Free-Standing Composite Film Based on Zinc Powder and Nanocellulose Achieving Dendrite-Free Anode of Aqueous Zinc–Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of NFC
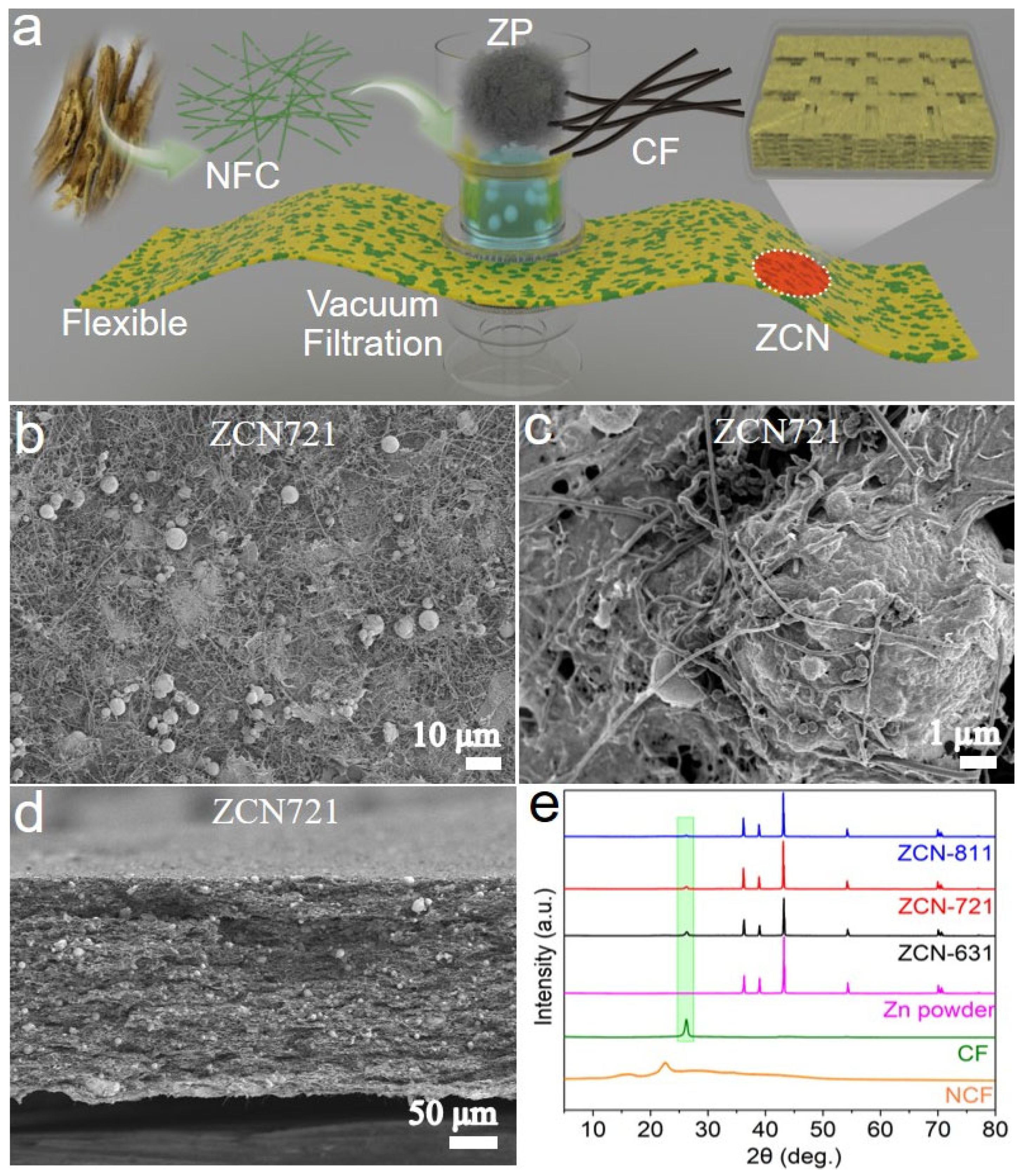
2.2. Fabrication of ZCN Films
2.3. Characterization and Electrochemical Measurements
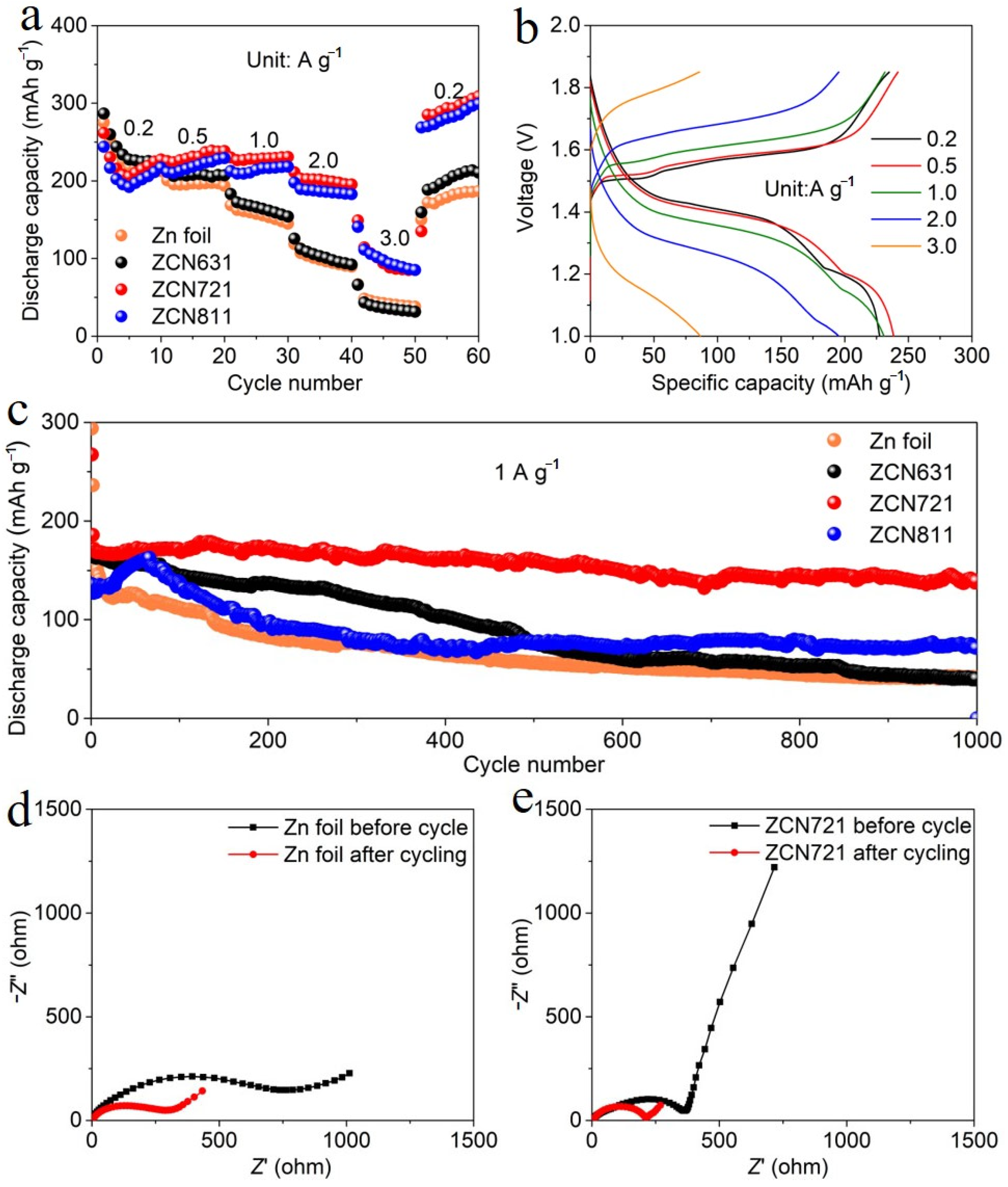
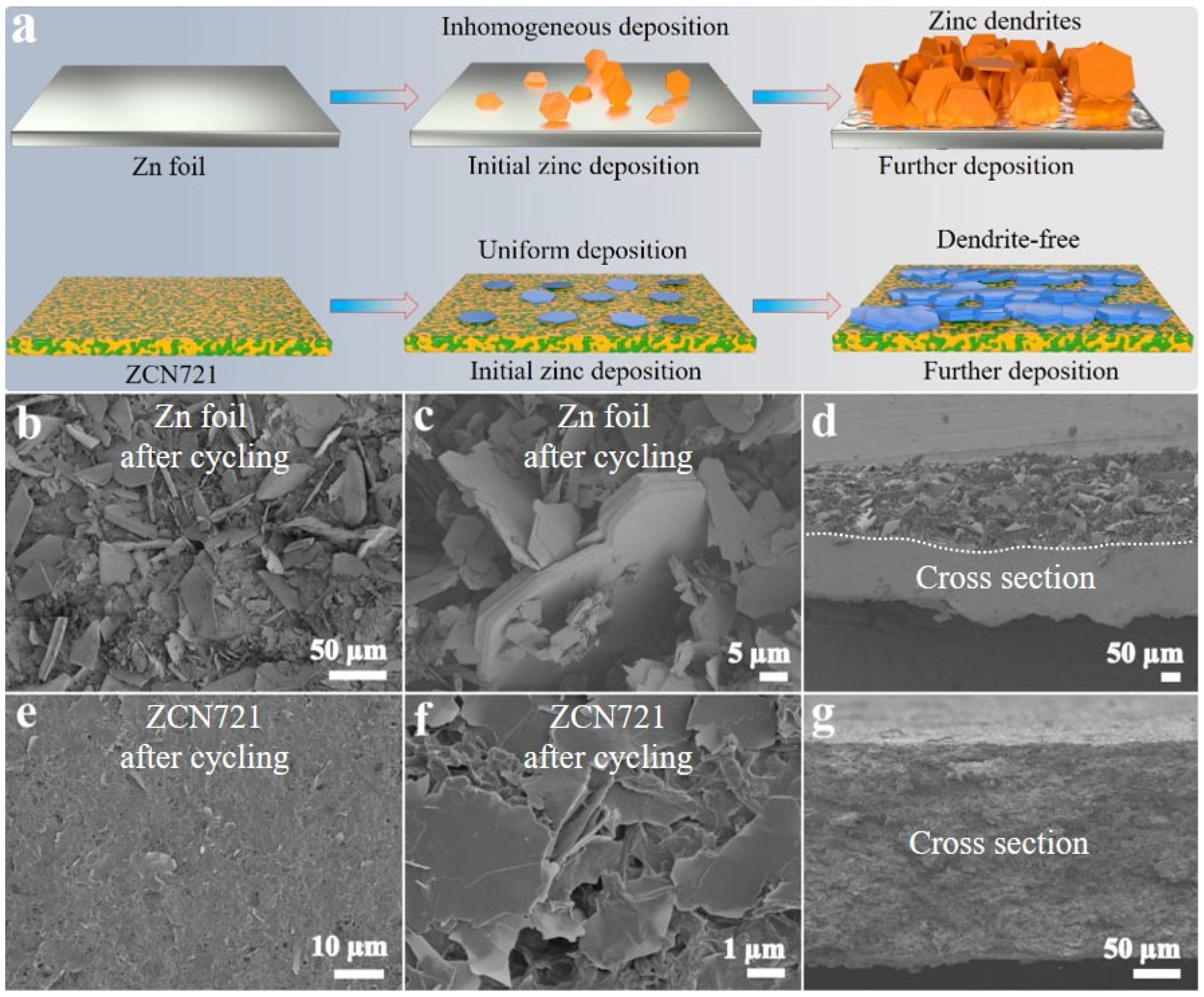
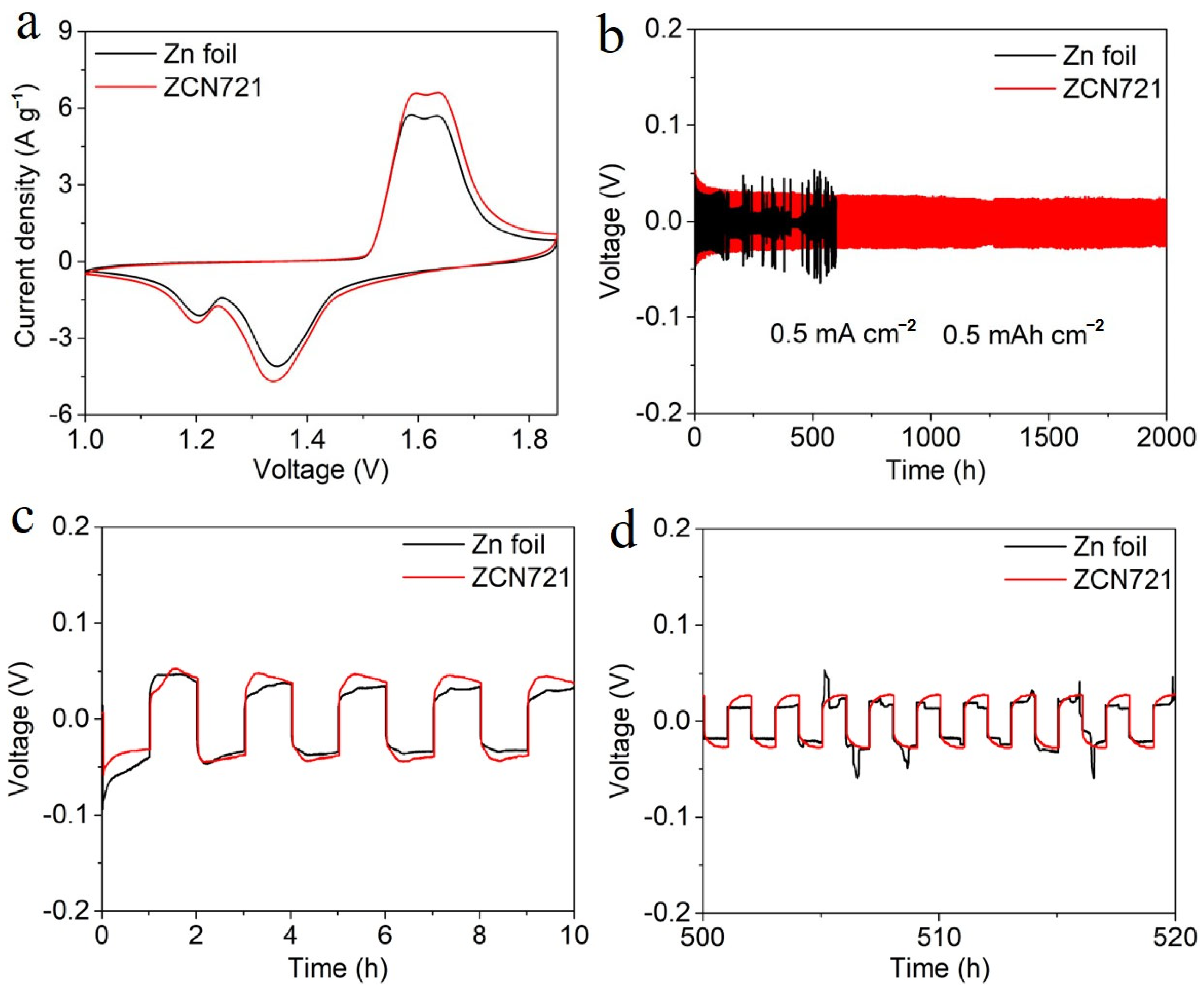
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Askarova, A.; Alekhina, T.; Popov, E.; Afanasev, P.; Mukhametdinova, A.; Smirnov, A.; Cheremisin, A.; Mukhina, E. Innovative technology for underground clean in situ hydrogen generation: Experimental and numerical insights for sustainable energy transition. Renew. Energy 2025, 240, 122259. [Google Scholar] [CrossRef]
- Bisen, D.; Chouhan, A.P.S.; Pant, M.; Chakma, S. Advancement of thermochemical conversion and the potential of biomasses for production of clean energy: A review. Renew. Sustain. Energy Rev. 2025, 208, 115016. [Google Scholar] [CrossRef]
- Ray, A.; Bhonsle, A.K.; Singh, J.; Trivedi, J.; Atray, N. Examining alternative carbon resources for sustainable energy generation: A comprehensive review. Next Energy 2025, 6, 100194. [Google Scholar] [CrossRef]
- Guelfo, J.L.; Ferguson, P.L.; Beck, J.; Chernick, M.; Doria-Manzur, A.; Faught, P.W.; Flug, T.; Gray, E.P.; Jayasundara, N.; Knappe, D.R. Lithium-ion battery components are at the nexus of sustainable energy and environmental release of per-and polyfluoroalkyl substances. Nat. Commun. 2024, 15, 5548. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Guan, P.; Li, M.; Wan, T.; Hu, L.; Zhang, S.; Liu, C.; Su, D.; Liu, Y.; et al. Designing MXene-wrapped AgCl@carbon core shell cathode for robust quasi-solid-state Ag-Zn battery with ultralong cycle life. Energy Storage Mater. 2023, 60, 102836. [Google Scholar] [CrossRef]
- Tong, Z.; Lv, C.; Bai, G.D.; Yin, Z.W.; Zhou, Y.; Li, J.T. A review on applications and challenges of carbon nanotubes in lithium-ion battery. Carbon Energy 2025, 7, e643. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, S.; Ju, J.; Liu, T.; Zheng, Y.; Xu, J.; Wang, Y.; Li, J.; Zhao, J.; Ma, J. A cathode homogenization strategy for enabling long-cycle-life all-solid-state lithium batteries. Nat. Energy 2024, 9, 1084–1094. [Google Scholar] [CrossRef]
- Xu, H.; Yang, W.; Li, M.; Liu, H.; Gong, S.; Zhao, F.; Li, C.; Qi, J.; Wang, H.; Peng, W. Advances in aqueous zinc ion batteries based on conversion mechanism: Challenges, strategies, and prospects. Small 2024, 20, 2310972. [Google Scholar] [CrossRef]
- Zhu, J.; Tie, Z.; Bi, S.; Niu, Z. Towards more sustainable aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2024, 136, e202403712. [Google Scholar] [CrossRef]
- Bai, Y.; Qin, Y.; Hao, J.; Zhang, H.; Li, C.M. Advances and perspectives of ion-intercalated vanadium oxide cathodes for high-performance aqueous zinc ion battery. Adv. Funct. Mater. 2024, 34, 2310393. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, Y.; Lu, Y.; Liang, T.; Zhou, L.; Fan, J.; Kuo, Y.; Guan, P.; Wan, T.; Hu, L.; et al. Rechargeable Zn-MnO2 batteries: Progress, challenges, rational design, and perspectives. ChemElectroChem 2024, 11, e202300495. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, K.; Yang, H.; Yang, W. Advancements in achieving high reversibility of zinc anode for alkaline zinc-based batteries. Adv. Mater. 2024, 36, 2306154. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Zhang, W.; Joshi, N.; Yang, Y. Recent advances in anode design for mild aqueous Zn-ion batteries. Energy Storage Mater. 2024, 64, 103075. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.-F.; Li, Z.-X.; Wang, P.-F.; Xie, Y.; Yi, T.-F. Carbon-based nanomaterials for stabilizing zinc metal anodes towards high-performance aqueous zinc-ion batteries. Energy Storage Mater. 2024, 67, 103300. [Google Scholar] [CrossRef]
- Zhao, L.-L.; Zhao, S.; Zhang, N.; Wang, P.-F.; Liu, Z.-L.; Xie, Y.; Shu, J.; Yi, T.-F. Construction of stable Zn metal anode by inorganic functional protective layer toward long-life aqueous Zn-ion battery. Energy Storage Mater. 2024, 71, 103628. [Google Scholar] [CrossRef]
- Peng, H.; Wang, D.; Zhang, F.; Yang, L.; Jiang, X.; Zhang, K.; Qian, Z.; Yang, J. Improvements and Challenges of Hydrogel Polymer Electrolytes for Advanced Zinc Anodes in Aqueous Zinc-Ion Batteries. ACS Nano 2024, 18, 21779–21803. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, R.; Lu, Q.; Liu, P.; Hu, L.; Zhang, K.; Bai, P.; Xu, R.; Cao, X.; Sun, Z. Recent progress and perspectives on highly utilized Zn metal anode-Towards marketable aqueous Zn-ion batteries. Energy Storage Mater. 2024, 72, 103689. [Google Scholar] [CrossRef]
- He, H.; Qu, W.; Lian, J.; Yan, Y.; Chen, C.; Xiong, Q.; Zhang, G.; Zhang, M. Progressive deposition on carbon nanofiber films enables dendrite-free zinc plating. Chem. Eng. J. 2024, 487, 150563. [Google Scholar] [CrossRef]
- Yan, L.; Zhai, Q.; Zhang, S.; Li, Z.; Kang, Q.; Gao, X.; Jin, C.; Liu, T.; Ma, T.; Lin, Z. The burgeoning zinc powder anode for aqueous zinc metal batteries: From electrode preparation to performance enhancement. Adv. Energy Mater. 2024, 14, 2401328. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, J.; Zhang, S.; Feng, Z.; Liu, C.; Zhu, R.; Liu, Y.; Guan, P.; Li, M.; Han, Z.; et al. Long-Life flexible mild Ag-Zn fibrous battery with bifunctional gel electrolyte. Chem. Eng. J. 2004, 480, 148334. [Google Scholar] [CrossRef]
- Chen, M.; Yang, M.; Han, X.; Chen, J.; Zhang, P.; Wong, C.P. Suppressing rampant and vertical deposition of cathode intermediate product via pH regulation toward large-capacity and high-durability Zn//MnO2 batteries. Adv. Mater. 2024, 36, 2304997. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, S.; Chen, M.; Lu, B.; Zhang, X.; Liang, S.; Zhou, J. Tailoring grain boundary stability of zinc-titanium alloy for long-lasting aqueous zinc batteries. Nat. Commun. 2023, 14, 7080. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, Z.; Fan, L.; Zhao, C.; Chen, A.; Wang, M.; Li, M.; Lu, X.; Zhang, J.; Zhang, Y. Construct robust epitaxial growth of (101) textured zinc metal anode for long life and high capacity in mild aqueous zinc-ion batteries. Adv. Mater. 2024, 36, 2305988. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Z.; Yang, Y.; Zhang, H.; Lai, G.; Lu, B.; Zhou, P.; Chen, L.; Zhou, J. Bifunctional self-segregated electrolyte realizing high-performance zinc-iodine batteries. InfoMat 2024, 6, e12620. [Google Scholar] [CrossRef]
- Ma, H.; Chen, H.; Chen, M.; Li, A.; Han, X.; Ma, D.; Zhang, P.; Chen, J. Biomimetic and biodegradable separator with high modulus and large ionic conductivity enables dendrite-free zinc-ion batteries. Nat. Commun. 2025, 16, 1014. [Google Scholar] [CrossRef]
- Fang, X.; Yuan, Y.; Wang, Q.; Ji, C.; Wu, Y.; Liu, H.; Jiang, J.; Ma, A. Effect of Zinc Powder Reduced Graphene Oxide on the Corrosion Resistance of Waterborne Inorganic Zinc-Rich Coatings. Coatings 2024, 14, 1321. [Google Scholar] [CrossRef]
- Kang, L.; Cui, M.; Jiang, F.; Gao, Y.; Luo, H.; Liu, J.; Liang, W.; Zhi, C. Nanoporous CaCO3 coatings enabled uniform Zn stripping/plating for long-life zinc rechargeable aqueous batteries. Adv. Energy Mater. 2018, 8, 1801090. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Le, S.; Yan, B.; Mao, Y.; Chi, D.; Zhu, M.; Jia, H.; Zhao, G.; Zhu, X.; Zhang, N. N-doped δ-MnO2 coated N-doped carbon cloth as stable cathode for aqueous zinc-ion batteries. Int. J. Electrochem. Sci. 2023, 18, 1–8. [Google Scholar] [CrossRef]
- Chen, J.; Chen, M.; Chen, H.; Yang, M.; Han, X.; Ma, D.; Zhang, P.; Wong, C.-P. Wood-inspired anisotropic hydrogel electrolyte with large modulus and low tortuosity realizing durable dendrite-free zinc-ion batteries. Proc. Natl. Acad. Sci. USA 2024, 121, e2322944121. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Qin, R.; Liu, X.; Fang, P.; Zheng, D.; Tong, Y.; Lu, X. Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries. Adv. Mater. 2019, 31, 1903675. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Yu, H.; Liu, W.; Kuang, G.; Mei, L.; Wu, Z.; Wei, W.; Ji, X.; Qu, B. A multifunctional artificial interphase with fluorine-doped amorphous carbon layer for ultra-stable Zn anode. Adv. Funct. Mater. 2022, 32, 2205600. [Google Scholar] [CrossRef]
- Xu, T.; Du, H.; Liu, H.; Liu, W.; Zhang, X.; Si, C.; Liu, P.; Zhang, K. Advanced nanocellulose-based composites for flexible functional energy storage devices. Adv. Mater. 2021, 33, 2101368. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, L.; Yanilmaz, M.; Lu, X.; Yang, K.; Hu, K.; Liu, Y.; Zhang, X. From nature, requite to nature: Bio-based cellulose and its derivatives for construction of green zinc batteries. Chem. Eng. J. 2023, 454, 140311. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Fan, L.; Shuai, Y.; Zhang, N. Ultrathin and super-tough membrane for anti-dendrite separator in aqueous zinc-ion batteries. Cell Rep. Phys. Sci. 2022, 3, 100824. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, M.; Chen, M.; Zhang, G.; Han, X.; Chen, J.; Ma, D.; Zhang, P. Ion-sieving effect enabled by sulfonation of cellulose separator realizing dendrite-free Zn deposition. Adv. Funct. Mater. 2024, 34, 2315444. [Google Scholar] [CrossRef]
- Chen, M.; Gong, Y.; Zhao, Y.; Song, Y.; Tang, Y.; Zeng, Z.; Liang, S.; Zhou, P.; Lu, B.; Zhang, X. Spontaneous grain refinement effect of rare earth zinc alloy anodes enables stable zinc batteries. Natl. Sci. Rev. 2024, 11, nwae205. [Google Scholar] [CrossRef]
- Xiao, Y.; Su, T.; Wang, T.; Xiang, W.; Tang, S.; Yu, J.S. Interfacial micro-electric field induced by phosphorus-doped g-C3N4 for highly reversible dendrite-free zinc metal anode. Chem. Eng. J. 2025, 512, 162391. [Google Scholar] [CrossRef]
- Shen, P.; Pu, X.; Zhang, X.; Liu, Y.; Han, D.; Sun, X.; Wang, H.-g. A porphyrin-functionalized conjugated microporous polymer coating for stable dendritic-free aqueous zinc ion batteries. Chem. Eng. J. 2024, 493, 152440. [Google Scholar] [CrossRef]
- Dong, J.; Duan, J.; Cao, R.; Zhang, W.; Fang, K.; Yang, H.; Liu, Y.; Shen, Z.; Li, F.; Liu, R.; et al. Dendrite-free Zn deposition initiated by nanoscale inorganic–organic coating-modified 3D host for stable Zn-ion battery. SusMat 2024, 4, e189. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, D.; Ouyang, K.; Shen, S.; Yang, M.; Wang, Y.; Zhao, J.; Mi, H.; Zhang, P. 3D artificial array interface engineering enabling dendrite-free stable Zn metal anode. Nano-Micro Lett. 2023, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kou, Y.; Hao, Q.; Chen, F.; Chen, X.; Li, N. Improving Zn ion transport behavior and uniform deposition using artificial ZnOHF coated film for deeply rechargeable Zn metal anodes. Electrochim. Acta 2023, 443, 141928. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, X.; Xu, W.; Chen, W.; Zhao, K.; Wang, Y.; Hong, S.; Wu, Q.; Li, M.C.; et al. Synergic effect of dendrite-free and zinc gating in lignin-containing cellulose nanofibers-mXene layer enabling long-cycle-life zinc metal batteries. Adv. Sci. 2022, 9, e2202380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tong, H.; Wu, Y.; Chen, X.; Wu, C.; Xu, Z.; Shen, L.; Zhang, X. A dendrite-free Zn anode Co-modified with In and ZnF2 for long-life Zn-ion capacitors. ACS Appl. Mater. Interfaces 2022, 14, 46665–46672. [Google Scholar] [CrossRef]
- Huang, X.; Cao, H.; Liu, Y.; Hu, Q.; Zheng, Q.; Zhao, J.; Lin, D.; Xu, B. Na superionic conductor-type compounds as protective layers for dendrites-free aqueous Zn-ion batteries. J. Colloid Interface Sci. 2023, 629, 3–11. [Google Scholar] [CrossRef]
- Meng, H.; Ran, Q.; Dai, T.Y.; Shi, H.; Zeng, S.P.; Zhu, Y.F.; Wen, Z.; Zhang, W.; Lang, X.Y.; Zheng, W.T.; et al. Surface-alloyed nanoporous zinc as reversible and stable anodes for high-performance aqueous zinc-ion battery. Nano-Micro Lett. 2022, 14, 128. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, J.; Li, Y.; Hansen, E.J.; Sun, D.; Wang, H.; Wang, L.; Liu, J. Regulating zinc nucleation sites and electric field distribution to achieve high-performance zinc metal anode via surface texturing. Small 2023, 19, e2206634. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Chen, M.; Chen, J. Free-Standing Composite Film Based on Zinc Powder and Nanocellulose Achieving Dendrite-Free Anode of Aqueous Zinc–Ion Batteries. Materials 2025, 18, 2696. https://doi.org/10.3390/ma18122696
Wang G, Chen M, Chen J. Free-Standing Composite Film Based on Zinc Powder and Nanocellulose Achieving Dendrite-Free Anode of Aqueous Zinc–Ion Batteries. Materials. 2025; 18(12):2696. https://doi.org/10.3390/ma18122696
Chicago/Turabian StyleWang, Guanwen, Minfeng Chen, and Jizhang Chen. 2025. "Free-Standing Composite Film Based on Zinc Powder and Nanocellulose Achieving Dendrite-Free Anode of Aqueous Zinc–Ion Batteries" Materials 18, no. 12: 2696. https://doi.org/10.3390/ma18122696
APA StyleWang, G., Chen, M., & Chen, J. (2025). Free-Standing Composite Film Based on Zinc Powder and Nanocellulose Achieving Dendrite-Free Anode of Aqueous Zinc–Ion Batteries. Materials, 18(12), 2696. https://doi.org/10.3390/ma18122696







