic acid lithium ion sieve with strong structure stability and large adsorption capacity. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 133960. [Google Scholar] [CrossRef]
- Hilton, J. European science editing. Eur. Sci. Ed. 2012, 38, 81. [Google Scholar]
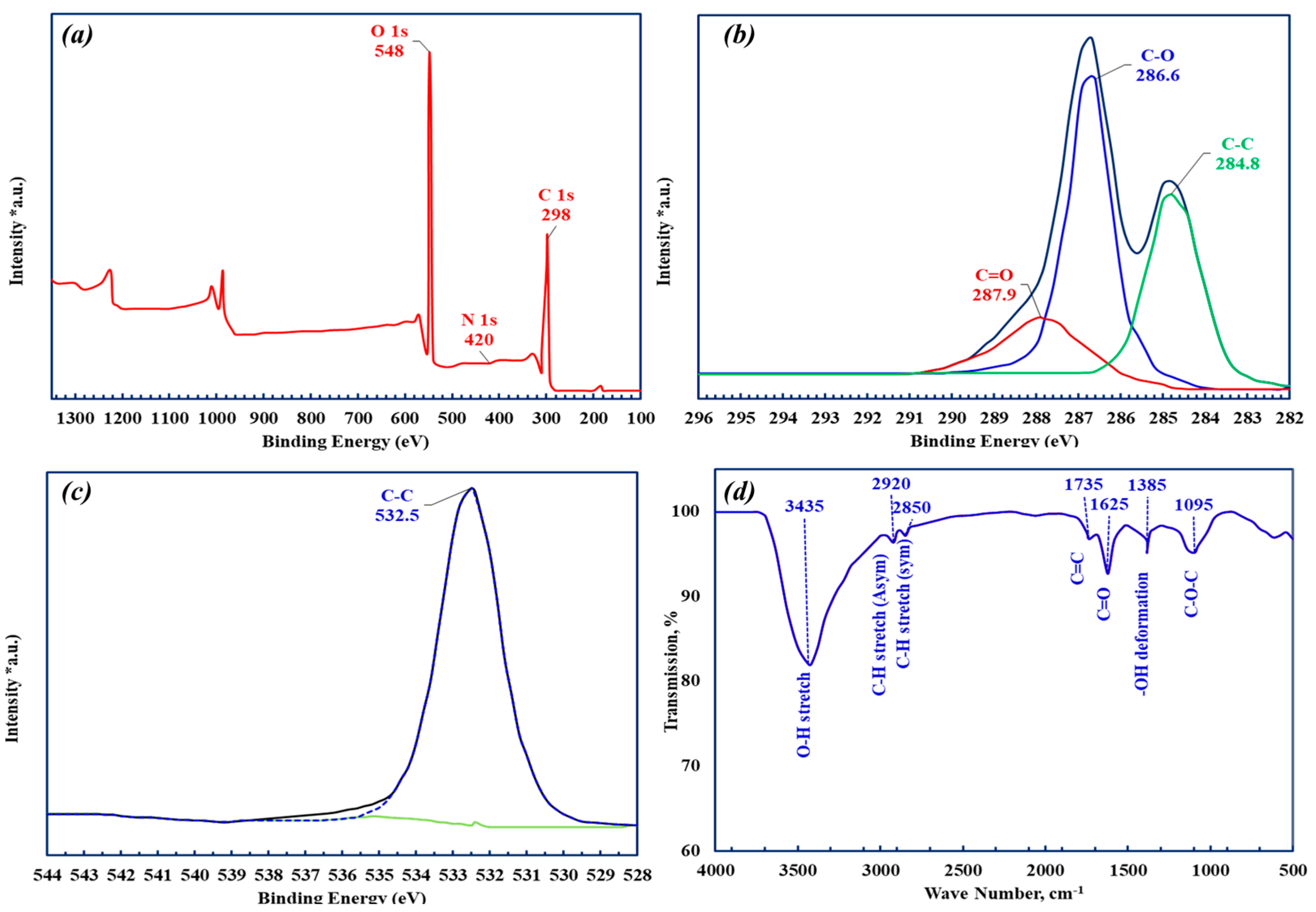
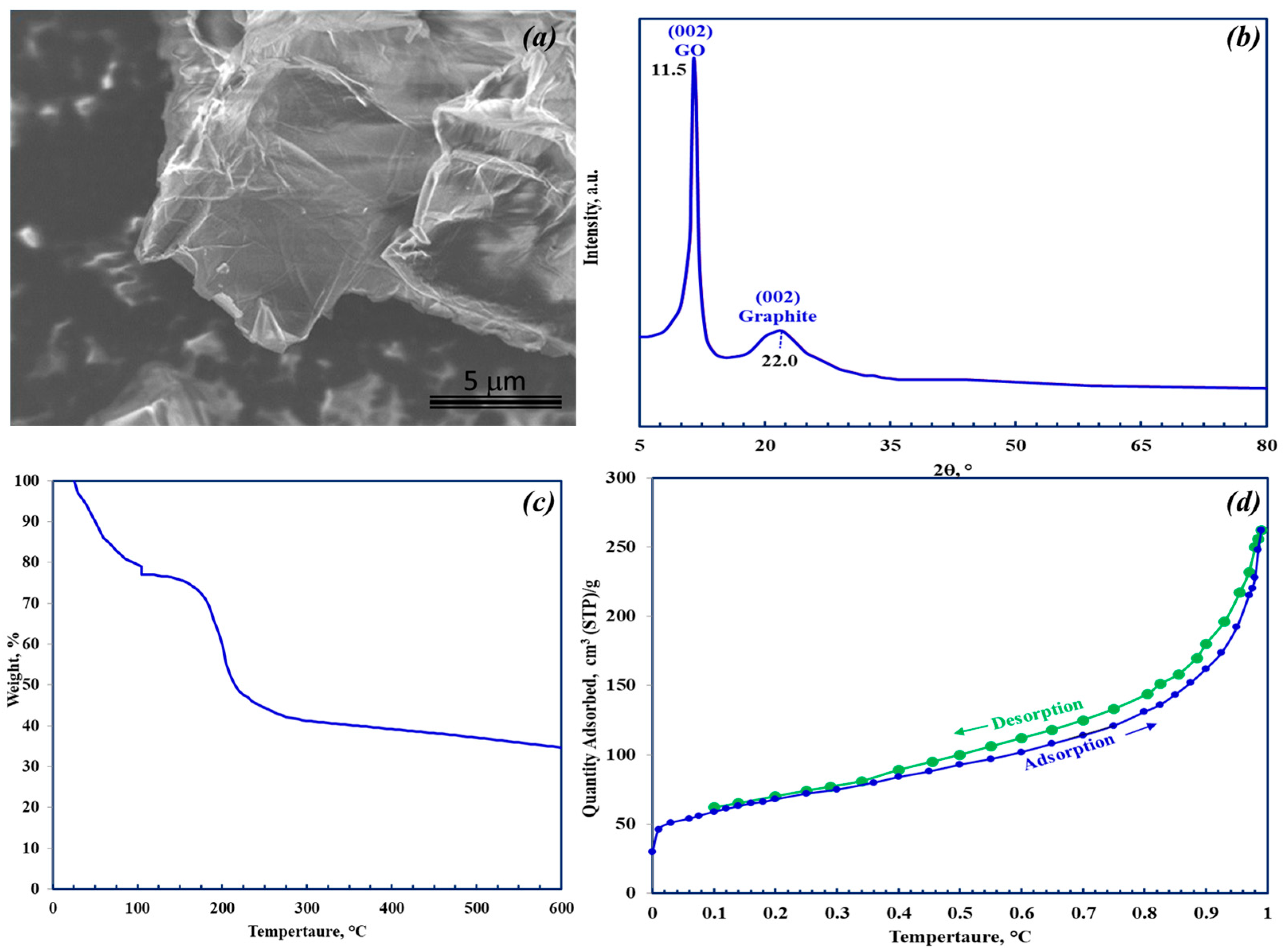




| Name | Peak BE | FWHM eV | Area (P) CPS.eV | Atomic % |
|---|---|---|---|---|
| C 1s | 259.00 | 4.26 | 691,735 | 62.17 |
| O 1s | 532.30 | 3.20 | 1,045,249 | 36.98 |
| Kinetic Model | Parameters | Fitting Quality |
|---|---|---|
| Pseudo-First Order | qe = 52.47 mg/g K1 = −0.002 | R2 = 0.871 SSR = 8.71 |
| Pseudo-Second Order | qe = 92.00 mg/g K2 = 0.003 | R2 = 0.997 SSR = 0.001 |
| Elovich | α = 50.00 β = 0.053 | R2 = 0.922 SSR = 530.17 |
| Intra-Particle Diffusion | KIP = 9.73 C = 26.24 | R2 = 0.761 SSR = 1621.86 |
| Avrami | qe = 84.64 mg/g Kav = 0.211 nAV = 0.942 | R2 = 0.997 SSR = 22.38 |
| Isotherm Model | Isotherm Parameters | SSE | R2 | ||
|---|---|---|---|---|---|
| P1 | P2 | P3 | |||
| Langmuir | KL = 1.99 | aL = 0.01 | qmax = 179 mg/g | 35.72 | 1.00 |
| Freundlich | aF = 8.79 | bF = 0.50 | - | 145.9 | 0.99 |
| Redlich–Peterson | KR = 2.44 | aR =0.032 | bR = 0.85 | 20.91 | 1.00 |
| Sips | KLF = 3.05 | aLF = 0.01 | nLF = 0.87 | 22.19 | 1.00 |
| Tempkin | B = 38.97 | aT = 0.11 | - | 101.2 | 0.99 |
| Adsorbent | Maximum Capacity (mg/g) | Isotherm Model | Ref. |
|---|---|---|---|
| Lithium/aluminum layered double hydroxides | 7.3 | Sips | [41] |
| Granulated H4Mn5O12 (HMO) ion sieve (CTS) | 12 | - | [12] |
| Adsorbent derived from spinel lithium manganese oxide | 23 | - | [42] |
| Hollow hemispherical mixed matrix lithium adsorbent | 25.7 | Freundlich | [17] |
| Spherical layer-structured H2TiO3 ion sieve | 30 | - | [11] |
| Magnetically recyclable Fe-doped Mn oxide spinel | 34.8 | Langmuir | [14] |
| Nano-metatitanic acid lithium ion sieve | 47 | Langmuir | [43] |
| Iron-doped lithium ion-sieves | 53 | - | [44] |
| Modified activated carbon MnO2 nanocomposites | 89 | - | [15] |
| Date pits impregnated with cellulose nanocrystals and ionic liquid | 99 | Freundlich | [17] |
| Graphene oxide | 179 | Redlich–Peterson | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Nada, A.; Abdala, A.; McKay, G.; Zuhara, S. Lithium Adsorption Using Graphene Oxide: Modeling, Regeneration, and Mechanistic Insights. Materials 2025, 18, 3211. https://doi.org/10.3390/ma18143211
Abu-Nada A, Abdala A, McKay G, Zuhara S. Lithium Adsorption Using Graphene Oxide: Modeling, Regeneration, and Mechanistic Insights. Materials. 2025; 18(14):3211. https://doi.org/10.3390/ma18143211
Chicago/Turabian StyleAbu-Nada, Abdulrahman, Ahmed Abdala, Gordon McKay, and Shifa Zuhara. 2025. "Lithium Adsorption Using Graphene Oxide: Modeling, Regeneration, and Mechanistic Insights" Materials 18, no. 14: 3211. https://doi.org/10.3390/ma18143211
APA StyleAbu-Nada, A., Abdala, A., McKay, G., & Zuhara, S. (2025). Lithium Adsorption Using Graphene Oxide: Modeling, Regeneration, and Mechanistic Insights. Materials, 18(14), 3211. https://doi.org/10.3390/ma18143211









