High-Performance Electrochromic Energy Storage Devices Based on Hexagonal WO3 and SnO2/PB Composite Films
Abstract
1. Introduction
2. Results and Discussion
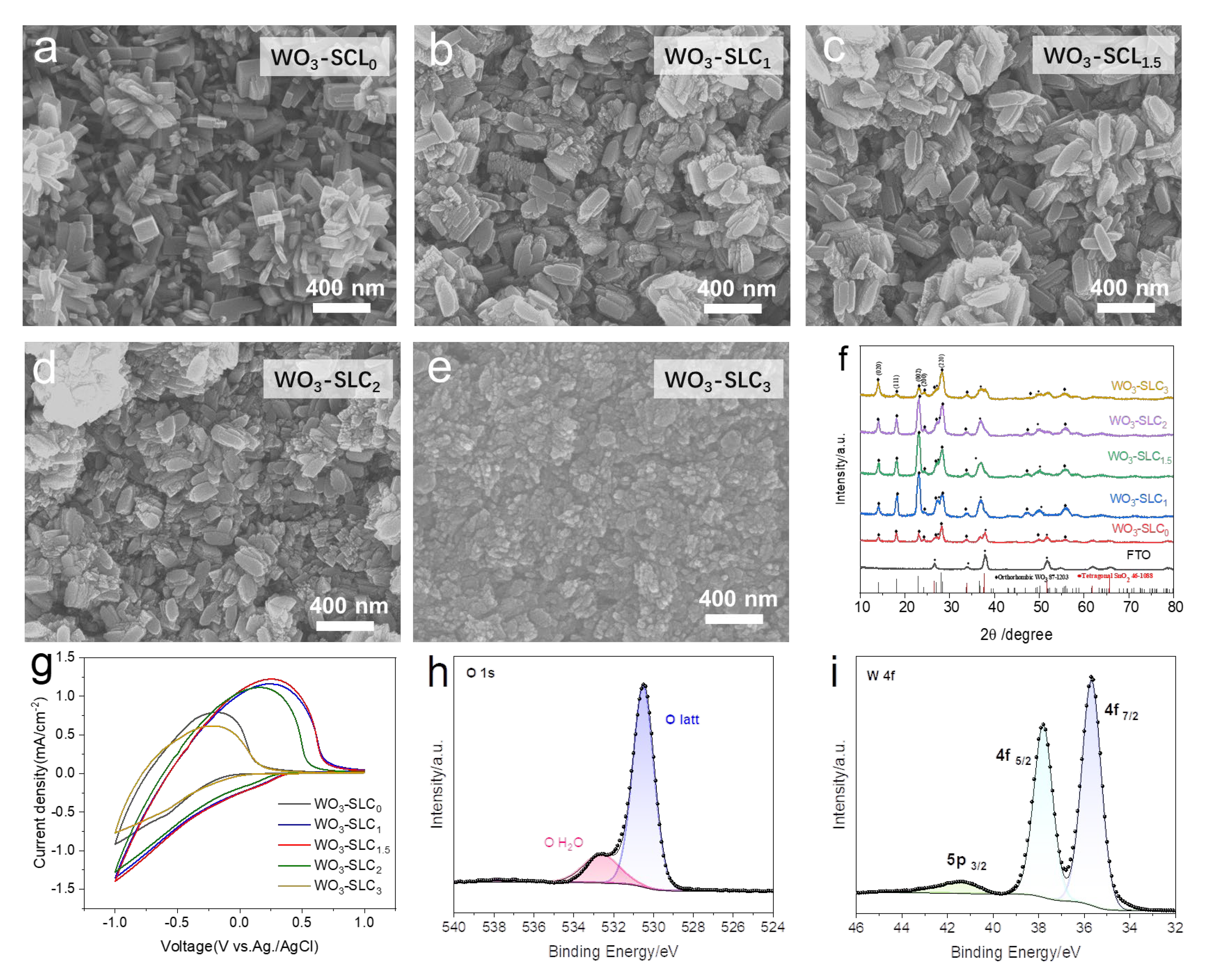
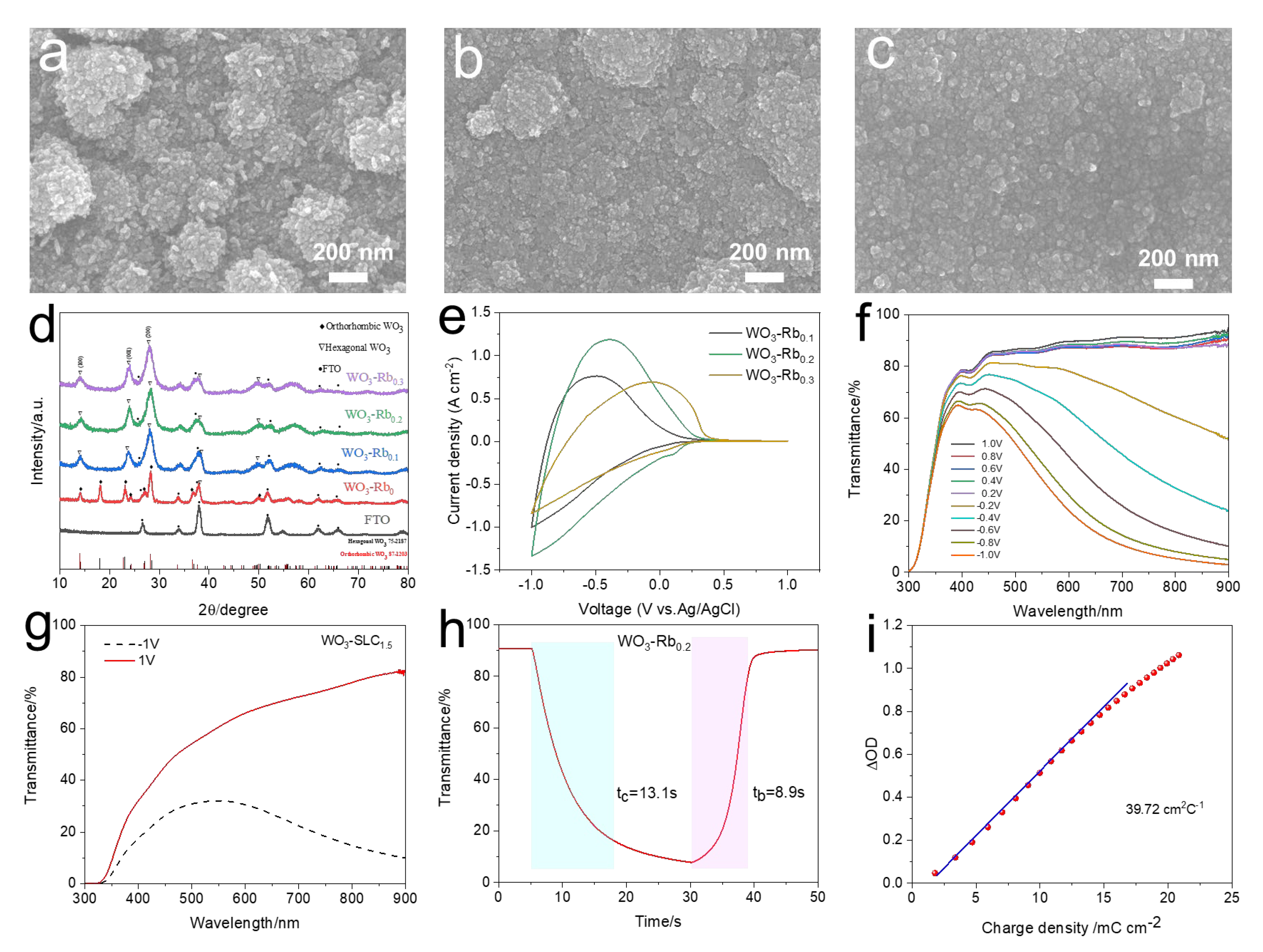
2.1. Fabrication and Characterization of WO3 Cathodic Films
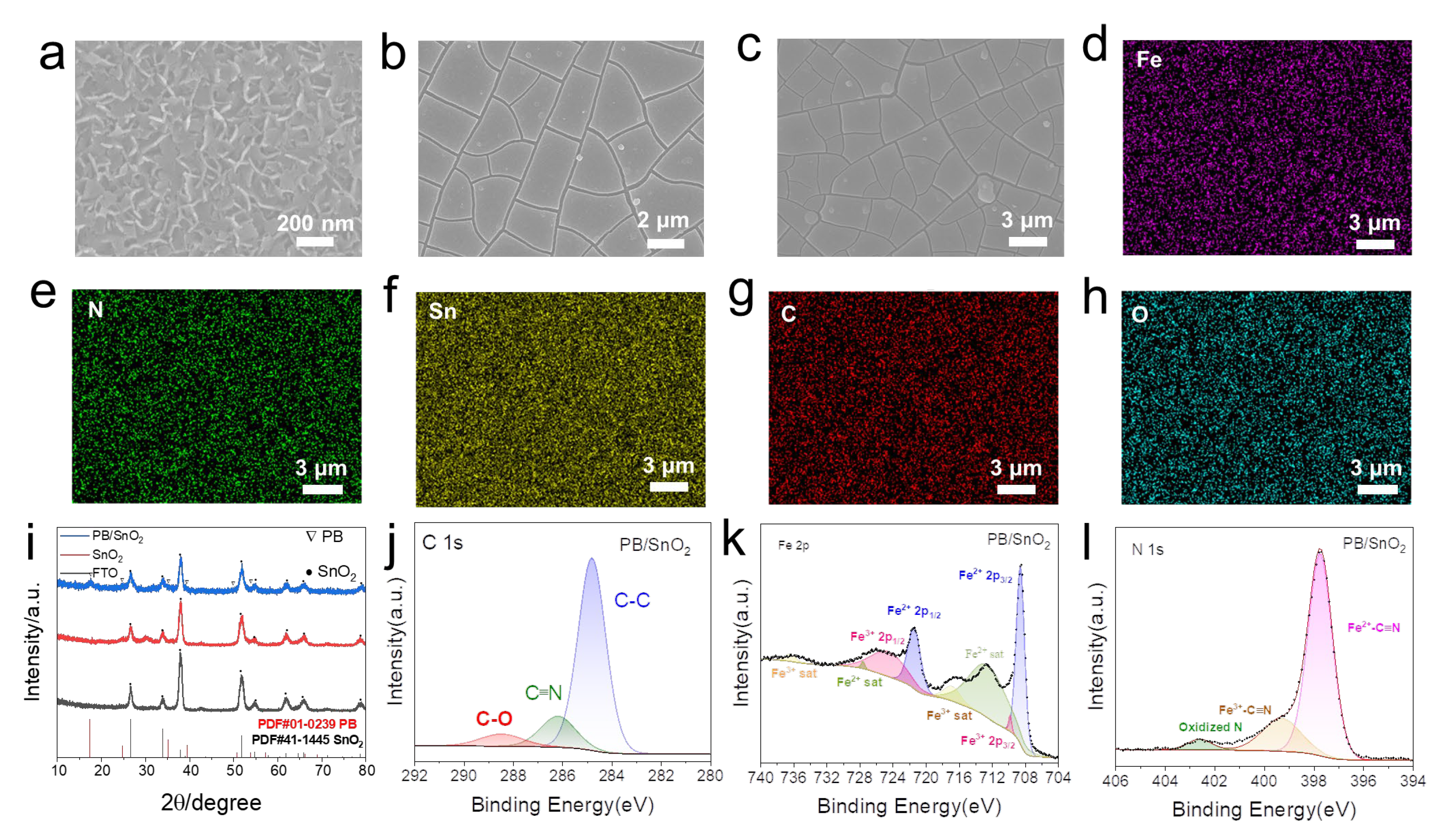
2.2. Fabrication and Characterization of SnO2/PB Anodic Films
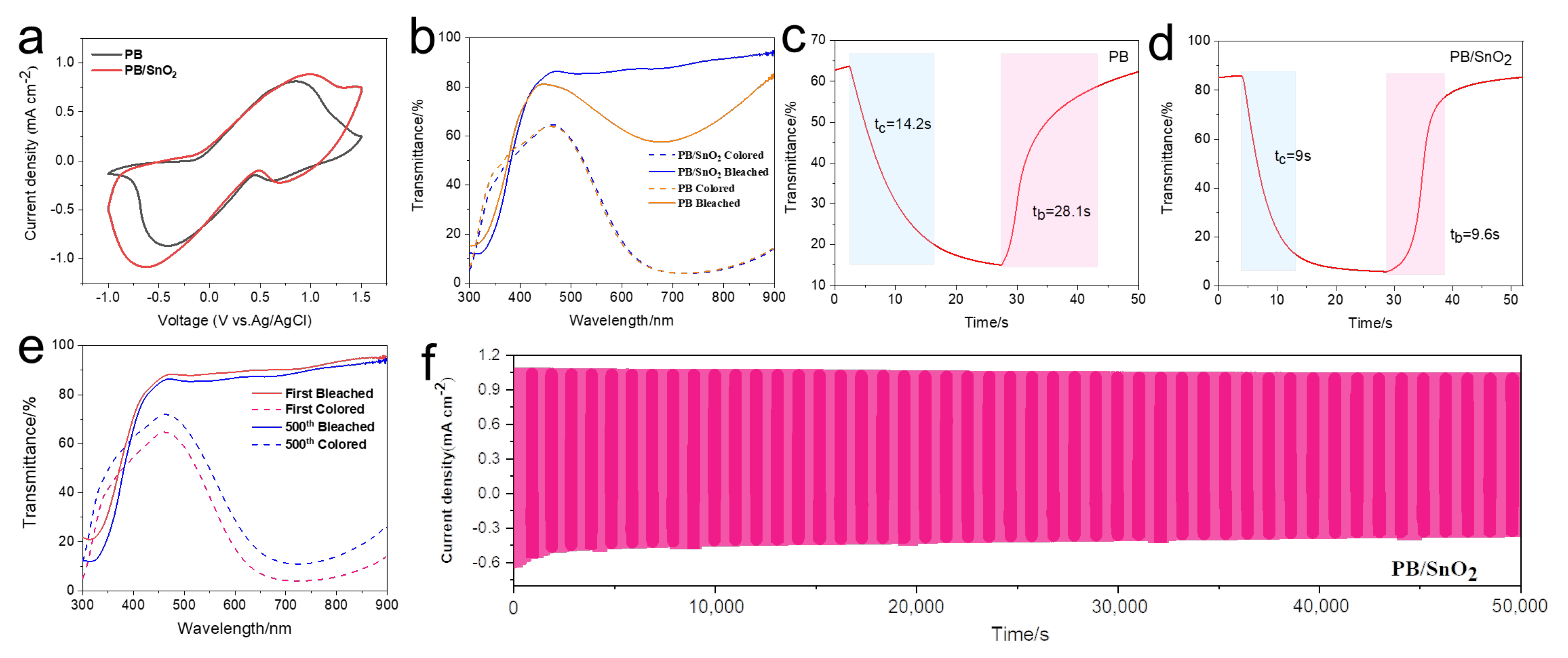
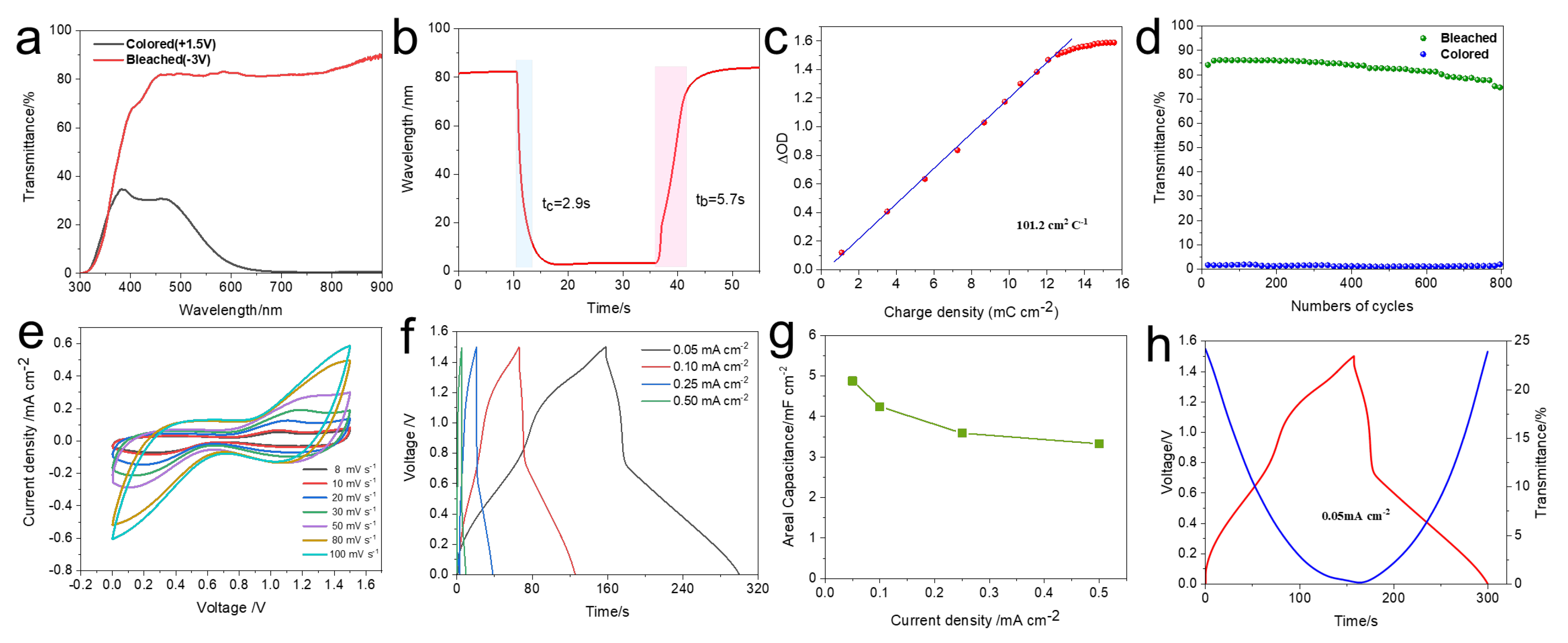
2.3. Assembly and Performance Characterization of Electrochromic Devices
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Preparation of WO3 Film
4.3. Preparation of SnO2 Films
4.4. Preparation of PB/SnO2 Films
4.5. EESD Assembly
4.6. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, M.; Chen, C.; Yu, A.; Feng, Y.; Cui, H.; Zhou, R.; Zhuang, Y.; Hu, X.; Liu, S.; Zhao, Q. Multilayer step-like microstructured flexible pressure sensing system integrated with patterned electrochromic display for visual detection. ACS Nano 2025, 19, 19488–19496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, Q.; Fan, F.; Wang, Q.; Su, Y.; Liu, M.; Zhu, F.; Zhao, D. Dual-band electrochromic smart window for dynamic switching between radiative cooling and solar heating. Adv. Sci. 2025, early view. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Huang, B.; Liu, P.; Ma, L.; Liu, L.; Chen, J.; Li, H.; Wang, C. Integrating transparent zinc mesh and anti-freezing hydrogel electrolyte toward durable zinc anode-based electrochromic devices. Adv. Mater. Technol. 2025, early view. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Li, H.; Qiao, L. Rapid response and wide-band regulation of an electrochromic device achieved by ultra-small V2O5 nanodots and a Zn2+/Li+ electrolyte. Inorg. Chem. Front. 2025, advance article. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, N.-Z.; Wu, Z.-A.; Liu, J.; Guan, K.-L.; Zhang, Z.-L.; Wan, H.-Z.; Wang, H.; Sun, D.-Y.; Xie, A. Nanofullerene regulated electric field to achieve stable Sn metal anode for aqueous Sn batteries. Rare Met. 2025, 44, 3869–3880. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, J.; Wang, Y.; Hu, L.; Tang, S.; Wan, Z.; Jia, C.; Weng, X.; Deng, L. A light-weight, thin-thickness, flexible multifunctional electrochromic device integrated with variable optical, thermal management and energy storage. Electrochim. Acta 2022, 435, 141274. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, R.; Luo, J.; Malik, H.A.; Wan, Z.; Jia, C.; Weng, X.; Xie, J.; Deng, L.; Yao, X. Self-healing dynamically cross linked versatile polymer electrolyte: A novel approach towards high performance, flexible electrochromic devices. Electrochim. Acta 2019, 320, 134489. [Google Scholar] [CrossRef]
- Xiao, L.; Lv, Y.; Lin, J.; Hu, Y.; Dong, W.; Guo, X.; Fan, Y.; Zhang, N.; Zhao, J.; Wang, Y.; et al. WO3-based electrochromic distributed bragg reflector: Toward electrically tunable microcavity luminescent device. Adv. Opt. Mater. 2018, 6, 1700791. [Google Scholar] [CrossRef]
- Arvizu, M.A.; Qu, H.Y.; Cindemir, U.; Qiu, Z.; Rojas-Gonzalez, E.A.; Primetzhofer, D.; Granqvist, C.G.; Osterlund, L.; Niklasson, G.A. Electrochromic WO3 thin films attain unprecedented durability by potentiostatic pretreatment. J. Mater. Chem. A 2019, 7, 2908–2918. [Google Scholar] [CrossRef]
- Cho, H.; Min, J.; Won, D.; Kwon, J.; Ko, S.H. Selective photo-thermal conversion of tungsten oxide sol precursor for electrochromic smart window applications. Acta Mater. 2020, 201, 528–534. [Google Scholar] [CrossRef]
- Hu, C.; Li, L.; Zhou, J.; Li, B.; Zhao, S.; Zou, C. Enhanced contrast of WO3-based smart windows by continuous li-ion insertion and metal electroplating. ACS Appl. Mater. Interfaces 2022, 14, 32253–32260. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, H.; Pei, S.; Xie, S.; You, S. Oxygen-deficient WO3−x nanoplate array film photoanode for efficient photoelectrocatalytic water decontamination. Chem. Eng. J. 2020, 381, 122740. [Google Scholar] [CrossRef]
- Son, M.; Shin, D.; Lee, C.S. Facile fabrication of trimodal switchable mirror device with zero transmittance in the black state. Adv. Mater. Interfaces 2020, 8, 2001416. [Google Scholar] [CrossRef]
- Koo, B.-R.; Jo, M.-H.; Kim, K.-H.; Ahn, H.-J. Amorphous-quantized WO3·H2O films as novel flexible electrode for advanced electrochromic energy storage devices. Chem. Eng. J. 2021, 424, 130383. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Seo, H. Intercalation of barium into monoclinic tungsten oxide nanoplates for enhanced photoelectrochemical water splitting. Chem. Eng. J. 2019, 355, 784–796. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, W.; Mao, X.; Zhu, H.; Wang, D. Na2SO4-assisted synthesis of hexagonal-phase WO3 nanosheet assemblies with applicable electrochromic and adsorption properties. J. Mater. Chem. A 2013, 1, 1261–1269. [Google Scholar] [CrossRef]
- Li, H.; Shi, G.; Wang, H.; Zhang, Q.; Li, Y. Self-seeded growth of nest-like hydrated tungsten trioxide film directly on FTO substrate for highly enhanced electrochromic performance. J. Mater. Chem. A 2014, 2, 11305–11310. [Google Scholar] [CrossRef]
- Gao, C.; Guo, X.; Nie, L.; Wu, X.; Peng, L.; Chen, J.; Ding, W. Structure design and performance research of WO3 hydrogen gasochromic film prepared by solvothermal synthesis assisted with electrodeposition of seed layer. Adv. Mater. Interfaces 2022, 9, 2101355. [Google Scholar] [CrossRef]
- Pan, J.; Zheng, R.; Wang, Y.; Ye, X.; Wan, Z.; Jia, C.; Weng, X.; Xie, J.; Deng, L. A high-performance electrochromic device assembled with hexagonal WO3 and NiO/PB composite nanosheet electrodes towards energy storage smart window. Sol. Energy Mater. Sol. Cells 2020, 207, 110337. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Y.; Zheng, R.; Wang, M.; Wan, Z.; Jia, C.; Weng, X.; Xie, J.; Deng, L. Directly grown high-performance WO3 films by a novel one-step hydrothermal method with significantly improved stability for electrochromic applications. J. Mater. Chem. A 2019, 7, 13956–13967. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.; Zhang, J.; Zhang, Z. Fabrication of vertical orthorhombic/hexagonal tungsten oxide phase junction with high photocatalytic performance. Appl. Catal. B Environ. 2017, 207, 207–217. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, Z.; Zuo, Y.; Zhou, H.; Sun, D.; Li, Y.; Yan, Y.; Feng, T.; Xie, A. Self-seeded growth of hexagonal-phase WO3 film by a one-step hydrothermal method for high-performance electrochromic energy storage devices. J. Power Sources 2025, 633, 236350. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; He, Z.; Yi, Y.; Wang, M.; Luo, Z.; Liu, Q.; Huang, J.; Zhong, X.; Du, K.; et al. All-solid-state electrochromic Li-ion hybrid supercapacitors for intelligent and wide-temperature energy storage. Chem. Eng. J. 2021, 414, 128892. [Google Scholar] [CrossRef]
- Luo, Y.; Jin, H.; Lu, Y.; Zhu, Z.; Dai, S.; Huang, L.; Zhuang, X.; Liu, K.; Huang, L. Potential gradient-driven fast-switching electrochromic device. ACS Energy Lett. 2022, 7, 1880–1887. [Google Scholar] [CrossRef]
- Zou, X.; Wang, Y.; Tan, Y.; Pan, J.; Niu, J.; Jia, C. Achieved RGBY four colors changeable electrochromic pixel by coelectrodeposition of iron hexacyanoferrate and molybdate hexacyanoferrate. ACS Appl. Mater. Interfaces 2020, 12, 29432–29442. [Google Scholar] [CrossRef]
- Liu, X.D.; Yang, Q.; Yuan, L.; Qi, D.; Wei, X.; Zhou, X.; Chen, S.; Cao, L.; Zeng, Y.; Jia, J.; et al. Oxygen vacancy-rich WO3 heterophase structure: A trade-off between surface-limited pseudocapacitance and intercalation-limited behaviour. Chem. Eng. J. 2021, 425, 131431. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, M.; Mei, Z.; Diao, X. Novel prussian white@MnO2-based inorganic electrochromic energy storage devices with integrated flexibility, multicolor, and long life. ACS Appl. Mater. Interfaces 2022, 14, 48833–48843. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Zheng, Y.; Cui, Y. High performance electrochromic energy storage devices based on Mo-doped crystalline/amorphous WO3 core-shell structures. Sol. Energy Mater. Sol. Cells 2022, 235, 111488. [Google Scholar] [CrossRef]
- Mak, A.K.; Uysal, D.; Alevli, M.; Karabulut, M.; Öztürk, O.; Tuna, Ö. Structural, optical, and electrochromic properties of Nb-doped WO3 thin films. J. Alloys Compd. 2025, 1031, 181040. [Google Scholar] [CrossRef]
- Zhao, F.; Zeng, Y.; Cheng, Z.; Shi, G.; Liu, Q.; Liu, Y.; Han, G. Cathode/Anode electrodes for large-area bifunctional electrochemical devices prepared by a novel Na3Cit-assisted chemical deposition method. Chem. Eng. J. 2024, 485, 149350. [Google Scholar] [CrossRef]
- Xu, B.; Chen, J.; Li, P.; Ouyang, Y.; Ma, Y.; Wang, H.; Li, H. Transparent metal oxide interlayer enabling durable and fast-switching zinc anode-based electrochromic devices. Nanoscale 2023, 15, 19629–19637. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, J.; Lv, C.; Hu, C.; Guan, H.; Wang, J.; Shi, Y.; Song, J.; Watanabe, A.; Ge, X. General and scalable preparation of Prussian blue analogues on arbitrary conductive substrates and their derived metal phosphides as highly efficient and ultra-long-life bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2021, 420, 129972. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, D.; Li, Z.; Zhang, B.; Sun, X.; Shao, Z.; Chen, C.; Wang, X.; Hao, L.; Wang, X.; et al. Chemical bath deposition of mesoporous SnO2 to improve interface adhesion and device operational stability. Chem. Eng. J. 2022, 443, 136308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Z.; Wang, Z.; Yan, Y.; Feng, T.; Xie, A. High-Performance Electrochromic Energy Storage Devices Based on Hexagonal WO3 and SnO2/PB Composite Films. Materials 2025, 18, 2871. https://doi.org/10.3390/ma18122871
Wang Y, Zhang Z, Wang Z, Yan Y, Feng T, Xie A. High-Performance Electrochromic Energy Storage Devices Based on Hexagonal WO3 and SnO2/PB Composite Films. Materials. 2025; 18(12):2871. https://doi.org/10.3390/ma18122871
Chicago/Turabian StyleWang, Yi, Zilong Zhang, Ze Wang, Yujie Yan, Tong Feng, and An Xie. 2025. "High-Performance Electrochromic Energy Storage Devices Based on Hexagonal WO3 and SnO2/PB Composite Films" Materials 18, no. 12: 2871. https://doi.org/10.3390/ma18122871
APA StyleWang, Y., Zhang, Z., Wang, Z., Yan, Y., Feng, T., & Xie, A. (2025). High-Performance Electrochromic Energy Storage Devices Based on Hexagonal WO3 and SnO2/PB Composite Films. Materials, 18(12), 2871. https://doi.org/10.3390/ma18122871






