Decellularized Extracellular Matrices for Skin Wound Treatment
Abstract
1. Introduction
2. Skin Wound Healing
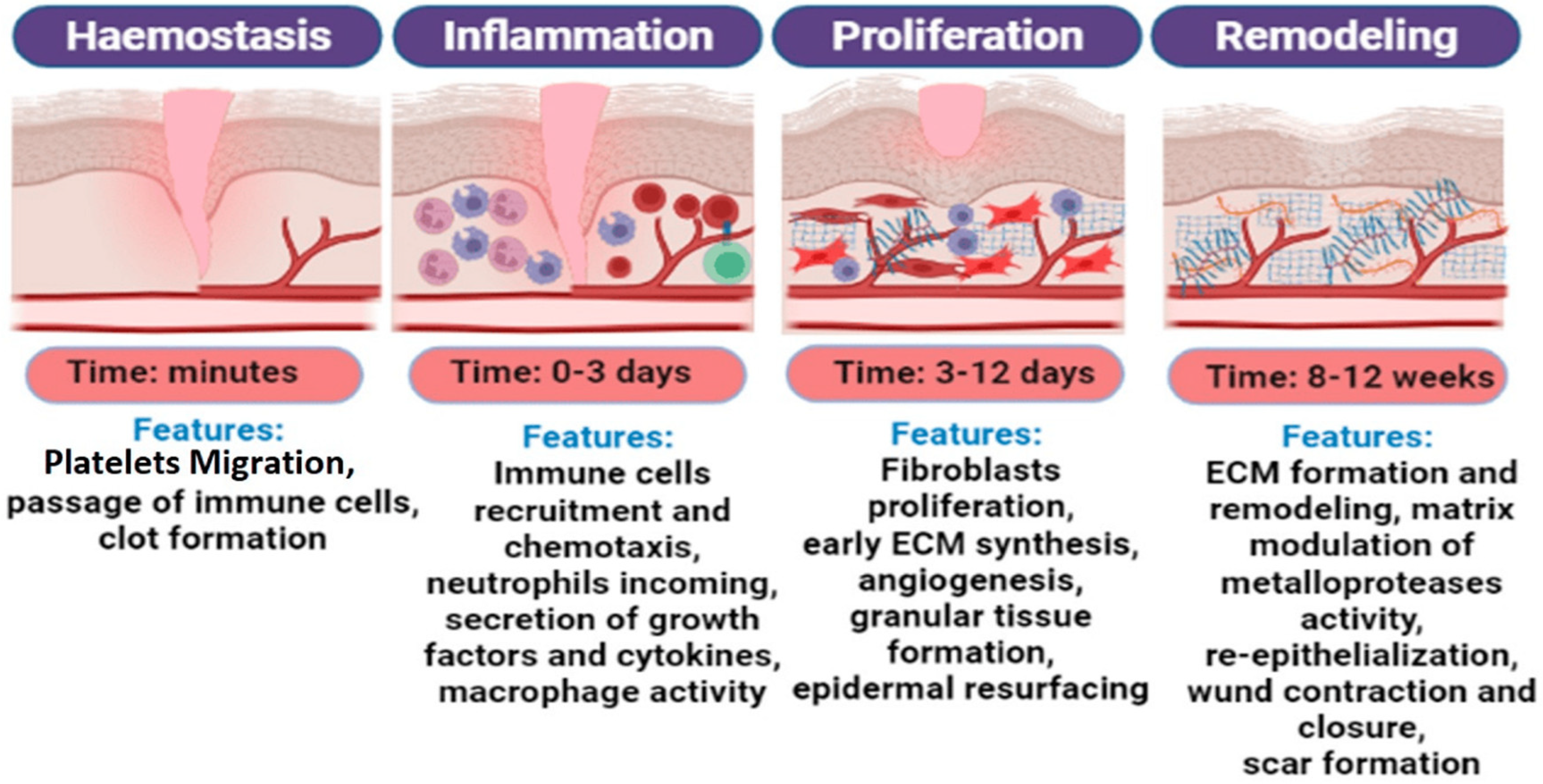
3. Effect of Extracellular Matrix Components on Skin Wound Healing
3.1. Role of Collagen in Wound Healing
3.2. Role of Elastin in Wound Healing
3.3. Role of Laminin in Wound Healing
3.4. Role of Fibronectin in Wound Healing
3.5. Role of Polysaccharides in Wound Healing (Glycosaminoglycans)
3.6. Role of ECM-Contained Growth Factors in Wound Healing
4. Decellularized Biomaterials for Skin Wound Healing
4.1. Acellular Dermal Matrix (ADM)
| Allogeneic (HADM) | Xenogeneic (e.g., Cattle, Pigs) | Ref. |
|---|---|---|
| The collagen amino acid species, quantity, and arrangement are consistent across individuals. | Low homology with humans; a small number of amino acids differ from those in human type I collagen. | [133] |
| No alpha-Gal antigen and rarely causes immune rejection. | Contains alpha-Gal antigen, which can trigger hyperacute and chronic immune rejection. | [33,134] |
| 1. The pore structure is the same as the human body; 2. Contains human cytokines; 3. Containing human cell recognition signals, with a stronger affinity for human cells, making it more conducive to cell attachment. | 1. Pore structure close to human body; 2. Xenogeneic cytokines; 3. Xenogeneic cellular recognition signals. | [133,135] |
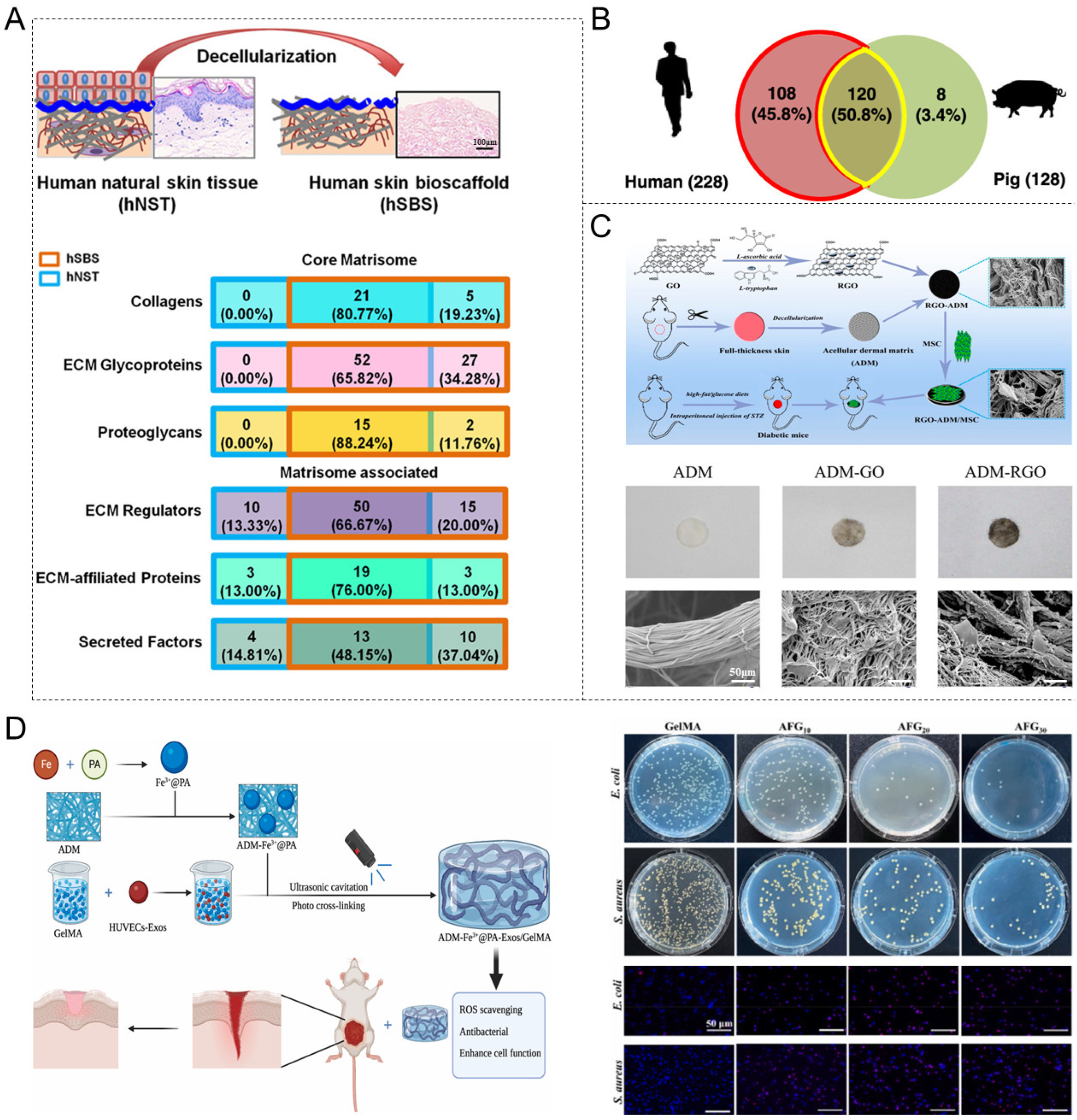
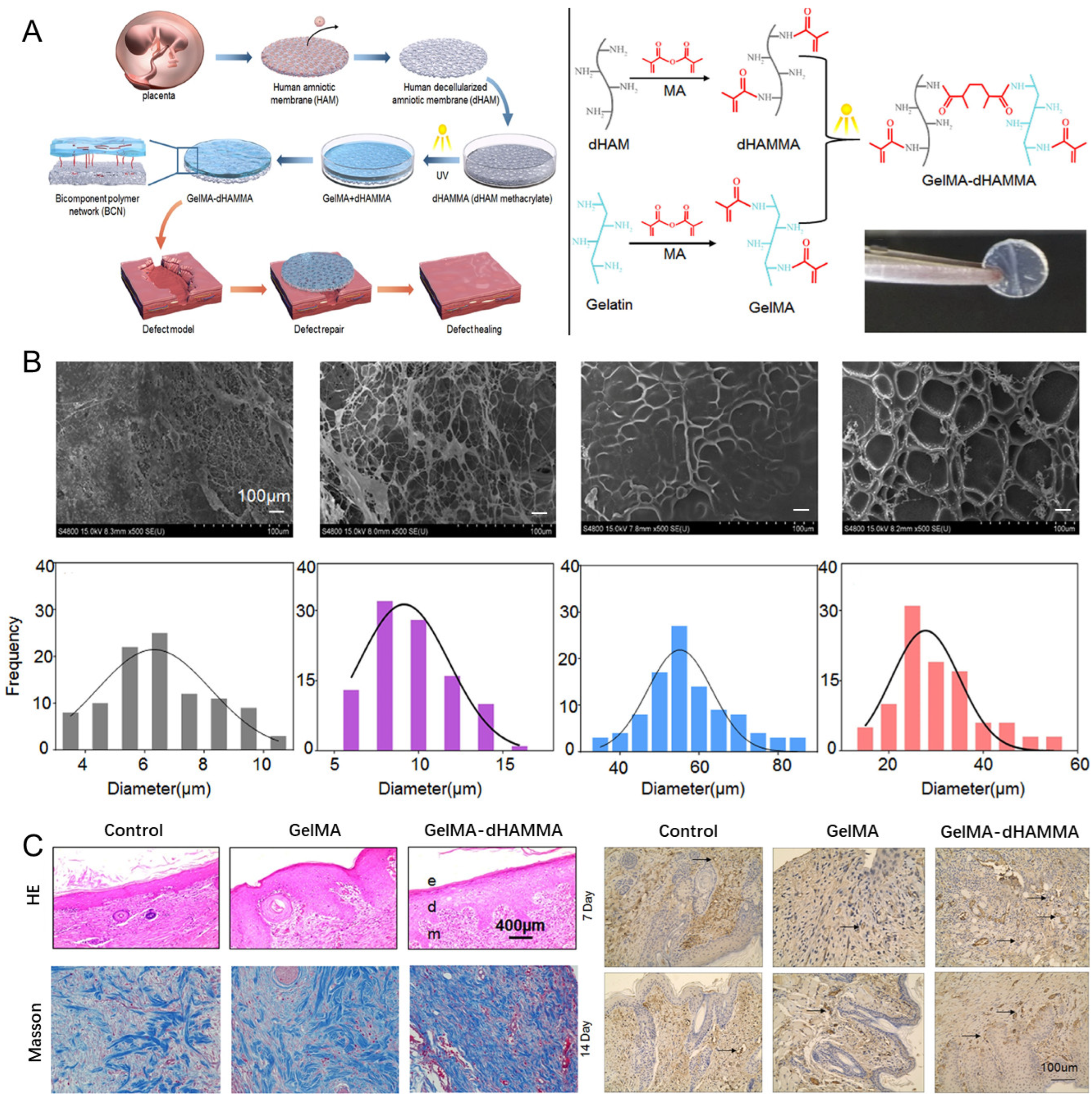
4.2. Acellular Amniotic Membrane (AAM)
4.3. Decellularized Small Intestinal Submucosa (DSIS)

4.4. Decellularized Adipose Tissue (DAT)

4.5. Decellularized Fish Skin
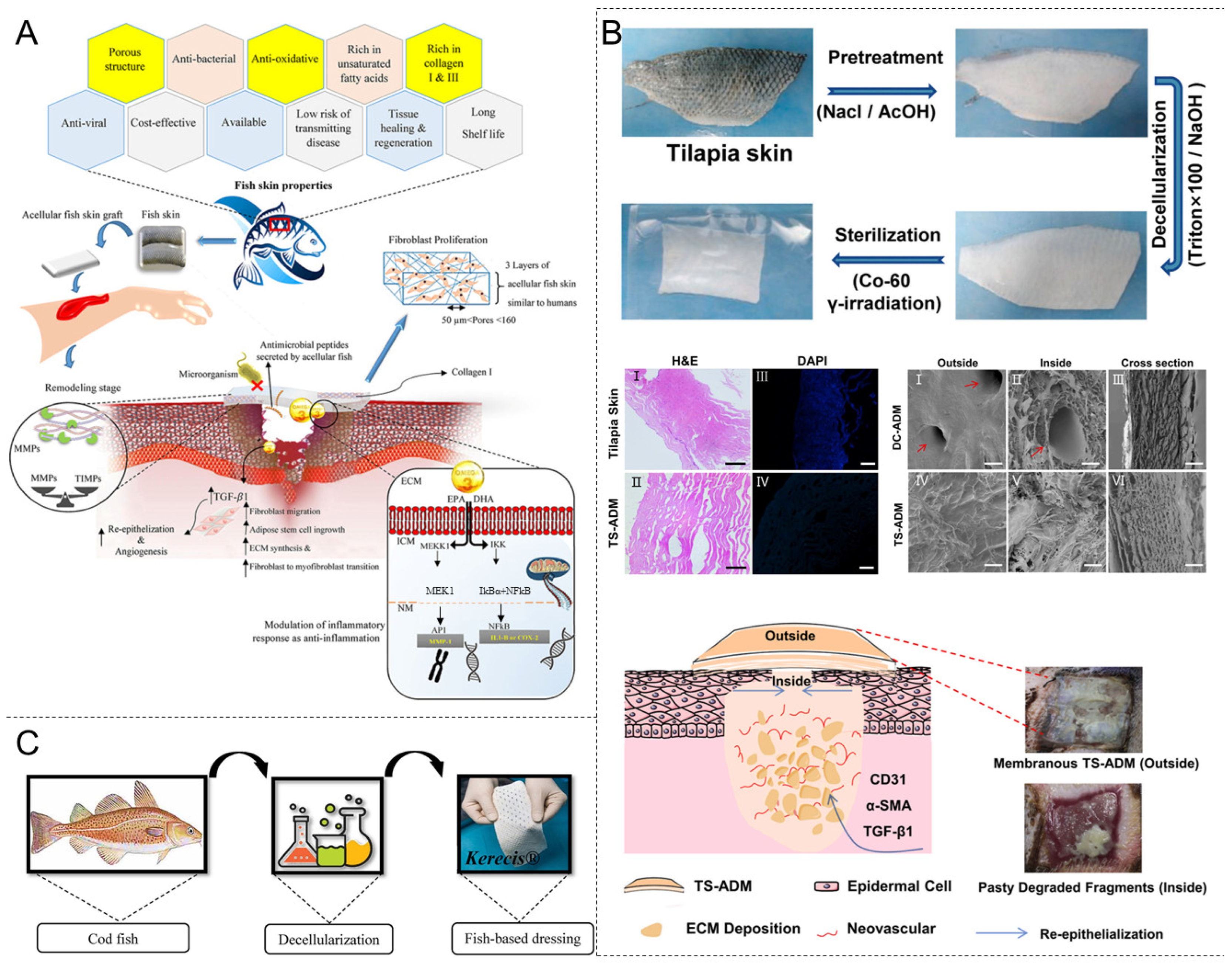
4.6. Others
| dECM | Structure | Immunogenicity | Degradation Rate | Mechanical Modulus | Advantage | Disadvantage |
|---|---|---|---|---|---|---|
| Acellular dermal matrix (ADM) [101,202] | A dense collagen fiber network, preserving the basement membrane structure. | Extremely low (xenogeneic ADM may still contain a small number of α-Gal antigens) | Relatively slow (up to several months after cross-linking) | High (10–30 MPa) | It has wide clinical applications, high mechanical strength, and good biocompatibility, which supports cell infiltration and angiogenesis. | The aperture of ADM is small, and its porosity is low, which is not conducive to cell migration and proliferation, resulting in prolonged healing and scar formation time, and hindering the regeneration of the subcutaneous fat layer; the use of xenogeneic sources may cause mild immune reactions; the source of human materials is limited. |
| Acellular amniotic membrane (AAM) [203] | Ultra-thin layered structure. | Extremely low (with natural immunity exemption characteristics, with very few residual antigens) | Relatively fast (within a few weeks to 2 months) | Low (0.1–1 MPa) | It expresses the secretion of leukocyte protease inhibitors and elafin, which have anti-inflammatory properties; it produces the antimicrobial peptide β-defensin; its transparency facilitates the observation of the healing process. | Poor mechanical strength; prone to tearing; difficult to use in load-bearing areas. |
| Decellularized small intestinal submucosa (DSIS) [204,205] | Multi-layer collagen + elastin with preserved growth factors (VEGF, FGF, etc.). | Low | Moderate (2–4 months) | Medium (2–10 MPa) | The antimicrobial peptides produced during the degradation process of DSIS can inhibit both Gram-positive and Gram-negative bacteria. Compared with other grafts, there are fewer infections and complications after implantation. | Limited source (extracted from pig small intestine); uneven thickness; insufficient long-term mechanical support. |
| Decellularized adipose tissue (DAT) [171] | Loose porous mesh structure. | Extremely low (without α-Gal antigen) | Fast (within a few weeks to 1 month) | Low (0.5–3 MPa) | Easy to obtain; promotes the regeneration of adipose tissue and the reconstruction of blood vessels. | Poor mechanical properties: rapid degradation requires cross-linking; risk of lipid residue. |
| Decellularized Fish Skin [185,206] | Orderly arranged collagen Type I bundles. | Extremely low (without α-Gal antigen) | Slow (3 to 6 months, and even longer after cross-linking) | Moderately high (5–20 MPa) | It reduces the risk of zoonotic disease spread and circumvents religious restrictions associated with different mammalian species. The Omega-3 unsaturated fatty acids in the fish-skin ECM have antiviral and antibacterial effects and are essential regulators of the inflammatory response. | Clinical data is limited; chemical cross-linking may be necessary to enhance stability; long-term biocompatibility needs to be verified. |
| Urinary bladder matrix (UBM) [207] | Multilayer composite structure. | Low | Moderate (2–3 months) | Medium (3–15 MPa) | The structure exhibits strong biomimetic properties, making it suitable for urinary system repair, and supports the differentiation of multiple cell lineages. | The preparation is complex, requiring layered processing; mechanical anisotropy may impact the application scenarios. |
5. Decellularized Matrix Materials for Skin Wound Repair
5.1. Burn Wounds
5.2. Diabetic Wounds
5.3. Infected Wounds
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| dECM | Decellularized extracellular matrix |
| ECM | Extracellular matrix |
| SEM | Scanning electron microscope |
| H&E | Hematoxylin and eosin |
| DAPI | 4′,6-diamidino-2-phenylindole |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| FDA | Food and Drug Administration |
| PMA | Pre-market approval |
| CE | European Conformity |
| HCT/Ps | Human cells, tissues, and cellular and tissue-based products |
| NMPA | National Medical Products Administration |
| MDR | Medical device regulation |
| TGA | Therapeutic Goods Administration |
| α-Gal | Alpha-galactosidase |
| CS | Chitosan |
| PCL | Polycaprolactone |
| UA | Usnic acid |
| ROS | Reactive oxygen species |
| TIMP | Tissue inhibitor of metalloproteinase |
| EL | Elastin |
| LM | Laminin |
| FN | Fibronectin |
| EDB-FN | Extra domain-B fibronectin |
| GAGs | Glycosaminoglycans |
| MMPs | Matrix metalloproteinases |
| TSP-1 | Thrombospondin-1 |
| DFU | Diabetic foot ulcer |
| AgNPs | Silver nanoparticles |
| Col I/AgNP | Collagen–silver nanoparticle |
| SF | Silk fibroin |
| RGD | Arginine–glycine–aspartic acid |
| HBDs | Heparin-binding domains |
| GF | Growth factor |
| HA | Hyaluronic acid |
| CS | Chondroitin sulfate |
| DS | Dermatan sulfate |
| KS | Keratan sulfate |
| HS | Heparan sulfate |
| HP | Heparin |
| MHC | Major histocompatibility complex |
| MSCs | Mesenchymal stem cells |
| RGO | Reduced graphene oxide |
| ADM | Acellular dermal matrix |
| HADM | Human acellular dermal matrix |
| PA | Protocatechualdehyde |
| GelMA | Gelatin methacrylate |
| AAM | Acellular amniotic membrane |
| AMSC | Adipose-derived mesenchymal stem cells |
| HAM | Human amniotic membrane |
| dHAM | Human decellularized amniotic membrane |
| MA | Methacrylic anhydride |
| dHAMMA | dHAM methacrylate |
| a-SMA | a-smooth muscle actin |
| DSIS | Decellularized small intestinal submucosa |
| PLGA | Poly(lactic-co-glycolic acid) |
| IL-10 | Interleukin-10 |
| TA | Tannic acid |
| DAT | Decellularized adipose tissue |
| ASCs | Adipose-derived stem cells |
| CD31 | Platelet endothelial cell-adhesion molecule-1 |
| TS-ADM | Tilapia-skin acellular dermal matrix |
| DC-ADM | Porcine acellular dermal matrix |
| bFGF | Basic fibroblast growth factor |
| UBM | Urinary bladder matrix |
| ACM | Acellular cartilage matrix |
| ABM | Acellular bone matrix |
| ACS | Acellular corneal stroma |
| PCNA | Proliferating cell nuclear antigen |
| K19 | Keratin 19 |
| PDGF | Platelet-derived growth factor |
| EGF | Epidermal growth factor |
| FGF | Fibroblast growth factor |
| ROS | Reactive oxygen species |
| VEGF | Vascular endothelial growth factor |
| TGF-β | Transforming growth factor-β |
References
- Li, R.T.; Liu, K.; Huang, X.; Li, D.; Ding, J.X.; Liu, B.; Chen, X.S. Bioactive Materials Promote Wound Healing through Modulation of Cell Behaviors. Adv. Sci. 2022, 9, 2105152. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.T.; Yu, J.F.; Wang, C.F.; Chen, S.; Li, Q.; Guo, K.; Qing, R.K.; Wang, G.F.; Ren, J.A. Micro-Gel Ensembles for Accelerated Healing of Chronic Wound via pH Regulation. Adv. Sci. 2022, 9, 2201254. [Google Scholar] [CrossRef] [PubMed]
- Heras, K.L.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef]
- Xu, P.-Y.; Kankala, R.K.; Li, Y.-W.; Wang, S.-B.; Chen, A.-Z. Synergistic chemo-/photothermal therapy based on supercritical technology-assisted chitosan–indocyanine green/luteolin nanocomposites for wound healing. Regen. Biomater. 2022, 9, rbac072. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, P.; Ge, P.; Chen, L.; Chen, Y.; Kankala, R.K.; Wang, S.; Chen, A. NIR-responsive carrier-free nanoparticles based on berberine hydrochloride and indocyanine green for synergistic antibacterial therapy and promoting infected wound healing. Regen. Biomater. 2023, 10, rbad076. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.G.; da Silva, L.P.; Cerqueira, M.T.; Ibañez, R.; Murphy, C.M.; Reis, R.L.; Marques, A.P. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022, 150, 22–33. [Google Scholar] [CrossRef]
- He, L.N.; Di, D.H.; Chu, X.H.; Liu, X.L.; Wang, Z.Y.; Lu, J.Y.; Wang, S.L.; Zhao, Q.F. Photothermal antibacterial materials to promote wound healing. J. Control. Release 2023, 363, 180–200. [Google Scholar] [CrossRef]
- Jiang, S.; Zhuang, Y.; Cai, M.; Wang, X.; Lin, K. Decellularized extracellular matrix: A promising strategy for skin repair and regeneration. Eng. Regen. 2023, 4, 357–374. [Google Scholar] [CrossRef]
- Vilaça-Faria, H.; Noro, J.; Reis, R.L.; Pirraco, R.P. Extracellular matrix-derived materials for tissue engineering and regenerative medicine: A journey from isolation to characterization and application. Bioact. Mater. 2024, 34, 494–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Z.; Zheng, Y.; Zhou, F.; Zhao, J.; Zhai, Q.; Zhang, Z.; Liu, T.; Chen, Y.; Qi, S. 3D-printed dermis-specific extracellular matrix mitigates scar contraction via inducing early angiogenesis and macrophage M2 polarization. Bioact. Mater. 2022, 10, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; Moya-Rull, D.; Marco, A.; Amato, G.; Ullate-Agote, A.; Tarantino, C.; Gallo, M.; Esporrín-Ubieto, D.; Centeno, A.; Vilas-Zornoza, A. Natural Hydrogels Support Kidney Organoid Generation and Promote In Vitro Angiogenesis. Adv. Mater. 2024, 36, 2400306. [Google Scholar] [CrossRef]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef]
- Hussein, K.H.; Ahmadzada, B.; Correa, J.C.; Sultan, A.; Wilken, S.; Amiot, B.; Nyberg, S.L. Liver tissue engineering using decellularized scaffolds: Current progress, challenges, and opportunities. Bioact. Mater. 2024, 40, 280–305. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.-F.; Wang, Y.; Duan, Y.-Y.; Luo, S.-C.; Zhang, J.-T.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Modeling dECM-based inflammatory cartilage microtissues in vitro for drug screening. Compos. Part B Eng. 2023, 250, 110437. [Google Scholar] [CrossRef]
- Xu, P.; Kankala, R.K.; Wang, S.; Chen, A. Decellularized extracellular matrix-based composite scaffolds for tissue engineering and regenerative medicine. Regen. Biomater. 2024, 11, rbad107. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.-M.; Kang, K.-S.; Woo, H.-M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef]
- Friedrich, E.E.; Lanier, S.T.; Niknam-Bienia, S.; Arenas, G.A.; Rajendran, D.; Wertheim, J.A.; Galiano, R.D. Residual sodium dodecyl sulfate in decellularized muscle matrices leads to fibroblast activation in vitro and foreign body response in vivo. J. Tissue Eng. Regen. Med. 2018, 12, e1704–e1715. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ahn, J.; Kang, H.K.; Kim, M.-S.; Kim, N.-G.; Kook, M.G.; Choi, S.W.; Jeon, N.L.; Woo, H.-M.; Kang, K.-S. Development of highly functional bioengineered human liver with perfusable vasculature. Biomaterials 2021, 265, 120417. [Google Scholar] [CrossRef] [PubMed]
- Bühler, N.E.M.; Schulze-Osthoff, K.; Königsrainer, A.; Schenk, M. Controlled processing of a full-sized porcine liver to a decellularized matrix in 24 h. J. Biosci. Bioeng. 2015, 119, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Gzik-Zroska, B.; Joszko, K.; Wolański, W.; Suchoń, S.; Burkacki, M.; Ples, M.; Malachowski, J.; Tomaszewski, M.; Szarek, A.; Stradomski, G.; et al. Assessment of the Impact of Decellularization Methods on Mechanical Properties of Biocomposites Used as Skin Substitute. Materials 2021, 14, 4785. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L.; et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019, 10, 5658. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.-S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.-B.; Lee, H.; Kim, J.H.; Cho, D.-W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Kalva, S.N.; Augustine, R.; Al Mamun, A.; Dalvi, Y.B.; Vijay, N.; Hasan, A. Active agents loaded extracellular matrix mimetic electrospun membranes for wound healing applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102500. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Jiang, X.; Dai, B.; Zhang, L.; Zhu, Y. Decellularized extracellular matrix materials for treatment of ischemic cardiomyopathy. Bioact. Mater. 2024, 33, 460–482. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, J.; Xu, Y.; Yi, K.; Li, F.; Zhou, H.; Wang, H.; Chan, H.F.; Lao, Y.-H.; Lv, S. Stem cell-derived hepatocyte therapy using versatile biomimetic nanozyme incorporated nanofiber-reinforced decellularized extracellular matrix hydrogels for the treatment of acute liver failure. Bioact. Mater. 2023, 28, 112–131. [Google Scholar] [CrossRef]
- Wang, B.; Tang, Q.; Yang, Q.; Li, M.; Zeng, S.; Yang, X.; Xiao, Z.; Tong, X.; Lei, L.; Li, S. Functional acellular matrix for tissue repair. Mater. Today Bio 2023, 18, 100530. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The role of the extracellular matrix (ECM) in wound healing: A review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, X.; Liu, X.; Wen, G.; Yu, Y. Recent advances in decellularized biomaterials for wound healing. Mater. Today Bio 2023, 19, 100589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Li, W.; Liu, X.; Ma, L.; Zhao, T.; Ding, Q.; Ding, C.; Liu, W. Polysaccharide-based hydrogel promotes skin wound repair and research progress on its repair mechanism. Int. J. Biol. Macromol. 2023, 248, 125949. [Google Scholar] [CrossRef]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A comprehensive review of natural compounds for wound healing: Targeting bioactivity perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Dis. 2019, 20, 207–217. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Ahmad, N. In vitro and in vivo characterization methods for evaluation of modern wound dressings. Pharmaceutics 2022, 15, 42. [Google Scholar] [CrossRef]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The extracellular matrix in skin inflammation and infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef]
- Solarte David, V.A.; Güiza-Argüello, V.R.; Arango-Rodríguez, M.L.; Sossa, C.L.; Becerra-Bayona, S.M. Decellularized tissues for wound healing: Towards closing the gap between scaffold design and effective extracellular matrix remodeling. Front. Bioeng. Biotechnol. 2022, 10, 821852. [Google Scholar] [CrossRef]
- Nyström, A.; Bruckner-Tuderman, L. Matrix molecules and skin biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Walraven, M.; Hinz, B. Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer. Matrix Biol. 2018, 71, 205–224. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Neill, T.; Iozzo, R.V. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75, 12–26. [Google Scholar] [CrossRef]
- Apte, S.S.; Parks, W.C. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015, 44, 1–6. [Google Scholar] [CrossRef]
- Gardeazabal, L.; Izeta, A. Elastin and collagen fibres in cutaneous wound healing. Exp. Dermatol. 2024, 33, e15052. [Google Scholar] [CrossRef]
- Mohanty, S.; Roy, S. Bioactive Hydrogels Inspired by Laminin: An Emerging Biomaterial for Tissue Engineering Applications. Macromol. Biosci. 2024, 24, 2400207. [Google Scholar] [CrossRef]
- Sriram, R.; Gopal, V. Aging Skin and Natural Bioactives that Impede Cutaneous Aging: A Narrative Review. Indian J. Dermatol. 2023, 68, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Padhi, A.; Nain, A.S. ECM in differentiation: A review of matrix structure, composition and mechanical properties. Ann. Biomed. Eng. 2020, 48, 1071–1089. [Google Scholar] [CrossRef] [PubMed]
- Patrawalla, N.Y.; Kajave, N.S.; Albanna, M.Z.; Kishore, V. Collagen and Beyond: A Comprehensive Comparison of Human ECM Properties Derived from Various Tissue Sources for Regenerative Medicine Applications. J. Funct. Biomater. 2023, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, S.; Wairkar, S. Collagen fabricated delivery systems for wound healing: A new roadmap. Biomater. Adv. 2022, 142, 213152. [Google Scholar] [CrossRef]
- Lwin, S.M.; McGrath, J.A. Restoring type VII collagen in skin. Med 2022, 3, 273–275. [Google Scholar] [CrossRef]
- Abas, M.; El Masry, M.; Elgharably, H. Collagen in diabetic wound healing. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Bagchi, D., Das, A., Roy, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 393–401. [Google Scholar]
- Bagci, I.S.; Gurevich, I.; Dolorito, J.A.; Tripathi, P.; Momin, N.S.; Sun, A.; La, T.; Sridhar, K.; Marinkovich, M.P. Collagen VII’s Dual Mesenchymal and Epithelial Origins: Implications for Molecular Corrective Therapies. J. Investig. Dermatol. 2025, 145, 230–232. [Google Scholar] [CrossRef]
- Woodley, D.; Hou, Y.; Tang, X.; Tan, C.; Zhang, K.; Bainvoll, L.; Li, W.; Chen, M. Topical type VII collagen increased elastic fiber formation, accelerated wound closure and reduced scarring of diabetic pigskin wounds. J. Investig. Dermatol. 2023, 143, S260. [Google Scholar] [CrossRef]
- He, Y.; Yin, S.; Xu, R.; Zhao, Y.; Du, Y.; Duan, Z.; Fan, D. Recombinant Type XVII Collagen Inhibits EGFR/MAPK/AP-1 and Activates TGF-β/Smad Signaling to Enhance Collagen Secretion and Reduce Photoaging. Cosmetics 2025, 12, 59. [Google Scholar] [CrossRef]
- Löffek, S.; Hurskainen, T.; Jackow, J.; Sigloch, F.C.; Schilling, O.; Tasanen, K.; Bruckner-Tuderman, L.; Franzke, C.-W. Transmembrane collagen XVII modulates integrin dependent keratinocyte migration via PI3K/Rac1 signaling. PLoS ONE 2014, 9, e87263. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Popovic, Z.; Chu, C.; Krämer, B.K.; Hocher, B. Endostatin in Renal and Cardiovascular Diseases. Kidney Dis. 2021, 7, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Khoshneviszadeh, M.; Henneicke, S.; Pirici, D.; Senthilnathan, A.; Morton, L.; Arndt, P.; Kaushik, R.; Norman, O.; Jukkola, J.; Dunay, I.R.; et al. Microvascular damage, neuroinflammation and extracellular matrix remodeling in Col18a1 knockout mice as a model for early cerebral small vessel disease. Matrix Biol. 2024, 128, 39–64. [Google Scholar] [CrossRef]
- Qu, Y.; Cao, C.; Wu, Q.; Huang, A.; Song, Y.; Li, H.; Zuo, Y.; Chu, C.; Li, J.; Man, Y. The dual delivery of KGF and b FGF by collagen membrane to promote skin wound healing. J. Tissue Eng. Regen. Med. 2018, 12, 1508–1518. [Google Scholar] [CrossRef]
- Fu, C.; Fan, Y.; Liu, G.; Li, W.; Ma, J.; Xiao, J. One-step fabrication of an injectable antibacterial collagen hydrogel with in situ synthesized silver nanoparticles for accelerated diabetic wound healing. Chem. Eng. J. 2024, 480, 148288. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, J.C.; de Torre, I.G.; Ibañez-Fonseca, A.; Alonso, M. Bioactive scaffolds based on elastin-like materials for wound healing. Adv. Drug Deliv. Rev. 2018, 129, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Sarangthem, V.; Singh, T.D.; Dinda, A.K. Emerging role of elastin-like polypeptides in regenerative medicine. Adv. Wound Care 2021, 10, 257–269. [Google Scholar] [CrossRef]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin biomaterials in dermal repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef]
- Tian, D.-M.; Wan, H.-H.; Chen, J.-R.; Ye, Y.-B.; He, Y.; Liu, Y.; Tang, L.-Y.; He, Z.-Y.; Liu, K.-Z.; Gao, C.-J. In-situ formed elastin-based hydrogels enhance wound healing via promoting innate immune cells recruitment and angiogenesis. Mater. Today Bio 2022, 15, 100300. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Gomes, A.C.; Cavaco-Paulo, A. Novel silk fibroin/elastin wound dressings. Acta Biomater. 2012, 8, 3049–3060. [Google Scholar] [CrossRef]
- Staubli, S.M.; Cerino, G.; De Torre, I.G.; Alonso, M.; Oertli, D.; Eckstein, F.; Glatz, K.; Cabello, J.C.R.; Marsano, A. Control of angiogenesis and host response by modulating the cell adhesion properties of an Elastin-Like Recombinamer-based hydrogel. Biomaterials 2017, 135, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Gao, W.; Shi, M.; Tang, F.; Fu, X.; Chen, X. Coaxial nanofibrous scaffolds mimicking the extracellular matrix transition in the wound healing process promoting skin regeneration through enhancing immunomodulation. J. Mater. Chem. B 2021, 9, 1395–1405. [Google Scholar] [CrossRef]
- Bao, L.; Li, J.; Solimani, F.; Didona, D.; Patel, P.M.; Li, X.; Qian, H.; Ishii, N.; Hashimoto, T.; Hertl, M. Subunit-specific reactivity of autoantibodies against laminin-332 reveals direct inflammatory mechanisms on keratinocytes. Front. Immunol. 2021, 12, 775412. [Google Scholar] [CrossRef]
- Hamill, K.J.; Paller, A.S.; Jones, J.C. Adhesion and migration, the diverse functions of the laminin α3 subunit. Dermatol. Clin. 2010, 28, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Tsuruta, D.; Ishii, M.; Jones, J.C.; Kobayashi, H. Laminin-332 and-511 in skin. Exp. Dermatol. 2008, 17, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Wegner, J.; Loser, K.; Apsite, G.; Nischt, R.; Eckes, B.; Krieg, T.; Werner, S.; Sorokin, L. Laminin α5 in the keratinocyte basement membrane is required for epidermal–dermal intercommunication. Matrix Biol. 2016, 56, 24–41. [Google Scholar] [CrossRef]
- Sampaolo, S.; Napolitano, F.; Tirozzi, A.; Reccia, M.G.; Lombardi, L.; Farina, O.; Barra, A.; Cirillo, F.; Melone, M.A.B.; Gianfrancesco, F. Identification of the first dominant mutation of LAMA5 gene causing a complex multisystem syndrome due to dysfunction of the extracellular matrix. J. Med. Genet. 2017, 54, 710–720. [Google Scholar] [CrossRef]
- Mukherjee, C.; Saleem, S.; Das, S.; Biswas, S.C.; Bhattacharyya, D. Human placental laminin: Role in neuronal differentiation, cell adhesion and proliferation. J. Biosci. 2020, 45, 93. [Google Scholar] [CrossRef]
- Caissie, R.; Gingras, M.; Champigny, M.-F.; Berthod, F. In vivo enhancement of sensory perception recovery in a tissue-engineered skin enriched with laminin. Biomaterials 2006, 27, 2988–2993. [Google Scholar] [CrossRef]
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 2018, 9, 2163. [Google Scholar] [CrossRef]
- Gimeno-LLuch, I.; Benito-Jardón, M.; Guerrero-Barberà, G.; Burday, N.; Costell, M. The role of the fibronectin synergy site for skin wound healing. Cells 2022, 11, 2100. [Google Scholar] [CrossRef] [PubMed]
- Patten, J.; Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 2021, 170, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mao, X.; Cong, J.; Zhang, Q.; Chen, W.; Yan, K.; Huang, Y.; Su, D.; Xiang, Q. Recombinantly expressed rhFEB remodeled the skin defect of db/db mice. Appl. Microbiol. Biotechnol. 2024, 108, 183. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; Qian, J. Effects of domain unfolding and catch-like dissociation on the collective behavior of integrin–fibronectin bond clusters. Acta Mech. Sin. 2021, 37, 229–243. [Google Scholar] [CrossRef]
- Ahn, S.; Sharma, U.; Kasuba, K.C.; Strohmeyer, N.; Müller, D.J. Engineered Biomimetic Fibrillar Fibronectin Matrices Regulate Cell Adhesion Initiation, Migration, and Proliferation via α5β1 Integrin and Syndecan-4 Crosstalk. Adv. Sci. 2023, 10, 2300812. [Google Scholar] [CrossRef]
- Vogel, V. Unraveling the mechanobiology of extracellular matrix. Annu. Rev. Physiol. 2018, 80, 353–387. [Google Scholar] [CrossRef] [PubMed]
- Kraft, S.; Klemis, V.; Sens, C.; Lenhard, T.; Jacobi, C.; Samstag, Y.; Wabnitz, G.; Kirschfink, M.; Wallich, R.; Hänsch, G.M. Identification and characterization of a unique role for EDB fibronectin in phagocytosis. J. Mol. Med. 2016, 94, 567–581. [Google Scholar] [CrossRef]
- Anderegg, U.; Halfter, N.; Schnabelrauch, M.; Hintze, V. Collagen/glycosaminoglycan-based matrices for controlling skin cell responses. Biol. Chem. 2021, 402, 1325–1335. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell–extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 2023, 24, 495–516. [Google Scholar] [CrossRef]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical application of glycosaminoglycans: A review. J. Tissue Eng. Regen. Med. 2018, 12, e23–e41. [Google Scholar] [CrossRef]
- Yang, P.; Lu, Y.; Gou, W.; Qin, Y.; Tan, J.; Luo, G.; Zhang, Q. Glycosaminoglycans’ ability to promote wound healing: From native living macromolecules to artificial Biomaterials. Adv. Sci. 2024, 11, 2305918. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.; Silvestre, A.J.; Vilela, C.; Freire, C.S. A guide to polysaccharide-based hydrogel bioinks for 3D bioprinting applications. Int. J. Mol. Sci. 2022, 23, 6564. [Google Scholar] [CrossRef]
- Hao, Y.; Li, H.; Guo, J.; Wang, D.; Zhang, J.; Liu, J.; Yang, C.; Zhang, Y.; Li, G.; Liu, J. Bio-Inspired Antioxidant Heparin-Mimetic Peptide Hydrogel for Radiation-Induced Skin Injury Repair. Adv. Healthc. Mater. 2023, 12, 2203387. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Quan, G.; Hou, A.; Yang, P.; Peng, T.; Gu, Y.; Qin, W.; Liu, R.; Ma, X.; Pan, X. Strategy for hypertrophic scar therapy: Improved delivery of triamcinolone acetonide using mechanically robust tip-concentrated dissolving microneedle array. J. Control. Release 2019, 306, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhu, Y.; Qin, X.; Chai, S.; Liu, G.; Su, W.; Lv, Q.; Li, D. Magnetic biohybrid microspheres for protein purification and chronic wound healing in diabetic mice. Chem. Eng. J. 2021, 425, 130671. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, L.; Luo, Y.; Cai, Z.; Zeng, H.; Wang, T.; Liu, Z.; Chen, Y.; Sheng, X.; Mandlate, A.E.d.G. Charge-Driven Self-Assembled Microspheres Hydrogel Scaffolds for Combined Drug Delivery and Photothermal Therapy of Diabetic Wounds. Adv. Funct. Mater. 2023, 33, 2214036. [Google Scholar] [CrossRef]
- Lee, W.H.; Rho, J.G.; Yang, Y.; Lee, S.; Kweon, S.; Kim, H.-M.; Yoon, J.; Choi, H.; Lee, E.; Kim, S.H. Hyaluronic acid nanoparticles as a topical agent for treating psoriasis. ACS Nano 2022, 16, 20057–20074. [Google Scholar] [CrossRef]
- Leung, C.M.; Dhand, C.; Mayandi, V.; Ramalingam, R.; Lim, F.P.; Barathi, V.A.; Dwivedi, N.; Orive, G.; Beuerman, R.W.; Ramakrishna, S. Wound healing properties of magnesium mineralized antimicrobial nanofibre dressings containing chondroitin sulphate–a comparison between blend and core–shell nanofibres. Biomater. Sci. 2020, 8, 3454–3471. [Google Scholar] [CrossRef]
- Murphy, A.R.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomater. 2017, 54, 1–20. [Google Scholar] [CrossRef]
- Kim, H.N.; Elgundi, Z.; Lin, X.; Fu, L.; Tang, F.; Moh, E.S.; Jung, M.; Chandrasekar, K.; Bartlett-Tomasetig, F.; Foster, C. Engineered short forms of perlecan enhance angiogenesis by potentiating growth factor signalling. J. Control. Release 2023, 362, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Takaya, K.; Aramaki-Hattori, N.; Sakai, S.; Okabe, K.; Asou, T.; Kishi, K. Fibroblast growth factor 7 suppresses fibrosis and promotes epithelialization during wound healing in mouse fetuses. Int. J. Mol. Sci. 2022, 23, 7087. [Google Scholar] [CrossRef] [PubMed]
- Won, K.-J.; Lee, R.; Choi, S.-H.; Kim, J.-H.; Hwang, S.-H.; Nah, S.-Y. Gintonin-Induced Wound-Healing-Related Responses Involve Epidermal-Growth-Factor-like Effects in Keratinocytes. Int. J. Mol. Sci. 2023, 24, 14094. [Google Scholar] [CrossRef] [PubMed]
- Xiaojie, W.; Banda, J.; Qi, H.; Chang, A.K.; Bwalya, C.; Chao, L.; Li, X. Scarless wound healing: Current insights from the perspectives of TGF-β, KGF-1, and KGF-2. Cytokine Growth Factor Rev. 2022, 68, 116. [Google Scholar] [CrossRef]
- Bang, F.S.; Leeberg, V.; Ding, M.; Dreyer, C.H. The effect of VEGF stimulation in diabetic foot ulcers: A systematic review. Wound Repair Regen. 2024, 32, 384–392. [Google Scholar] [CrossRef]
- Gowda, S.; Weinstein, D.A.; Blalock, T.D.; Gandhi, K.; Mast, B.A.; Chin, G.; Schultz, G.S. Topical application of recombinant platelet-derived growth factor increases the rate of healing and the level of proteins that regulate this response. Int. Wound J. 2015, 12, 564–571. [Google Scholar] [CrossRef]
- Frazão, L.P.; Vieira de Castro, J.; Nogueira-Silva, C.; Neves, N.M. Decellularized human chorion membrane as a novel biomaterial for tissue regeneration. Biomolecules 2020, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Fu, S.; Luan, J. Regenerated fat induced by a decellularized adipose matrix can survive long-term in vivo. Acta Biomater. 2025, 191, 233–243. [Google Scholar] [CrossRef]
- Wang, C.; Li, G.; Cui, K.; Chai, Z.; Huang, Z.; Liu, Y.; Chen, S.; Huang, H.; Zhang, K.; Han, Z. Sulfated glycosaminoglycans in decellularized placenta matrix as critical regulators for cutaneous wound healing. Acta Biomater. 2021, 122, 199–210. [Google Scholar] [CrossRef]
- Walters, J.; Cazzell, S.; Pham, H.; Vayser, D.; Reyzelman, A. Healing rates in a multicenter assessment of a sterile, room temperature, acellular dermal matrix versus conventional care wound management and an active comparator in the treatment of full-thickness diabetic foot ulcers. Eplasty 2016, 16, e10. [Google Scholar]
- Williams, M.; Holewinski, J. Use of a human acellular dermal wound matrix in patients with complex wounds and comorbidities. J. Wound Care 2015, 24, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, S.; Ferrari, M.; Sotgiu, M.A.; Piu, G.; Solinas, M.G.; Usai, N.; Bulla, A.; Serra, P.L.; Grieco, F.; Montella, A. Objective Non-Invasive Bio-Parametric Evaluation of Regenerated Skin: A Comparison of Two Acellular Dermal Substitutes. Life 2024, 14, 121. [Google Scholar] [CrossRef]
- Corrêa, F.B.; Castro, J.C.; Almeida, I.R.; Farina-Junior, J.A.; Coltro, P.S. Evaluation of contraction of the split-thickness skin graft using three dermal matrices in the treatment of burn contractures: A randomised clinical trial. Wound Repair Regen. 2022, 30, 222–231. [Google Scholar] [CrossRef]
- Hoinoiu, T.; Grujic, D.; Prilipceanu, G.; Folescu, R.; Hoinoiu, B.; Bratu, T.; Poroch, V.; Grujic, L. The Use of Collagen-Glycosaminoglycan Biodegradable Matrix (Integra®) in the Management of Neck Postburn Hypertrophic Scars and Contractures. Appl. Sci. 2020, 10, 3731. [Google Scholar] [CrossRef]
- Yoo, B.W.; Kong, Y.T.; Chae, S.W.; Kim, K.N.; Song, B.; Kim, J. Comparison of the Characteristics of Three Acellular Dermal Matrices Subjected to Distinct Processing Methods Using Five Types of Histochemical Staining. Aesthetic Plast. Surg. 2023, 47, 1315–1323. [Google Scholar] [CrossRef]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Santema, T.B.; Poyck, P.P.; Ubbink, D.T. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2016, 2017, CD011255. [Google Scholar] [CrossRef] [PubMed]
- Piejko, M.; Radziun, K.; Bobis-Wozowicz, S.; Waligórska, A.; Zimoląg, E.; Nessler, M.; Chrapusta, A.; Madeja, Z.; Drukała, J. Adipose-derived stromal cells seeded on Integra® dermal regeneration template improve post-burn wound reconstruction. Bioengineering 2020, 7, 67. [Google Scholar] [CrossRef]
- Xue, S.-L.; Liu, K.; Parolini, O.; Wang, Y.; Deng, L.; Huang, Y.-C. Human acellular amniotic membrane implantation for lower third nasal reconstruction: A promising therapy to promote wound healing. Burns Trauma 2018, 6, 34. [Google Scholar] [CrossRef]
- Cazzell, S.; Vayser, D.; Pham, H.; Walters, J.; Reyzelman, A.; Samsell, B.; Dorsch, K.; Moore, M. A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active acellular dermal matrix comparator. Wound Repair Regen. 2017, 25, 483–497. [Google Scholar] [CrossRef]
- Agostinis, C.; Spazzapan, M.; Vuerich, R.; Balduit, A.; Stocco, C.; Mangogna, A.; Ricci, G.; Papa, G.; Zacchigna, S.; Bulla, R. Differential capability of clinically employed dermal regeneration scaffolds to support vascularization for tissue bioengineering. Biomedicines 2021, 9, 1458. [Google Scholar] [CrossRef] [PubMed]
- Negron, L.; Lun, S.; May, B.C. Ovine forestomach matrix biomaterial is a broad spectrum inhibitor of matrix metalloproteinases and neutrophil elastase. Int. Wound J. 2014, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, L.; Pianigiani, E.; Ierardi, F.; Lorenzini, G.; Casella, D.; Liso, F.G.; De Pascalis, A.; Cinotti, E.; Rubegni, P. The use of human acellular dermal matrices in advanced wound healing and surgical procedures: State of the art. Dermatol. Ther. 2021, 34, e14987. [Google Scholar] [CrossRef]
- Luthringer, M.; Mukherjee, T.; Arguello-Angarita, M.; Granick, M.S.; Alvarez, O.M. Human-derived acellular dermal matrix grafts for treatment of diabetic foot ulcers: A systematic review and meta-analysis. Wounds 2020, 32, 57–65. [Google Scholar]
- Dolivo, D.; Xie, P.; Hou, C.; Li, Y.; Phipps, A.; Mustoe, T.; Hong, S.; Galiano, R. Application of decellularized human reticular allograft dermal matrix promotes rapid re-epithelialization in a diabetic murine excisional wound model. Cytotherapy 2021, 23, 672–676. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hwang, K.T.; Kim, K.H.; Sung, I.H.; Kim, S.W. Application of acellular human dermis and skin grafts for lower extremity reconstruction. J. Wound Care 2019, 28, S12–S17. [Google Scholar] [CrossRef]
- Nazempour, M.; Mehrabani, D.; Mehdinavaz-Aghdam, R.; Hashemi, S.S.; Derakhshanfar, A.; Zare, S.; Zardosht, M.; Moayedi, J.; Vahedi, M. The effect of allogenic human Wharton’s jelly stem cells seeded onto acellular dermal matrix in healing of rat burn wounds. J. Cosmet. Dermatol. 2020, 19, 995–1001. [Google Scholar] [CrossRef]
- Saricilar, E.C.; Huang, S. Comparison of porcine and human acellular dermal matrix outcomes in wound healing: A deep dive into the evidence. Arch. Plast. Surg. 2021, 48, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, S.; Wang, W.; Yun, Z.; Lv, L.; Chai, M.; Wu, Z.; Zhu, Y.; Ma, J.; Leng, L. Matrisome provides a supportive microenvironment for skin functions of diverse species. ACS Biomater. Sci. Eng. 2020, 6, 5720–5733. [Google Scholar] [CrossRef]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging innovative wound dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef]
- Chen, X.; Yang, R.; Wang, J.; Ruan, S.; Lin, Z.; Xin, Q.; Yang, R.; Xie, J. Porcine acellular dermal matrix accelerates wound healing through miR-124-3p. 1 and miR-139-5p. Cytotherapy 2020, 22, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Gierek, M.; Łabuś, W.; Kitala, D.; Lorek, A.; Ochała-Gierek, G.; Zagórska, K.M.; Waniczek, D.; Szyluk, K.; Niemiec, P. Human Acellular Dermal Matrix in Reconstructive Surgery—A Review. Biomedicines 2022, 10, 2870. [Google Scholar] [CrossRef]
- Campbell, K.T.; Burns, N.K.; Rios, C.N.; Mathur, A.B.; Butler, C.E. Human versus non-cross-linked porcine acellular dermal matrix used for ventral hernia repair: Comparison of in vivo fibrovascular remodeling and mechanical repair strength. Plast. Reconstr. Surg. 2011, 127, 2321–2332. [Google Scholar] [CrossRef]
- Pires, G.R.; Moss, W.D.; Hosein, R.C.; Overschmidt, B.T.; Magno-Padron, D.A.; Agarwal, J.P.; McFarland, M.M.; Casucci, T.; Kwok, A.C. Comparison of Human, Porcine, and Bovine Acellular Dermal Matrix in Prepectoral Breast Reconstruction: A Scoping Review. Ann. Plast. Surg. 2022, 89, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Shi, P.; Deng, X.; Jin, Y.; Liu, H.; Chen, M.; Han, X.; Liu, H. Dynamic multiphoton imaging of acellular dermal matrix scaffolds seeded with mesenchymal stem cells in diabetic wound healing. J. Biophotonics 2018, 11, e201700336. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, Y.; Chu, J.; Wang, X.; Yan, W.; Zhang, Q.; Liu, H. Reduced graphene oxide incorporated acellular dermal composite scaffold enables efficient local delivery of mesenchymal stem cells for accelerating diabetic wound healing. ACS Biomater. Sci. Eng. 2019, 5, 4054–4066. [Google Scholar] [CrossRef]
- Xiang, K.; Chen, J.; Guo, J.; Li, G.; Kang, Y.; Wang, C.; Jiang, T.; Zhang, M.; Jiang, G.; Yuan, M. Multifunctional ADM hydrogel containing endothelial cell-exosomes for diabetic wound healing. Mater. Today Bio 2023, 23, 100863. [Google Scholar] [CrossRef]
- Ayaz, M.; Najafi, A.; Karami, M. Thin split thickness skin grafting on human acellular dermal matrix scaffold for the treatment of deep burn wounds. Int. J. Organ Transplant. Med. 2021, 12, 44–51. [Google Scholar]
- Aghayan, H.R.; Hosseini, M.S.; Gholami, M.; Mohamadi-Jahani, F.; Tayanloo-Beik, A.; Alavi-Moghadam, S.; Payab, M.; Goodarzi, P.; Abdollahi, M.; Larijani, B. Mesenchymal stem cells’ seeded amniotic membrane as a tissue-engineered dressing for wound healing. Drug Deliv. Transl. Res. 2022, 12, 538–549. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Farahani, M.; Tayebi, T.; Jafari, A.; Biniazan, F.; Modaresifar, K.; Moravvej, H.; Bahrami, S.; Redl, H.; Tayebi, L. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 431–440. [Google Scholar] [CrossRef]
- Schmiedova, I.; Dembickaja, A.; Kiselakova, L.; Nowakova, B.; Slama, P. Using of amniotic membrane derivatives for the treatment of chronic wounds. Membranes 2021, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Wilshaw, S.-P.; Kearney, J.; Fisher, J.; Ingham, E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng. Part A 2008, 14, 463–472. [Google Scholar] [CrossRef]
- Kshersagar, J.; Kshirsagar, R.; Desai, S.; Bohara, R.; Joshi, M. Decellularized amnion scaffold with activated PRP: A new paradigm dressing material for burn wound healing. Cell Tissue Bank. 2018, 19, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xiao, C.; Miao, Y.; Wang, J.; Chen, R.; Fan, Z.; Hu, Z. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res. Ther. 2021, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chang, C.; Qian, C.; Xiao, W.; Zhu, H.; Guo, J.; Meng, Z.; Cui, W.; Ge, Z. Photo-crosslinkable amniotic membrane hydrogel for skin defect healing. Acta Biomater. 2021, 125, 197–207. [Google Scholar] [CrossRef]
- Dadkhah Tehrani, F.; Firouzeh, A.; Shabani, I.; Shabani, A. A review on modifications of amniotic membrane for biomedical applications. Front. Bioeng. Biotechnol. 2021, 8, 606982. [Google Scholar] [CrossRef]
- Su, Y.N.; Zhao, D.Y.; Li, Y.H.; Yu, T.Q.; Sun, H.; Wu, X.Y.; Zhou, X.Q.; Li, J. Human amniotic membrane allograft, a novel treatment for chronic diabetic foot ulcers: A systematic review and meta-analysis of randomised controlled trials. Int. Wound J. 2020, 17, 753–764. [Google Scholar] [CrossRef]
- Álvaro-Afonso, F.J.; García-Álvarez, Y.; Lázaro-Martínez, J.L.; Kakagia, D.; Papanas, N. Advances in Dermoepidermal Skin Substitutes for Diabetic Foot Ulcers. Curr. Vasc. Pharmacol. 2020, 18, 182–192. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Wang, Y.; Ma, S.-Q.; Huang, Y.-Q.; Jing, W.; Wei, P.-F.; Yu, X.-Q.; Zhao, B. Mechanically active small intestinal submucosa hydrogel for accelerating chronic wound healing. J. Mater. Chem. B 2022, 10, 6279–6286. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, J.; Sun, T.; Tang, K.; Xiong, Z.; Ren, Z.; Yao, S.; Chen, K.; Yang, F.; Zhu, F. Diverse preparation methods for small intestinal submucosa (SIS): Decellularization, components, and structure. J. Biomed. Mater. Res. A 2019, 107, 689–697. [Google Scholar] [CrossRef]
- Parmaksiz, M.; Elcin, A.E.; Elcin, Y.M. Decellularization of bovine small intestinal submucosa and its use for the healing of a critical-sized full-thickness skin defect, alone and in combination with stem cells, in a small rodent model. J. Tissue Eng. Regen. Med. 2017, 11, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, X.; Chao, N.-N.; Qin, T.-W.; Ding, W.; Zhang, Y.; Sang, J.-W.; Luo, J.-C. Preparation and characterization of pro-angiogenic gel derived from small intestinal submucosa. Acta Biomater. 2016, 29, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, J.Y.; Kim, H.; Shin, J.; Shin, Y.; Yoo, Y.T.; Kim, H.-Y. Fabrication of electropsun PLGA and small intestine submucosa-blended nanofibrous membranes and their biocompatibility for wound healing. Fibers Polym. 2017, 18, 231–239. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Tan, J.; Nie, R.; Song, Y.-T.; Zhou, X.-L.; Feng, Z.-Y.; Huang, K.; Zou, C.-Y.; Yuan, Q.-J.; Zhao, L.-M. Acceleration of wound healing by composite small intestinal submucosa hydrogels through immunomodulation. Compos. Part B Eng. 2023, 254, 110550. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Y.; Ullah, M.W.; Ou, H.; Zhang, W.; Xiong, L.; Zhang, X. Cryogenic free-form extrusion bioprinting of decellularized small intestinal submucosa for potential applications in skin tissue engineering. Biofabrication 2019, 11, 035023. [Google Scholar] [CrossRef]
- Hodde, J.P.; Hiles, M.C.; Metzger, D.W. Characterization of the local wound environment following treatment of chronic leg ulcers with SIS wound matrix. J. Tissue Viability 2020, 29, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Tanaka, R. Porcine small intestinal submucosa alters the biochemical properties of wound healing: A narrative review. Biomedicines 2022, 10, 2213. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, Y.; Zhong, Y.; Zhang, L.; Zhang, X.; Guo, Z.; Wang, F.; Chen, X.; Tong, H.; Fan, J. Combining decellularized adipose tissue with decellularized adventitia extravascular matrix or small intestinal submucosa matrix for the construction of vascularized tissue-engineered adipose. Acta Biomater. 2023, 170, 567–579. [Google Scholar] [CrossRef]
- Pal, S.; Chaudhari, R.; Baurceanu, I.; Hill, B.J.; Nagy, B.A.; Wolf, M.T. Extracellular Matrix Scaffold-Assisted Tumor Vaccines Induce Tumor Regression and Long-Term Immune Memory. Adv. Mater. 2024, 36, 2309843. [Google Scholar] [CrossRef]
- Mohiuddin, O.A.; Campbell, B.; Poche, J.N.; Thomas-Porch, C.; Hayes, D.A.; Bunnell, B.A.; Gimble, J.M. Decellularized adipose tissue: Biochemical composition, in vivo analysis and potential clinical applications. Cell Biol. Transl. Med. 2020, 1212, 57–70. [Google Scholar]
- Liu, K.; He, Y.; Lu, F. Research progress on the immunogenicity and regeneration of acellular adipose matrix: A mini review. Front. Bioeng. Biotechnol. 2022, 10, 881523. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Naranjo, J.D.; Londono, R.; Badylak, S.F. Biologic scaffolds. Cold Spring Harb. Perspect. Med. 2017, 7, a025676. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Han, Y. Tissue-Engineered Soft-Tissue Reconstruction Using Noninvasive Mechanical Preconditioning and a Shelf-Ready Allograft Adipose Matrix. Plast. Reconstr. Surg. 2020, 146, 98e. [Google Scholar] [CrossRef]
- Kokai, L.E.; Schilling, B.K.; Chnari, E.; Huang, Y.-C.; Imming, E.A.; Karunamurthy, A.; Khouri, R.K.; D’Amico, R.A.; Coleman, S.R.; Marra, K.G. Injectable allograft adipose matrix supports adipogenic tissue remodeling in the nude mouse and human. Plast. Reconstr. Surg. 2019, 143, 299e–309e. [Google Scholar] [CrossRef]
- Guo, X.; Zeng, A.; Zhu, L. Research progresson decellularized adipose tissue extracellular matrixscaffolds. Chin. J. Plast. Surg. 2019, 6, 89–92. [Google Scholar]
- Yang, J.-Z.; Qiu, L.-H.; Xiong, S.-H.; Dang, J.-L.; Rong, X.-K.; Hou, M.-M.; Wang, K.; Yu, Z.; Yi, C.-G. Decellularized adipose matrix provides an inductive microenvironment for stem cells in tissue regeneration. World J. Stem Cells 2020, 12, 585–603. [Google Scholar] [CrossRef]
- Dong, J.; Yu, M.; Zhang, Y.; Yin, Y.; Tian, W. Recent developments and clinical potential on decellularized adipose tissue. J. Biomed. Mater. Res. A 2018, 106, 2563–2574. [Google Scholar] [CrossRef]
- Yang, X.; Jin, L.; Xu, M.; Liu, X.; Tan, Z.; Liu, L. Adipose tissue reconstruction facilitated with low immunogenicity decellularized adipose tissue scaffolds. Biomed. Mater. 2024, 19, 035023. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I. Signalling role of adipose tissue: Adipokines and inflammation in obesity. Biochem. Soc. Trans. 2005, 33, 1078–1081. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.; Cha, M.; Kim, S.H.; Jung, Y. The regeneration of large-sized and vascularized adipose tissue using a tailored elastic scaffold and dECM hydrogels. Int. J. Mol. Sci. 2021, 22, 12560. [Google Scholar] [CrossRef]
- Yu, C.; Bianco, J.; Brown, C.; Fuetterer, L.; Watkins, J.F.; Samani, A.; Flynn, L.E. Porous decellularized adipose tissue foams for soft tissue regeneration. Biomaterials 2013, 34, 3290–3302. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.-W.; Tang, S.-L.; Zhang, Y.; Yang, J.-Q.; Wang, Z.-L.; Xie, H.-Q.; Lv, Q. Hydrogel from acellular porcine adipose tissue accelerates wound healing by inducing intradermal adipocyte regeneration. J. Investig. Dermatol. 2019, 139, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Kornmuller, A.; Brown, C.; Hoare, T.; Flynn, L.E. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials 2017, 120, 66–80. [Google Scholar] [CrossRef]
- Li, S.; Poche, J.N.; Liu, Y.; Scherr, T.; McCann, J.; Forghani, A.; Smoak, M.; Muir, M.; Berntsen, L.; Chen, C. Hybrid Synthetic-Biological hydrogel system for adipose tissue regeneration. Macromol. Biosci. 2018, 18, 1800122. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, J.; Bai, S. Biocompatibility of injectable hydrogel from decellularized human adipose tissue in vitro and in vivo. J. Biomed. Mater. Res. B 2019, 107, 1684–1694. [Google Scholar] [CrossRef]
- Giatsidis, G.; Succar, J.; Haddad, A.; Lago, G.; Schaffer, C.; Wang, X.; Schilling, B.; Chnari, E.; Matsumine, H.; Orgill, D.P. Preclinical optimization of a shelf-ready, injectable, human-derived, decellularized allograft adipose matrix. Tissue Eng. Part A 2019, 25, 271–287. [Google Scholar] [CrossRef]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Ha, D.-H.; Jang, J.; Han, H.H.; Rhie, J.-W.; Cho, D.-W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2015, 62, 164–175. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, J.S.; Kim, J.S.; Choi, Y.C.; Cho, Y.W. Injectable and thermosensitive soluble extracellular matrix and methylcellulose hydrogels for stem cell delivery in skin wounds. Biomacromolecules 2016, 17, 4–11. [Google Scholar] [CrossRef]
- Xia, Z.; Guo, X.; Yu, N.; Zeng, A.; Si, L.; Long, F.; Zhang, W.; Wang, X.; Zhu, L.; Liu, Z. The application of decellularized adipose tissue promotes wound healing. Tissue Eng. Regen. Med. 2020, 17, 863–874. [Google Scholar] [CrossRef]
- Woo, C.H.; Choi, Y.C.; Choi, J.S.; Lee, H.Y.; Cho, Y.W. A bilayer composite composed of TiO2-incorporated electrospun chitosan membrane and human extracellular matrix sheet as a wound dressing. J. Biomater. Sci. Polym. Ed. 2015, 26, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Locurcio, L.L.; Breen, M.; Haq, J. Can a Single-Stage Approach Using a Dermal Regeneration Template Lead to Satisfactory Scalp Defect Reconstruction After Skin Cancer Excision? J. Oral Maxillofac. Surg. 2024, 82, 341–346. [Google Scholar] [CrossRef]
- Luze, H.; Nischwitz, S.P.; Smolle, C.; Zrim, R.; Kamolz, L.-P. The use of acellular fish skin grafts in burn wound management—A systematic review. Medicina 2022, 58, 912. [Google Scholar] [CrossRef]
- Yun, T.; Shin, S.; Bang, K.; Lee, M.; Cho, J.-A.; Baek, M. Skin wound healing rate in fish depends on species and microbiota. Int. J. Mol. Sci. 2021, 22, 7804. [Google Scholar] [CrossRef] [PubMed]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Cherry, I.; Tarhini, L.; Doan, M.; De Buys Roessingh, A. Exploring the Place of Fish Skin Grafts with Omega-3 in Pediatric Wound Management. J. Clin. Med. 2023, 13, 112. [Google Scholar] [CrossRef]
- Zhao, C.; Feng, M.; Gluchman, M.; Ma, X.; Li, J.; Wang, H. Acellular fish skin grafts in the treatment of diabetic wounds: Advantages and clinical translation. J. Diabetes 2024, 16, e13554. [Google Scholar] [CrossRef]
- Lv, K.; Wang, L.; He, X.; Li, W.; Han, L.; Qin, S. Application of tilapia skin acellular dermal matrix to induce acute skin wound repair in rats. Front. Bioeng. Biotechnol. 2022, 9, 792344. [Google Scholar] [CrossRef]
- Li, D.; Sun, W.Q.; Wang, T.; Gao, Y.; Wu, J.; Xie, Z.; Zhao, J.; He, C.; Zhu, M.; Zhang, S. Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112202. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, J.H.; Jeong, H.G.; Wee, S.Y. The utility of novel fish-skin derived acellular dermal matrix (Kerecis) as a wound dressing material. J. Wound Manag. Res. 2021, 17, 39–47. [Google Scholar] [CrossRef]
- Badois, N.; Bauër, P.; Cheron, M.; Hoffmann, C.; Nicodeme, M.; Choussy, O.; Lesnik, M.; Poitrine, F.C.; Fromantin, I. Acellular fish skin matrix on thin-skin graft donor sites: A preliminary study. J. Wound Care 2019, 28, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Heitzmann, W.; Enzmann, J.; von Kohout, M.; Mattern, M.M.; Akkan, J.; Fuchs, P.C.; Schiefer, J.L. Accelerated wound healing of enzymatically debrided deep dermal burn wounds after the use of fish skin (Kerecis Omega3 Wound®) in comparison to Suprathel®. Burns 2025, 51, 107471. [Google Scholar] [CrossRef] [PubMed]
- Bual, R.; Labares, M., Jr.; Valle, K.D.D.; Pague, J., Jr.; Bantilan, Z.C.; Ducao, P.G.; Alimasag, J.; Acibar, C. Characterization of decellularized extracellular matrix from milkfish (Chanos chanos) skin. Biomimetics 2022, 7, 213. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, H.; Zhang, H.; Zhang, Z.; Chen, G.; Zhao, Y. Bio-printed hydrogel textiles based on fish skin decellularized extracellular matrix for wound healing. Engineering 2023, 25, 120–127. [Google Scholar] [CrossRef]
- Esmaeili, A.; Biazar, E.; Ebrahimi, M.; Heidari Keshel, S.; Kheilnezhad, B.; Saeedi Landi, F. Acellular fish skin for wound healing. Int. Wound J. 2023, 20, 2924–2941. [Google Scholar] [CrossRef]
- Alizadeh, M.; Rezakhani, L.; Alizadeh, A. Characterization of the decellularized ovine pericardium for skin tissue engineering. J. Shahrekord Univ. Med. Sci. 2020, 22, 173–180. [Google Scholar] [CrossRef]
- Cui, H.; Chai, Y.; Yu, Y. Progress in developing decellularized bioscaffolds for enhancing skin construction. J. Biomed. Mater. Res. A 2019, 107, 1849–1859. [Google Scholar] [CrossRef]
- Wu, X.; He, W.; Mu, X.; Liu, Y.; Deng, J.; Liu, Y.; Nie, X. Macrophage polarization in diabetic wound healing. Burns Trauma 2022, 10, tkac051. [Google Scholar] [CrossRef]
- Dziki, J.L.; Wang, D.S.; Pineda, C.; Sicari, B.M.; Rausch, T.; Badylak, S.F. Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J. Biomed. Mater. Res. A 2017, 105, 138–147. [Google Scholar] [CrossRef]
- Sadtler, K.; Sommerfeld, S.D.; Wolf, M.T.; Wang, X.; Majumdar, S.; Chung, L.; Kelkar, D.S.; Pandey, A.; Elisseeff, J.H. Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostasis and injury. Semin. Immunol. 2017, 29, 14–23. [Google Scholar] [CrossRef]
- Zhang, Q.; Johnson, J.A.; Dunne, L.W.; Chen, Y.; Iyyanki, T.; Wu, Y.; Chang, E.I.; Branch-Brooks, C.D.; Robb, G.L.; Butler, C.E. Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater. 2016, 35, 166–184. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Paltoo, A.; Kelly, R.W.; Sallenave, J.M.; Bocking, A.D.; Challis, J.R.G. Expression of Natural Antimicrobials by Human Placenta and Fetal Membranes. Placenta 2007, 28, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.-R.; Gong, M.; Da, L.-C.; Bai, L.; Li, X.-Q.; Chen, K.-F.; Li-Ling, J.; Yang, Z.-M.; Xie, H.-Q. Tissue engineered esophagus scaffold constructed with porcine small intestinal submucosa and synthetic polymers. Biomed. Mater. 2014, 9, 015012. [Google Scholar] [CrossRef]
- Sarikaya, A.; Record, R.; Wu, C.-C.; Tullius, B.; Badylak, S.; Ladisch, M. Antimicrobial Activity Associated with Extracellular Matrices. Tissue Eng. 2002, 8, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ebersole, J. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral. Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef]
- Paige, J.T.; Kremer, M.; Landry, J.; Hatfield, S.A.; Wathieu, D.; Brug, A.; Lightell, D.J.; Spiller, K.L.; Woods, T.C. Modulation of inflammation in wounds of diabetic patients treated with porcine urinary bladder matrix. Regen. Med. 2019, 14, 269–277. [Google Scholar] [CrossRef]
- Dai, C.; Shih, S.; Khachemoune, A. Skin substitutes for acute and chronic wound healing: An updated review. J. Dermatol. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef]
- Férnandez-Guarino, M.; Naharro-Rodriguez, J.; Bacci, S. Disturbances in the Skin Homeostasis: Wound Healing, an Undefined Process. Cosmetics 2024, 11, 90. [Google Scholar] [CrossRef]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional hydrogels for treatment of chronic wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605. [Google Scholar] [CrossRef]
- Turner, N.J.; Badylak, S.F. The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv. Wound Care 2015, 4, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, Y.; Shu, B.; Xie, X.; Yang, R.; Zhang, L.; Ruan, S.; Lin, Y.; Lin, Z.; Shen, R. The effect of porcine ADM to improve the burn wound healing. Int. J. Clin. Exp. Pathol. 2013, 6, 2280–2291. [Google Scholar]
- Chen, Y.; Hu, M.; Hu, H.; Ji, S.; Huang, L.; Wei, W.; Zhao, K.; Teng, C. Fabrication of an Adhesive Small Intestinal Submucosa Acellular Matrix Hydrogel for Accelerating Diabetic Wound Healing. ACS Omega 2023, 8, 46653–46662. [Google Scholar] [CrossRef] [PubMed]
- Martinson, M.; Martinson, N. A comparative analysis of skin substitutes used in the management of diabetic foot ulcers. J. Wound Care 2016, 25, S8–S17. [Google Scholar] [CrossRef]
- Chandika, P.; Khan, F.; Heo, S.-Y.; Kim, Y.-M.; Yi, M.; Jung, W.-K. Enhanced wound-healing capability with inherent antimicrobial activities of usnic acid incorporated poly (ε-caprolactone)/decellularized extracellular matrix nanofibrous scaffold. Biomater. Adv. 2022, 140, 213046. [Google Scholar] [CrossRef]
- Rose, L.F.; Chan, R.K. The burn wound microenvironment. Adv. Wound Care 2016, 5, 106–118. [Google Scholar] [CrossRef]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn wound healing: Clinical complications, medical care, treatment, and dressing types: The current state of knowledge for clinical practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Bucan, V.; Vogt, P.M.; Krezdorn, N. A short history of skin grafting in burns: From the gold standard of autologous skin grafting to the possibilities of allogeneic skin grafting with immunomodulatory approaches. Medicina 2021, 57, 225. [Google Scholar] [CrossRef]
- Wang, L.; Hao, C.; Zhou, B.; Fu, X.; Li, J.; Wang, Z.; Rong, Z. Application of Heparinized Selective Acellular Sheepskin in Wound-healing Promotion of Deep Second-degree Burns. Wounds 2016, 28, 438–447. [Google Scholar]
- Takami, Y.; Yamaguchi, R.; Ono, S.; Hyakusoku, H. Clinical application and histological properties of autologous tissue-engineered skin equivalents using an acellular dermal matrix. J. Nippon Med. Sch. 2014, 81, 356–363. [Google Scholar] [CrossRef]
- Van Netten, J.J.; Bus, S.A.; Apelqvist, J.; Lipsky, B.A.; Hinchliffe, R.J.; Game, F.; Rayman, G.; Lazzarini, P.A.; Forsythe, R.O.; Peters, E.J. Definitions and criteria for diabetic foot disease. Diabetes Metab. Res. Rev. 2020, 36, e3268. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Tan, T.-W.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers: A review. Jama 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Guo, X.; Mu, D.; Gao, F. Efficacy and safety of acellular dermal matrix in diabetic foot ulcer treatment: A systematic review and meta-analysis. Int. J. Surg. 2017, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.N.; Karimizade, A.; Enderami, S.E.; Abasi, M.; Talebpour Amiri, F.; Jafarirad, A.; Mellati, A. The effect of source animal age, decellularization protocol, and sterilization method on bovine acellular dermal matrix as a scaffold for wound healing and skin regeneration. Artif. Organs 2023, 47, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Magden, G.K.; Vural, C.; Bayrak, B.Y.; Ozdogan, C.Y.; Kenar, H. Composite sponges from sheep decellularized small intestinal submucosa for treatment of diabetic wounds. J. Biomater. Appl. 2021, 36, 113–127. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, M.; Karvar, M.; Aoki, S.; Endo, Y.; Hamaguchi, R.; Ma, C.; Matar, D.Y.; Orgill, D.P.; Panayi, A.C. The three-dimensional structure of porcine bladder scaffolds alters the biology of murine diabetic wound healing. Adv. Skin Wound Care 2022, 35, 1–10. [Google Scholar] [CrossRef]
- Jiang, Y.-L.; Wang, Z.-L.; Fan, Z.-X.; Wu, M.-J.; Zhang, Y.; Ding, W.; Huang, Y.-Z.; Xie, H.-Q. Human adipose-derived stem cell-loaded small intestinal submucosa as a bioactive wound dressing for the treatment of diabetic wounds in rats. Biomater. Adv. 2022, 136, 212793. [Google Scholar] [CrossRef]
- Isaac, A.L.; Tritto, M. Use and efficacy of porcine urinary bladder matrix for tissue regeneration: A review. Wounds 2023, 35, E339–E375. [Google Scholar]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2020, 8, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lei, Y.; Li, Y.; Zhang, M.; Sun, J.; Zhu, M.-Q.; Wang, J. Dual-crosslinked bioadhesive hydrogel as NIR/pH stimulus-responsiveness platform for effectively accelerating wound healing. J. Colloid Interface Sci. 2023, 637, 20–32. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, L.; Li, R.; Shao, J.; Lu, L.; Yang, P.; Zhao, A.; Liu, Y. Ellagic acid–cyclodextrin inclusion complex-loaded thiol–ene hydrogel with antioxidant, antibacterial, and anti-inflammatory properties for wound healing. ACS Appl. Mater. Interfaces 2023, 15, 4959–4972. [Google Scholar] [CrossRef]
- Han, W.; Zhou, B.; Yang, K.; Xiong, X.; Luan, S.; Wang, Y.; Xu, Z.; Lei, P.; Luo, Z.; Gao, J. Biofilm-inspired adhesive and antibacterial hydrogel with tough tissue integration performance for sealing hemostasis and wound healing. Bioact. Mater. 2020, 5, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fang, H.; Zheng, S.; Li, L.; Jiao, Z.; Wang, H.; Nie, Y.; Liu, T.; Song, K. A biological functional hybrid scaffold based on decellularized extracellular matrix/gelatin/chitosan with high biocompatibility and antibacterial activity for skin tissue engineering. Int. J. Biol. Macromol. 2021, 187, 840–849. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, T.; Joshi, A.; Gugulothu, S.B.; Choudhury, S.; Singh, N. Light-Mediated 3D Printing of Micro-Pyramid-Decorated Tailorable Wound Dressings with Endogenous Growth Factor Sequestration for Improved Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 327–337. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, L.; Lin, C.; Xin, X.; Liu, Y.; Leng, J. 4D printed continuous fiber reinforced shape memory polymer composites with enhanced mechanical properties and shape memory effects. Compos. Part A Appl. Sci. Manuf. 2024, 180, 108085. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Meng, F.; Xu, Z.; Hao, L.; Yao, Y.; Zhu, H.; Wang, C.; Wu, J.; Bian, S.; et al. 4D printed hydrogel scaffold with swelling-stiffening properties and programmable deformation for minimally invasive implantation. Nat. Commun. 2024, 15, 1587. [Google Scholar] [CrossRef]
- Joshi, A.; Choudhury, S.; Gugulothu, S.B.; Visweswariah, S.S.; Chatterjee, K. Strategies to Promote Vascularization in 3D Printed Tissue Scaffolds: Trends and Challenges. Biomacromolecules 2022, 23, 2730–2751. [Google Scholar] [CrossRef]
| Components | Percentages | Major Function | Ref. |
|---|---|---|---|
| Collagen(s) | 50–90 | Synthesized by fibroblasts, it regulates cell migration and promotes re-epithelialization. | [49] |
| Elastin | 0.6–7.9 | Maintains skin elasticity. | [49] |
| Laminin | <1.0 | It helps maintain tissue structure and promotes cell adhesion and differentiation, enabling cells to adhere to the basement membrane. | [50] |
| Fibronectin | <1.0 | It participates in wound healing, including platelet diffusion and the migration of white blood cells to injured tissues. It helps to promote elastin deposition and the mechanical strength of ECM. | [46] |
| Glycosaminoglycan(s) | <1.0 | A gel-like network maintains the biomechanical properties of the tissue by binding functional proteins. | [51] |
| Others | <1.0 | Including growth factors, etc., to stimulate and regulate the cell migration, proliferation, and differentiation behaviors necessary for angiogenesis and tissue regeneration. | [52] |
| Decellularized Materials | Product | Application | Ref. |
|---|---|---|---|
| Acellular dermal matrix (ADM) | DermACELL AWM® LifeNet Health, Virginia Beach, Virginia, USA | Normal and chronic wounds | [111] |
| GRAFTJACKET®RTM Stryker, Memphis, Tennessee, USA | Normal and chronic wounds | [112] | |
| Integra® Integra LifeSciences, Princeton, New Jersey, USA | Normal and chronic wounds | [113,114,115] | |
| Alloderm® Allergan Aesthetics, Branchburg, New Jersey, USA | Normal and chronic wounds | [116,117] | |
| Dermagraft® Organogenesis, Canton, Massachusetts, USA | Diabetic foot ulcers | [118] | |
| PermaDerm™ Regenicin, Madison, Wisconsin, USA | Burn wounds | [117] | |
| Decellularized adipose tissue (DAT) | Integra® DRT Integra LifeSciences, Princeton, New Jersey, USA | Normal and chronic wounds | [119] |
| Decellularized fish skin | Kerecis® Omega3 MicroGraft™ Kerecis, Isafjordur, Iceland | Normal and diabetic | [117] |
| Acellular amniotic membrane (AAM) | SURFFIXX® NorthStar Medical Radioisotopes/Tissue Regenix Group, Madison, Wisconsin, USA | Normal, chronic and infectious wounds | [120] |
| AmnioBand® Musculoskeletal Transplant Foundation (MTF Biologics), Edison, New Jersey, USA | Normal, chronic and infectious wounds | [120] | |
| EpiFix® MiMedx Group, Inc., Marietta, Georgia, USA | Normal, chronic and infectious wounds | [120] | |
| Biovance® Acelity/Integra LifeSciences, San Diego, California, USA | Normal, chronic and infectious wounds | [120] | |
| Decellularized small intestine submucosa (DSIS) | OASIS® Wound Matrix Smith & Nephew, Fort Worth, Texas, USA | Diabetic foot ulcers | [121] |
| Acellular fetal bovine dermis | PriMatrix® Integra LifeSciences, Princeton, New Jersey, USA | Diabetic | [117,122] |
| Decellularized forestomach matrix | Endoform® Aroa Biosurgery, Auckland, New Zealand | Normal and chronic wounds | [123] |
| Wound Type | Decellularized Materials | Author | Method | Results | Conclusion |
|---|---|---|---|---|---|
| Burn wound | Acellular dermal matrix (ADM) | Chen et al. [213] | Seventy healthy Wistar rats were inflicted with a 2 cm second-degree burn and divided into two groups; one group was treated with porcine ADM and the other with povidone–iodine cream. Biopsies were taken on day 1, 3, 5, 7, 10, 14, 21 for histopathological and biochemical analysis to test PCNA, K19, integrin-beta 1, PDGF, EGF, and FGF. | The results revealed relatively better and faster regeneration after the treatment of porcine ADM, along with a significantly increased synthesis of collagen in the experimental group. PCNA, K19, and integrin-beta 1 showed an increase and then tapered off, and were stronger in the experimental group than in the control group during the 21 days after burns. PDGF, EGF, and FGF levels increased on day 3, peaked on day 5, and then started to decrease, while a significantly enhanced expression of relevant growth factors was observed in the experimental group. | Porcine ADM stimulates collagen synthesis, stem-cell proliferation and differentiation, and expression of related growth factors, ultimately promoting burn wound healing. |
| Diabetic wound | Acellular dermal matrix (ADM) | S. Cazzell et al. [121] | Diabetic foot ulcer subjects. A total of 53 subjects in the D-ADM arm, 56 in the conventional care arm, and 23 in the GJ-ADM arm (2:2:1 ratio). Subjects were followed through 24 weeks with major endpoints at weeks 12, 16, and 24. | Single application D-ADM subjects showed significantly greater wound-closure rates than conventional care at all three endpoints while all applications D-ADM displayed a significantly higher healing rate than conventional care at week 16 and week 24. GJ-ADM did not show a significantly greater healing rate over conventional care at any of these time points. | D-ADM demonstrated significantly greater wound healing, larger wound area reduction, and a better capability of keeping healed wounds closed than conventional care in the treatment of chronic DFUs. |
| Diabetic wound | Decellularized small intestine submucosa (DSIS) | Chen et al. [214] | The diabetes model was established using C57BL/6 mice, and circular full-thickness skin wounds with a diameter of 10 mm were created on the back skin of the model mice. The diabetic mice were divided into three groups (n = 15): (1) control (PBS); (2) rhbFGF; and (3) SISAM@HN. The cutaneous wounds were photographed and measured at 0, 3, 7, and 14 days. | SISAM@HN is known to promote cell proliferation, aid collagen deposition, reduce inflammation, and stimulate blood-vessel growth. | The SIS acellular matrix-containing HA hydrogel was able to adhere to wound sites, promote cell proliferation, and facilitate angiogenesis, making it a promising biomaterial for wound dressing in the clinical therapy of diabetic skin defects. |
| Diabetic wound | Urinary bladder matrix (UBM) | M. Martinson et al. [215] | Medicare claims data from 2011 to 2014 were used to identify beneficiaries with diabetes and foot ulcers. Patients treated with one of four types of skin substitute (Apligraf, Dermagraft, OASIS, and MatriStem) were identified. The skin substitutes were compared in terms of episode length, amputation rate, skin substitute utilization, and skin substitute costs. | The percentage of DFUs that healed at 90 days was UBM 62%; SIS 63%; HML 58%; and HSL 58%. Over the entire time, UBM was non-inferior to SIS (p < 0.001), and both were significantly better than HML or HSL (p < 0.005 in all four tests). Medicare reimbursements for skin substitutes per DFU episode for UBM and SIS appeared to be equivalent. Both were less than HML or HSL (p < 0.0005 in all four tests). | UBM and SIS were associated with both shorter DFU episode lengths and lower payer reimbursements compared to HML and HSL, while HML was less costly than HSL but equivalent in terms of healing. |
| Infective wound | Decellularized extracellular matrix (dECM) | P. Chandika et al. [216] | Establish a diabetic mouse model using ICR mice—full-thickness 2.25 cm2 square excision wounds. The mice were divided into control, PCL, PCLU, PE4:6, and PEU4:6 groups and were treated accordingly over 21 days. | PEU 4:6 nanofibrous scaffold enhanced re-epithelialization, dermal tissue maturation, and complete wound closure. | Composite nanofiber PEU scaffolds have great potential in treating infected total wounds. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, R.; Pan, R.; He, L.; Dai, Y.; Jiang, Y.; He, S.; Li, B.; Li, Y. Decellularized Extracellular Matrices for Skin Wound Treatment. Materials 2025, 18, 2752. https://doi.org/10.3390/ma18122752
Liang R, Pan R, He L, Dai Y, Jiang Y, He S, Li B, Li Y. Decellularized Extracellular Matrices for Skin Wound Treatment. Materials. 2025; 18(12):2752. https://doi.org/10.3390/ma18122752
Chicago/Turabian StyleLiang, Rui, Ruliang Pan, Li He, Yu Dai, Yuting Jiang, Shujun He, Baoguo Li, and Yuli Li. 2025. "Decellularized Extracellular Matrices for Skin Wound Treatment" Materials 18, no. 12: 2752. https://doi.org/10.3390/ma18122752
APA StyleLiang, R., Pan, R., He, L., Dai, Y., Jiang, Y., He, S., Li, B., & Li, Y. (2025). Decellularized Extracellular Matrices for Skin Wound Treatment. Materials, 18(12), 2752. https://doi.org/10.3390/ma18122752







