Effects of Forging Temperature and Micro-Arc Coatings on the Static/Stress Corrosion Resistance of AZ80 Magnesium Alloy
Abstract
1. Introduction
2. Materials and Methods
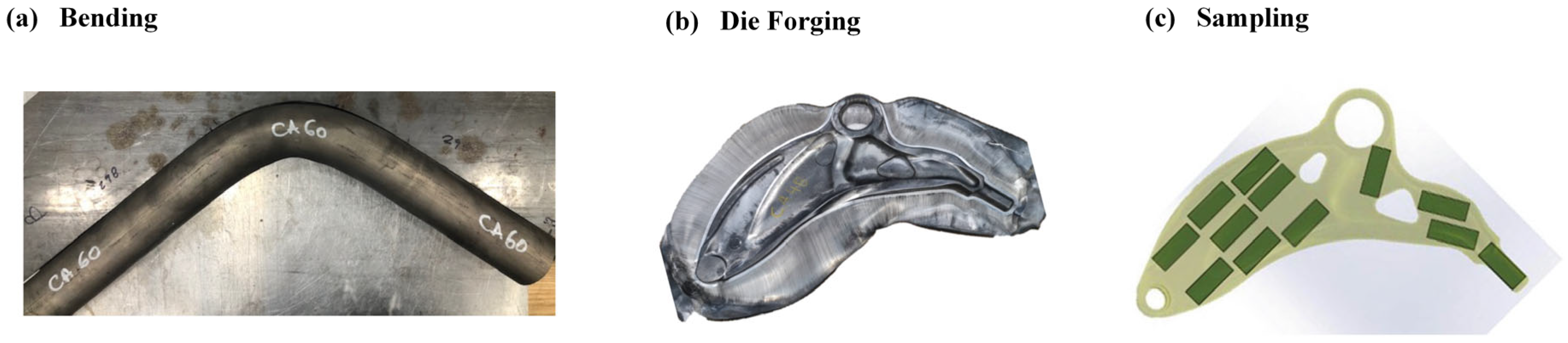
2.1. Experimental Materials and Specimen Preparation
2.2. Preparation of MAO and MCC Coatings
2.3. Experimental Methods
2.3.1. Salt Spray Accelerated Corrosion Test
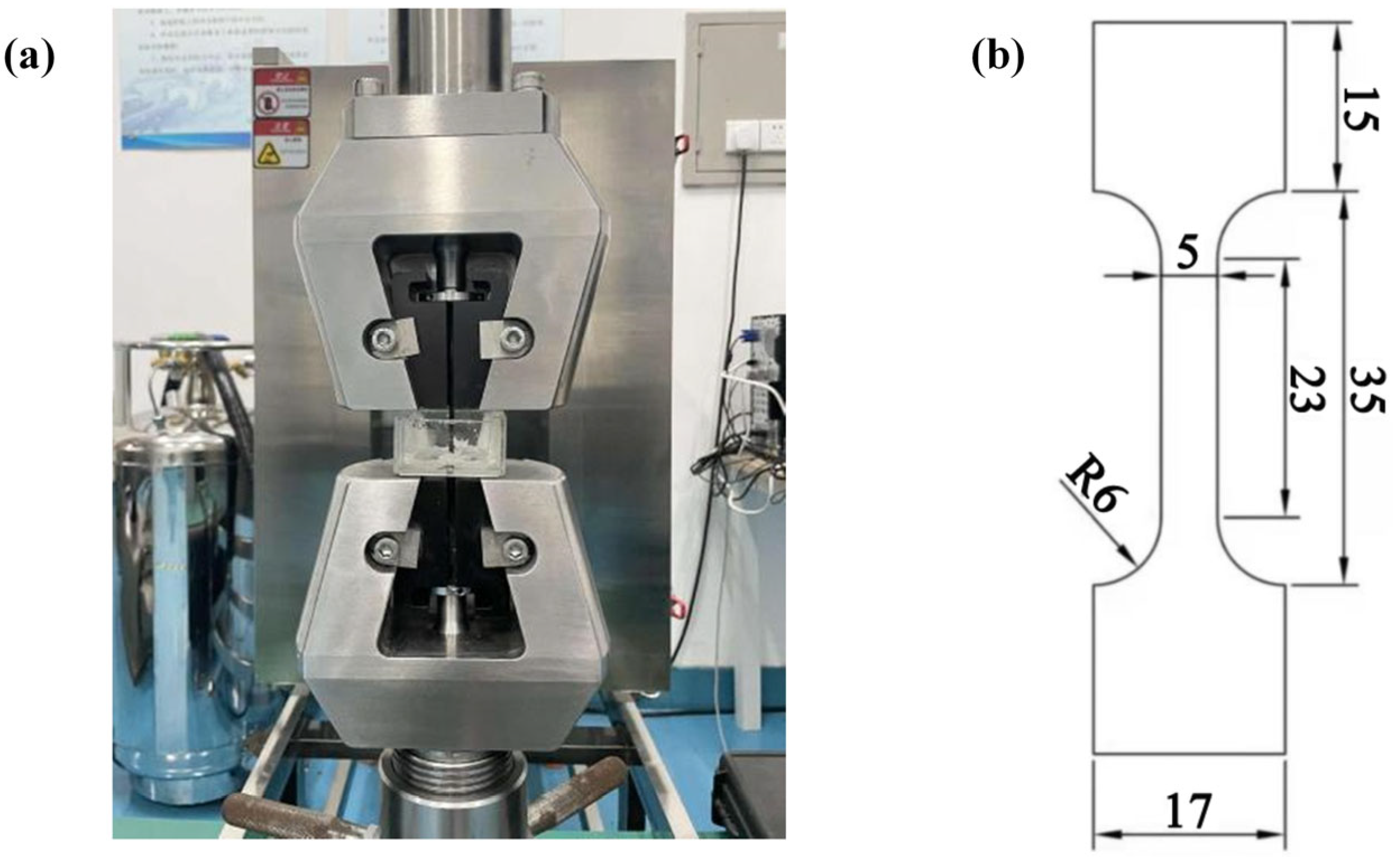
2.3.2. Stress Corrosion Cracking Test
3. Results and Discussion
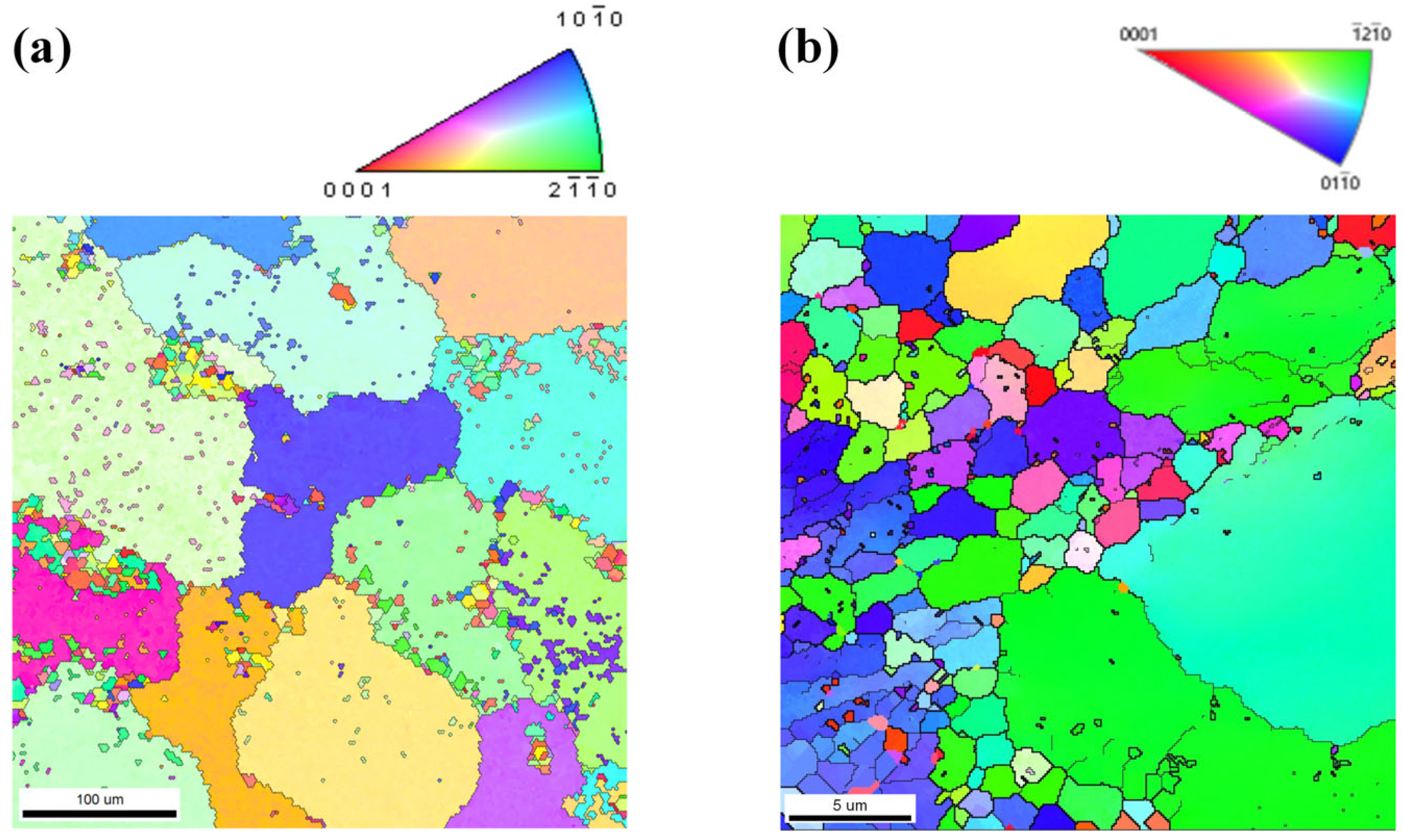
3.1. Microstructure
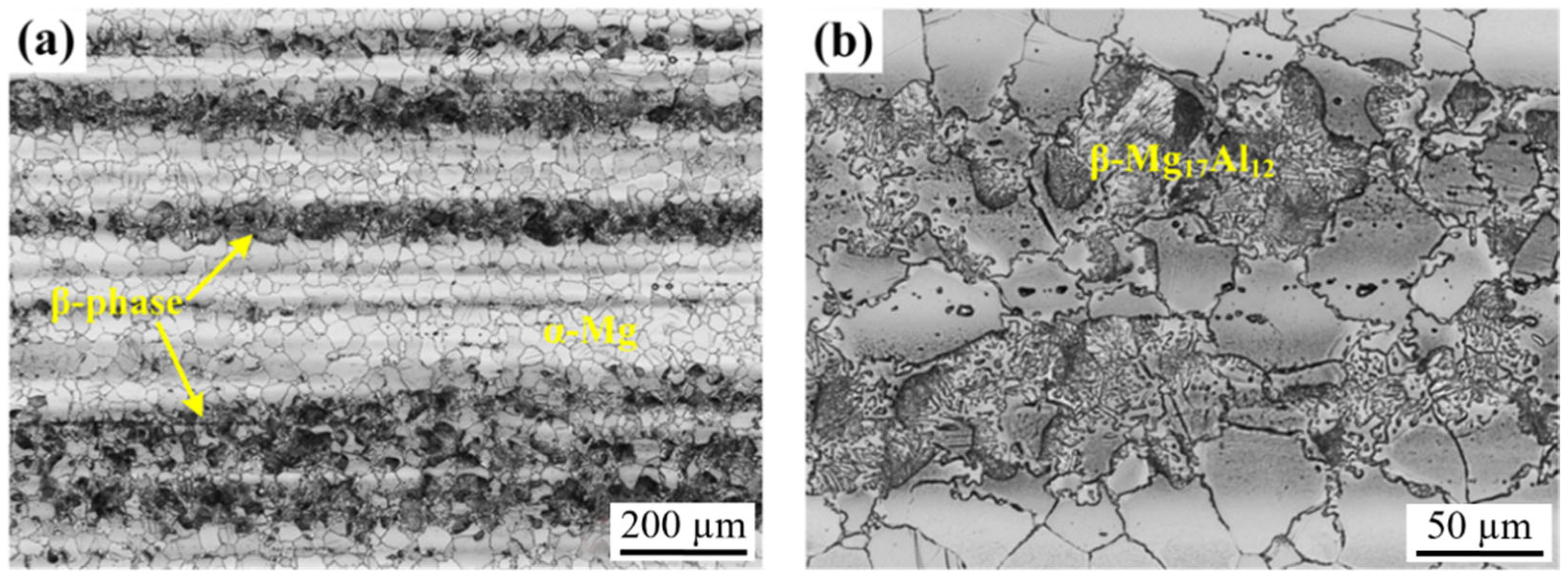
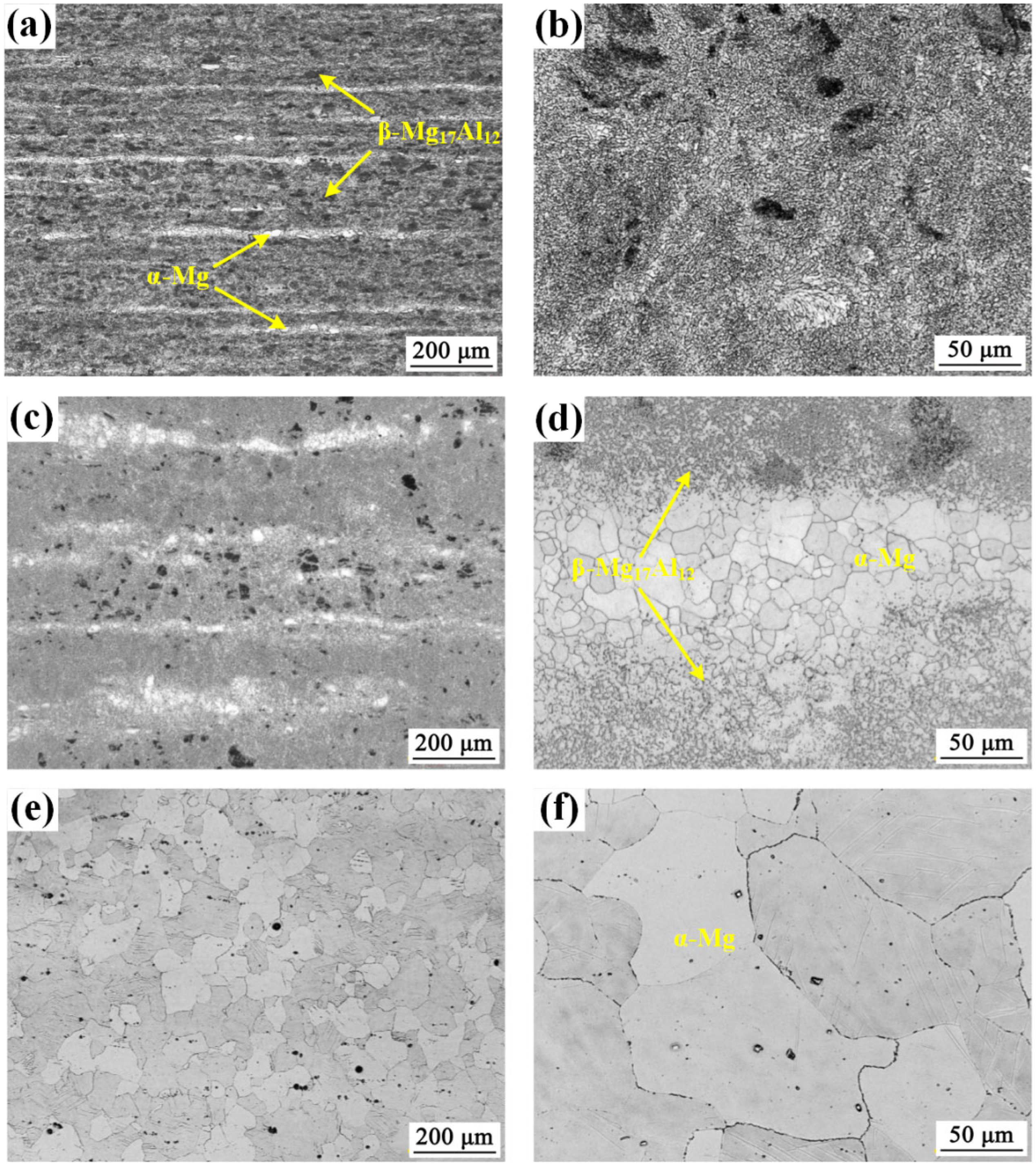
3.1.1. Microstructure Evolution of AZ80 Magnesium Alloy
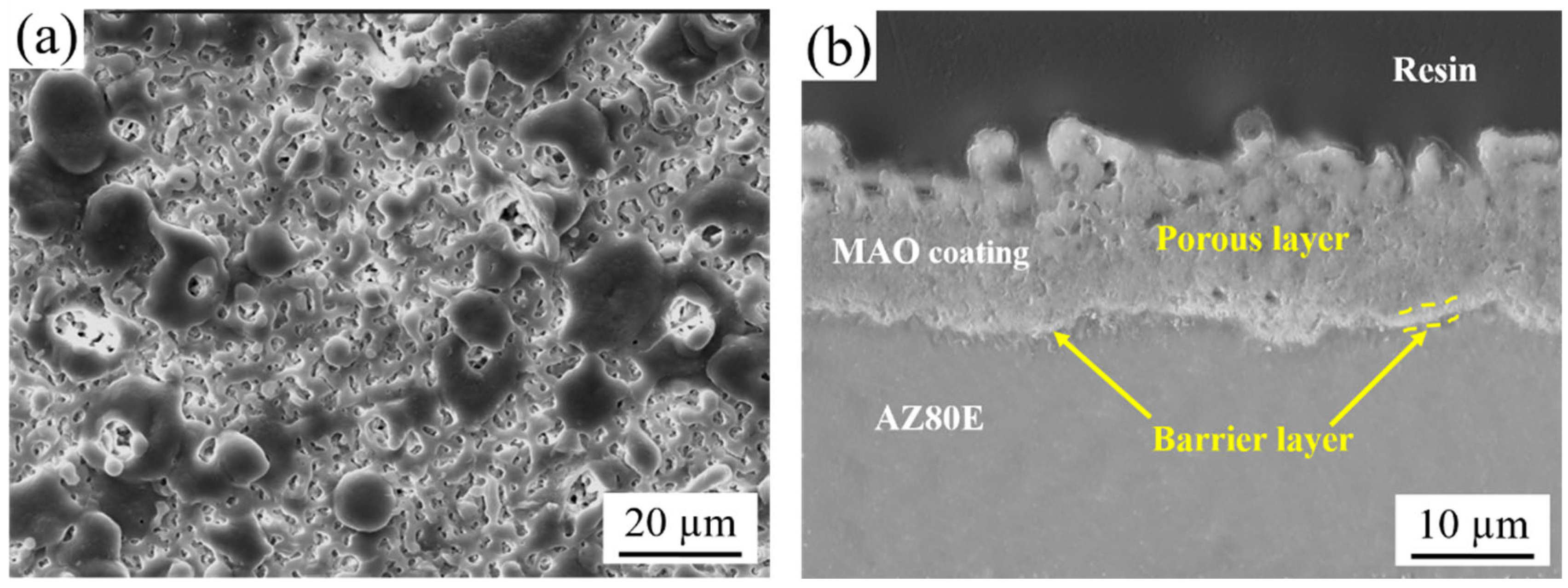
3.1.2. Microstructure of MAO and MCC Coatings
3.2. Accelerated Corrosion Behavior in Salt Spray
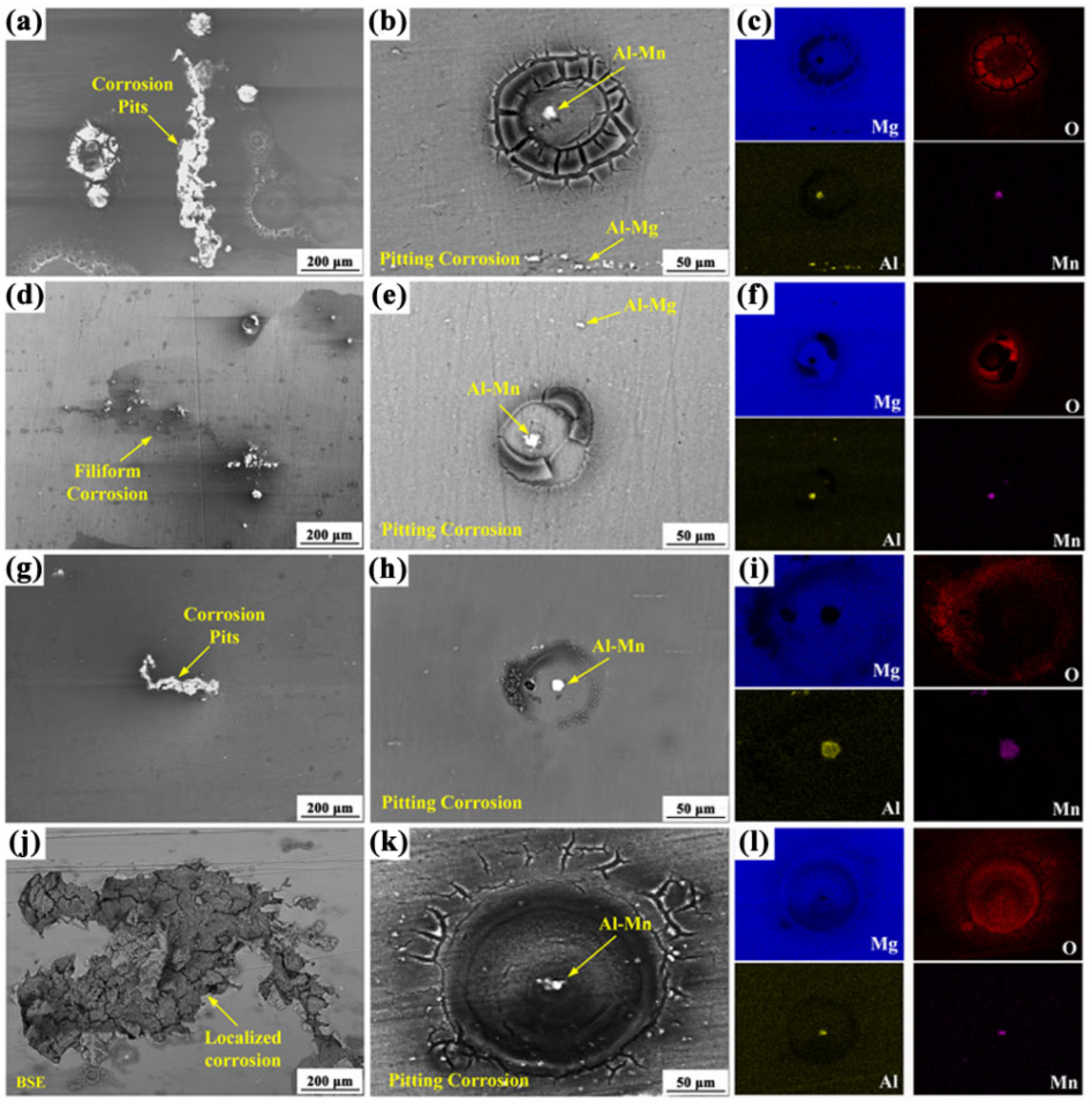
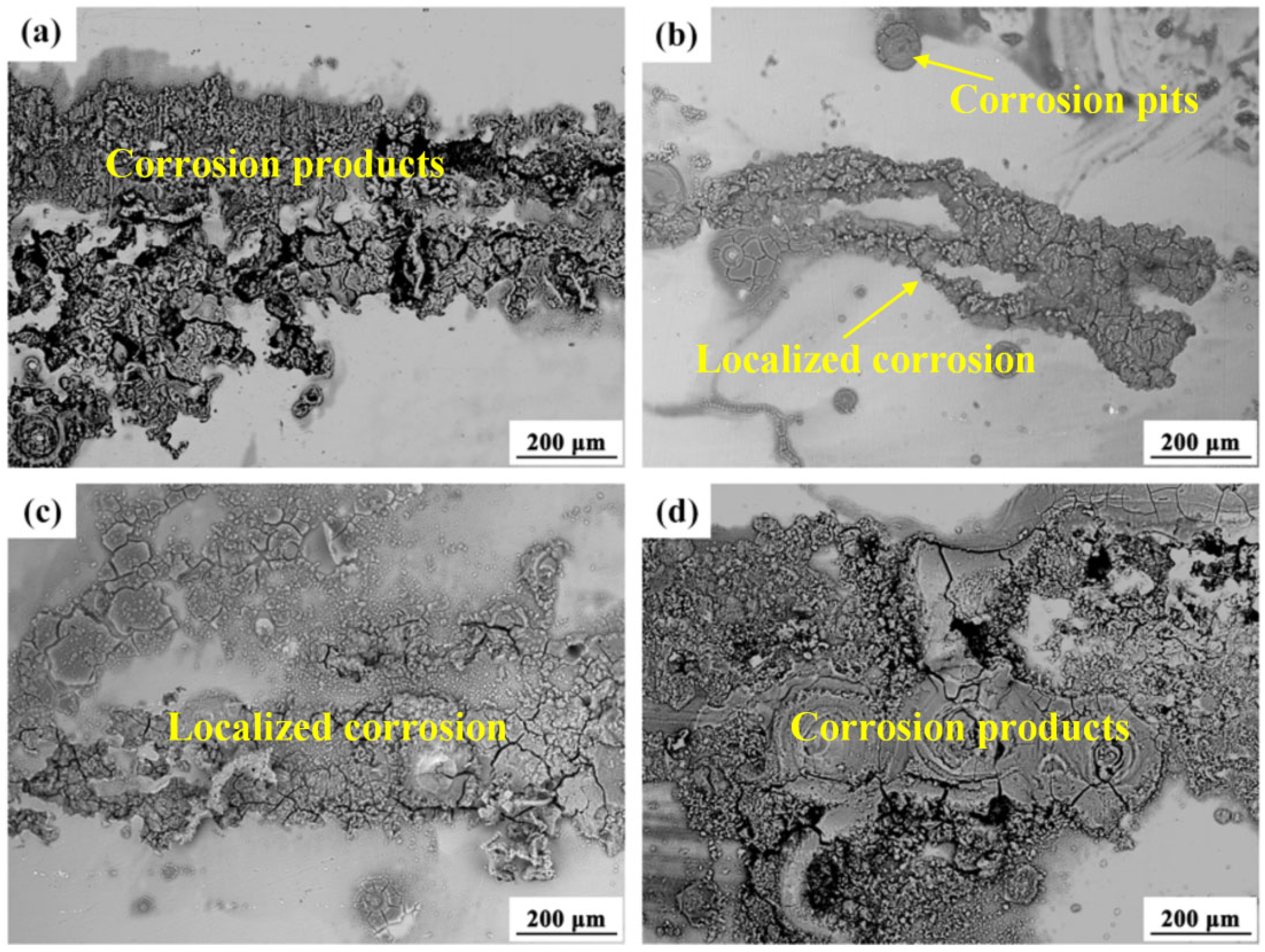
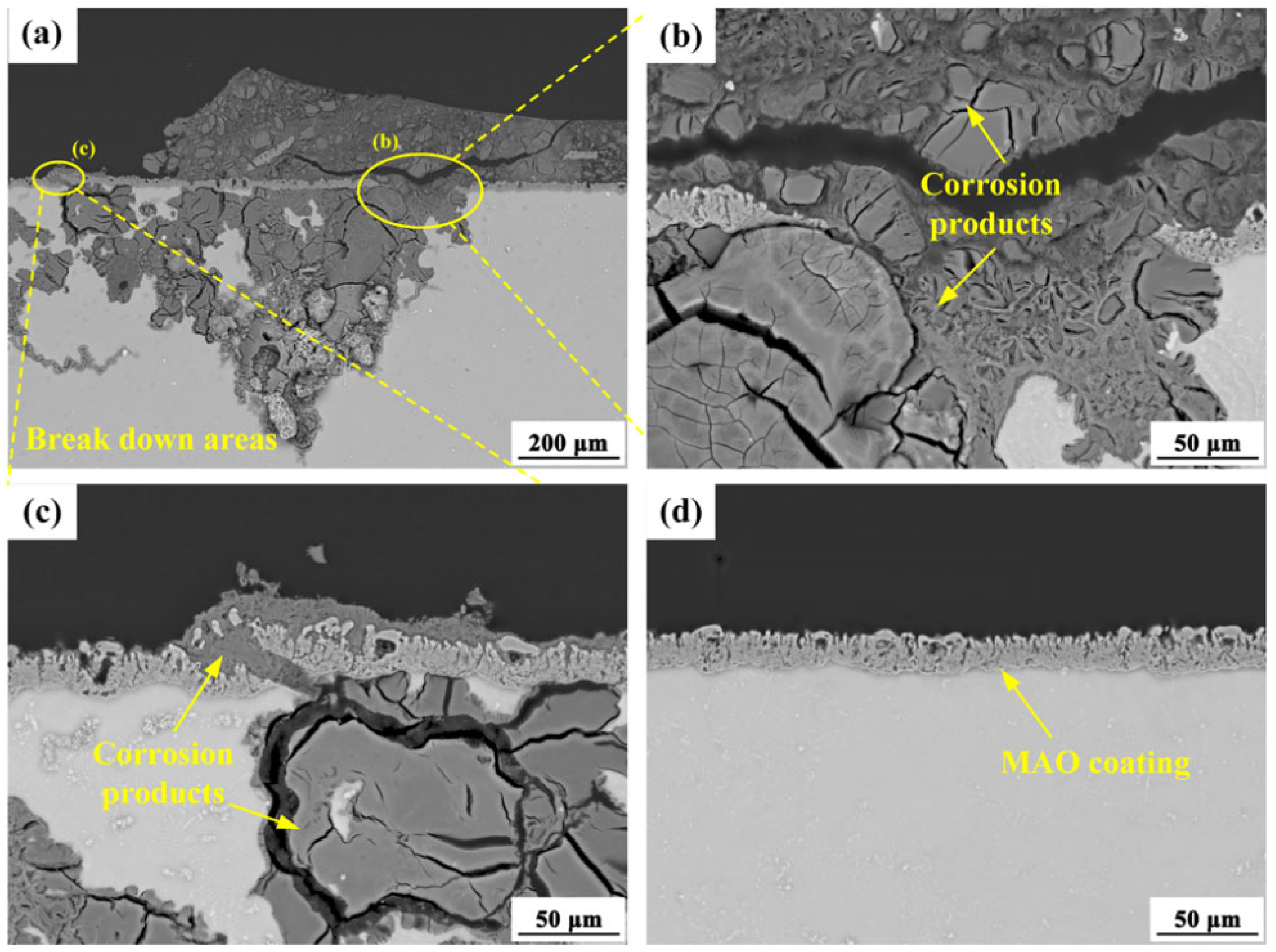
3.2.1. Corrosion Behavior of AZ80 Alloys and MAO Coating
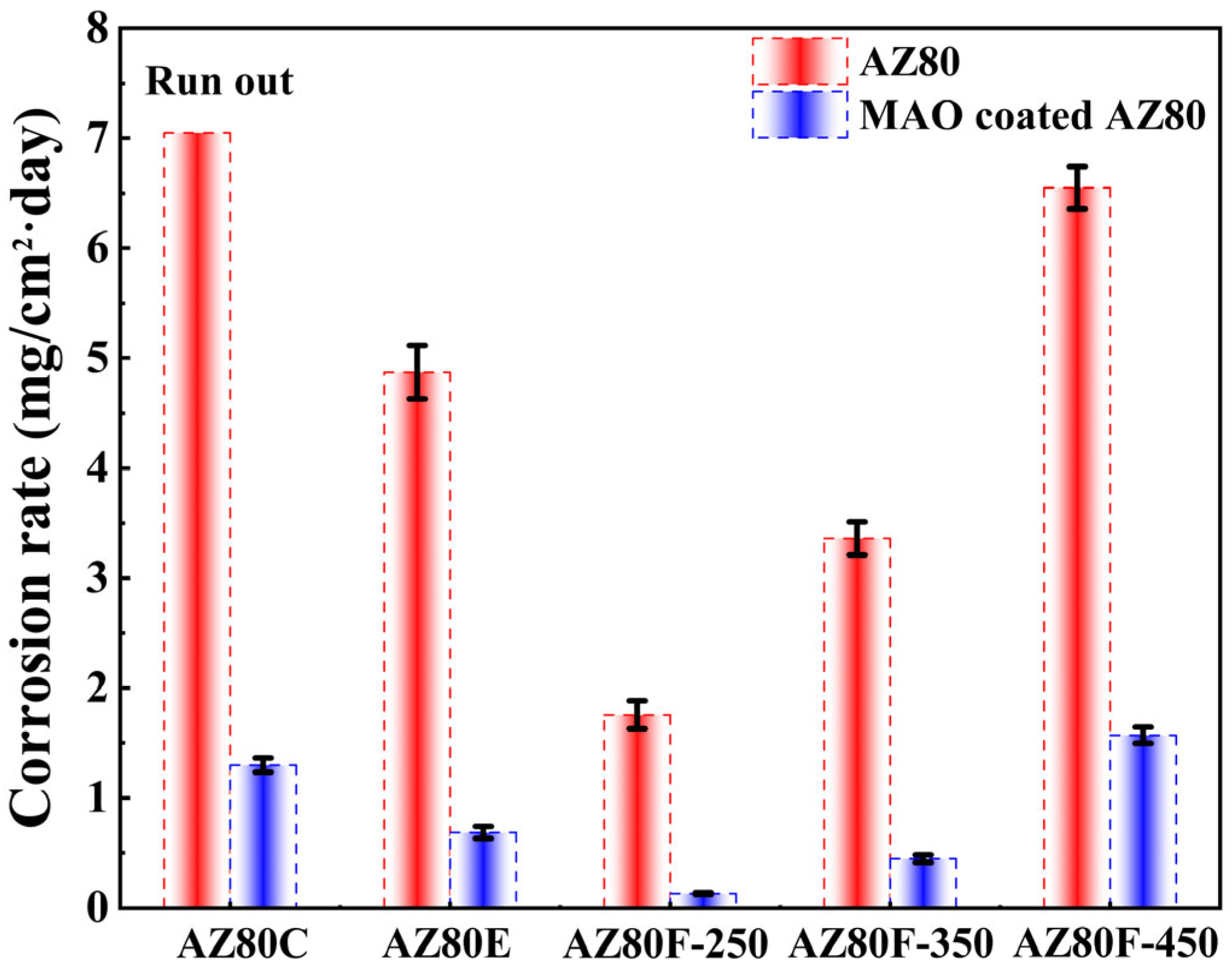
3.2.2. Mass Loss Measurement
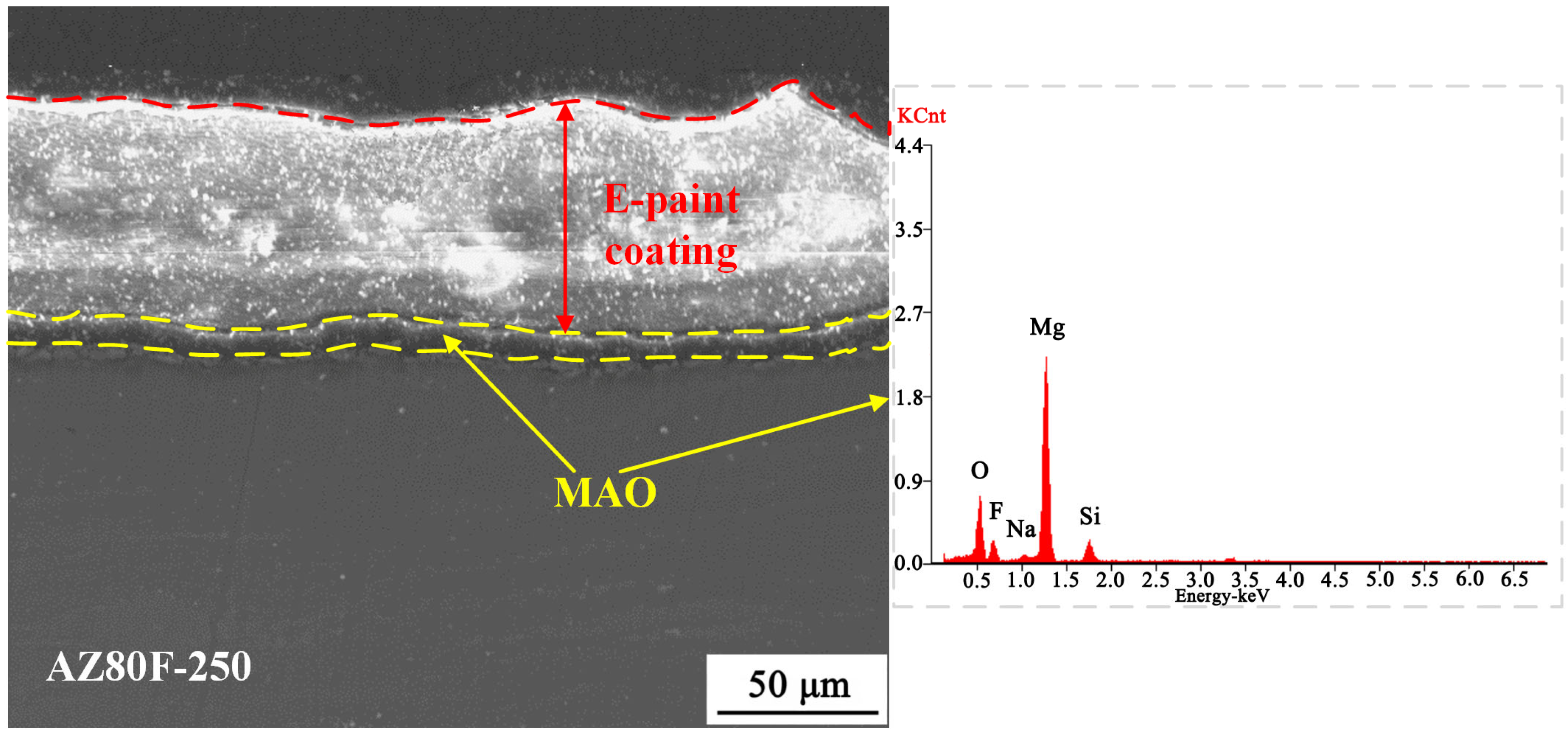
3.2.3. Corrosion Behavior of MCC Coating
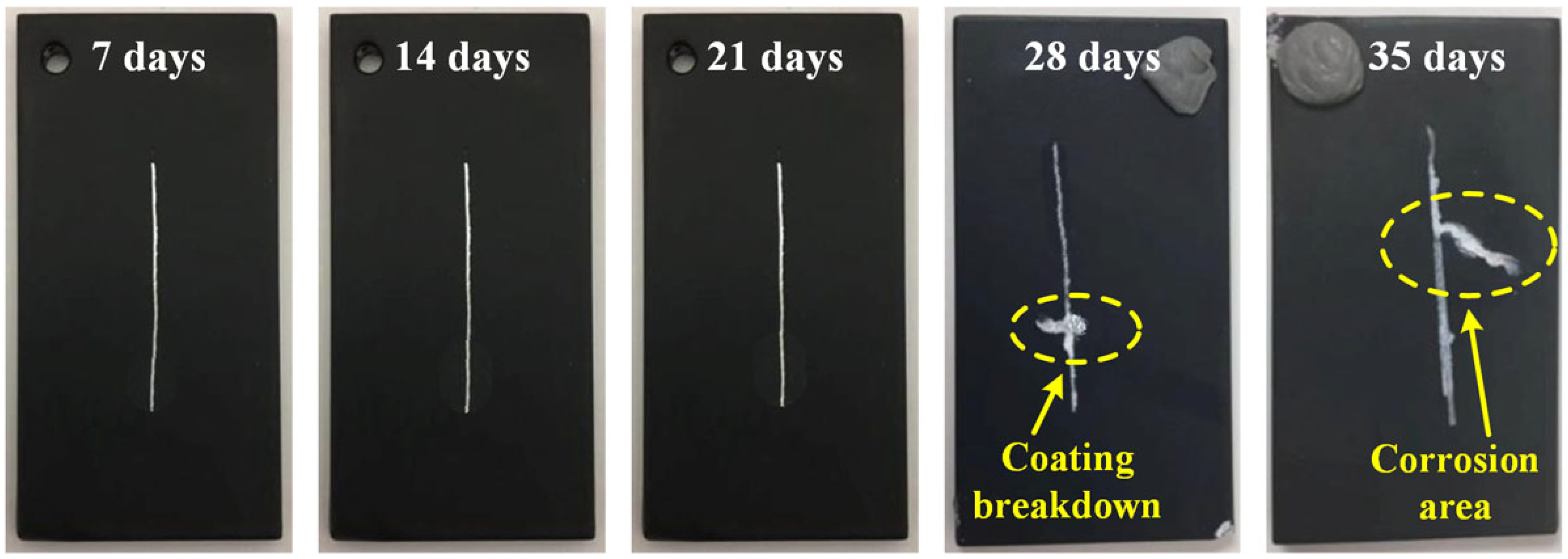
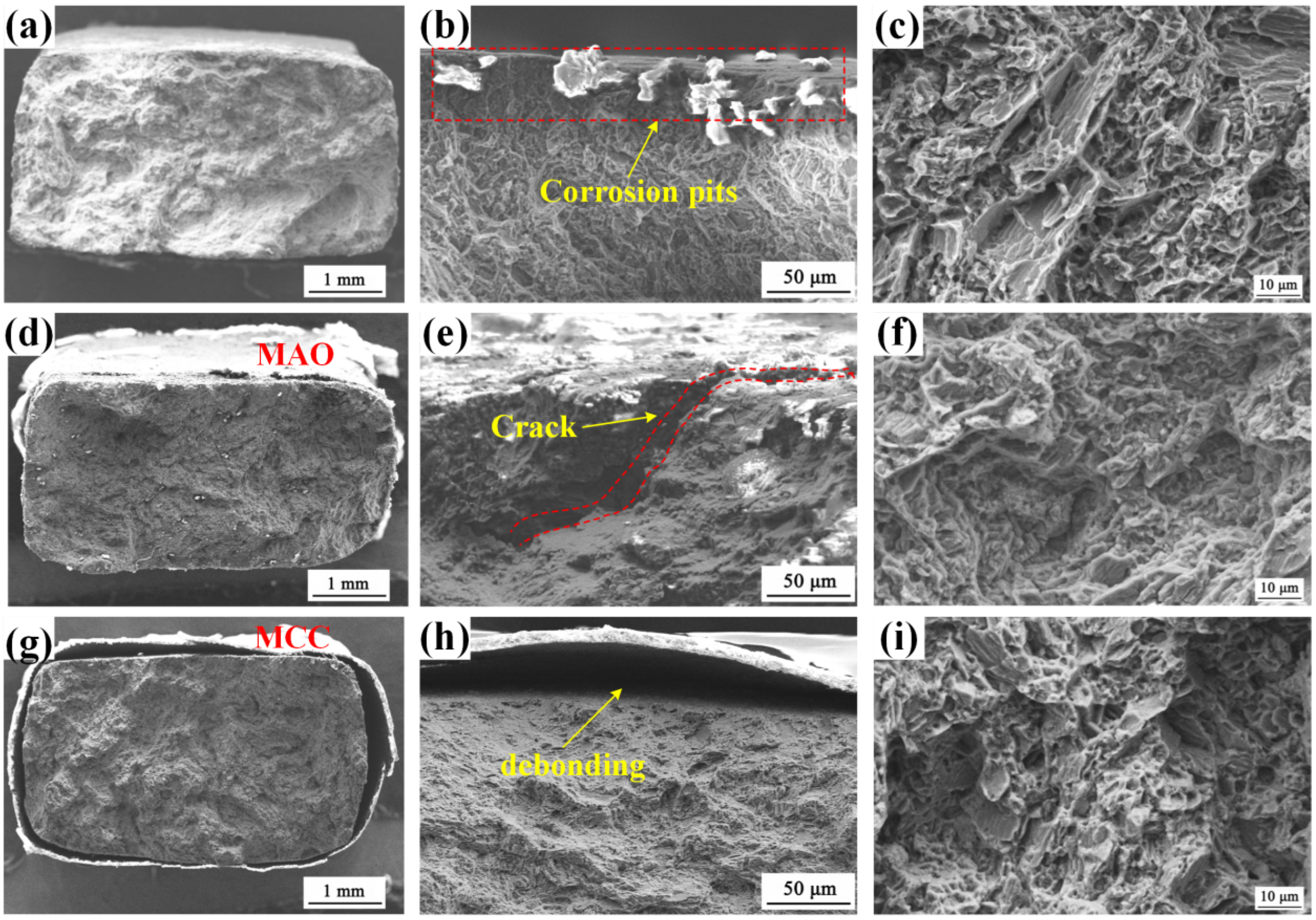
3.3. Stress Corrosion Cracking Behavior
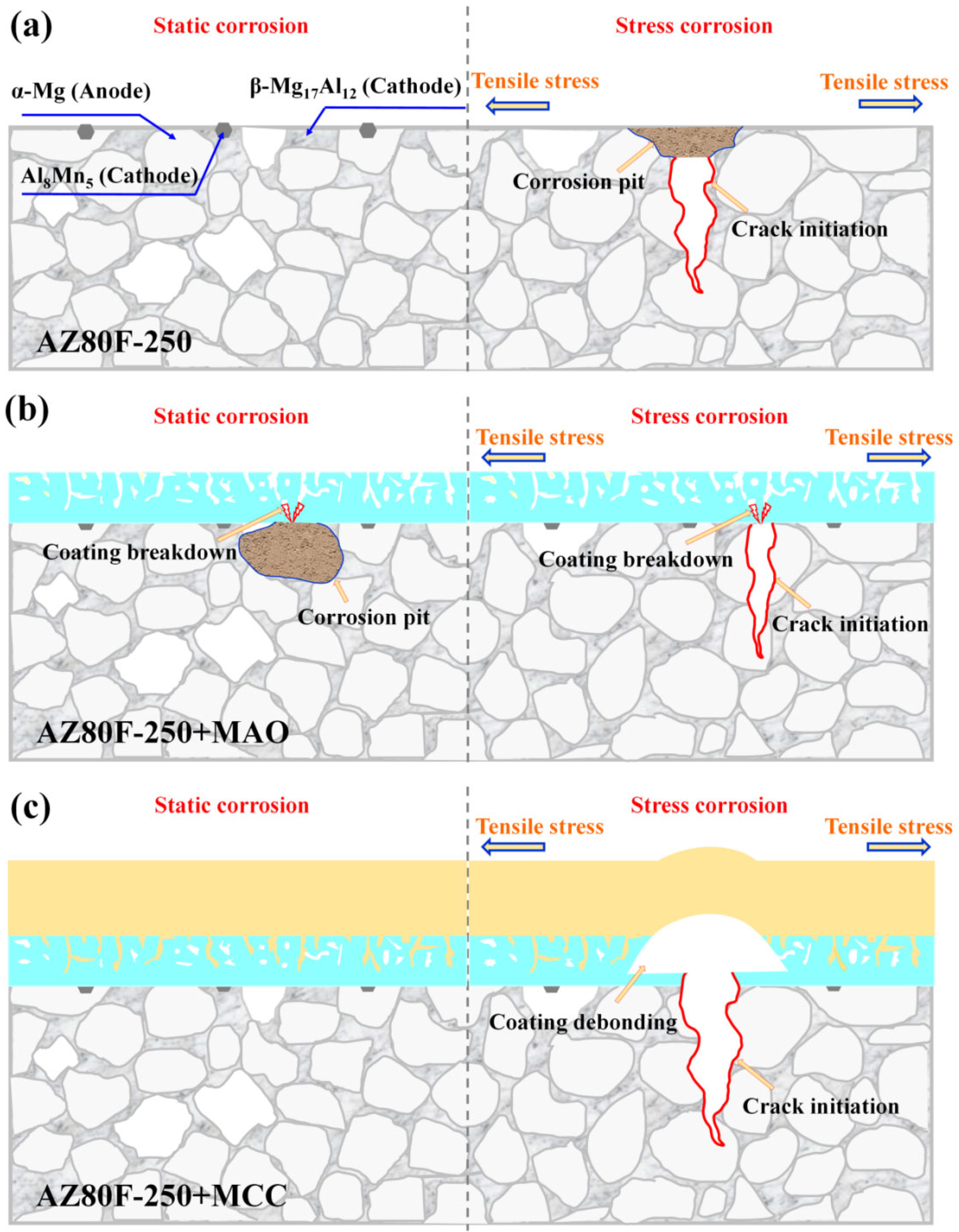
3.4. Analysis of Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Satya Prasad, S.; Prasad, S.; Verma, K.; Mishra, R.; Singh, V. The role and significance of magnesium in modern day research—A review. J. Magnes. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Z.; Han, S.; Gu, Y.; Zhu, Z.; Zhang, H. Evolution, limitations, advantages, and future challenges of magnesium alloys as materials for aerospace applications. J. Alloys Compd. 2024, 1008, 176707. [Google Scholar] [CrossRef]
- He, H.; Huang, S.; Yi, Y.; Guo, W. Simulation and experimental research on isothermal forging with semi-closed die and multi-stage-change speed of large AZ80 magnesium alloy support beam. J. Mater. Process. Technol. 2017, 246, 198–204. [Google Scholar] [CrossRef]
- Jonghun, Y.; Junghwan, L. Process design of warm-forging with extruded Mg-8Al-0.5Zn alloy for differential case in automobile transmission. Int. J. Precis. Eng. Manuf. 2015, 16, 841–846. [Google Scholar]
- Alhosseini, A.; Karbasi, M.; Chaharmahali, R.; Fardosi, A.; Kaseem, M. An overview of electrochemical, non-electrochemical and analytical approaches for studying corrosion in magnesium and its alloys. J. Magnes. Alloys 2024, 12, 3516–3542. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Wang, M.; Sun, C.; Wang, X.; Li, J.R.; Li, J.Y. Corrosion behavior of AZ91 magnesium alloys in harsh marine atmospheric environment in south China sea. J. Mater. Res. Technol. 2025, 35, 2477–2486. [Google Scholar] [CrossRef]
- Hong, B.; Bao, D.; Yuan, C.; Jiang, L.; Li, Q.; Zhang, X.; Li, X.; Deng, Z. Microstructure, wear and corrosion behavior of AZ91D magnesium alloys fabricated by laser surface-modification. Surf. Coat. Technol. 2025, 498, 131863. [Google Scholar] [CrossRef]
- Cao, J.; Ma, B.; Xu, C.; Li, L.; Li, X.; Wang, X. Research progress on superhydrophobic surface corrosion prevention of magnesium alloys: A review. Mater. Today Commun. 2024, 41, 110962. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Techno. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Dubey, D.; Kadali, K.; Panda, S.; Kumar, A.; Jayant, J.; Mondal, K.; Singh, S. Comparative study on the stress corrosion cracking susceptibility of AZ80 and AZ31 magnesium alloys. Mater. Sci. Eng. A 2020, 792, 139793. [Google Scholar] [CrossRef]
- Jiang, J.; Geng, X.; Zhang, X. Stress corrosion cracking of magnesium alloys: A review. J. Magnes. Alloys 2023, 11, 1906–1930. [Google Scholar] [CrossRef]
- Aiken, T.A.; McPolin, D.; Russell, M.; Madden, M.; Bagnall, L. Physical and mechanical performance of magnesium-based construction boards: A comparative study. Constr. Build. Mater. 2021, 270, 121397. [Google Scholar] [CrossRef]
- Wan, Y.; Tang, B.; Gao, Y.; Tang, L.; Sha, G.; Zhang, B.; Liang, N.; Liu, C.; Jiang, S.; Chen, Z.; et al. Bulk nanocrystalline high-strength magnesium alloys prepared via rotary swaging. Acta Mater. 2020, 200, 274–286. [Google Scholar] [CrossRef]
- Ramezani, S.M.; Zarei-Hanzaki, A.; Abedi, H.R.; Salandari-Rabori, A.; Minarik, P. Achievement of fine-grained bimodal microstructures and superior mechanical properties in a multi-axially forged GWZ magnesium alloy containing LPSO structures. J. Alloys Compd. 2019, 793, 134–145. [Google Scholar] [CrossRef]
- Toscano, D.; Shaha, S.K.; Behravesh, B.; Jahed, H.; Williams, B. Effect of forging on the low cycle fatigue behavior of cast AZ31B alloy. Mater. Sci. Eng. A 2017, 706, 342–356. [Google Scholar] [CrossRef]
- Wang, J.; Pang, X.; Jahed, H. Surface protection of Mg alloys in automotive applications: A review. AIMS Mater. Sci. 2019, 6, 567–600. [Google Scholar] [CrossRef]
- Xiong, Y.; Jiang, Y. Compressive deformation of rolled AZ80 magnesium alloy along different material orientations. J. Mater. Sci. 2020, 55, 4043–4053. [Google Scholar] [CrossRef]
- You, S.; Huang, Y.; Kainer, K.U.; Hort, N. Recent research and development on wrought magnesium alloys. J. Magnes. Alloys 2017, 5, 239–253. [Google Scholar] [CrossRef]
- Gryguc, A.; Behravesh, S.B.; Shaha, S.K.; Jahed, H.; Wells, M.; Williams, B.; Su, X. Multiaxial cyclic behavior of extruded and forged AZ80 Mg alloy. Int. J. Fatigue 2019, 127, 324–337. [Google Scholar] [CrossRef]
- Gryguc, A.; Behravesh, S.B.; Jahed, H. Effect of thermomechanical processing defects on fatigue and fracture behavior of forged magnesium. Frat. Integrita Strut. 2021, 55, 213–227. [Google Scholar]
- Xiong, Y.; Shen, Y.; He, L.; Yang, Z.; Song, R. Stress corrosion cracking behavior of LSP/MAO treated magnesium alloy during SSRT in a simulated body fluid. J. Magnes. Alloys 2020, 822, 153707. [Google Scholar] [CrossRef]
- Srinivasan, P.B.; Blawert, C.; Dietzel, W. Effect of plasma electrolytic oxidation coating on the stress corrosion cracking behaviour of wrought AZ61 magensium alloy. Corros. Sci. 2008, 50, 2415–2418. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, H.; Yao, B.; Qin, Z.; Zhang, Q. Corrosion resistance property of the ceramic coating obtained through microarc oxidation on the AZ31 magnesium alloy surfaces. Surf. Coat. Technol. 2007, 201, 4905–4908. [Google Scholar] [CrossRef]
- Azqadan, E.; Anousheh, A.; Jahed, H. The effect of Mg17Al12 intermetallic compound on dynamic recrystallization of cast-forged AZ80 magnesium alloy. J. Alloys Compd. 2025, 1010, 177336. [Google Scholar] [CrossRef]
- Sager, C.A.; Yakubtsov, I.; MacDonald, W.D.; Shook, S.; Diak, B.J.; Niewczas, M. Physical metallurgy of Mg AZ80 alloys for forging applications. Mater. Soc. Annu. Meet. 2009, 12, 405–410. [Google Scholar]
- Xue, Y.; Pang, X.; Jiang, B.; Jahed, H. Corrosion and corrosion fatigue performances of micro-arc oxidation coating on AZ31B cast magnesium alloy. Mater. Corros. 2019, 70, 268–280. [Google Scholar] [CrossRef]
- Xue, Y.; Pang, X.; Jiang, B.; Jahed, H.; Wang, D. Characterization of the corrosion performances of as-cast Mg-Al and Mg-Zn magnesium alloys with microarc oxidation coatings. Mater. Corros. 2020, 71, 992–1006. [Google Scholar] [CrossRef]
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM: West Conshohocken, PA, USA, 2019.
- ASTM G1-03; Standard Practice for Preparing Cleaning, and Evaluating Corrosion Test Specimen. ASTM: West Conshohocken, PA, USA, 2003.
- ASTM D1654-24; Standard Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive Environments. ASTM: West Conshohocken, PA, USA, 2024.
- Wang, L.; Mostaed, E.; Cao, X.; Huang, G.; Fabrizi, A.; Bonollo, F.; Chi, C.; Vedani, M. Effect of texture and grain size on mechanical properties of AZ80 magnesium alloys at lower temperatures. Mater. Des. 2016, 89, 1–8. [Google Scholar] [CrossRef]
- Prakash, P.; Toscano, D.; Shaha, S.K.; Wells, M.A.; Jahed, H.; Williams, B.W. Effect of temperature on the hot deformation behavior of AZ80 magnesium alloy. Mater. Sci. Eng. A 2020, 794, 139923. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Liao, W.; Guo, W.; Ye, B.; Jiang, H.; Ding, W. Effect of homogenization on the microstructure and mechanical properties of the repetitive-upsetting processed AZ91D alloy. J. Mater. Sci. Technol. 2017, 33, 935–940. [Google Scholar] [CrossRef]
- Gao, T.; Xiang, H.; Cai, C.; Xiong, Z.; He, X.; Guo, Y.; Tang, W. Effects of different hot extrusion processes on corrosion behavior of Mg-6Gd-1Zn-0.5Zr alloy in 3.5 wt.% sodium chloride solution. J. Alloys Compd. 2025, 1010, 177982. [Google Scholar] [CrossRef]
- Xie, P.; Blawert, C.; Serdechnova, M.; Konchakova, N.; Shulha, T.; Wu, T.; Zheludkevich, M.L. Effect of low concentration electrolytes on the formation and corrosion resistance of PEO coatings on AM50 magnesium alloy. J. Magnes. Alloys 2024, 12, 1386–1405. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate-fluoride solutions and evaluation of corrosion resistance. Appl. Surf. Sci. 2005, 246, 229–238. [Google Scholar] [CrossRef]
- Andreatta, F.; Apachitei, I.; Kodentsov, A.A.; Dzwonczyk, J.; Duszczyk, J. Volta potential of second phase particles in extruded AZ80 magnesium alloy. Electrochim. Acta 2006, 51, 3551–3557. [Google Scholar] [CrossRef]
- Tang, L.; Jiang, F.; Teng, J.; Fu, D.; Zhang, H. Strain path dependent evolutions of microstructure and texture in AZ80 magnesium alloy during hot deformation. J. Alloys Compd. 2019, 806, 292–301. [Google Scholar] [CrossRef]
- Kwak, T.Y.; Kim, W.J. Hot compression behavior of the 1wt% calcium containing Mg-8Al-0.5Zn (AZ80) alloy fabricated using electromagnetic casting technology. Mater. Sci. Eng. A 2014, 615, 222–230. [Google Scholar] [CrossRef]
- Ambat, R.; Aung, N.N.; Zhou, W. Evaluation of microstructural effects on corrosion behaviour of AZ91D magnesium alloy. Corros. Sci. 2000, 42, 1433–1455. [Google Scholar] [CrossRef]
- He, Y.; Wu, L.; Yao, W.; Yuan, Y.; Wu, R.; Wu, G.; Wang, J.; Pan, F. Analysis of the evolution process of corrosion products of pure magnesium based on the phase angle. Corros. Sci. 2024, 240, 112492. [Google Scholar] [CrossRef]
- Ben-Haroush, M.; Ben-Hamu, G.; Eliezer, D.; Wagner, L. The relation between microstructure and corrosion behavior of AZ80 Mg alloy following different extrusion temperatures. Corros. Sci. 2008, 50, 1766–1778. [Google Scholar] [CrossRef]
- Tian, G.; Wang, J.; Wang, S.; Xue, C.; Yang, X.; Su, H. An ultra-light Mg-Li alloy with exceptional elastic modulus, high strength, and corrosion-resistance. Mater. Today Commun. 2023, 35, 105623. [Google Scholar] [CrossRef]
- Padekar, B.S.; Singh Raman, R.K.; Raja, V.S.; Paul, L. Stress corrosion cracking of a recent rare-earth containing magnesium alloy, EV31A, and a common Al-containing alloy, AZ91E. Corros. Sci. 2013, 71, 1–9. [Google Scholar] [CrossRef]
- Luo, S.; Fu, A.; Liu, M.; Xue, Y.; Lv, N.; Pan, Y. Stress corrosion cracking behavior and mechanism of super 13Cr stainless steel in simulated O2/CO2 containing 3.5 wt% NaCl solution. Eng. Fail. Anal. 2021, 130, 105748. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Du, X.; Mao, P.; Zhou, L.; Wei, Z.; Li, J. Effect of microstructure evolution on the corrosion behavior of extrusion-shearing Mg-3Zn-XGa-0.6Zr alloys. J. Alloys Compd. 2025, 1010, 177838. [Google Scholar] [CrossRef]
- Chen, L.; Tseng, C.; Qiu, Y.; Yang, J.; Chang, C.; Wang, X.; Li, W. A layer-by-layer assembled coating for improved stress corrosion cracking on biomedical magnesium alloy in cell culture medium. Surf. Coat. Technol. 2020, 403, 126427. [Google Scholar] [CrossRef]
- Nachtsheim, J.; Ma, S.; Burja, J.; Markert, B. In vitro evaluation of stress corrosion cracking susceptibility of PEO-coated rare-earth magnesium alloy WE43. Surf. Coat. Technol. 2024, 477, 130391. [Google Scholar] [CrossRef]
- Choudhary, L.; Singh Raman, R.K.; Hofstetter, J.; Uggowitzer, P.J. In-vitro characterization of stress corrosion cracking of aluminium-free magnesium alloys for temporary bio-implant applications. Mater. Sci. Eng. C 2014, 42, 629–636. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Corrosion resistance of WE43 and AZ91D magnesium alloys with phosphate PEO coatings. Corros. Sci. 2008, 50, 1744–1752. [Google Scholar] [CrossRef]
- Gaalen, K.; Quinn, C.; Weiler, M.; Gremse, F.; Benn, F.; McHugh, P.E.; Vaughan, T.J.; Kopp, A. Predicting localised corrosion and mechanical performance of a PEO surface modified rare earth magnesium alloy for implant use through insilico modelling. Bioact. Mater. 2023, 26, 437–451. [Google Scholar]
- Zou, Y.; Wang, Y.; Wei, D.; Du, Q.; Ouyang, J.; Jia, D.; Zhou, Y. In-situ SEM analysis of brittle plasma electrolytic oxidation coating bonded to plastic aluminum substrate: Microstructure and fracture behaviors. Mater. Charact. 2019, 156, 109851. [Google Scholar] [CrossRef]
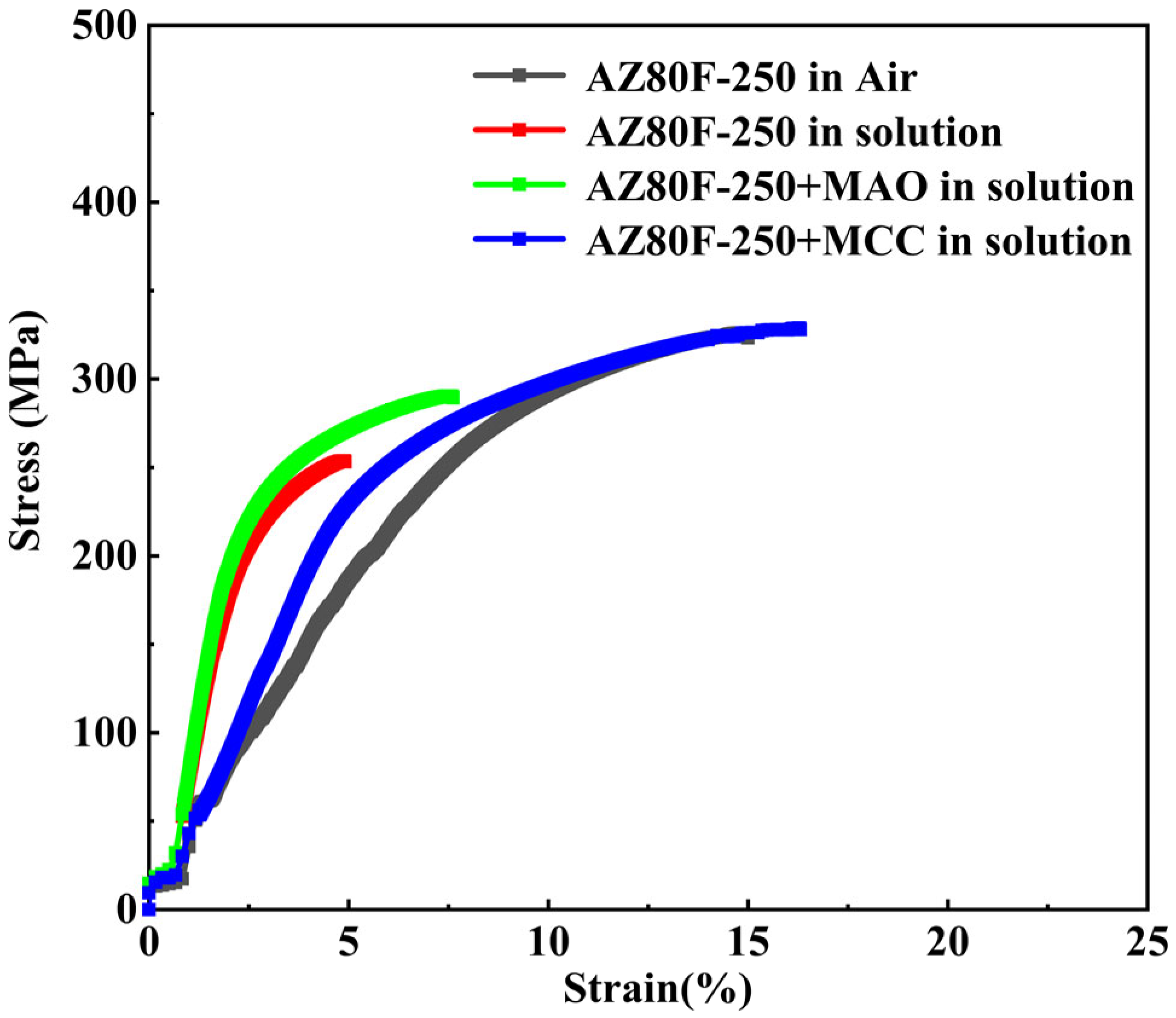
| Specimen | Corrosion Environment | Strain Rate/s−1 | UTS/MPa | Tf/h | ε/% | Ie/% |
|---|---|---|---|---|---|---|
| AZ80F-250 | Air | 10−5 | 324 | 3.9 | 14.9 | 0 |
| AZ80F-250 | 3.5 wt.% NaCl solution | 10−5 | 253 | 1.3 | 4.9 | 67.1 |
| AZ80F-250 + MAO | 10−5 | 289 | 2.1 | 7.6 | 48.9 | |
| AZ80F-250 + MCC | 10−5 | 328 | 4.0 | 16.3 | −0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Zhang, J.; Shen, Y.; Qiao, Y.; Luo, S.; Wang, D. Effects of Forging Temperature and Micro-Arc Coatings on the Static/Stress Corrosion Resistance of AZ80 Magnesium Alloy. Materials 2025, 18, 2590. https://doi.org/10.3390/ma18112590
Xue Y, Zhang J, Shen Y, Qiao Y, Luo S, Wang D. Effects of Forging Temperature and Micro-Arc Coatings on the Static/Stress Corrosion Resistance of AZ80 Magnesium Alloy. Materials. 2025; 18(11):2590. https://doi.org/10.3390/ma18112590
Chicago/Turabian StyleXue, Yuna, Jie Zhang, Yi Shen, Yongpeng Qiao, Sheji Luo, and Di Wang. 2025. "Effects of Forging Temperature and Micro-Arc Coatings on the Static/Stress Corrosion Resistance of AZ80 Magnesium Alloy" Materials 18, no. 11: 2590. https://doi.org/10.3390/ma18112590
APA StyleXue, Y., Zhang, J., Shen, Y., Qiao, Y., Luo, S., & Wang, D. (2025). Effects of Forging Temperature and Micro-Arc Coatings on the Static/Stress Corrosion Resistance of AZ80 Magnesium Alloy. Materials, 18(11), 2590. https://doi.org/10.3390/ma18112590






