Binder-Less Molybdenum Doped CoO Based Integrated Electrodes Fabricated by Electric Discharge Corrosion for High-Efficiency Supercapacitors
Abstract
1. Introduction
2. Materials Sources and Methods
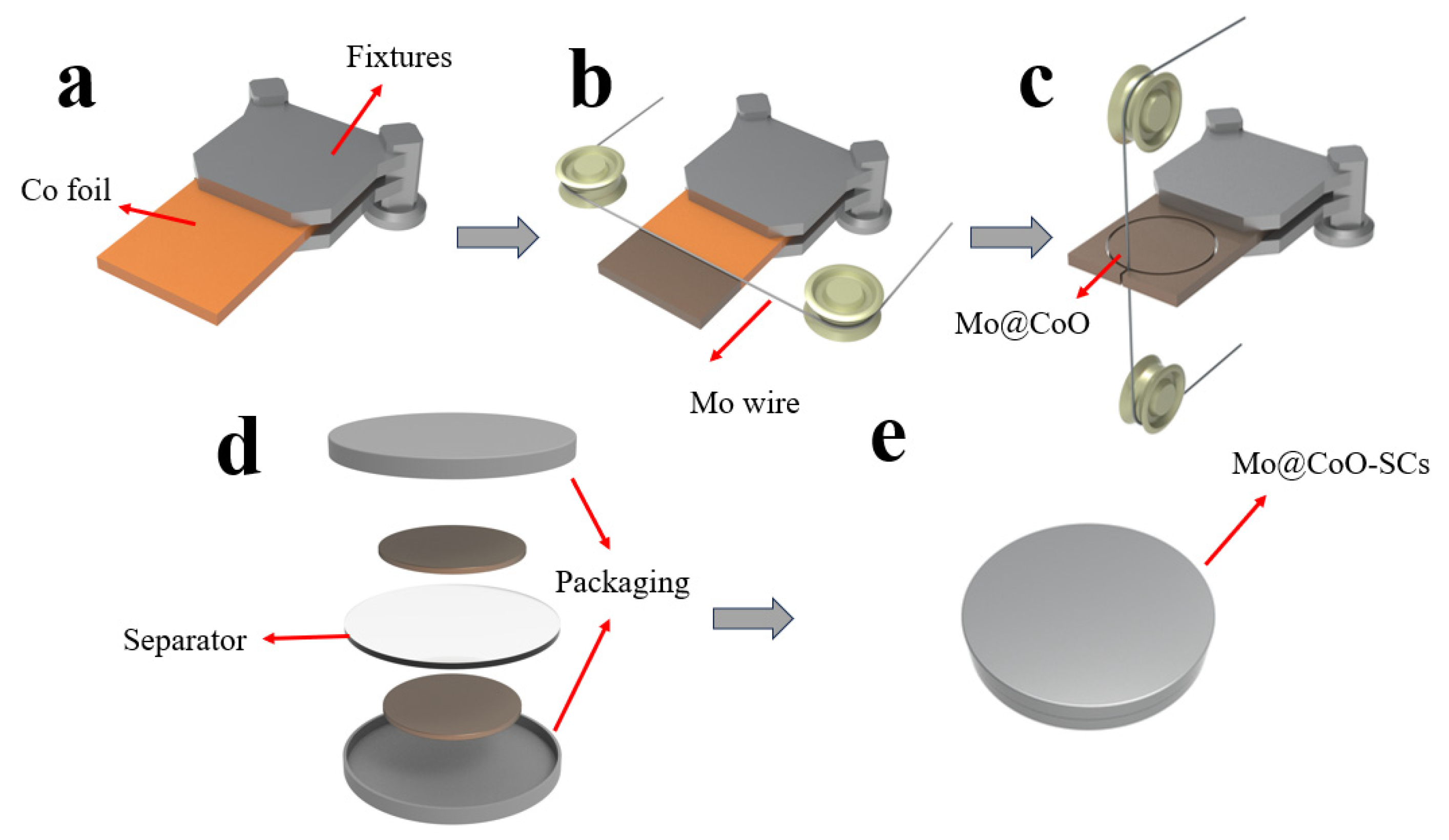
2.1. Materials Sources and Samples Preparation
2.2. Characterization Methods for Electrode Materials
2.3. Electrochemical Characterization
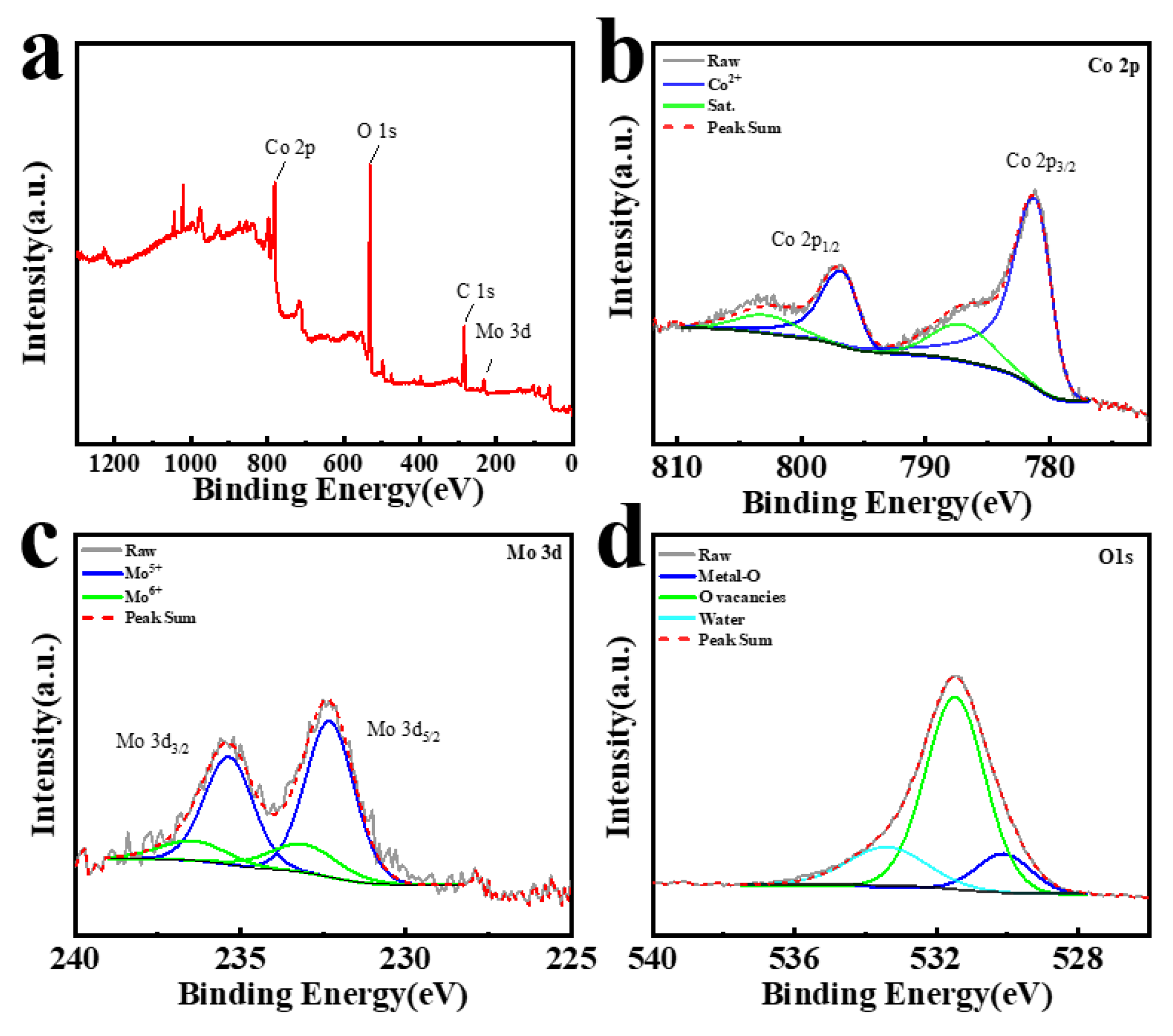
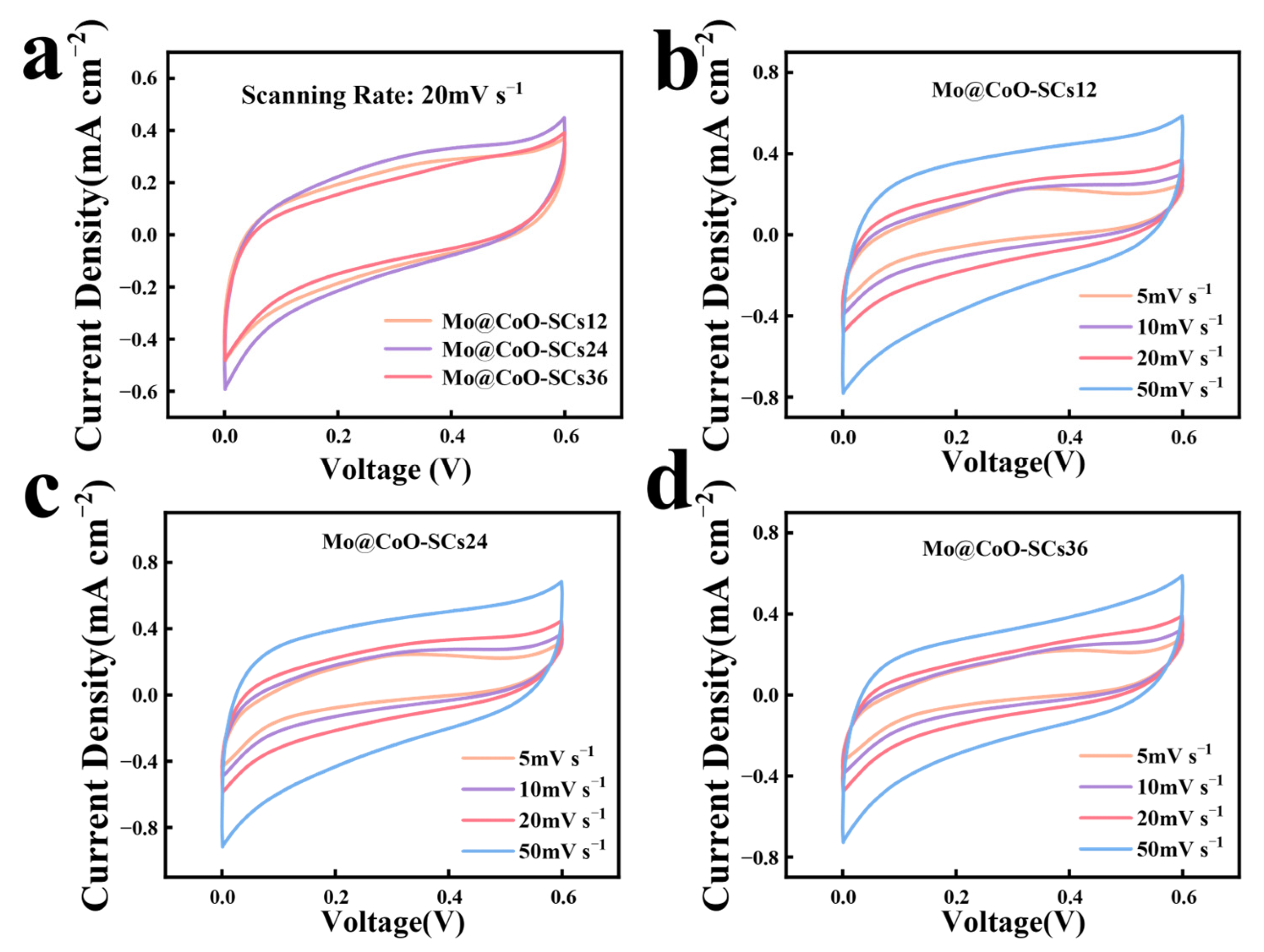
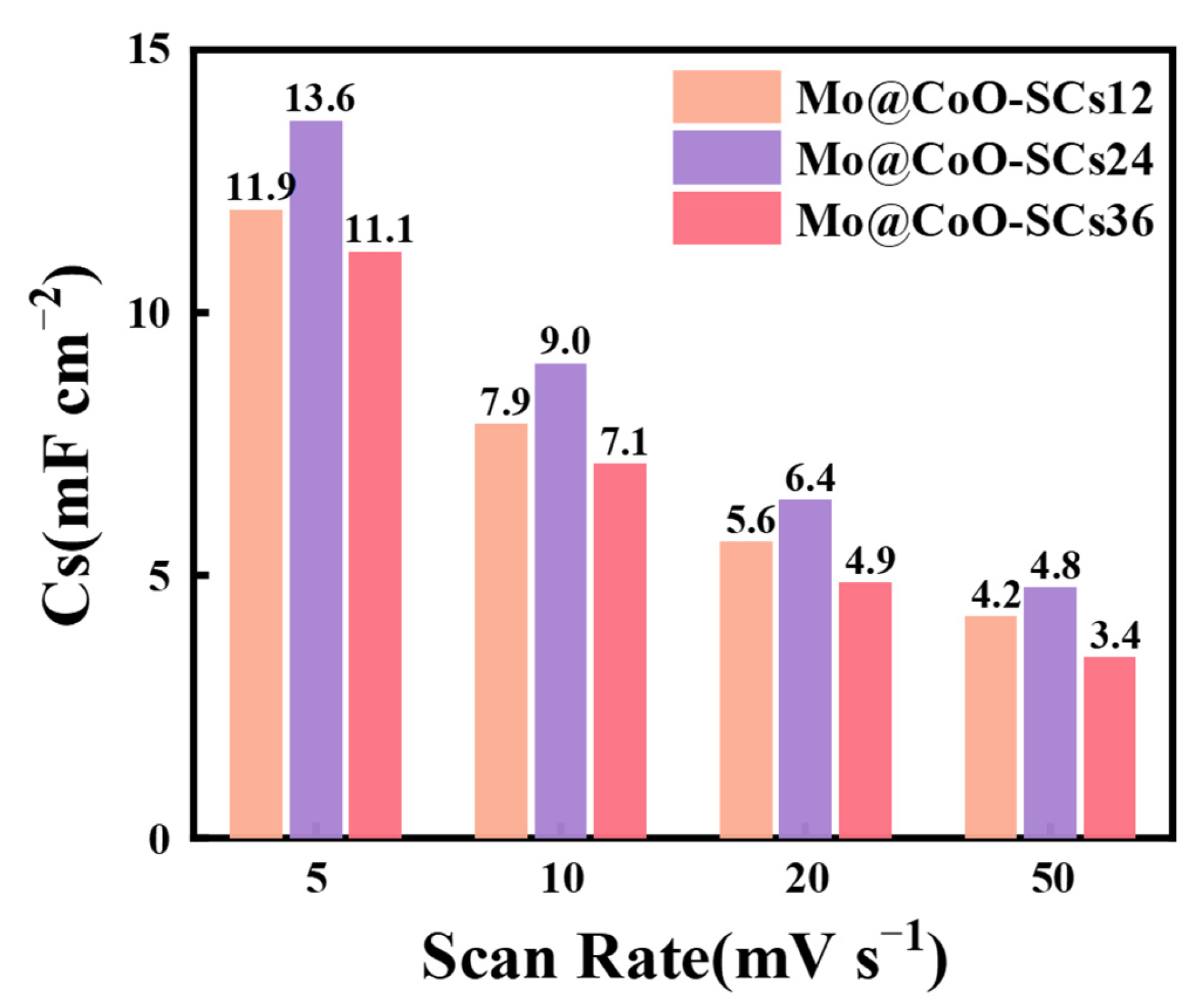
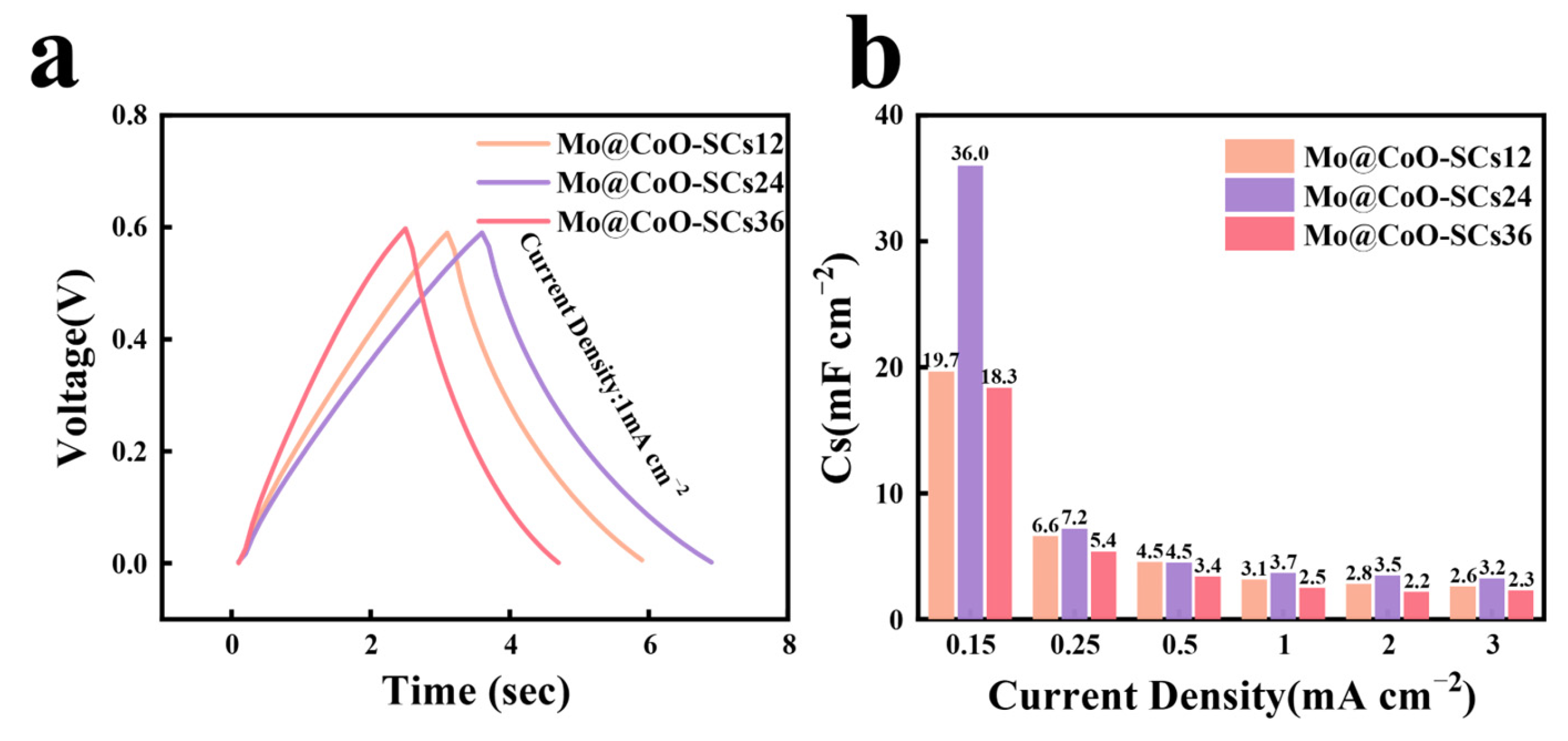
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Gu, M.; Chen, X. Supercapacitors for renewable energy applications: A review. Micro Nano Eng. 2023, 21, 100229. [Google Scholar] [CrossRef]
- Shaheen, I.; Hussain, I.; Zahra, T.; Javed, M.S.; Shah, S.S.A.; Khan, K.; Hanif, M.B.; Assiri, M.A.; Said, Z.; Arifeen, W.U. Recent advancements in metal oxides for energy storage materials: Design, classification, and electrodes configuration of supercapacitor. J. Energy Storage 2023, 72, 108719. [Google Scholar] [CrossRef]
- Dutta, A.; Mitra, S.; Basak, M.; Banerjee, T. A comprehensive review on batteries and supercapacitors: Development and challenges since their inception. Energy Storage 2023, 5, e339. [Google Scholar] [CrossRef]
- Devi, R.; Tapadia, K.; Maharana, T. Casting of carbon cloth enrobed polypyrrole electrode for high electrochemical performances. Heliyon 2020, 6, e03122. [Google Scholar] [CrossRef]
- Chen, M.; Chen, R.; Zhitomirsky, I.; He, G.; Shi, K. Redox-active molecules for aqueous electrolytes of energy storage devices: A review on fundamental aspects, current progress, and prospects. Mater. Sci. Eng. R Rep. 2024, 161, 100865. [Google Scholar] [CrossRef]
- Yu, M.; Peng, Y.; Wang, X.; Ran, F. Emerging Design Strategies Toward Developing Next-Generation Implantable Batteries and Supercapacitors. Adv. Funct. Mater. 2023, 33, 2301877. [Google Scholar] [CrossRef]
- Pan, Z.; Yu, S.; Wang, L.; Li, C.; Meng, F.; Wang, N.; Zhou, S.; Xiong, Y.; Wang, Z.; Wu, Y.J.N. Recent advances in porous carbon materials as electrodes for supercapacitors. Nanomaterials 2023, 13, 1744. [Google Scholar] [CrossRef]
- Riaz, A.; Sarker, M.R.; Saad, M.H.M.; Mohamed, R. Review on comparison of different energy storage technologies used in micro-energy harvesting, WSNs, low-cost microelectronic devices: Challenges and recommendations. Sensors 2021, 21, 5041. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yang, G.; Kim, C.-H.; Mahajan, R.L.; Lee, S.-Y.; Park, S.-J. Flexible solid-state hybrid supercapacitors for the internet of everything (IoE). Energy Environ. Sci. 2022, 15, 2233–2258. [Google Scholar] [CrossRef]
- Yadlapalli, R.T.; Alla, R.R.; Kandipati, R.; Kotapati, A. Super capacitors for energy storage: Progress, applications and challenges. J. Energy Storage 2022, 49, 104194. [Google Scholar] [CrossRef]
- Manohar, A.; Vijayakanth, V.; Vattikuti, S.P.; Kim, K.H. Electrochemical investigation on nickel-doped spinel magnesium ferrite nanoparticles for supercapacitor applications. Mater. Chem. Phys. 2023, 301, 127601. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Reddy, V.S.; Zhen, Y.; Ramakrishna, S.; Chellappan, V. Metal Organic Framework Derived Zirconia–Carbon Nanoporous Mat for Integrated Strain Sensor Powered by Solid-State Supercapacitor. Adv. Fiber Mater. 2023, 5, 1404–1416. [Google Scholar] [CrossRef]
- Elsehsah, K.A.A.A.; Noorden, Z.A.; Saman, N.M. Current Insights and Future Prospects of Graphene Aerogel-Enhanced Supercapacitors: A Systematic Review. Heliyon 2024, 10, e37071. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wei, L.; Chen, R.; Wu, Q.; Li, J. Reviews and Prospectives of Co3O4-Based Nanomaterials for Supercapacitor Application. ChemistrySelect 2020, 5, 5268–5288. [Google Scholar] [CrossRef]
- Jia, J.; Zhu, Y.; Das, P.; Ma, J.; Wang, S.; Zhu, G.; Wu, Z.-S. Advancing MXene-based integrated microsystems with micro-supercapacitors and/or sensors: Rational design, key progress, and challenging perspectives. J. Mater. 2023, 9, 1242–1262. [Google Scholar] [CrossRef]
- Bhojane, P. Recent advances and fundamentals of Pseudocapacitors: Materials, mechanism, and its understanding. J. Energy Storage 2022, 45, 103654. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Y.; Yan, X.; Zhang, W.; Zhang, M.; Huang, X.; Pan, J.; Shahnavaz, Z.; Moradian, J.M. Porous CoO/carbon foam composites synthesized by solvothermal method for supercapacitor and enhanced microwave absorption applications. Diam. Relat. Mater. 2023, 138, 110270. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Hussain, C.M. Green supercapacitors: Latest developments and perspectives in the pursuit of sustainability. Renew. Sustain. Energy Rev. 2024, 195, 114324. [Google Scholar] [CrossRef]
- Lim, J.M.; Jang, Y.S.; Nguyen, H.V.T.; Kim, J.S.; Yoon, Y.; Park, B.J.; Seo, D.H.; Lee, K.-K.; Han, Z.; Ostrikov, K.K. Advances in high-voltage supercapacitors for energy storage systems: Materials and electrolyte tailoring to implementation. Nanoscale Adv. 2023, 5, 615–626. [Google Scholar] [CrossRef]
- Pholauyphon, W.; Charoen-amornkitt, P.; Suzuki, T.; Tsushima, S. Guidelines for supercapacitor electrochemical analysis: A comprehensive review of methodologies for finding charge storage mechanisms. J. Energy Storage 2024, 98, 112833. [Google Scholar] [CrossRef]
- Sengupta, S.; Kundu, M. Recent Advances of Sustainable Electrode Materials for Supercapacitor Devices. In Sustainable Energy Storage in the Scope of Circular Economy: Advanced Materials and Device Design; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 145–158. [Google Scholar]
- Park, H.W.; Roh, K.C. Recent advances in and perspectives on pseudocapacitive materials for supercapacitors—A review. J. Power Sources 2023, 557, 232558. [Google Scholar] [CrossRef]
- Gopi, C.V.M.; Alzahmi, S.; Al-Haik, M.Y.; Kumar, Y.A.; Hamed, F.; Haik, Y.; Obaidat, I.M. Recent advances in pseudocapacitive electrode materials for high energy density aqueous supercapacitors: Combining transition metal oxides with carbon nanomaterials. Mater. Today Sustain. 2024, 28, 100981. [Google Scholar] [CrossRef]
- Jayakumar, S.; Santhosh, P.C.; Mohideen, M.M.; Radhamani, A. A comprehensive review of metal oxides (RuO2, Co3O4, MnO2 and NiO) for supercapacitor applications and global market trends. J. Alloys Compd. 2023, 976, 173170. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The development of pseudocapacitor electrodes and devices with high active mass loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Jeon, S.; Jeong, J.H.; Yoo, H.; Yu, H.K.; Kim, B.-H.; Kim, M.H. RuO2 nanorods on electrospun carbon nanofibers for supercapacitors. ACS Appl. Nano Mater. 2020, 3, 3847–3858. [Google Scholar] [CrossRef]
- Chandrashekhar, R.; Yadav, A.A. Spray-deposited cobalt-doped RuO2 electrodes for high-performance supercapacitors. Electrochim. Acta 2023, 437, 141521. [Google Scholar]
- Chung, M.-Y.; Lo, C.-T. High-performance binder-free RuO2/electrospun carbon fiber for supercapacitor electrodes. Electrochim. Acta 2020, 364, 137324. [Google Scholar] [CrossRef]
- Xiao, X.; Duan, X.; Song, Z.; Deng, X.; Deng, W.; Hou, H.; Zheng, R.; Zou, G.; Ji, X. High-Throughput Production of Cheap Mineral-Based Heterostructures for High Power Sodium Ion Capacitors. Adv. Funct. Mater. 2022, 32, 2110476. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, G.; Deng, X.; Zou, K.; Xiao, X.; Momen, R.; Massoudi, A.; Deng, W.; Hu, J.; Hou, H. Ultra-low-dose pre-metallation strategy served for commercial metal-ion capacitors. Nano-Micro Lett. 2022, 14, 53. [Google Scholar] [CrossRef]
- Eswaramoorthy, N.; Rajendran, S.; Kumar, B.A.; Nallusamy, S.; Rengasamy, M.; Selvaraj, Y.; Sangaraju, S.; Krishnan, T.; Kumaresan, G.; Rajaram, K. Influence of ZnO/MWCNTs based hybrid electrodes for boosting the performance of photovoltaic and supercapacitor devices. Mater. Chem. Phys. 2024, 316, 129049. [Google Scholar] [CrossRef]
- Ahmed, F.; Almutairi, G.; AlOtaibi, B.; Kumar, S.; Arshi, N.; Hussain, S.G.; Umar, A.; Ahmad, N.; Aljaafari, A. Binder-free electrode based on ZnO nanorods directly grown on aluminum substrate for high performance supercapacitors. Nanomaterials 2020, 10, 1979. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Wen, L.; Javed, M.S.; Ahmad, A.; Nazir, M.S.; Assiri, M.A.; Imran, M.; Bocchetta, P. Binder-free porous 3D-ZnO hexagonal-cubes for electrochemical energy storage applications. Materials 2022, 15, 2250. [Google Scholar] [CrossRef] [PubMed]
- Shembade, U.V.; Dhas, S.D.; Gurav, S.R.; Sonkawade, R.G.; Wategaonkar, S.B.; Ghatage, S.R.; Gaikwad, M.A.; Kim, J.H.; Parale, V.G.; Park, H.-H. Acid substitutions for WO3 nanostructures synthesis by the hydrothermal route and its effect on physio-chemical and electrochemical properties for supercapacitors. J. Energy Storage 2023, 72, 108432. [Google Scholar] [CrossRef]
- Anikpa, P.; Mee, A.; Nwanya, A.; Nkele, A.C.; Malavekar, D.; Osuji, R.; Nwulu, N.; Lokhande, C.; Ezema, F.I. Asymmetric supercapacitor performance of hydrothermally-synthesized MWCNT-WO3 composite electrode. J. Energy Storage 2024, 81, 110439. [Google Scholar] [CrossRef]
- Karmakar, R.; Sinha, S.; Das, A.K.; Dey, S.; Dutta, B.; Pramanik, S.; Kuiri, P.K.; Basu, S.; Meikap, A.K. Observation of synergistic effects in multiphase tungsten oxide (WO3) nanocomposite and its role in enhanced supercapacitive and photoluminescence properties. Mater. Chem. Phys. 2023, 305, 127915. [Google Scholar] [CrossRef]
- Amate, R.U.; Morankar, P.J.; Chavan, G.T.; Teli, A.M.; Desai, R.S.; Dalavi, D.S.; Jeon, C.-W. Bi-functional electrochromic supercapacitor based on hydrothermal-grown 3D Nb2O5 nanospheres. Electrochim. Acta 2023, 459, 142522. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Chen, H.; Xu, Y.-X.; An, C.-S.; Xiang, K.-X. Facile preparation of Nb2O5 microspheres and their excellent electrochemical performance in aqueous zinc-ion hybrid supercapacitors. Rare Met. 2022, 41, 3129–3141. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, S.; Zhang, W.; Wang, M.; Feng, F. Controllable synthesis of gossamer-like Nb2O5-RGO nanocomposite and its application to supercapacitor. J. Nanoparticle Res. 2020, 22, 57. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Song, Z.; Xiao, X.; Cao, Z.; Xiong, D.; Deng, W.; Hou, H.; Yang, Y.; Zou, G. Enabling Electron Delocalization by Conductor Heterostructure for Highly Reversible Sodium Storage. Adv. Funct. Mater. 2024, 34, 2312905. [Google Scholar] [CrossRef]
- Jangu, S.; Kumar, S.; Deepika, K.N.; Jacob, C.; Pradhan, D. Effect of microwave power and Cu doping on MnO2 nanostructures and its supercapacitor performance. ACS Appl. Electron. Mater. 2023, 5, 3078–3092. [Google Scholar] [CrossRef]
- Devi, R.; Kumar, V.; Kumar, S.; Bulla, M.; Sharma, S.; Sharma, A. Electrochemical analysis of MnO2 (α, β, and γ)-based electrode for high-performance supercapacitor application. Appl. Sci. 2023, 13, 7907. [Google Scholar] [CrossRef]
- Feng, Y.; Qu, H.; Wang, Y.; Wang, L.; Wang, Y.; Yang, D.; Ding, B.; Sun, Y.; Guo, J.; Dai, S. Facile Synthesis of Ag-Doped Urchin-like MnO2 on Carbon Cloth for Supercapacitors. Materials 2024, 17, 1312. [Google Scholar] [CrossRef] [PubMed]
- Kuzhandaivel, H.; Paramasivam, K.; Manickam, S.; Nallathambi, K.S. Nickel-doped CuO/Cu/Cu2O nanocomposite as an efficient electrode for electrochemical non-enzymatic glucose sensor and asymmetric supercapacitor. J. Appl. Electrochem. 2023, 53, 1869–1886. [Google Scholar] [CrossRef]
- Dabir, M.; Masoudpanah, S.; Bafghi, M.S.; Mamizadeh, M. Tuning the composition of CuO/Cu2O composite powders via solution combustion method for supercapacitor application. Mater. Sci. Eng. B 2024, 306, 117479. [Google Scholar] [CrossRef]
- Kumar, A.; Thomas, A.; Garg, M.; Perumal, G.; Grewal, H.S.; Arora, H.S. High performance CuO@ brass supercapacitor electrodes through surface activation. J. Mater. Chem. A 2021, 9, 9327–9336. [Google Scholar] [CrossRef]
- Kunhikrishnan, L.; Shanmugam, R. A facile route to synthesize CuO sphere-like nanostructures for supercapacitor electrode application. J. Mater. Sci. Mater. Electron. 2020, 31, 21528–21539. [Google Scholar] [CrossRef]
- Xi, Y.; Xiao, Z.; Lv, H.; Sun, H.; Zhai, S.; An, Q. Construction of CuO/Cu-nanoflowers loaded on chitosan-derived porous carbon for high energy density supercapacitors. J. Colloid Interface Sci. 2023, 630, 525–534. [Google Scholar] [CrossRef]
- Shah, A.; Senapati, S.; Murthy, H.A.; Singh, L.R.; Mahato, M. Supercapacitor performance of NiO, NiO-MWCNT, and NiO–Fe-MWCNT composites. ACS Omega 2023, 8, 33380–33391. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Simonenko, E.P.; Kuznetsov, N.T. Hydrothermal Synthesis of a Cellular NiO Film on Carbon Paper as a Promising Way to Obtain a Hierarchically Organized Electrode for a Flexible Supercapacitor. Materials 2023, 16, 5208. [Google Scholar] [CrossRef]
- Dhas, S.D.; Maldar, P.S.; Patil, M.D.; Nagare, A.B.; Waikar, M.R.; Sonkawade, R.G.; Moholkar, A.V. Synthesis of NiO nanoparticles for supercapacitor application as an efficient electrode material. Vacuum 2020, 181, 109646. [Google Scholar] [CrossRef]
- Otun, K.O.; Xaba, M.S.; Zong, S.; Liu, X.; Hildebrandt, D.; El-Bahy, S.M.; El-Bahy, Z.M. Double linker MOF-derived NiO and NiO/Ni supercapacitor electrodes for enhanced energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 128019. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, P.; Chen, R.; Xie, W.; Xu, Z.; Yang, Y.; Liu, D.; Huang, F.; Zhuang, Z.; Zhitomirsky, I. Electric discharge direct writing of 3D Mo-MoOx pseudocapacitive micro-supercapacitors with designable patterns. Ceram. Int. 2023, 49, 22586–22594. [Google Scholar] [CrossRef]
- Xie, H.; Ma, S.; He, Z. Facile preparation of PANI/MoOx nanowires decorated MXene film electrodes for electrochemical supercapacitors. Electrochim. Acta 2023, 448, 142173. [Google Scholar] [CrossRef]
- Liu, D.; Xie, W.; Xu, Z.; Deng, P.; Wu, Z.; Zhitomirsky, I.; Wang, W.; Chen, R.; Zhou, L.; Xu, Y. Fabrication of Ni-Cr-FeOx ceramic supercapacitor electrodes and devices by one-step electric discharge ablation. J. Energy Storage 2023, 74, 109429. [Google Scholar] [CrossRef]
- Sun, L.; Cai, Y.; Haider, M.K.; Miyagi, D.; Zhu, C.; Kim, I.S. Structural design and optimization of metal-organic framework-derived FeOx@ C/rGO anode materials for constructing high-performance hybrid supercapacitors. Compos. Part B Eng. 2022, 236, 109812. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Z.; Xie, W.; Deng, P.; Xu, Y.; Xu, L.; Zhang, G.; Yang, Y.; Xie, G.; Zhitomirsky, I. Fabrication of Fe–Fe1−x O based 3D coplanar microsupercapacitors by electric discharge rusting of pure iron substrates. RSC Adv. 2023, 13, 26995–27005. [Google Scholar] [CrossRef]
- Chen, R.; Qin, J.; Xu, Z.; Lv, S.; Tao, Z.; He, J.; Zhou, P.; Shu, Z.; Zhuang, Z.; Wang, W. The impact of processing voltage of wire electric discharge machining on the performance of Mo doped V–VO0.2 based Archimedean micro-supercapacitors. RSC Adv. 2024, 14, 28543–28554. [Google Scholar] [CrossRef]
- Javed, M.S.; Najim, T.; Hussain, I.; Batool, S.; Idrees, M.; Mehmood, A.; Imran, M.; Assiri, M.A.; Ahmad, A.; Shah, S.S.A. 2D V2O5 nanoflakes as a binder-free electrode material for high-performance pseudocapacitor. Ceram. Int. 2021, 47, 25152–25157. [Google Scholar] [CrossRef]
- Zahra, T.; Barsoum, I.; Alharbi, F.; Ahmad, Z.; Somaily, H.; Abdullah, M.; Alqurashi, H.; Weinstein, I.A.; Henaish, A.; Farid, H.M.T. Fabrication of V2O5@ g-C3N4 nanocomposite by hydrothermal route for use as an improved electrochemical property in supercapacitor applications. J. Energy Storage 2024, 87, 111470. [Google Scholar] [CrossRef]
- Kim, K.-W.; Wu, K.; Park, B.; Jang, J.; Kwon, J.H.; Alam, A.; Lim, S.; Kim, S.H.; Kim, J.K.; Moon, H.C. 3D-printed, high-energy-density, current collector-free flexible micro-supercapacitors based on CNT@ V2O5 nanowires. J. Energy Storage 2024, 75, 109642. [Google Scholar] [CrossRef]
- Sharma, M.; Adalati, R.; Kumar, A.; Chawla, V.; Chandra, R. Elevated performance of binder-free Co3O4 electrode for the supercapacitor applications. Nano Express 2021, 2, 010002. [Google Scholar] [CrossRef]
- Bathula, C.; Rabani, I.; Ramesh, S.; Lee, S.-H.; Palem, R.R.; Ahmed, A.T.A.; Kim, H.S.; Seo, Y.-S.; Kim, H.-S. Highly efficient solid-state synthesis of Co3O4 on multiwalled carbon nanotubes for supercapacitors. J. Alloys Compd. 2021, 887, 161307. [Google Scholar] [CrossRef]
- Umar, A.; Akhtar, M.S.; Ibrahim, A.A.; Algadi, H.; Alhamami, M.A.; Ahmed, F.; Motlak, M.; Akbar, S. Electrospun Co3O4 nanofibers as potential material for enhanced supercapacitors and chemo-sensor applications. J. Mater. Res. Technol. 2022, 21, 5018–5031. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, L.; Qi, Z.; Zhang, Y.; Wang, G.; Gao, W.; Liu, W. Cationic and anionic defect decoration of CoO through Cu dopants and oxygen vacancy for a High-Performance supercapacitor. J. Colloid Interface Sci. 2023, 652, 1099–1107. [Google Scholar] [CrossRef]
- Gao, M.; Dong, X.; Mei, X.; Wang, K.; Wang, W.; Tang, Z.; Duan, W. Laser direct synthesis of Co/CoO modified graphene for high-performance microsupercapacitors. Chem. Eng. J. 2023, 471, 144609. [Google Scholar] [CrossRef]
- Lu, Z.; Cheng, J.; Zhang, L.; Yang, Q.; Pan, H.; Wu, D.; Gao, Y.; Huang, X.; Wang, T.; Chen, X. Free-standing hierarchical Co@ CoO/CNFs/Cu-foam composite based on electrochemical deposition as high-performance supercapacitor electrode. J. Alloys Compd. 2021, 856, 158075. [Google Scholar] [CrossRef]
- Yang, S.; Chen, R.; Huang, F.; Wang, W.; Zhitomirsky, I. 3D Binder-Free Mo@ CoO Electrodes Directly Manufactured in One Step via Electric Discharge Machining for In-Plane Microsupercapacitor Application. Micromachines 2024, 15, 1294. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Pandey, R.; Tan, W.K.; Kawamura, G.; Moshkalev, S.A.; Matsuda, A. Microwave-assisted dry synthesis of hybrid electrode materials for supercapacitors: Nitrogen-doped rGO with homogeneously dispersed CoO nanocrystals. J. Energy Storage 2023, 68, 107820. [Google Scholar] [CrossRef]
- Xu, J.; Yan, A.; Wang, X.; Wang, B.; Cheng, J. A review of cobalt monoxide and its composites for supercapacitors. Ceram. Int. 2021, 47, 22229–22239. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, S.; Xiao, Z.; Xu, F.; Xiang, C.; Sun, L.; Zou, Y. Graphene-supported Cu2O-CoO heterojunctions for high performance supercapacitors. J. Energy Storage 2024, 77, 110009. [Google Scholar] [CrossRef]
- Sun, X.; Lu, Y.; Li, T.; Zhao, S.; Gao, Z.; Song, Y.-Y. Metallic CoO/Co heterostructures stabilized in an ultrathin amorphous carbon shell for high-performance electrochemical supercapacitive behaviour. J. Mater. Chem. A 2019, 7, 372–380. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, C.; Liu, H.; Su, L.; Li, J.; Qin, H. One-step synthesis of needle-like CoO/C composite materials and their enhanced electrochemical performance for supercapacitor. Int. J. Electrochem. Sci. 2020, 15, 2272–2280. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, M.; Quan, Y.; Li, X.; Hu, X.; Yan, J.; Wang, Y.; Sun, M.; Li, S. A review of binder-free electrodes for advanced supercapacitors. J. Ind. Eng. Chem. 2024, 141, 1–31. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Wu, K.; Dai, G.; Chen, Q.; Zhou, L.; Zheng, J.; Ma, L.; Li, G.; Wang, W. Novel Ag-bridged dual Z-scheme g-C3N4/BiOI/AgI plasmonic heterojunction: Exceptional photocatalytic activity towards tetracycline and the mechanism insight. J. Environ. Sci. 2023, 131, 123–140. [Google Scholar] [CrossRef]
- Kumar, J.; Jung, H.J.; Neiber, R.R.; Soomro, R.A.; Kwon, Y.J.; Hassan, N.U.; Shon, M.; Lee, J.H.; Baek, K.Y.; Cho, K.Y. Recent advances in oxygen deficient metal oxides: Opportunities as supercapacitor electrodes. Int. J. Energy Res. 2022, 46, 7055–7081. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Zhang, Y.; Zheng, Y.; Liu, N.; Su, J.; Gao, Y. Interior and exterior decoration of transition metal oxide through Cu0/Cu+ Co-doping strategy for high-performance supercapacitor. Nano-Micro Lett. 2021, 13, 61. [Google Scholar] [CrossRef]
- Ahmed, R.; Nabi, G. Enhanced electrochemical performance of Cr-doped NiO nanorods for supercapacitor application. J. Energy Storage 2021, 33, 102115. [Google Scholar] [CrossRef]
- Pandey, P.; Bhowmick, S.; Qureshi, M. Magnetically triggered interplay of capacitive and diffusion contributions for boosted supercapacitor performance. ACS Appl. Mater. Interfaces 2023, 15, 39435–39447. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.; Shim, J.-J.; Chong, K.F. Cobalt-doped tungsten suboxides for supercapacitor applications. Chem. Eng. J. 2023, 473, 145341. [Google Scholar] [CrossRef]
- Xia, P.; Pan, J.; Zhang, Y.; Mao, M.; Ma, L.; Chen, J.; Zhang, L.; Wang, H.; Fan, H.; Gao, X. Highly sensitive detection of glucose at a novel non-enzyme electrochemical sensing based on Mo-doped CoO Nanosheets. Chem.–Asian J. 2024, 19, e202300951. [Google Scholar] [CrossRef]
- Zhou, R.; Bi, X.; Yuan, X.; Zhang, L.; Huang, Q. Significant Improvement of the Electrochemical Performance of CoO Nanoparticles Through Activating Synergistic Effects by Doping with Nickel. Eur. J. Inorg. Chem. 2020, 2020, 2889–2895. [Google Scholar] [CrossRef]
- Gerard, O.; Numan, A.; Krishnan, S.; Khalid, M.; Subramaniam, R.; Kasi, R. A review on the recent advances in binder-free electrodes for electrochemical energy storage application. J. Energy Storage 2022, 50, 104283. [Google Scholar] [CrossRef]
- How, Y.Y.; Numan, A.; Mustafa, M.N.; Walvekar, R.; Khalid, M.; Mubarak, N.M. A review on the binder-free electrode fabrication for electrochemical energy storage devices. J. Energy Storage 2022, 51, 104324. [Google Scholar] [CrossRef]
- Shen, K.; Zhai, S.; Wang, S.; Ru, Q.; Hou, X.; San Hui, K.; Nam Hui, K.; Chen, F. Recent progress in binder-free electrodes synthesis for electrochemical energy storage application. Batter. Supercaps 2021, 4, 860–880. [Google Scholar] [CrossRef]
- Lamiel, C.; Hussain, I.; Ma, X.; Zhang, K. Properties, functions, and challenges: Current collectors. Mater. Today Chem. 2022, 26, 101152. [Google Scholar] [CrossRef]
- Abdisattar, A.; Yeleuov, M.; Daulbayev, C.; Askaruly, K.; Tolynbekov, A.; Taurbekov, A.; Prikhodko, N. Recent advances and challenges of current collectors for supercapacitors. Electrochem. Commun. 2022, 142, 107373. [Google Scholar] [CrossRef]
- Adelowo, E.; Baboukani, A.R.; Chen, C.; Wang, C. Electrostatically sprayed reduced graphene oxide-carbon nanotubes electrodes for lithium-ion capacitors. C 2018, 4, 31. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, Q.; Du, X.; Zhang, J.; Li, W.; Lu, Y.; Zhang, D.; Qi, P.; Tang, Y. Synthesis of binder-free Co/CoO encapsulated in graphene as electrode material for electrochemical applications. J. Alloys Compd. 2019, 783, 363–370. [Google Scholar] [CrossRef]
- Wang, L.-H.; Teng, X.-L.; Qin, Y.-F.; Li, Q. High electrochemical performance and structural stability of CoO nanosheets/CoO film as self-supported anodes for lithium-ion batteries. Ceram. Int. 2021, 47, 5739–5746. [Google Scholar] [CrossRef]
- Qudeiri, J.E.A.; Zaiout, A.; Mourad, A.-H.I.; Abidi, M.H.; Elkaseer, A. Principles and characteristics of different EDM processes in machining tool and die steels. Appl. Sci. 2020, 10, 2082. [Google Scholar] [CrossRef]
- Manjaiah, M.; Narendranath, S.; Basavarajappa, S. A review on machining of titanium based alloys using EDM and WEDM. Rev. Adv. Mater. Sci 2014, 36, 89–111. [Google Scholar]
- Devi, M.B.; Birru, A.K.; Bannaravuri, P.K. The recent trends of EDM applications and its relevance in the machining of aluminium MMCs: A comprehensive review. Mater. Today Proc. 2021, 47, 6870–6873. [Google Scholar] [CrossRef]
- Kiyak, M.; Çakır, O. Examination of machining parameters on surface roughness in EDM of tool steel. J. Mater. Process. Technol. 2007, 191, 141–144. [Google Scholar] [CrossRef]
- Chen, R.; Guo, Z.; Liu, J.; Yue, T.M.; Chen, S. A numerical model and computer simulation for machining particle reinforced metal matrix composites in electric discharge machining. Mater. Res. Innov. 2015, 19, S1-392–S1-396. [Google Scholar] [CrossRef]
- Lee, H.T.; Tai, T.Y. Relationship between EDM parameters and surface crack formation. J. Mater. Process. Technol. 2003, 142, 676–683. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, W.; Arcelus, O.; Kim, J.-G.; Carrasco, J.; Yoo, S.J.; Zheng, W.; Wang, J.; Tian, H.; Zhang, H. Atomic-level energy storage mechanism of cobalt hydroxide electrode for pseudocapacitors. Nat. Commun. 2017, 8, 15194. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Wu, Q.; Li, J. Review of cobalt-based nanocomposites as electrode for supercapacitor application. Ionics 2022, 28, 989–1015. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Zhang, W.-D.; Xiao, H.-M.; Yang, Y.; Fu, S.-Y. Facile synthesis of metallic Co hierarchical nanostructured microspheres by a simple solvothermal process. J. Phys. Chem. C 2008, 112, 10073–10078. [Google Scholar] [CrossRef]
- Ma, K.; Liu, F.; Yuan, Y.; Liu, X.; Wang, J.; Xie, J.; Cheng, J. CoO microspheres and metallic Co evolved from hexagonal α-Co(OH)2 plates in a hydrothermal process for lithium storage and magnetic applications. Phys. Chem. Chem. Phys. 2018, 20, 595–604. [Google Scholar] [CrossRef]
- Qian, K.; Yan, Y.; Xi, S.; Wei, T.; Dai, Y.; Yan, X.; Kobayashi, H.; Wang, S.; Liu, W.; Li, R. Elucidating the strain–vacancy–activity relationship on structurally deformed Co@ CoO nanosheets for aqueous phase reforming of formaldehyde. Small 2021, 17, 2102970. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Qing, C.; Qu, G.; Ma, W.; Tang, Y. Controllable shell thickness of Co/CoO core-shell structure on 3D nickel foam with high performance supercapacitors. J. Alloys Compd. 2017, 726, 139–147. [Google Scholar] [CrossRef]
- Nazri, G.-A.; Julien, C. Far-infrared and Raman studies of orthorhombic MoO3 single crystal. Solid State Ion. 1992, 53, 376–382. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, H.; Pan, B.; Su, Y.; Wang, C.A.; Zhong, M. Binder-free carbon-coated nanocotton transition metal oxides integrated anodes by laser surface ablation for lithium-ion batteries. Surf. Interface Anal. 2019, 51, 874–881. [Google Scholar] [CrossRef]
- Song, T.; Qi, Y.; Jia, A.; Ta, N.; Lu, J.; Wu, P.; Li, X. Continuous hydrogenation of CO2-derived ethylene carbonate to methanol and ethylene glycol at Cu-MoOx interface with a low H2/ester ratio. J. Catal. 2021, 399, 98–110. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, S.; Ma, Z.; Kundu, M.; Tang, B.; Li, J.; Wang, X. Oxygen vacancies engineered self-supported B doped Co3O4 nanowires as an efficient multifunctional catalyst for electrochemical water splitting and hydrolysis of sodium borohydride. Chem. Eng. J. 2021, 404, 126474. [Google Scholar] [CrossRef]
- Vijaya, S.; Kennedy, L.J. From waste to energy storage: Post-consumer waste expanded polystyrene/rGO composite as a high performance self-standing electrode for coin cell supercapacitors. RSC Adv. 2024, 14, 689–699. [Google Scholar] [CrossRef]
- Zheng, S.; Tang, X.; Wu, Z.-S.; Tan, Y.-Z.; Wang, S.; Sun, C.; Cheng, H.-M.; Bao, X. Arbitrary-shaped graphene-based planar sandwich supercapacitors on one substrate with enhanced flexibility and integration. Acs Nano 2017, 11, 2171–2179. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, X.; Sang, Y.; Zhao, Z.; Zhou, K.; Liu, H.; Chen, S. Enhanced performance of layered titanate nanowire-based supercapacitor electrodes by nickel ion exchange. ACS Appl. Mater. Interfaces 2014, 6, 4578–4586. [Google Scholar] [CrossRef]
- Gopi, C.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kalla, R.M.N.; Kim, H.-J. One-pot synthesis of copper oxide–cobalt oxide core–shell nanocactus-like heterostructures as binder-free electrode materials for high-rate hybrid supercapacitors. Mater. Today Energy 2019, 14, 100358. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Lü, S.; Chen, Y.; Fan, H.; Wei, M.; Yang, L.; William, W.Y.; Meng, X. CoO@ CoS/Ni3S2 hierarchical nanostructure arrays for high performance asymmetric supercapacitor. Appl. Surf. Sci. 2020, 532, 147438. [Google Scholar] [CrossRef]
- Peighambardoust, N.S.; Aydemir, U. Blue TiO2 nanotube arrays as semimetallic materials with enhanced photoelectrochemical activity towards water splitting. Turk. J. Chem. 2020, 44, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.M. Electrochemical impedance spectroscopy in the characterisation and application of modified electrodes for electrochemical sensors and biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Marf, A.S.; Dannoun, E.M.; Brza, M.A.; Abdullah, R.M. The study of the degree of crystallinity, electrical equivalent circuit, and dielectric properties of polyvinyl alcohol (PVA)-based biopolymer electrolytes. Polymers 2020, 12, 2184. [Google Scholar] [CrossRef] [PubMed]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical interpretations of Nyquist plots for EDLC electrodes and devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Haye, E.; Miao, Y.; Pilloud, D.; Douard, C.; Boukherroub, R.; Pierson, J.-F.; Brousse, T.; Lucas, S.; Houssiau, L.; Pireaux, J.-J. Enhancing cycling stability and specific capacitance of vanadium nitride electrodes by tuning electrolyte composition. J. Electrochem. Soc. 2022, 169, 063503. [Google Scholar] [CrossRef]
- Basu, A.; Bhardwaj, M.; Gawli, Y.; Rode, C.; Ogale, S. A Robust Highly Flexible All–solid–state Micro Pseudocapacitor Based on Ternary Oxide CuCo2O4 having Ultrathin Porous Nanowall Type Morphology Blended with CNT. ChemistrySelect 2016, 1, 5159–5164. [Google Scholar] [CrossRef]
- Barik, R.; Raulo, A.; Jha, S.; Nandan, B.; Ingole, P.P. Polymer-derived electrospun Co3O4@ C porous nanofiber network for flexible, high-performance, and stable supercapacitors. ACS Appl. Energy Mater. 2020, 3, 11002–11014. [Google Scholar] [CrossRef]
- Kalasina, S.; Pattanasattayavong, P.; Suksomboon, M.; Phattharasupakun, N.; Wutthiprom, J.; Sawangphruk, M. A new concept of charging supercapacitors based on the photovoltaic effect. Chem. Commun. 2017, 53, 709–712. [Google Scholar] [CrossRef]
- Tian, Y.; Gong, J.; Zhu, W. Vertically-aligned Co(OH)2 Nanosheet Films for Flexible All-solid-state Electrochemical Supercapacitor. IOP Conf. Ser. Earth Environ. Sci. 2017, 94, 012131. [Google Scholar] [CrossRef]
- Afsahi, N.; Majumder, M.; Naseri, N. Printed flexible solid-state microsupercapacitor with highly-stable aqueous cobalt-based inks. Chem. Eng. J. 2024, 493, 152356. [Google Scholar] [CrossRef]
- Niu, X.; Zhu, G.; Yin, Z.; Dai, Z.; Hou, X.; Shao, J.; Huang, W.; Zhang, Y.; Dong, X. Fiber-based all-solid-state asymmetric supercapacitors based on Co3O4@ MnO2 core/shell nanowire arrays. J. Mater. Chem. A 2017, 5, 22939–22944. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Xu, Z.; Xu, Y.; Lei, T.; Liu, D.; Chen, C.; Wang, W.; Zhitomirsky, I.; Qu, M.; Zhang, G. Binder-Less Molybdenum Doped CoO Based Integrated Electrodes Fabricated by Electric Discharge Corrosion for High-Efficiency Supercapacitors. Materials 2025, 18, 80. https://doi.org/10.3390/ma18010080
Chen R, Xu Z, Xu Y, Lei T, Liu D, Chen C, Wang W, Zhitomirsky I, Qu M, Zhang G. Binder-Less Molybdenum Doped CoO Based Integrated Electrodes Fabricated by Electric Discharge Corrosion for High-Efficiency Supercapacitors. Materials. 2025; 18(1):80. https://doi.org/10.3390/ma18010080
Chicago/Turabian StyleChen, Ri, Zehan Xu, Yunying Xu, Tujun Lei, Dawei Liu, Chunlong Chen, Wenxia Wang, Igor Zhitomirsky, Muchao Qu, and Guoying Zhang. 2025. "Binder-Less Molybdenum Doped CoO Based Integrated Electrodes Fabricated by Electric Discharge Corrosion for High-Efficiency Supercapacitors" Materials 18, no. 1: 80. https://doi.org/10.3390/ma18010080
APA StyleChen, R., Xu, Z., Xu, Y., Lei, T., Liu, D., Chen, C., Wang, W., Zhitomirsky, I., Qu, M., & Zhang, G. (2025). Binder-Less Molybdenum Doped CoO Based Integrated Electrodes Fabricated by Electric Discharge Corrosion for High-Efficiency Supercapacitors. Materials, 18(1), 80. https://doi.org/10.3390/ma18010080





