The Potential of MN4-GPs (M = Mn, Fe, Co, Ni, Cu, Mo) as Adsorbents for the Efficient Separation of CH4 from CO2 and H2S
Abstract
1. Introduction
2. Calculation Methods
3. Results and Discussion
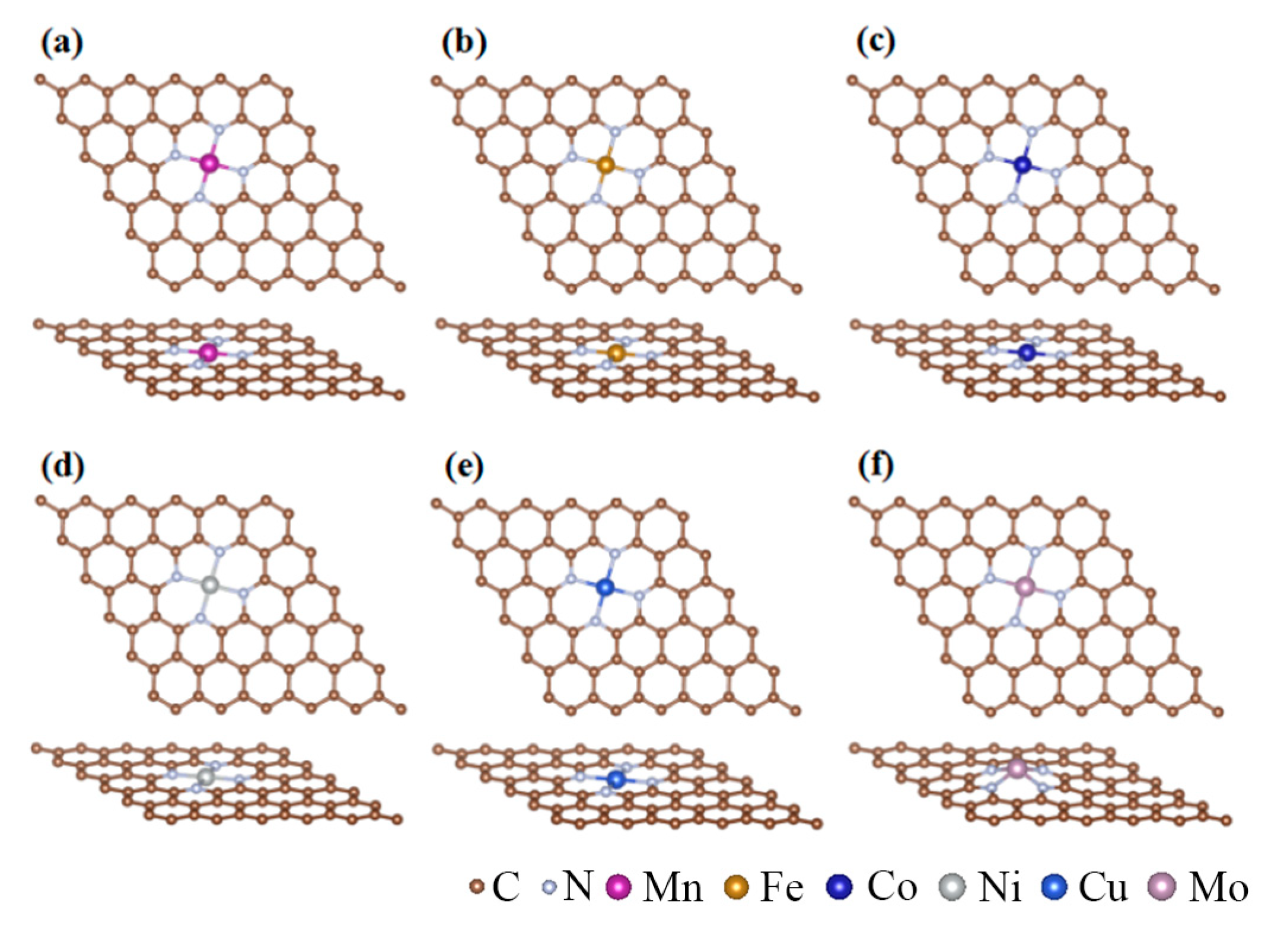
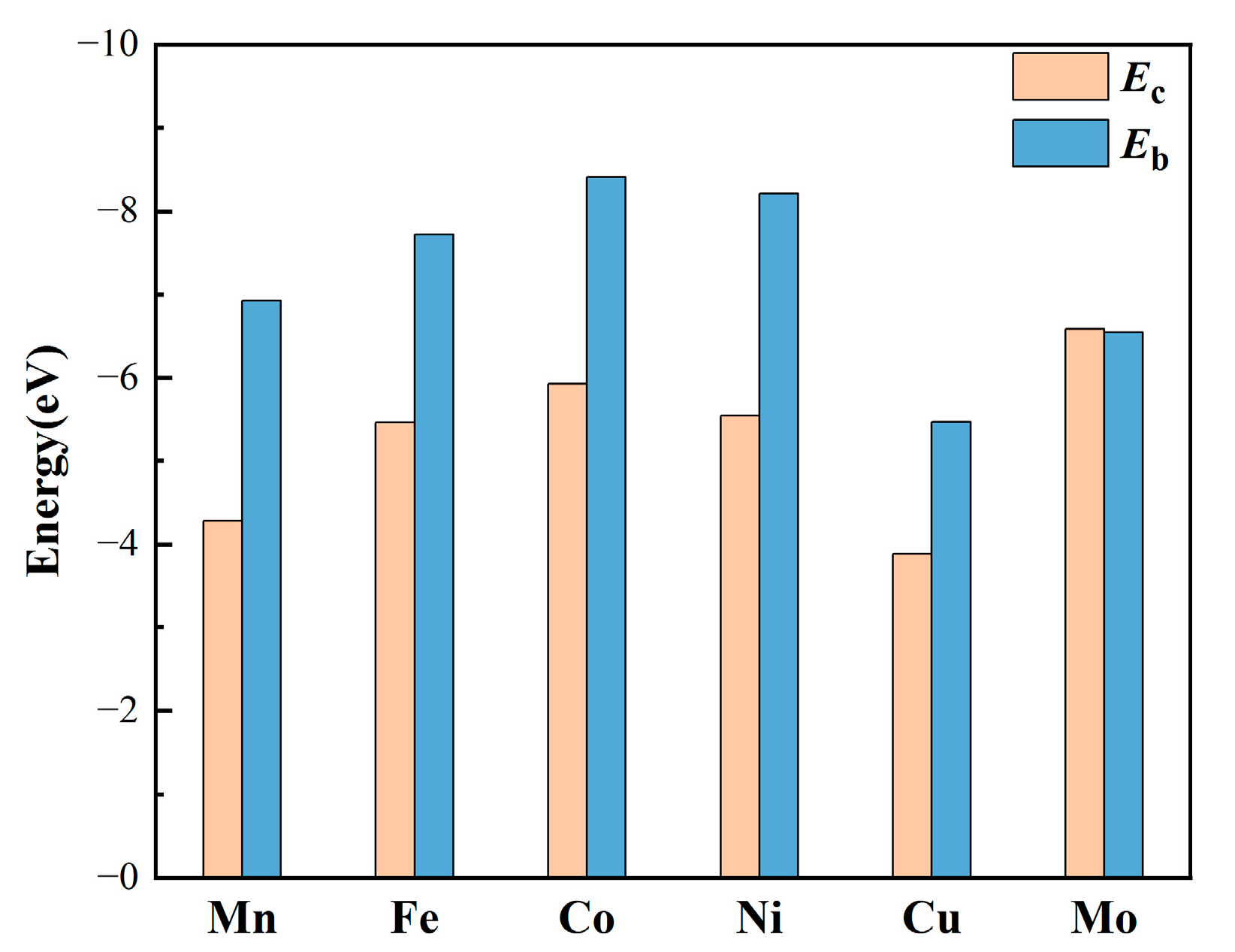
3.1. The Thermodynamic Stability of MN4-GPs
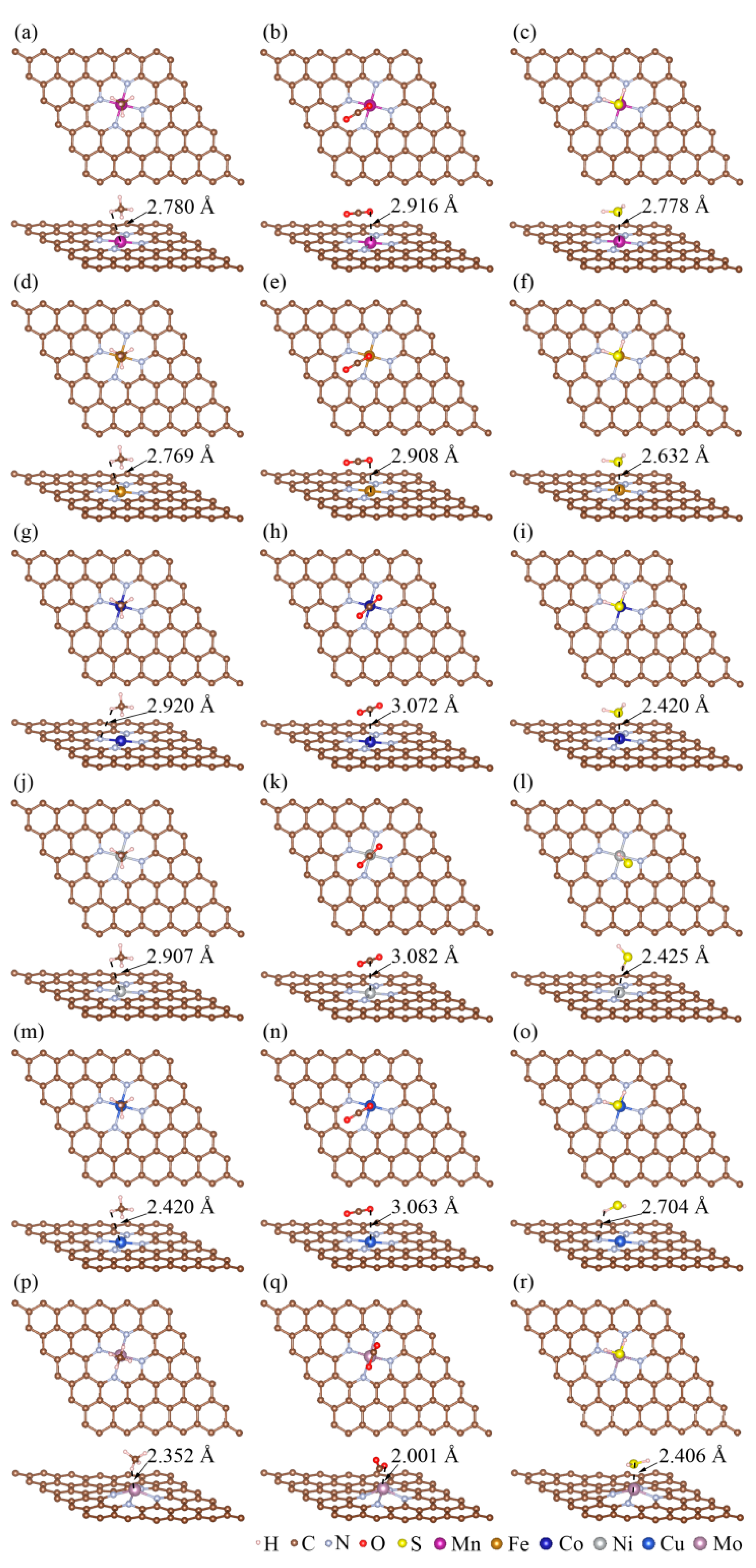
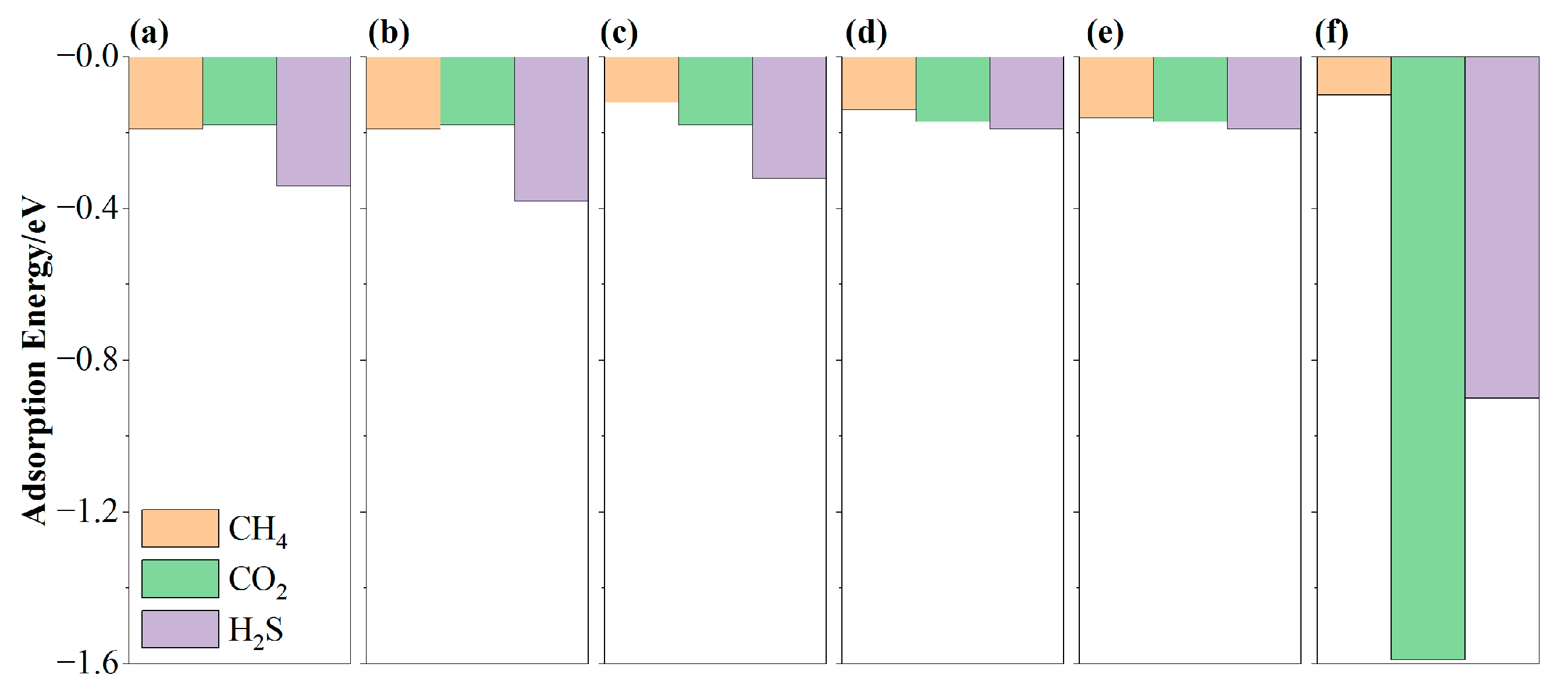
3.2. The Adsorption of CH4, CO2, and H2S on MN4-GPs
3.3. The Feasibility of MN4-GPs for Efficient Separation of CH4 from CO2 and H2S
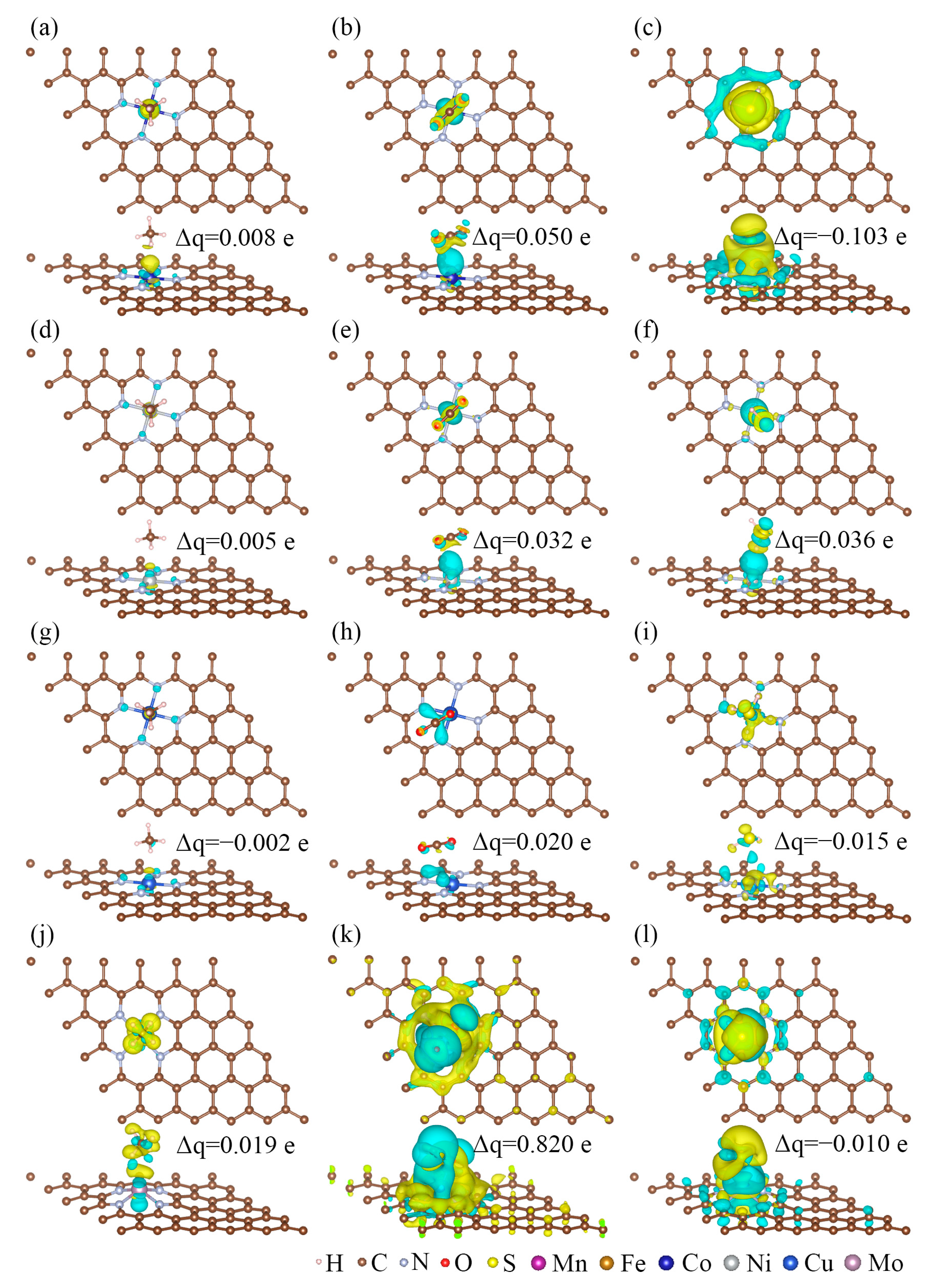
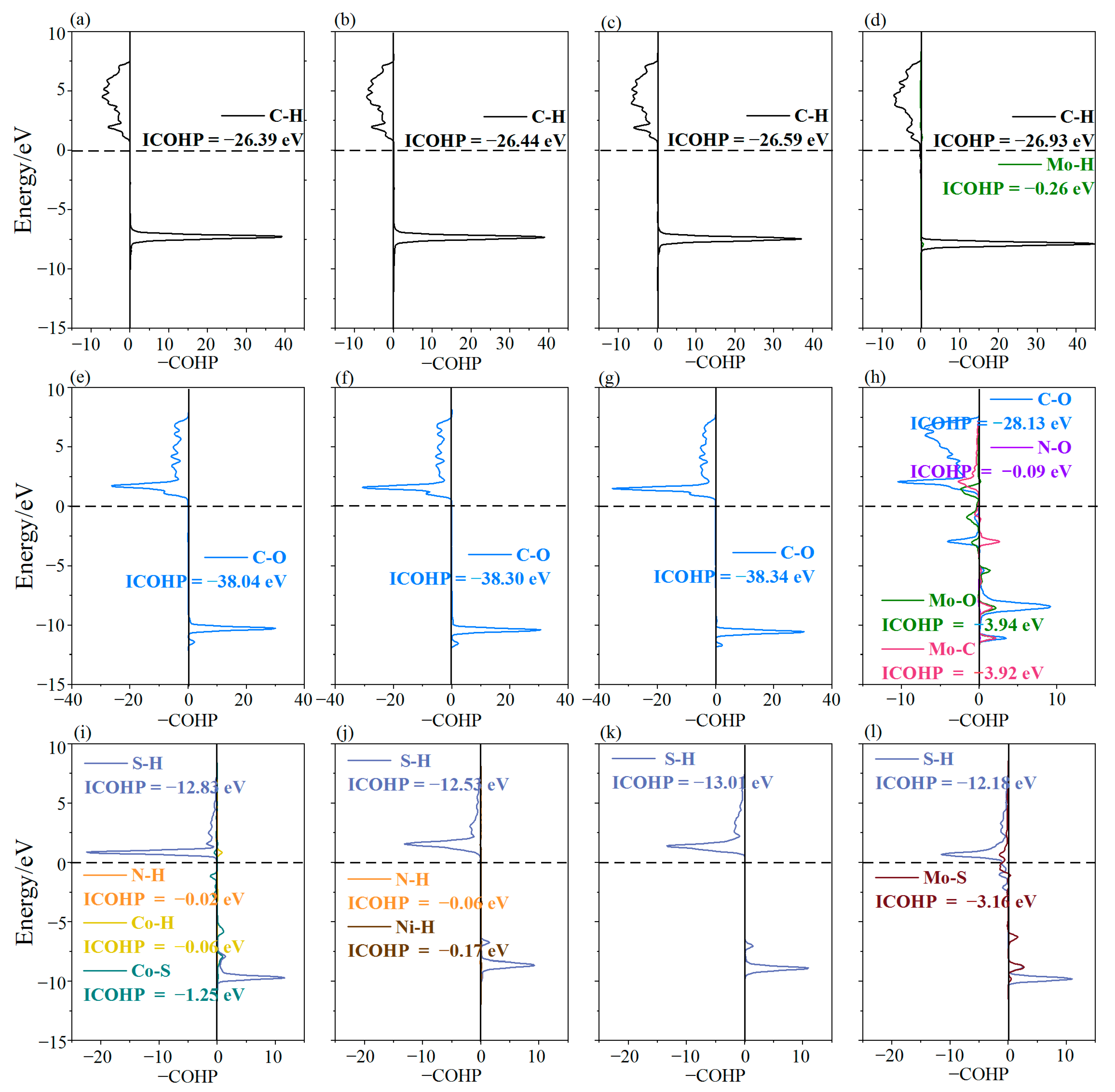
3.4. The Adsorption Mechanisms of CH4, CO2, and H2S on MN4-GPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aghel, B.; Behaein, S.; Wongwises, S.; Shadloo, M.S. A review of recent progress in biogas upgrading: With emphasis on carbon capture. Biomass Bioenergy 2022, 160, 106422. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.Á. The potential of microplastics as carriers of metals. Renew Energy 2024, 237, 121685. [Google Scholar] [CrossRef]
- An, B.; Ma, Y.; Han, X.; Schröder, M.; Yang, S. Activation and Catalysis of Methane over Metal–Organic Framework Materials. Acc. Mater. Res. 2025, 6, 77–88. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Sulfide—Pathological and Physiological Functions in Mammalian Cells. Cells 2023, 12, 2684. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Bai, P.; Luo, B.; Zheng, S.; Chen, C. Review of recent progress in the study of corrosion products of steels in a hydrogen sulphide environment. Corros. Sci. 2018, 139, 124–140. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, M.; Li, W.; Wen, F.; Dong, G.; Zou, L.; Zhang, Y. Development of a Predictive Model for Carbon Dioxide Corrosion Rate and Severity Based on Machine Learning Algorithms. Materials 2024, 16, 4046. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Zheng, S.; Qi, Y.; Xiong, M.; Zheng, Y. Effect of H2S/CO2 partial pressure ratio on the tensile properties of X80 pipeline steel. Int. J. Hydrogen Energy 2015, 40, 11925–11930. [Google Scholar] [CrossRef]
- Han, D.B.; Kim, Y.J.; Byun, H.; Cho, W.J.; Baek, Y. CO2 Methanation of Biogas over 20 wt% Ni-Mg-Al Catalyst: On the Effect of N2, CH4, and O2 on CO2 Conversion Rate. Catalysts 2020, 10, 1201. [Google Scholar] [CrossRef]
- Han, D.B.; Cho, W.J.; Baek, Y. CO2 Methanation of Biogas over Ni-Mg-Al: The Effects of Ni Content, Reduction Temperature, and Biogas Composition. Catalysts 2022, 9, 1054. [Google Scholar] [CrossRef]
- Azamat, J.; Khataee, A.; Joo, S.W. Functionalized graphene as a nanostructured membrane for removal of copper and mercury from aqueous solution: A molecular dynamics simulation study. J. Ind. Eng. Chem. 2014, 53, 112–117. [Google Scholar] [CrossRef]
- Bayat, G.; Saghatchi, R.; Azamat, J.; Khataee, A. Separation of methane from different gas mixtures using modified silicon carbide nanosheet: Micro and macro scale numerical studies. Chin. J. Chem. Eng. 2020, 28, 1268–1276. [Google Scholar] [CrossRef]
- Li, J.; Kuppler, R.J.; Zhou, H. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477. [Google Scholar] [CrossRef] [PubMed]
- Sokhanvaran, V.; Gomar, M.; Yeganegi, S. H2S separation from biogas by adsorption on functionalized MIL-47-X (X = −OH and − OCH3): A simulation study. Appl. Surf. Sci. 2019, 479, 1006–1013. [Google Scholar] [CrossRef]
- Oliveira, L.H.D.; Meneguin, J.G.; Pereira, M.V.; do Nascimento, J.F.; Arroyo, P.A. Adsorption of hydrogen sulfide, carbon dioxide, methane, and their mixtures on activated carbon. Chem. Eng. Commun. 2019, 206, 1533–1553. [Google Scholar] [CrossRef]
- Lin, J.Y.S. Molecular sieves for gas separation. Science 2016, 353, 121–122. [Google Scholar] [CrossRef]
- Dong, G.; Lee, Y.M. Microporous polymeric membranes inspired by adsorbent for gas separation. J. Mater. Chem. A 2017, 26, 13294–13319. [Google Scholar] [CrossRef]
- Aksu, G.O.; Erucar, I.; Haslak, Z.P.; Keskin, S. Exploring covalent organic frameworks for H2S+CO2 separation from natural gas using efficient computational approaches. J. CO2 Util. 2022, 62, 102077. [Google Scholar] [CrossRef]
- Malaescu, I.; Sfirloaga, P.; Bunoiu, O.M.; Marin, C.N. A Comparative Analysis of the Electrical Properties of Silicone Rubber Composites with Graphene and Unwashed Magnetite. Materials 2024, 17, 6006. [Google Scholar] [CrossRef]
- Janutiene, R.K.; Syzonenko, O.; Mažeika, D.; Gegeckiene, L.; Venyte, I.; Torpakov, A. Microstructure and Phase Composition of Ti-Al-C Materials Obtained by High Voltage Electrical Discharge/Spark Plasma Sintering. Materials 2024, 17, 115. [Google Scholar] [CrossRef]
- Abbas, Q.; Shinde, P.A.; Alami, A.H.; Mirzaeian, M.; Yadav, A.; Olabi, A.G.; Abdelkareem, M.A. Graphene Synthesis Techniques and Environmental Applications. Materials 2022, 15, 7804. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Lee, D.-J.; Park, S.-S. Estimation of Number of Graphene Layers Using Different Methods: A Focused Review. Materials 2021, 14, 4590. [Google Scholar] [CrossRef]
- Ghiasi, M.; Zeinali, P.; Gholami, S.; Zahedi, M. Separation of CH4, H2S, N2 and CO2 gases using four types of nanoporous graphene cluster model: A quantum chemical investigation. J. Mol. Model. 2021, 27, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hou, M.; Cen, W.; Jiao, W. A DFT study of H2S, H2O, SO2 and CH4 Adsorption Behavior on Graphene Surface Decorated with Alkaline Earth Metals. Surf. Sci. 2022, 726, 122178. [Google Scholar] [CrossRef]
- Pan, D.; Guo, L.; Zhang, J.; Chen, X.; Xue, Q.; Huang, H.; Li, J.; Zhang, Z.; Yu, W.; Chen, Z.; et al. Cutting sp2 clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J. Mater. Chem. 2012, 22, 3314–3318. [Google Scholar] [CrossRef]
- Rostamizadeh, M.; Sadatnia, B.; Norouzbahari, S.; Ghadimi, A. Enhancing the gas separation properties of mixed matrix membranes via impregnation of sieve phases with metal and nonmetal promoters. Sep. Purif. Technol. 2020, 245, 116859. [Google Scholar] [CrossRef]
- Ovsianytskyi, O.; Nam, Y.-S.; Tsymbalenko, O.; Lan, P.-T.; Moon, M.-W.; Lee, K.-B. Highly sensitive chemiresistive H2S gas sensor based on graphene decorated with Ag nanoparticles and charged impurities. Sens. Actuators B-Chem. 2018, 257, 278–285. [Google Scholar] [CrossRef]
- Tit, N.; Said, K.; Mahmoud, N.M.; Kouser, S.; Yamani, Z.H. Ab-initio investigation of adsorption of CO and CO2 molecules on graphene: Role of intrinsic defects on gas sensing. Appl. Surf. Sci. 2017, 334, 219–230. [Google Scholar] [CrossRef]
- Suhaimi, N.H.; Yeong, Y.F.; Jusoh, N.; Chew, T.L.; Bustam, M.A.; Mubashiret, M. RSM Modeling and Optimization of CO2 Separation from High CO2 Feed Concentration over Functionalized Membrane. Results Eng. 2022, 14, 1371. [Google Scholar] [CrossRef]
- Grasseschi, D.; Silva, W.C.; Paiva, R.D.S.; Starke, L.D.; do Nascimento, A.S. Surface coordination chemistry of graphene: Understanding the coordination of single transition metal atoms. Coord. Chem. Rev. 2020, 422, 213469. [Google Scholar] [CrossRef]
- Impeng, S.; Junkaew, A.; Maitarad, P.; Kungwan, N.; Zhang, D.S.; Shi, L.Y.; Namuangruk, S. A MnN4 moiety embedded graphene as a magnetic gas sensor for CO detection: A first principle study. Appl. Surf. Sci. 2019, 473, 820–827. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Q.; Wang, J.X.; Xu, L.N.; Zeng, W. Performance of Intrinsic and Modified Graphene for the Adsorption of H2S and CH4: A DFT Study. Nanomaterials 2020, 2, 299. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.G.; Peng, X.; Liu, K.; Ding, Z.Y. Adsorption of C2H2, CH4 and CO on Mn-doped graphene: Atomic, electronic, and gas-sensing properties. Physica E 2020, 119, 113959. [Google Scholar] [CrossRef]
- Nosheen, U.; Jalil, A.; Llyas, S.Z.; Ahmed, S.; Lllahi, A.; Rafiq, M.A. Ab-initio characterization of iron-embedded nitrogen-doped graphene as a toxic gas sensor. J. Comput. Electron. 2023, 22, 116–127. [Google Scholar] [CrossRef]
- Chen, S.Q.; Zhang, X.; Xiang, Y.C.; Fan, J.; Gan, L.Y. Computational screening of single transition metal atom embedded in nitrogen doped graphene for CH4 detection. Mater. Today Commun. 2022, 31, 103383. [Google Scholar] [CrossRef]
- Qu, L.; Fu, X.; Zhong, C.; Zhou, P.; Zhang, J. Equibiaxial Strained Oxygen Adsorption on Pristine Graphene, Nitrogen/Boron Doped Graphene, and Defected Graphene. Materials 2020, 13, 4945. [Google Scholar] [CrossRef]
- Liu, D.; Shi, Y.; Tao, L.; Yan, D.; Chen, R.; Wang, S. First-principles study of methanol adsorption on heteroatom-doped phosphorene. Chin. Chem. Lett. 2019, 30, 207–210. [Google Scholar] [CrossRef]
- Geng, W.; Liu, Y.; Xu, N.; Tang, G.; Kawazoe, Y.; Wang, V. Empowering materials science with VASPKIT: A toolkit for enhanced simulation and analysis. Nat. Protoc. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, T.; Wang, C. Analysis of the influence of impurity gas on the hydrogen storage performance of Ti/2C-BN. Int. J. Hydrogen Energy 2023, 48, 38389–38399. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 77, 3685. [Google Scholar] [CrossRef]
- Yang, L.; Fan, J.; Zhu, W. Copper monatomic wire supported on graphene nanoribbons as an electrocatalyst for nitric oxide reduction: Pre-adsorption mechanism of reactant. J. Mol. Model. 2023, 29, 384. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Han, N.; Su, Y.; Zhang, J.; Zhao, J. Schottky barrier at graphene/metal oxide interfaces: Insight from first-principles calculations. Sci. Rep. 2017, 7, 41771. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.Y.; Lee, M.W.; Ham, H.C.; Lee, K.-Y. Role of the Zn atomic arrangements in enhancing the activity and stability of the kinked Cu(2 1 1) site in CH3OH production by CO2 hydrogenation and dissociation: First-principles microkinetic modeling study. J. Catal. 2019, 373, 336–350. [Google Scholar] [CrossRef]
- Janthon, P.; Kozlov, S.M.; Viñes, F.; Limtrakul, J.; Lllas, F. Establishing the Accuracy of Broadly Used Density Functionals in Describing Bulk Properties of Transition Metals. J. Chem. Theory Comput. 2013, 9, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gao, Z.; Yang, W.; Li, A.; Ding, X. Adsorption characteristics of Co-anchored different graphene substrates toward O2 and NO molecules. Appl. Surf. Sci. 2019, 480, 779–791. [Google Scholar] [CrossRef]
- Zhang, Q.; Guan, J. Single-Atom Catalysts for Electrocatalytic Applications. Adv. Funct. Mater. 2020, 30, 2000768. [Google Scholar] [CrossRef]
- Tang, C.; Jiao, Y.; Shi, B.; Liu, J.-N.; Xie, Z.; Chen, X.; Zhang, Q.; Qiao, S.-Z. Coordination Tunes Selectivity: Two-Electron Oxygen Reduction on High-Loading Molybdenum Single-Atom Catalysts. Angew. Chem. Int. Ed. Engl. 2020, 59, 9171–9176. [Google Scholar] [CrossRef]
- Han, L.; Liu, X.; Chen, J.; Lin, R.; Liu, H.; Lv, F.; Bak, S.; Liang, Z.; Zhao, S.; Stavitski, E.; et al. Atomically Dispersed Molybdenum Catalysts for Efficient Ambient Nitrogen Fixation. Angew. Chem. Int. Edit. 2019, 58, 2321–2325. [Google Scholar] [CrossRef]
- Chen, W.; Pei, J.; He, C.; Wan, J.; Ren, H.; Zhu, Y.; Wang, Y.; Dong, J.; Tian, S.; Cheong, W.-C.; et al. Single Tungsten Atoms Supported on MOF-Derived N-Doped Carbon for Robust Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1800396. [Google Scholar] [CrossRef]
- Yong, Y.; Zhang, W.; Hou, Q.; Gao, R.; Yuan, X.; Hu, S.; Kuang, Y. Highly sensitive and selective gas sensors based on nanoporous CN monolayer for reusable detection of NO, H2S and NH3: A first-principles study. Appl. Surf. Sci. 2022, 606, 154806. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Li, Q.; Lin, J.; Guo, Z.; Zhang, X.; Lu, Z.; Ma, Y.; Huang, Y.; Tang, C. Fluorine doped porous boron nitride for efficient CO2 capture and separation: A DFT study. Appl. Surf. Sci. 2021, 556, 149775. [Google Scholar] [CrossRef]
- Yong, Y.; Cui, H.; Zhou, Q.; Su, X.; Kuang, Y.; Li, X. C2N monolayer as NH3 and NO sensors: A DFT study. Appl. Surf. Sci. 2019, 487, 488–495. [Google Scholar] [CrossRef]
- Braga, M.U.C.; Perin, G.H.; de Oliveira, L.H.; Arroyo, P.A. DFT calculations for adsorption of H2S and other natural gas compounds on (Fe, Co, Ni, Cu and Zn)–Y zeolite clusters. Microporous Mesoporous Mater. 2022, 331, 111643. [Google Scholar] [CrossRef]
- Zhan, S.; Ning, S.; Baao, J.; Wu, J.; Zhang, M.; Cui, J.; Duan, X.; Wang, X.; Li, Y. Comparative study on adsorption behaviors of CH4/CO2 and CH4/H2S in quartz nanopores from molecular perspectives: Implication for EGR in shale reservoirs. Colloid Surf. A 2025, 712, 136419. [Google Scholar] [CrossRef]
- Dronskowski, R.; Bloechl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Jameh, A.A.; Mohammadi, T.; Bakhtiari, O.; Mahdyarfar, M. Synthesis and modification of Zeolitic Imidazolate Framework (ZIF-8) nanoparticles as highly efficient adsorbent for H2S and CO2 removal from natural gas. J. Environ. Chem Eng. 2019, 7, 103058. [Google Scholar] [CrossRef]
- Daneshyar, A.; Ghaedi, M.; Sabzehmeidani, M.M.; Daneshyar, A. H2S adsorption onto Cu-Zn-Ni nanoparticles loaded activated carbon and Ni-Co nanoparticles loaded γ-Al2O3: Optimization and adsorption isotherms. J. Colloid Interface Sci. 2017, 490, 553–561. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.; Xian, X.; Jiang, Y.; Tang, J.; Liao, Q.; Li, H.; Chen, Y. Adsorption behavior and thermodynamic analysis of pure and binary CO2/CH4 mixture on shale. Gas Sci. Eng. 2025, 134, 205520. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Tian, X.; Rao, Z.; Wang, C.; Tang, R.; He, Y.; Luo, Y.; Fan, Q.; Fan, W.; Hu, Y. The Potential of MN4-GPs (M = Mn, Fe, Co, Ni, Cu, Mo) as Adsorbents for the Efficient Separation of CH4 from CO2 and H2S. Materials 2025, 18, 2907. https://doi.org/10.3390/ma18122907
Wei S, Tian X, Rao Z, Wang C, Tang R, He Y, Luo Y, Fan Q, Fan W, Hu Y. The Potential of MN4-GPs (M = Mn, Fe, Co, Ni, Cu, Mo) as Adsorbents for the Efficient Separation of CH4 from CO2 and H2S. Materials. 2025; 18(12):2907. https://doi.org/10.3390/ma18122907
Chicago/Turabian StyleWei, Shiqian, Xinyu Tian, Zhen Rao, Chunxia Wang, Rui Tang, Ying He, Yu Luo, Qiang Fan, Weifeng Fan, and Yu Hu. 2025. "The Potential of MN4-GPs (M = Mn, Fe, Co, Ni, Cu, Mo) as Adsorbents for the Efficient Separation of CH4 from CO2 and H2S" Materials 18, no. 12: 2907. https://doi.org/10.3390/ma18122907
APA StyleWei, S., Tian, X., Rao, Z., Wang, C., Tang, R., He, Y., Luo, Y., Fan, Q., Fan, W., & Hu, Y. (2025). The Potential of MN4-GPs (M = Mn, Fe, Co, Ni, Cu, Mo) as Adsorbents for the Efficient Separation of CH4 from CO2 and H2S. Materials, 18(12), 2907. https://doi.org/10.3390/ma18122907





