Biomaterials Based on Bee Products and Their Effectiveness in Soft Tissue Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
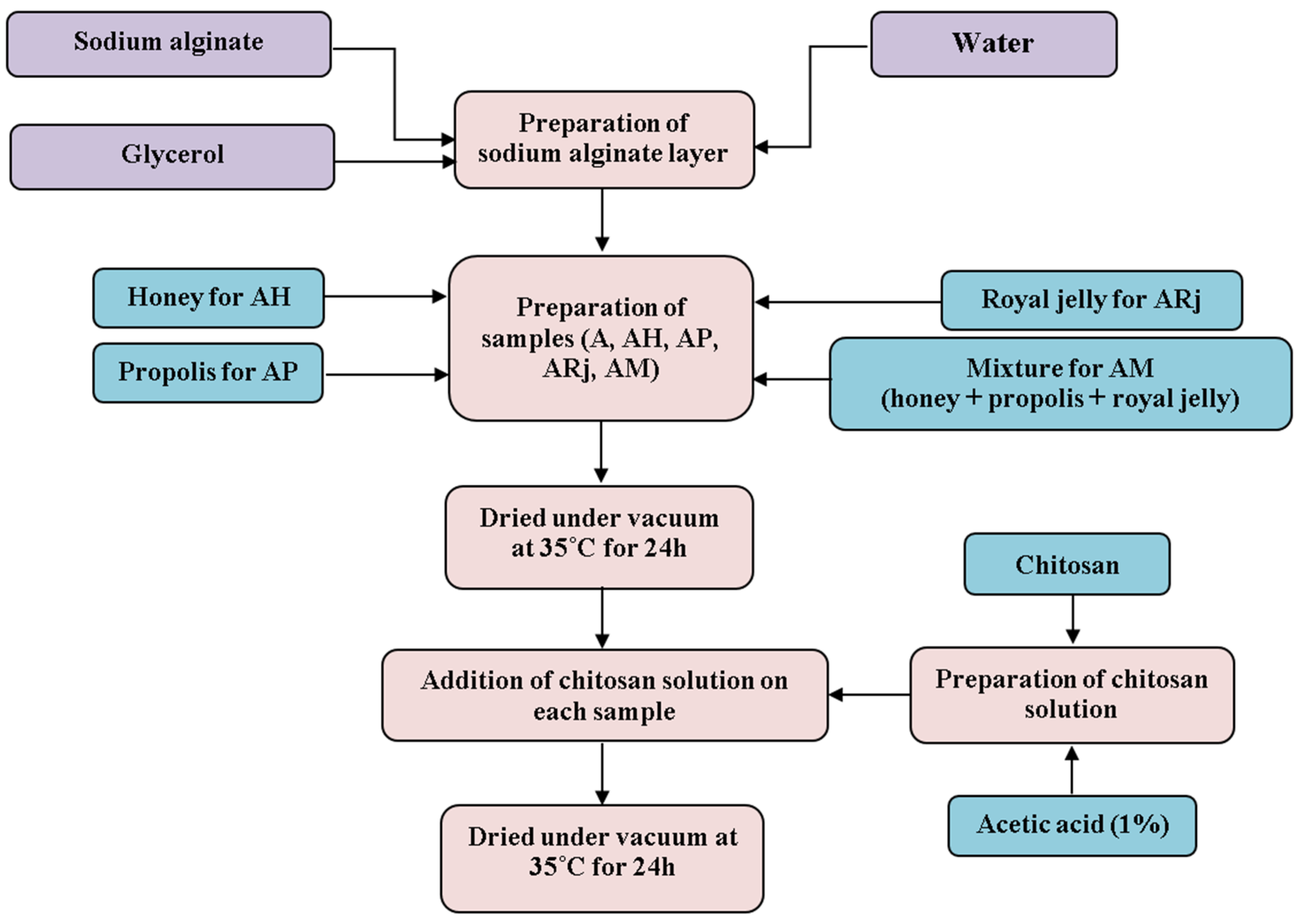
2.2. Synthesis of Biomaterials Based on Bee Products
2.3. Characterization of Biomaterials Based on Bee Products
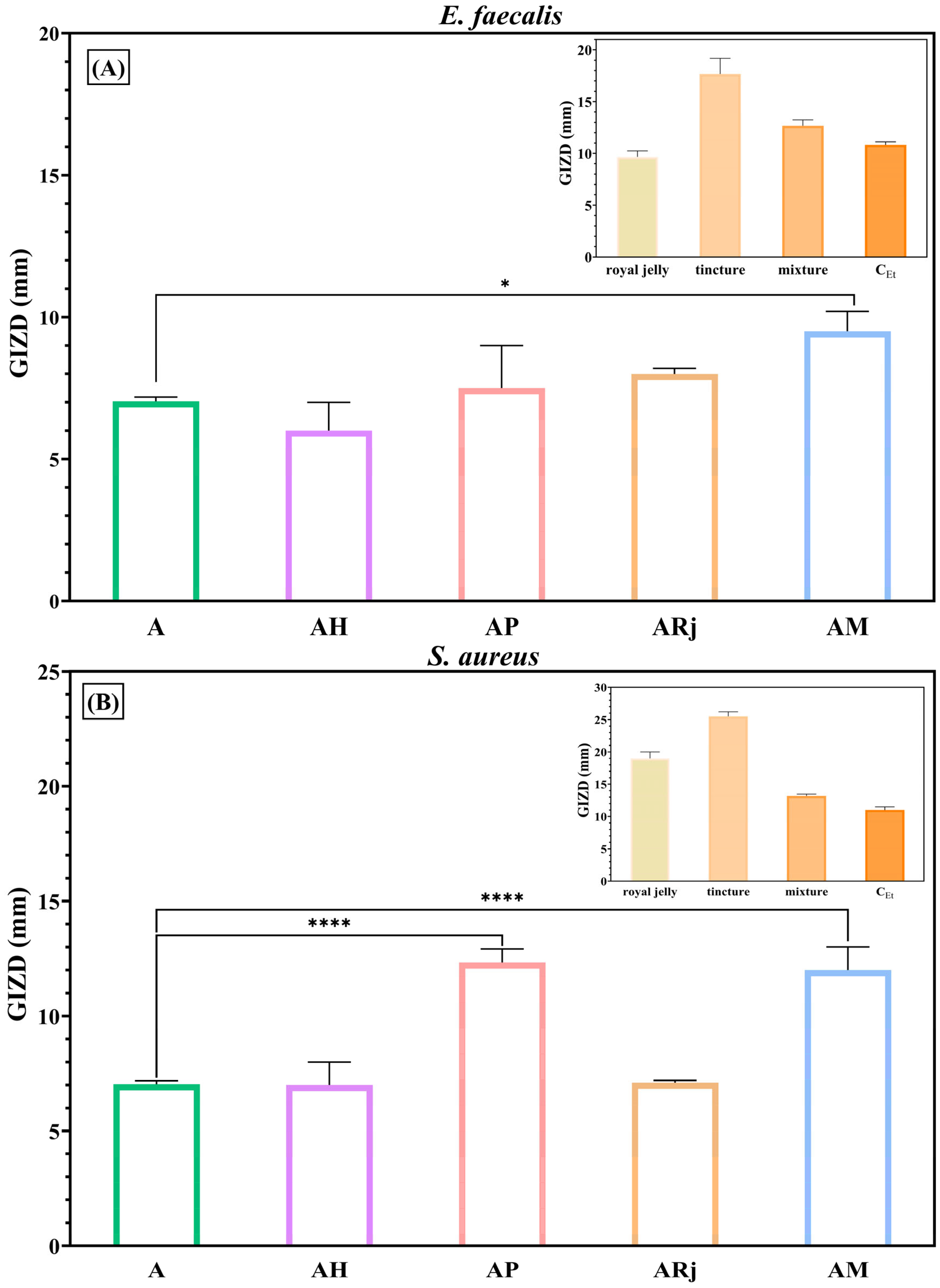
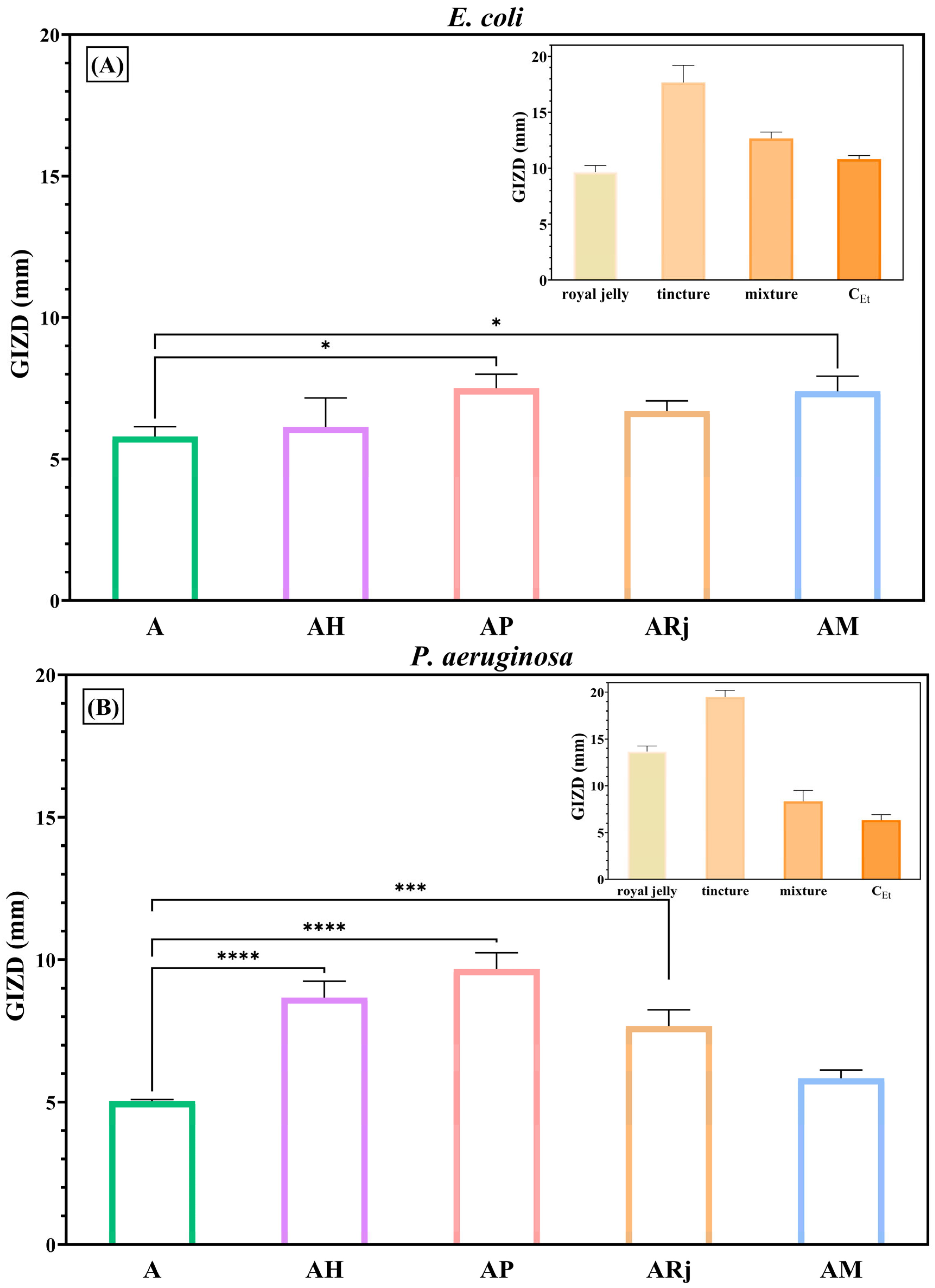
2.4. Antimicrobial Assessments
2.5. Biocompatibility Assays
2.5.1. Cell Culture
2.5.2. Indirect Cytotoxicity Tests
2.6. Statistical Analysis
3. Results and Discussion
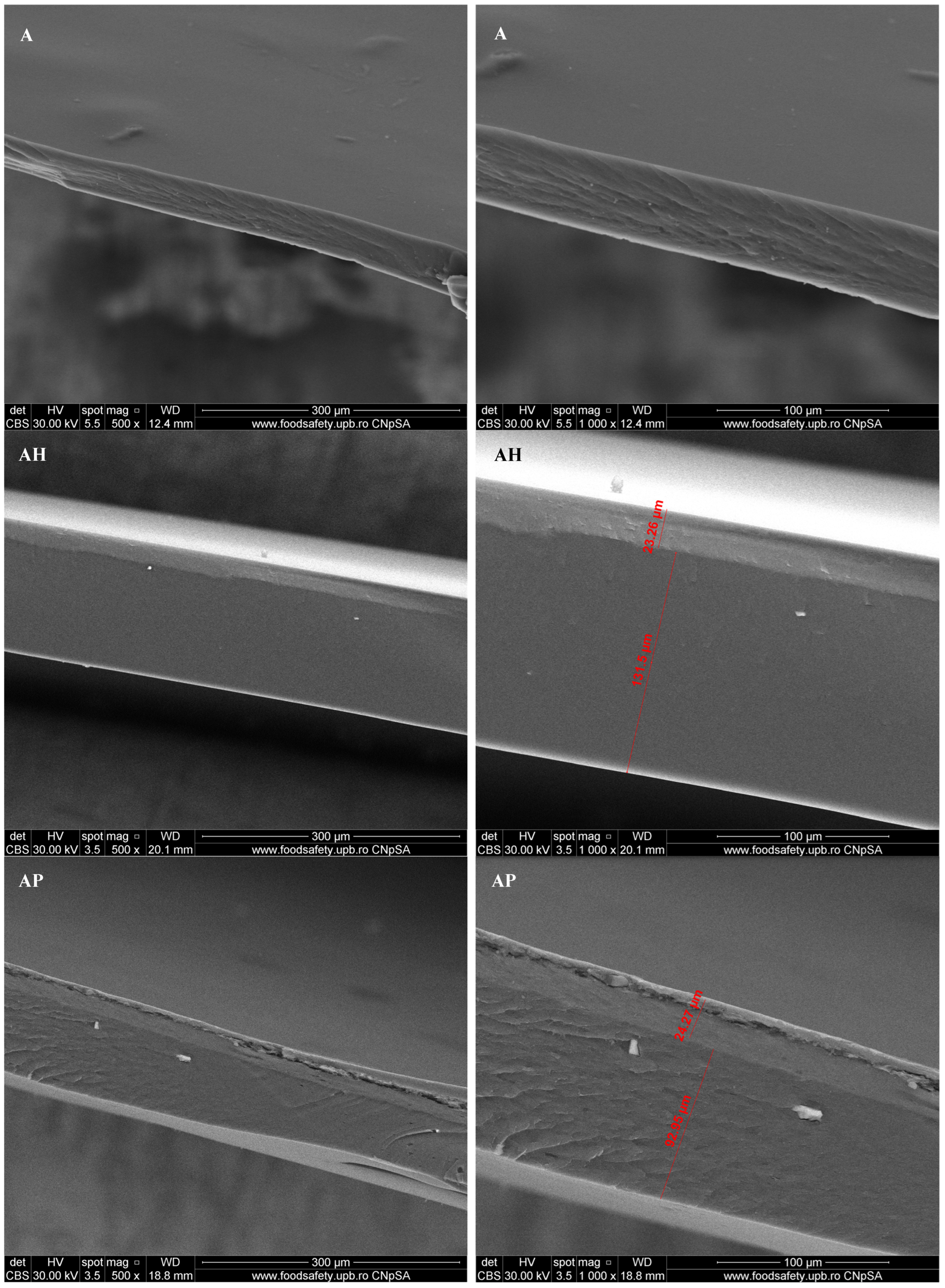
3.1. Scanning Electron Microscopy (SEM)
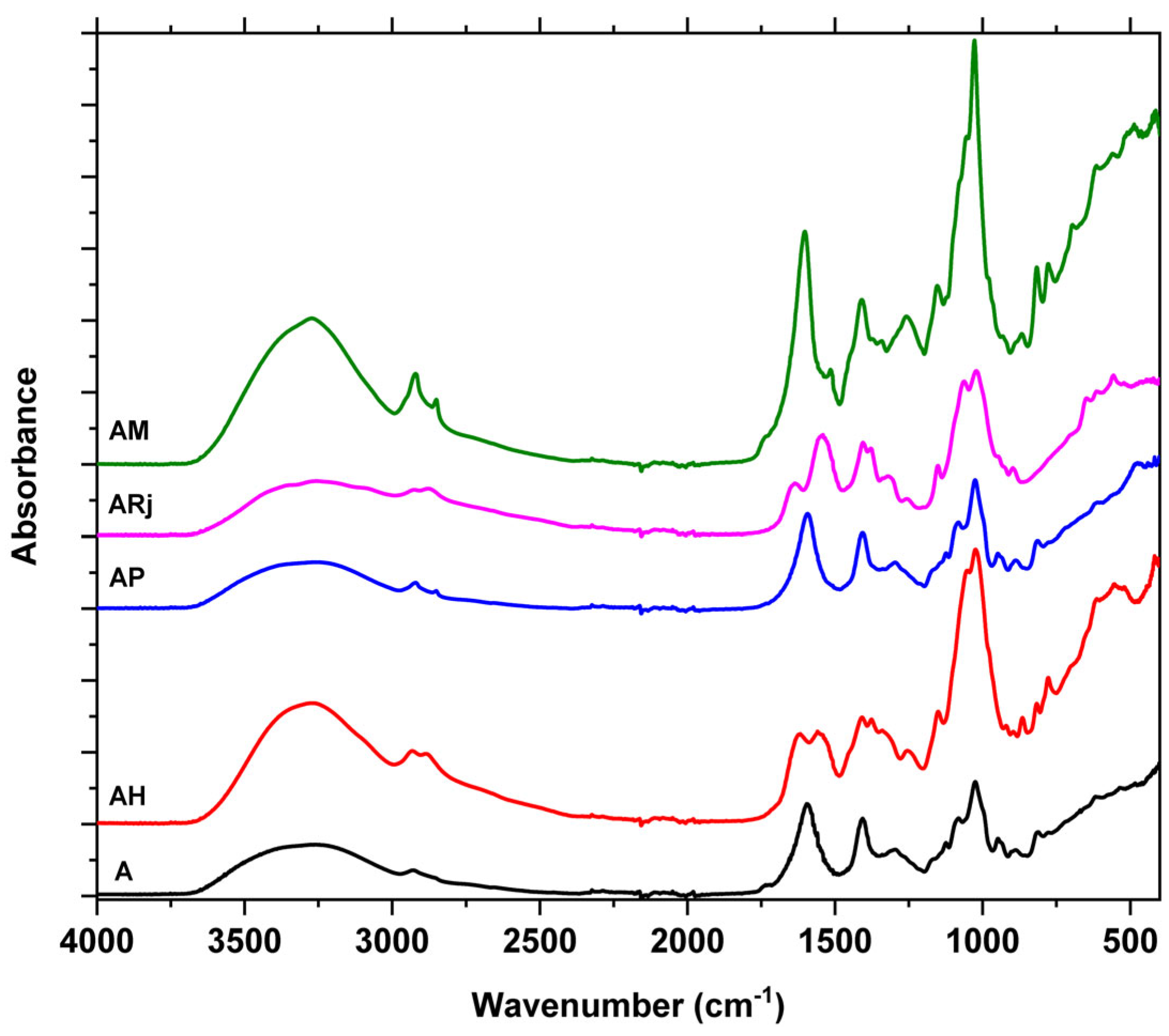
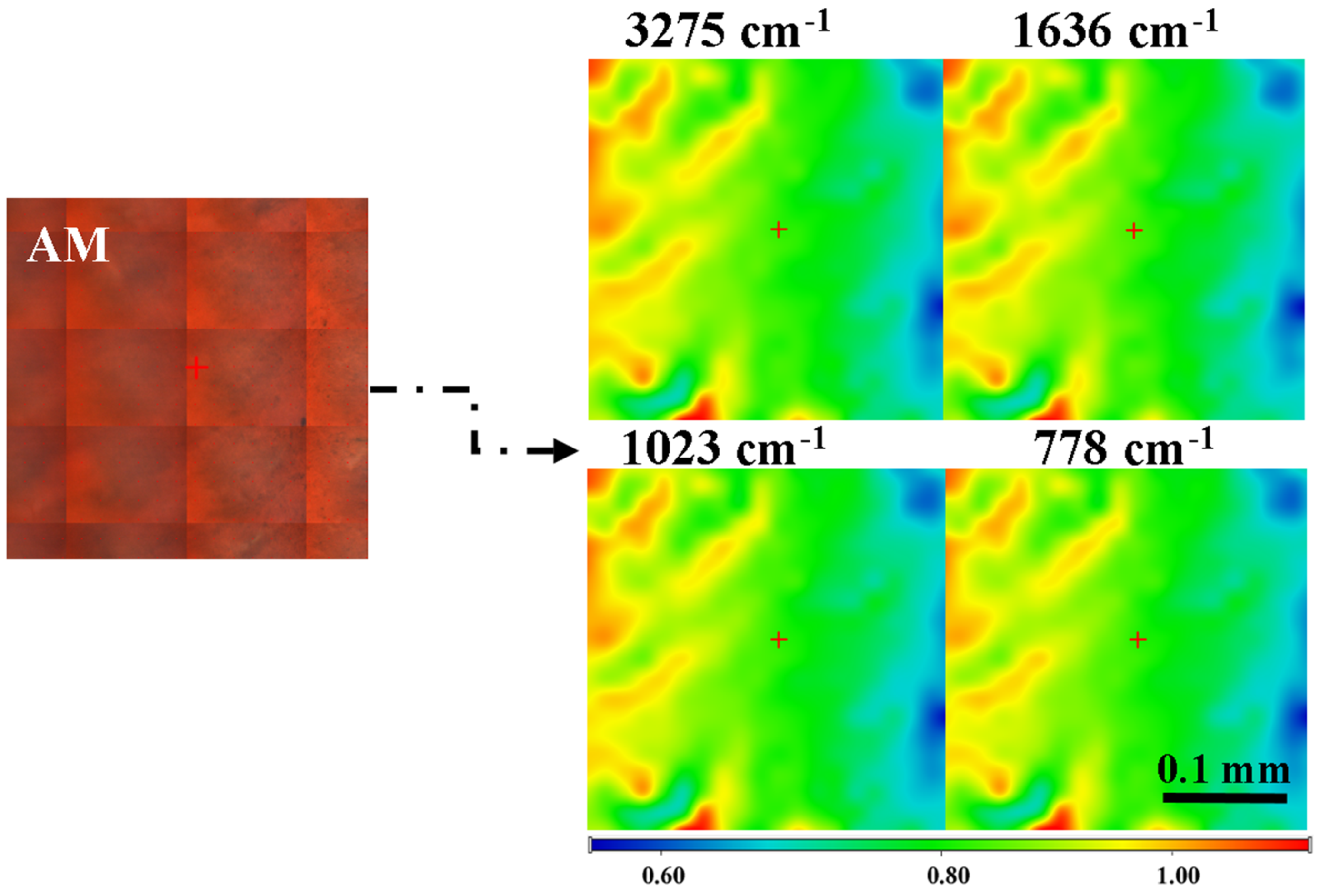
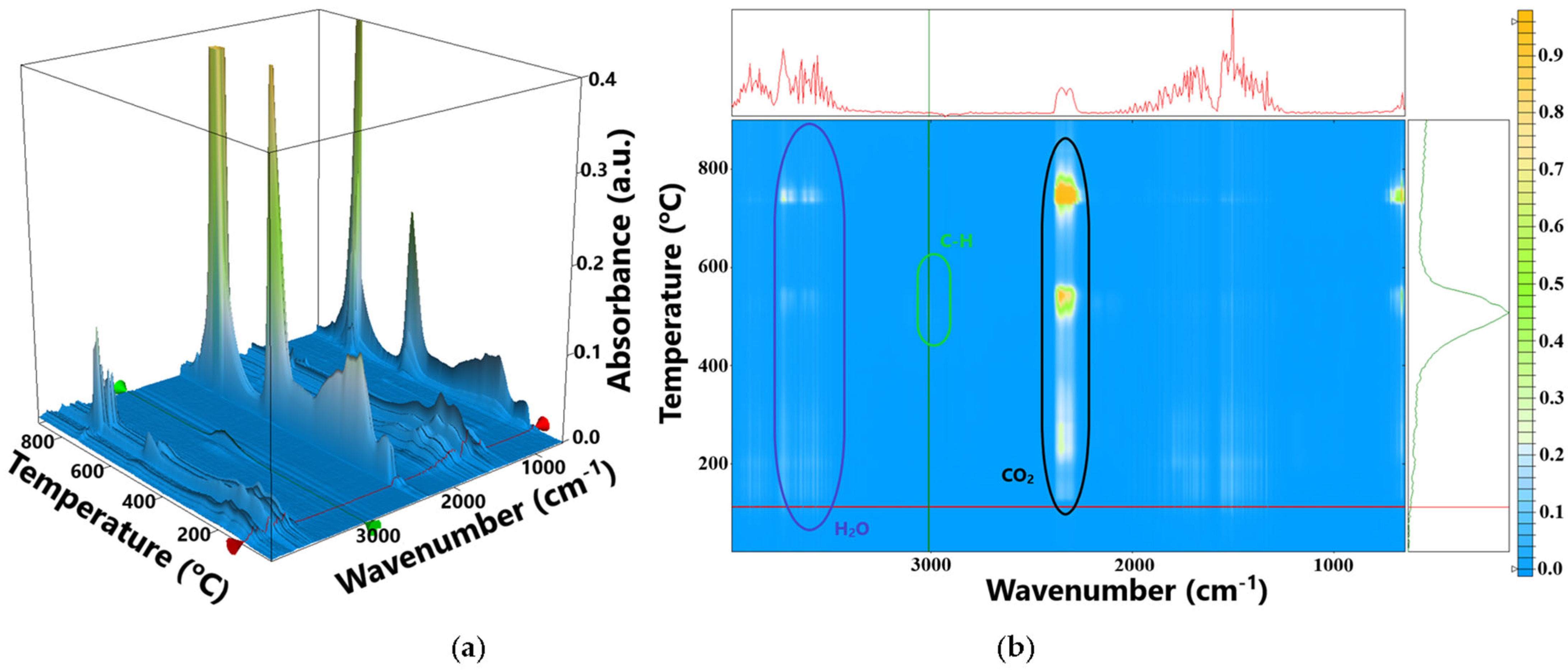
3.2. Fourier Transformed Infrared Spectroscopy (FTIR)
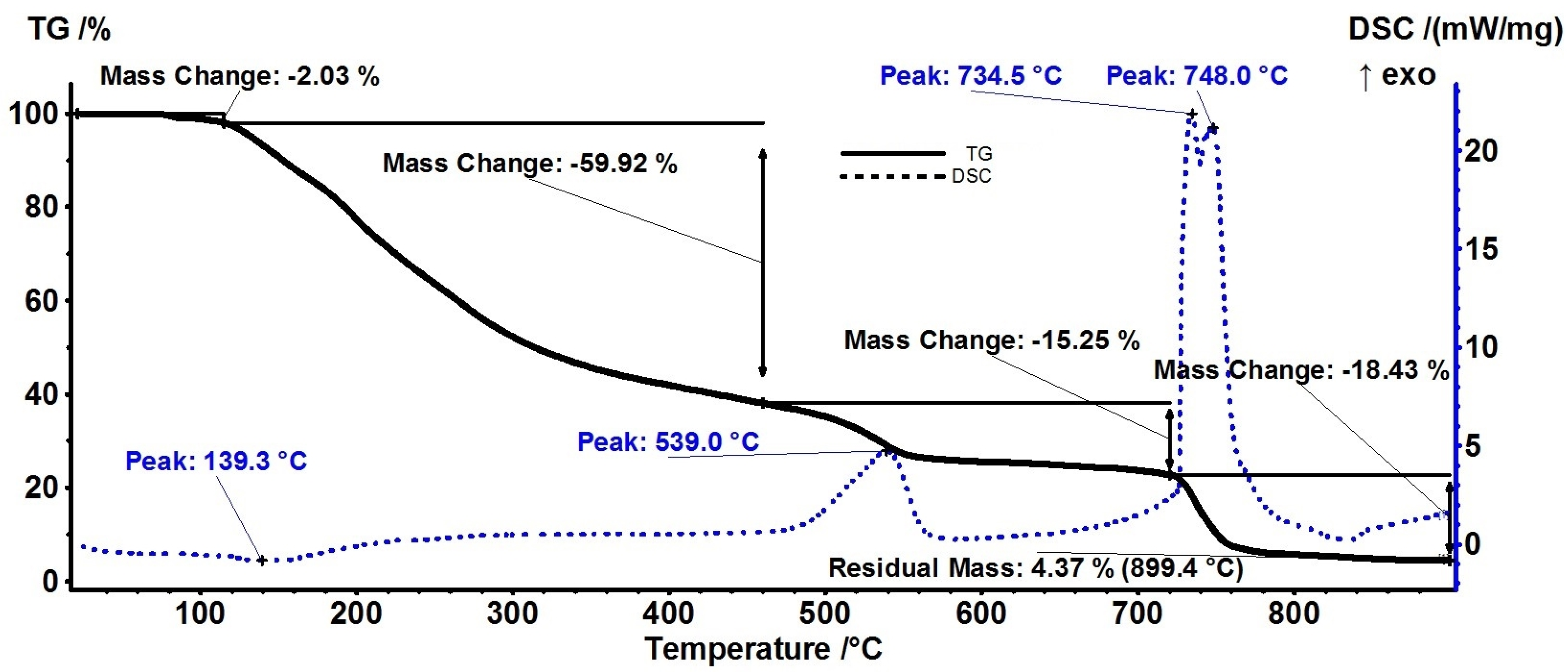
3.3. Thermal Analysis
3.4. Antimicrobial Activity
3.5. Biocompatibility and Cytotoxicity Assays
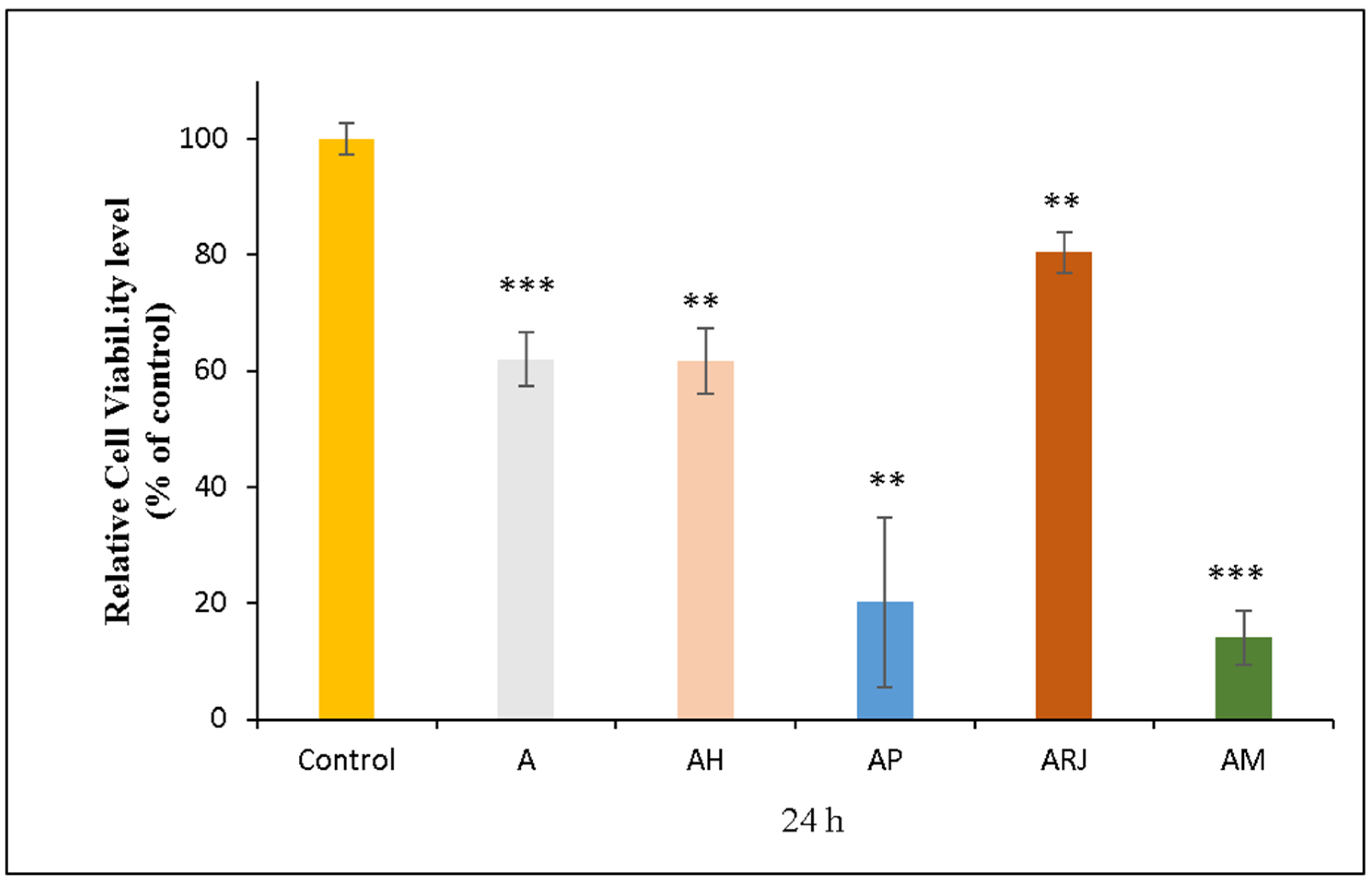
3.5.1. MTT Assay
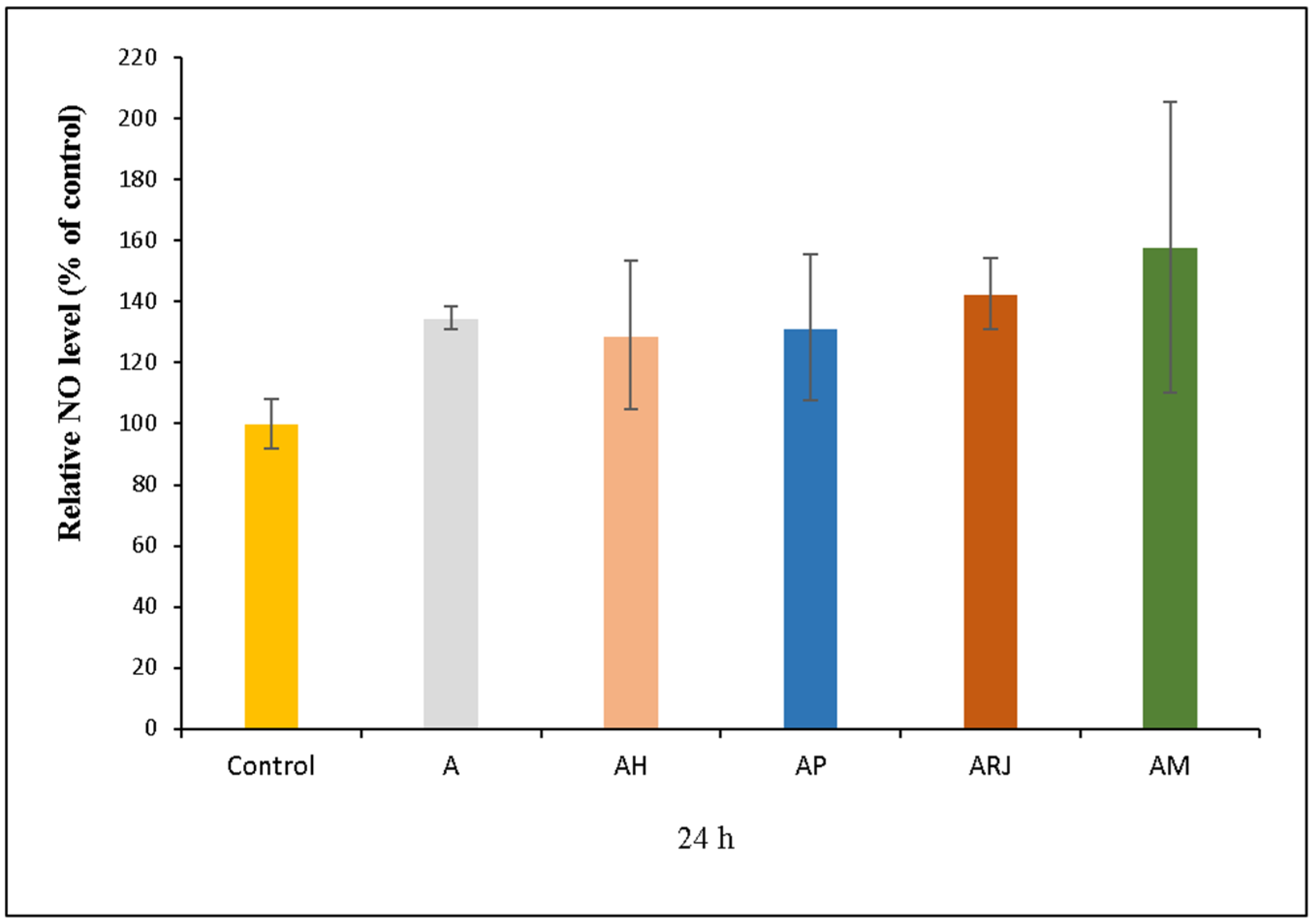
3.5.2. NO Assay
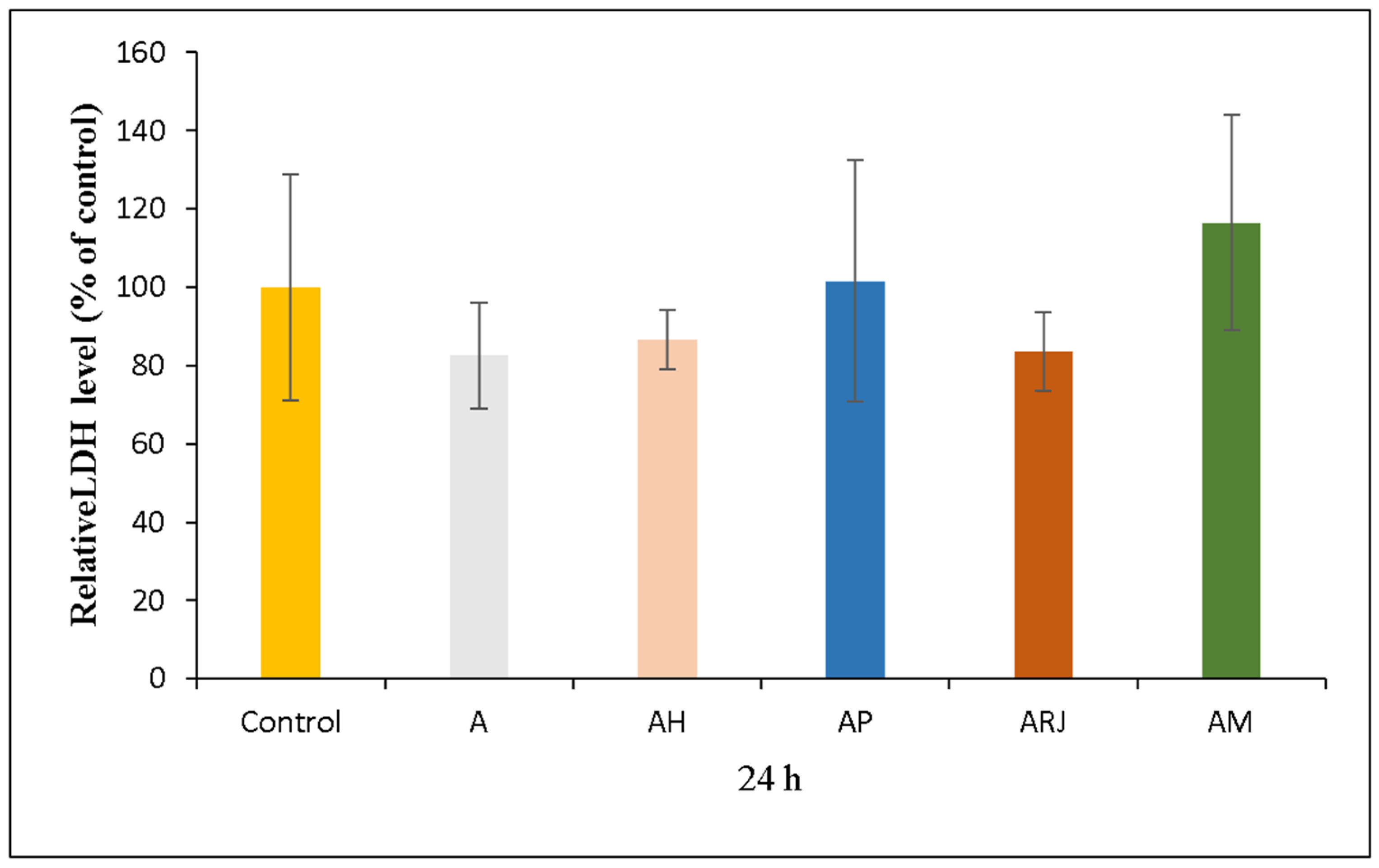
3.5.3. LDH Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maeso, L.; Antezana, P.E.; Arana, A.G.H.; Evelson, P.A.; Orive, G.; Desimone, M.F. Progress in the Use of Hydrogels for Antioxidant Delivery in Skin Wounds. Pharmaceutics 2024, 16, 524. [Google Scholar] [CrossRef] [PubMed]
- Iacopetti, I.; Perazzi, A.; Martinello, T.; Gemignani, F.; Patruno, M. Hyaluronic acid, Manuka honey and Acemannan gel: Wound-specific applications for skin lesions. Res. Vet. Sci. 2020, 129, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lacatusu, I.; Badea, N.; Murariu, A.; Oprea, O.; Bojin, D.; Meghea, A. Antioxidant Activity of Solid Lipid Nanoparticles Loaded with Umbelliferone. Soft Mater. 2013, 11, 75–84. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z.; Jiang, H.; Wu, M.; Dong, Z.; Chen, C.; Chen, F.; Zhao, G.; Ma, P. “Bamboo-like” strong and tough sodium alginate/polyacrylate hydrogel fiber with directional controlled release for wound healing promotion. Carbohydr. Polym. 2025, 347, 122761. [Google Scholar] [CrossRef]
- Song, J.; Zhang, C.; Kong, S.; Liu, F.; Hu, W.; Su, F.; Li, S. Novel chitosan based metal-organic polyhedrons/enzyme hybrid hydrogel with antibacterial activity to promote wound healing. Carbohydr. Polym. 2022, 291, 119522. [Google Scholar] [CrossRef]
- Mao, G.; Tian, S.; Shi, Y.; Yang, J.; Li, H.; Tang, H.; Yang, W. Preparation and evaluation of a novel alginate-arginine-zinc ion hydrogel film for skin wound healing. Carbohydr. Polym. 2023, 311, 120757. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Badea, G.; Oprea, O.; Mihaila, M.A.; Kaya, D.A.; Stan, R.; Meghea, A. Lipid nanocarriers based on natural oils with high activity against oxygen free radicals and tumor cell proliferation. Mat. Sci. Eng. C Mater. 2015, 56, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-based hydrogel wound dressing: From mechanism to applications, a review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef]
- Lan, X.; Du, T.; Zhuo, J.; Wang, T.; Shu, R.; Li, Y.; Zhang, W.; Ji, Y.; Wang, Y.; Yue, X.; et al. Advances of biomacromolecule-based antibacterial hydrogels and their performance evaluation for wound healing: A review. Int. J. Biol. Macromol. 2024, 279, 135577. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Makky, S.; Nofal, R.; Hebishy, M.; Agwa, M.M.; Aly, R.G.; Abo El-Naga, M.Y.; Heikal, Y.A.; Fayez, M.S.; Rezk, N.; et al. Enhancement of wound healing via topical application of natural products: In vitro and in vivo evaluations. Arab. J. Chem. 2022, 15, 103869. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.S.; Oprea, O.C.; Negreanu-Pirjol, T.; Roncea, F.N.; Prelipcean, A.M.; Craciunescu, O.; Iosageanu, A.; Artem, V.; Ranca, A.; Motelica, L.; et al. Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants 2023, 12, 951. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Prundeanu, M.; Berger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu-Pirjol, T.; Muntean, D.; Danciu, C. Properties of Salvia officinalis L. and Thymus serpyllum L. Extracts Free and Embedded into Mesopores of Silica and Titania Nanomaterials. Nanomaterials 2020, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.; Ares, A.M.; Stephen Elmore, J.; Bernal, J. Recent trends in the analysis of honey constituents. Food Chem. 2022, 387, 132920. [Google Scholar] [CrossRef] [PubMed]
- Nezhad-Mokhtari, P.; Javanbakht, S.; Asadi, N.; Ghorbani, M.; Milani, M.; Hanifehpour, Y.; Gholizadeh, P.; Akbarzadeh, A. Recent advances in honey-based hydrogels for wound healing applications: Towards natural therapeutics. J. Drug Deliv. Sci. Technol. 2021, 66, 102789. [Google Scholar] [CrossRef]
- El-Didamony, S.E.; Gouda, H.I.A.; Zidan, M.M.M.; Amer, R.I. Bee products: An overview of sources, biological activities and advanced approaches used in apitherapy application. Biotechnol. Rep. 2024, 44, e00862. [Google Scholar] [CrossRef]
- Firmanda, A.; Mahardika, M.; Fahma, F.; Gozan, M.; Pratama, A.W.; Mardawati, E.; Millar, A.; Rahmadanis; Amelia, D.; Ya Habib, A.A. Honey-loaded 3D bioprinted scaffolds: A promising fabrication with wound healing properties. Biocatal. Agric. Biotechnol. 2024, 59, 103247. [Google Scholar] [CrossRef]
- Tunç, M.; Solmaz, R. A preliminary hazard analysis in bee pollen and propolis decontamination process: From the beehive to the laboratory. Food Control 2024, 163, 110487. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Nan, S.; Li, J.; Pi, A.; Yan, L.; Xu, J.; Hao, Y. Therapeutic effect of propolis nanoparticles on wound healing. J. Drug Deliv. Sci. Technol. 2023, 82, 104284. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, M.; Xu, Y.; Peng, X.; Zhang, M.; Wang, Q.; Du, J.; Zhang, H.; Fu, L. Electrochemical Determination of Antioxidant Activity of Different Bee Products. Int. J. Electrochem. Sci. 2019, 14, 3663–3672. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Alshaer, W.; Hatmal, M.M.; Ibrahim, A.A.; Dellinger, A.L.; Nsairat, H.; Abdaljaleel, M.; Mustafa, M.Z.; Mohamud, R. Synthesis, characterization, and wound healing activity of alginate-based polymeric nanoparticles loaded with stingless bee honey. Biocatal. Agric. Biotechnol. 2024, 60, 103329. [Google Scholar] [CrossRef]
- Lotfinia, F.; Norouzi, M.R.; Ghasemi-Mobarakeh, L.; Naeimirad, M. Anthocyanin/Honey-Incorporated Alginate Hydrogel as a Bio-Based pH-Responsive/Antibacterial/Antioxidant Wound Dressing. J. Funct. Biomater. 2023, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Vasile, B.S.; Ficai, A.; Surdu, A.V.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; Volume 41, p. 352.
- Khan, K.; Malik, K.; Ahmad, M.; Qureshi, R.; Aziz, M.A.; Gul, S.; Al-Qahtani, W.H.; Khan, R. Diversity of melliferous Flora (Apiaries) in Honey and microscopic authentication using LM and SEM Techniques. Flora 2024, 312, 152477. [Google Scholar] [CrossRef]
- Cárdenas-Escudero, J.; Galán-Madruga, D.; Cáceres, J.O. FTIR-ATR detection method for emerging C3-plants-derivated adulterants in honey: Beet, dates, and carob syrups. Talanta 2023, 265, 124768. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Torkay, G.; İdil, N.; Akar, R.O.; Özbaş, Z.; Özkahraman, B. Propolis-loaded photocurable methacrylated pullulan films: Evaluation of mechanical, antibacterial, biocompatibility, wound healing and pro-angiogenic abilities. Int. J. Biol. Macromol. 2024, 282, 137071. [Google Scholar] [CrossRef]
- Chen, D.; Guo, C.; Lu, W.; Zhang, C.; Xiao, C. Rapid quantification of royal jelly quality by mid-infrared spectroscopy coupled with backpropagation neural network. Food Chem. 2023, 418, 135996. [Google Scholar] [CrossRef]
- Hashemirad, F.-S.; Behfar, M.; Kavoosi, G. Proximate composition, physico-chemical, techno-functional, amino acid profile, fatty acid profile, nutritional quality, antioxidant, anti-amylase and anti-lipase properties of bee bread, royal jelly, and bee propolis. LWT 2024, 200, 116190. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, V.-A.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Mustățea, G.; Ungureanu, E.L.; Dobre, A.A. Antibacterial activity of zinc oxide nanoparticles loaded with essential oils. Pharmaceutics 2023, 15, 2470. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.D.; Andronescu, E.; Holban, A.M. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Petrisor, G.; Oprea, O.C.; Trușcǎ, R.D.; Ficai, A.; Andronescu, E.; Hudita, A.; Holban, A.M. Antimicrobial Hydroxyethyl-Cellulose-Based Composite Films with Zinc Oxide and Mesoporous Silica Loaded with Cinnamon Essential Oil. Pharmaceutics 2024, 16, 1225. [Google Scholar] [CrossRef]
- Liang, H.; He, X.; Li, X.; Semiruomi, D.; Yan, F. Effect of Royal Gel addition to chitosan matrix for wound dress applications: Fabrication, characterization and artificial neural network analysis. Environ. Technol. Innov. 2023, 30, 103077. [Google Scholar] [CrossRef]
- Deng, J.L.; Liu, R.; Lu, Q.; Hao, P.Y.; Xu, A.Q.; Zhang, J.L.; Tan, J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Balázs, V.L.; Nagy-Radványi, L.; Filep, R.; Kerekes, E.; Kocsis, B.; Kocsis, M.; Farkas, A. In Vitro Antibacterial and Antibiofilm Activity of Hungarian Honeys against Respiratory Tract Bacteria. Foods 2021, 10, 1632. [Google Scholar] [CrossRef]
- Lukasiewicz, M.; Kowalski, S.; Makarewicz, M. Antimicrobial an antioxidant activity of selected Polish herbhoneys. LWT-Food Sci. Technol. 2015, 64, 547–553. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Green, K.J.; Lawag, I.L.; Locher, C.; Hammer, K.A. Correlation of the antibacterial activity of commercial manuka and Leptospermum honeys from Australia and New Zealand with methylglyoxal content and other physicochemical characteristics. PLoS ONE 2022, 17, e0272376. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, M.C.; Izah, S.C. Honey as a Natural Antimicrobial. Antibiotics 2025, 14, 255. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, J.R.; Wang, Y.B.; Li, T.T.; Zhao, J.; Zhang, C.H. Green tea polyphenols function as prooxidants to inhibit Pseudomonas aeruginosa and induce the expression of oxidative stress-related genes. Folia Microbiol. 2013, 58, 211–217. [Google Scholar] [CrossRef]
- Nagy-Radványi, L.; Balázs, V.L.; Kocsis, B.; Csikós, E.; Angyán, V.D.; Szabó, P.; Biró, V.; Kocsis, M.; Farkas, A. Antibacterial activity of Hungarian varietal honeys against respiratory pathogens as a function of storage time. Sci. Rep. 2024, 14, 10200. [Google Scholar] [CrossRef]
- Al-Waili, N.; Al-Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Salom, K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 2012, 9, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Pleeging, C.C.F.; Coenye, T.; Mossialos, D.; de Rooster, H.; Chrysostomou, D.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication. Antibiotics 2020, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Alandejani, T.; Marsan, J.; Ferris, W.; Slinger, R.; Chan, F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol. Head Neck Surg. 2009, 141, 114–118. [Google Scholar] [CrossRef]
- Tanaka, S.; Yasuda, T.; Hamada, Y.; Kawaguchi, N.; Fujishita, Y.; Mori, S.; Yokoyama, Y.; Yamamoto, H.; Kogo, M. Synthetic peptide SVVYGLR upregulates cell motility and facilitates oral mucosal wound healing. Peptides 2020, 134, 170405. [Google Scholar] [CrossRef] [PubMed]
- Millones-Gómez, P.A.; De la Garza-Ramos, M.A.; Urrutia-Baca, V.H.; Hernandez-Martinez, H.C.; Hernández Marín, D.A.; Minchón Medina, C.A. Cytotoxicity of Peruvian propolis and Psidium guajava on human gingival fibroblasts, PBMCs and HeLa cells. F1000Research 2022, 11, 430. [Google Scholar] [CrossRef]
- Gruber, S.; Nickel, A. Toxic or not toxic? The specifications of the standard ISO 10993-5 are not explicit enough to yield comparable results in the cytotoxicity assessment of an identical medical device. Front. Med. Technol. 2023, 5, 1195529. [Google Scholar] [CrossRef]
- Lin, Y.; Shao, Q.Q.; Zhang, M.; Lu, C.Y.; Fleming, J.; Su, S.K. Royal jelly-derived proteins enhance proliferation and migration of human epidermal keratinocytes in an in vitro scratch wound model. BMC Complement. Altern. Med. 2019, 19, 175. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Saramet, V.; Stan, M.S.; Ripszky Totan, A.; Țâncu, A.M.C.; Voicu-Balasea, B.; Enasescu, D.S.; Rus-Hrincu, F.; Imre, M. Analysis of Gingival Fibroblasts Behaviour in the Presence of 3D-Printed versus Milled Methacrylate-Based Dental Resins-Do We Have a Winner? J. Funct. Biomater. 2024, 15, 147. [Google Scholar] [CrossRef]
- Sánchez-Martán, V.; Morales, P.; Iriondo-DeHond, A.; Hospital, X.F.; Fernández, M.; Hierro, E.; Haza, A.I. Differential Apoptotic Effects of Bee Product Mixtures on Normal and Cancer Hepatic Cells. Antioxidants 2023, 12, 615. [Google Scholar] [CrossRef]
- Ranzato, E.; Martinotti, S.; Burlando, B. Honey exposure stimulates wound repair of human dermal fibroblasts. Burn. Trauma 2013, 1, 2321–3868. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. Honey as a topical antibacterial agent for treatment of infected wounds. World Wide Wounds 2001, 10, 1–13. [Google Scholar]


| Sample | Alginate | Honey | Propolis | Royal Jelly | Chitosan | Glycerol |
|---|---|---|---|---|---|---|
| A | 1 g | - | - | - | 0.4 g | 0.1 g |
| AH | 1 g | 3.5 g | - | - | 0.4 g | 0.1 g |
| AP | 1 g | - | 2 mL | - | 0.4 g | 0.1 g |
| ARj | 1 g | - | - | 0.5 g | 0.4 g | 0.1 g |
| AM | 1 g | 3.5 g | 2 mL | 0.5 g | 0.4 g | 0.1 g |
| Sample | T1% * | T5% * | T10% * | Mass Loss RT-115 °C | Mass Loss 115–460 °C | Mass Loss 460–720 °C |
|---|---|---|---|---|---|---|
| AM | 95.3 °C | 132.4 °C | 152.7 °C | 2.03% | 59.92% | 15.25% |
| A | 50.1 °C | 94.6 °C | 156.7 °C | 6.94% | 53.18% | 21.65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitru, C.D.; Neacșu, I.A.; Oprea, O.C.; Motelica, L.; Voicu Balasea, B.; Ilie, C.-I.; Marinescu, F.; Ripszky, A.; Pituru, S.-M.; Andronescu, E. Biomaterials Based on Bee Products and Their Effectiveness in Soft Tissue Regeneration. Materials 2025, 18, 2689. https://doi.org/10.3390/ma18122689
Dumitru CD, Neacșu IA, Oprea OC, Motelica L, Voicu Balasea B, Ilie C-I, Marinescu F, Ripszky A, Pituru S-M, Andronescu E. Biomaterials Based on Bee Products and Their Effectiveness in Soft Tissue Regeneration. Materials. 2025; 18(12):2689. https://doi.org/10.3390/ma18122689
Chicago/Turabian StyleDumitru, Corina Dana, Ionela Andreea Neacșu, Ovidiu Cristian Oprea, Ludmila Motelica, Bianca Voicu Balasea, Cornelia-Ioana Ilie, Florica Marinescu, Alexandra Ripszky, Silviu-Mirel Pituru, and Ecaterina Andronescu. 2025. "Biomaterials Based on Bee Products and Their Effectiveness in Soft Tissue Regeneration" Materials 18, no. 12: 2689. https://doi.org/10.3390/ma18122689
APA StyleDumitru, C. D., Neacșu, I. A., Oprea, O. C., Motelica, L., Voicu Balasea, B., Ilie, C.-I., Marinescu, F., Ripszky, A., Pituru, S.-M., & Andronescu, E. (2025). Biomaterials Based on Bee Products and Their Effectiveness in Soft Tissue Regeneration. Materials, 18(12), 2689. https://doi.org/10.3390/ma18122689










