The Influence of Process Parameters on Hydrogen-Terminated Diamond and the Enhancement of Carrier Mobility
Highlights
- Hydrogen-terminated diamonds with different conditions were prepared by microwave plasma chemical vapor deposition (CVD).
- Effect of process parameters on the electrical properties of hydrogen-terminated diamond.
- The electrical properties of hydrogen-terminated diamond prepared by different process parameters were analyzed and explained.
- The reasons for the enhancement of carrier mobility on the surface of hydrogen-terminated diamond were analyzed and explained.
Abstract
1. Introduction
2. Experiments and Methods
- (1)
- The sample was placed in the chamber and vacuumed to 0.5 Pa. Hydrogen was introduced to excite the microwave plasma, and the pressure and power were gradually increased to make the surface of the sample reach about 900 °C. Hydrogen plasma pre-etching was carried out for 10 min. In the process of homoepitaxial growth, on the one hand, the pre-etching process can remove most of the impurities on the surface, such as dust and the non-diamond phase, and improve the purity of the epitaxial layer. On the other hand, it can remove the stress damage caused by the surface polishing process to a certain extent, reduce the defect density of the epitaxial layer, and improve the crystal quality. Then, the homoepitaxial growth of the diamond was carried out by introducing different CH4 contents. The temperature was controlled at 950 °C–980 °C, and the growth was carried out at this temperature environment for 8h. Then, the CH4 was turned off and the hydrogen etching was carried out at 900 °C for 30 min. The homoepitaxial growth parameters of high-quality diamond layers are shown in Table 1.
- (2)
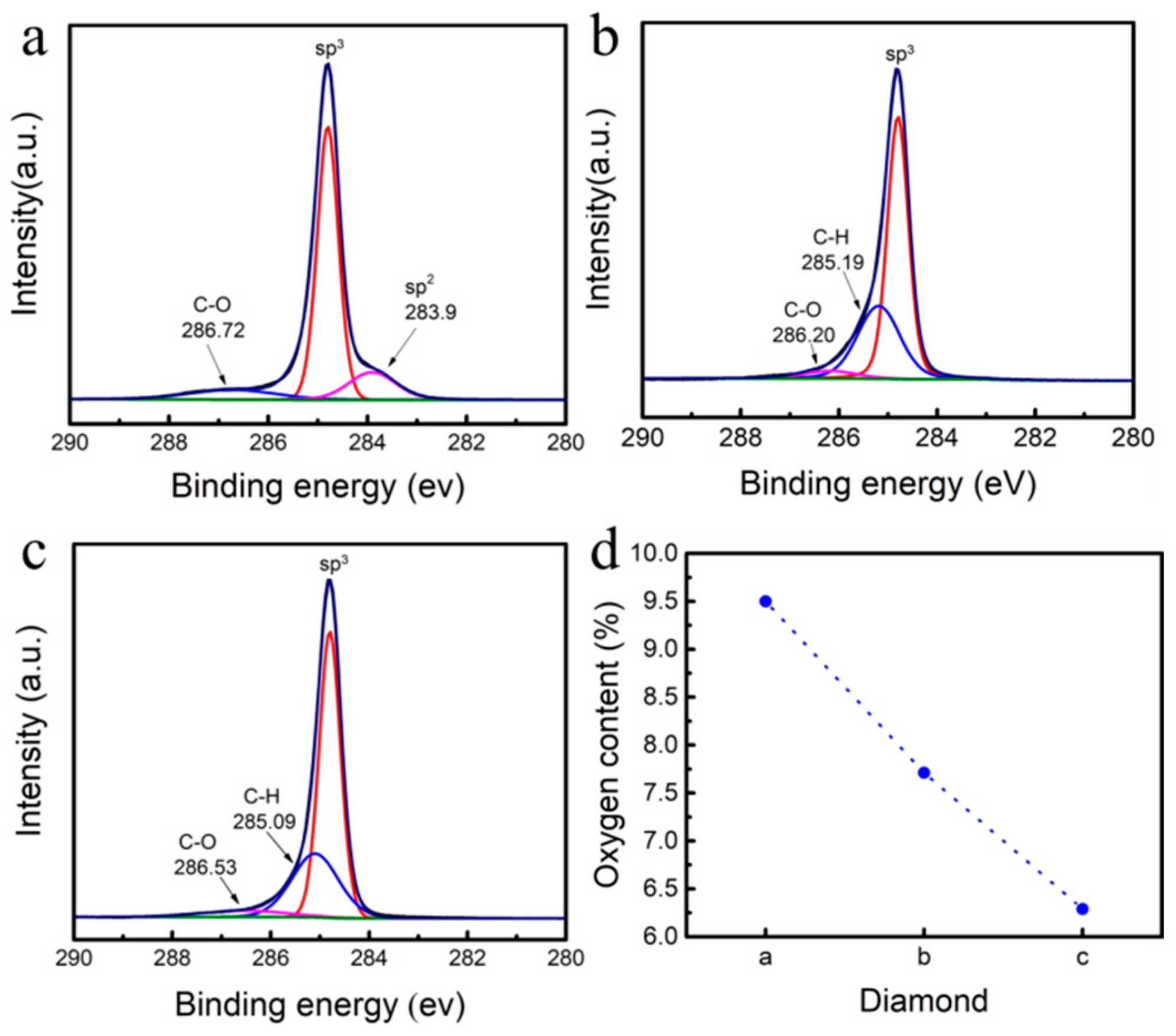
- Under the same conditions, 3% CH4 was selected for temperature-controlled growth, and the temperature was controlled at 950 °C–980 °C. After 8 h of growth, a high-purity single-crystal diamond homoepitaxial layer was grown on the substrate surface. After CH4 was turned off, hydrogen etching was carried out at 900 °C for 15 min, 30 min, and 45 min, respectively.
- (3)
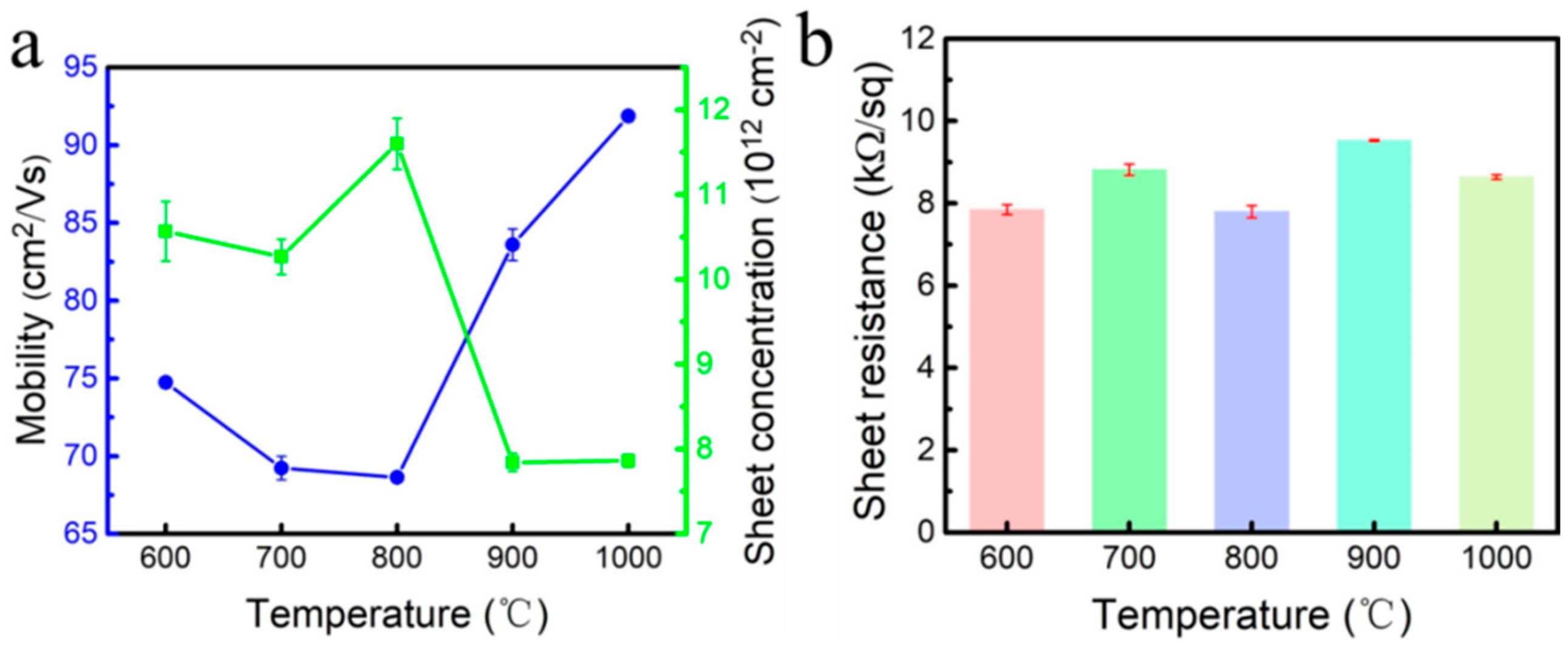
- The samples were placed in a 6 kW microwave plasma chemical vapor deposition (MPCVD) chamber and vacuumed to 0.5 Pa. Then, hydrogen was introduced to excite the plasma, and the power and pressure of the equipment were gradually increased. Hydrogen etching was performed at 600 °C, 700 °C, 800 °C, 900 °C, and 1000 °C, respectively. After the surface temperature of the sample reached the set temperature, the hydrogen etching was maintained at this temperature for 30 min. The temperature parameters of the hydrogen plasma treatment are shown in Table 2.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perez, G.; Maréchal, A.; Chicot, G.; Lefranc, P.; Jeannin, P.-O.; Eon, D.; Rouger, N. Diamond semiconductor performances in power electronics applications. Diam. Relat. Mater. 2020, 110, 108154. [Google Scholar] [CrossRef]
- Ding, H.-B.; Wu, L.-Y.; Feng, Y.-J.; Niu, R.; Liu, Y.-M.; Zhong, G.-H.; Yang, C.-L.; Lin, H.-Q. Superconductivity at 117 K inH-doped diamond. Mater. Today Phys. 2023, 35, 101115. [Google Scholar] [CrossRef]
- Mosińska, L.; Popielarski, P.; Fabisiak, K.; Dychalska, A. Effects of hydrogen termination of CVD diamond layers. Opt. Mater. 2020, 101, 109676. [Google Scholar] [CrossRef]
- Crawford, K.G.; Maini, I.; Macdonald, D.A. Surface transfer doping of diamond: A review. Prog. Surf. Sci. 2021, 96, 100613. [Google Scholar] [CrossRef]
- Bormashov, V.; Buga, S.; Kuznetsov, M.; Terentiev, S.; Semenov, A.; Blank, V. Electrical properties of the high quality boron-doped synthetic single-crystal diamonds grown by the temperature gradient method. Diam. Relat. Mater. 2013, 35, 19–23. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, Q.; Qiu, M.; Jia, Z.; Yang, G.; Nishimura, K.; Lin, C.-T.; Sun, X.; Jiang, N.; Hu, Y. Temperature dependence of two-dimensional hole gas on hydrogen-terminated diamond surface. Diam. Relat. Mater. 2023, 139, 110414. [Google Scholar] [CrossRef]
- Li, F.N.; Li, Y.; Bao, H.W.; Wang, H.X.; Ma, F. Fabrication of hydroxyl terminateddiamond by high-voltage hydroxide ion treatments. Appl. Surf. Sci. 2023, 622, 156909. [Google Scholar] [CrossRef]
- Kageura, T.; Sasama, Y.; Yamada, K.; Kimura, K.; Onoda, S.; Takahide, Y. Surface transfer doping of hydrogen-terminated diamond probed by shallow nitrogen-vacancy centers. Carbon 2024, 229, 119404. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.F.; Liu, G.P.; Ren, Z.Y.; Zhang, J.C.; Hao, Y. Mobility of two-dimen-sional hole gas in H-terminated diamond. Phys. Status Solidi RRL 2018, 12, 1700401. [Google Scholar] [CrossRef]
- Lew, C.T.-K.; Dontschuk, N.; Broadway, D.A.; Tetienne, J.-P.; McCallum, J.C.; Hollenberg, L.C.L.; Johnson, B.C. Investigation of charge carrier trapping in H-terminated diamond devices. Appl. Phys. Lett. 2020, 117, 143507. [Google Scholar] [CrossRef]
- Yang, N.; Yu, S.; Macpherson, J.V.; Einaga, Y.; Zhao, H.; Swain, G.; Greg, M.; Jiang, X. Conductive diamond: Synthesis, properties, and electrochemical applications. Chem. Soc. Rev. 2019, 48, 157–204. [Google Scholar] [CrossRef] [PubMed]
- Kubovic, M.; Kasu, M.; Kageshima, H. Sorption properties of NO2 gas and its strong influence on hole concentration of H-terminated diamond surfaces. Appl. Phys. Lett 2010, 96, 052101. [Google Scholar] [CrossRef]
- Kubovic, M.; Kasu, M.; Kageshima, H.; Maeda, F. Electronic and surface properties of H-terminated diamond surface affected by NO2 gas. Diam. Relat. Mater. 2010, 19, 889–893. [Google Scholar] [CrossRef]
- Wade, T.; Geis, M.W.; Fedynyshyn, T.H.; Vitale, S.A.; Varghese, J.O.; Lennon, D.M.; Nemanich, R.J.; Hollis, M.A. Effect of surface roughness and H–termination chemistry on diamond’s semiconducting surface conductance. Diam. Relat. Mater. 2017, 76, 79–85. [Google Scholar] [CrossRef]
- Yianni, S.A.; Creedon, D.L.; Schenk, A.K.; Xing, K.; Akhgar, G.; Hoxley, D.I.; Ley, L.; McCallum, J.C.; Pakes, C.I.; Yianni, S.A.; et al. Correlation between electronic micro-roughness and surface topography in two-dimensional surface conducting hydrogen-terminated diamond. Diam. Relat. Mater. 2021, 116, 108377. [Google Scholar] [CrossRef]
- Jia, C.; Jia, D.; Zhang, H.; Zhuang, Q.; Ou, T.; Jiang, D.; Zhang, F.; Zhang, S.; Meng, Y. First principles investigation of thermodynamic property of diamond under high pressure and high temperature. Phys. B: Condens. Matter 2023, 674, 415556. [Google Scholar] [CrossRef]
- Zhao, H.; Li, B.; Wang, Z.; Liu, Y.; Guo, Q.; Wang, S.; Teng, Y.; Chen, L.; Ma, H.; Jia, X. Effect of nitrogen–hydrogen co-doping on diamond growth. Int. J. Refract. Met. Hard Mater. 2023, 117, 106410. [Google Scholar] [CrossRef]
- Gong, M.; Wang, Q.; Gao, N.; Li, H. Structural and electronic properties of nitrogen-terminated diamond (100) surfaces. Diam. Relat. Mater. 2021, 120, 108601. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Gou, L. Dielectric behavior and defects of nitrogen-containing single crystal diamond films. Diam. Relat. Mater. 2024, 49, 111642. [Google Scholar] [CrossRef]
- Vikharev, A.; Lobaev, M.; Gorbachev, A.; Radishev, D.; Isaev, V.; Bogdanov, S. Investigation of homoepitaxial growth by microwave plasma CVD providing high growth rate and high quality of diamond simultaneously. Mater. Today Commun. 2020, 22, 100816. [Google Scholar] [CrossRef]
- Takano, Y.; Takenouchi, T.; Ishii, S.; Ueda, S.; Okutsu, T.; Sakaguchi, I.; Umezawa, H.; Kawarada, H.; Tachiki, M. Superconducting properties of homoepitaxial CVD diamond. Diam. Relat. Mater. 2007, 16, 911–914. [Google Scholar] [CrossRef]
- Tallaire, A.; Achard, J.; Silva, F.; Sussmann, R.; Gicquel, A. Homoepitaxial deposition of high-quality thick diamond films: Effect of growth parameters. Diam. Relat. Mater. 2005, 14, 249–254. [Google Scholar] [CrossRef]
- Chen, J.; Shinei, C.; Inoue, J.; Abe, H.; Ohshima, T.; Sekiguchi, T.; Teraji, T. Appearance of spectral dip in the cathodoluminescence spectrum of negatively charged nitrogen-vacancy centers in diamonds. Diam. Relat. Mater. 2024, 148, 111476. [Google Scholar] [CrossRef]
- Lee, N.; Badzian, A. A study on surface morphologies of (001) homoepitaxial diamond films. Diam. Relat. Mater. 1997, 6, 130–145. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, W.; Zhang, L.; He, S.; Wang, H.; Yan, S.; Ma, W.; Guo, C.; Wang, Y. Evolution of High-Quality Homoepitaxial CVD Diamond Films Induced by Methane Concentration. Coatings 2021, 11, 888. [Google Scholar] [CrossRef]
- Crawford, K.G.; Tallaire, A.; Li, X.; Macdonald, D.A.; Qi, D.; Moran, D.A.J. The role of hydrogen plasma power on surface roughness and carrier transport in transfer-doped H-diamond. Diam. Relat. Mater. 2018, 84, 48–54. [Google Scholar] [CrossRef]
- Kuntumalla, M.K.; Michaelson, S.; Hoffman, A. Nitrogen, oxygen, and hydrogen bonding and thermal stability of ambient exposed nitrogen-terminated H-diamond (111) surfaces studied by XPS and HREELS. Surf. Sci. 2024, 749, 122555. [Google Scholar] [CrossRef]
- Oliveira, E.F.; Neupane, M.R.; Li, C.; Kannan, H.; Zhang, X.; Puthirath, A.B.; Shah, P.B.; Birdwell, A.G.; Ivanov, T.G.; Vajtai, R.; et al. A reactive molecular dynamics study of the hydrogenation of diamond surfaces. Comput. Mater. Sci. 2021, 200, 110859. [Google Scholar] [CrossRef]
- Varga, M.; Izak, T.; Vretenar, V.; Kozak, H.; Holovsky, J.; Artemenko, A.; Hulman, M.; Skakalova, V.; Lee, D.S.; Kromka, A. Diamond/carbon nanotube composites: Raman, FTIR and XPS spectroscopic studies. Carbon 2017, 111, 54–61. [Google Scholar] [CrossRef]
- Fischer, A.; Bhattacharya, A.; Hardy, A.; Grotjohn, T.; Ponce, F. The effect of step-flow growth on the surface morphology and optical properties of thick diamond films. Diam. Relat. Mater. 2023, 140, 110507. [Google Scholar] [CrossRef]
- Hu, W.; Chen, K.; Tao, T.; Yu, X.; Zhou, J.; Xie, Z.; Liu, B.; Zhang, R. High-rate growth of single-crystal diamond with an atomically flat surface by microwave plasma chemical vapor deposition. Thin Solid Films 2022, 763, 139571. [Google Scholar] [CrossRef]
- Oda, K.; Kaneko, J.; Chayahara, A.; Watanabe, H.; Umezawa, H.; Kobayakawa, Y. Evaluation of the effect of oxygen addition on charge carrier transport properties in single-crystal diamond growth. Diam. Relat. Mater. 2024, 148, 111441. [Google Scholar] [CrossRef]
- Liu, J.; Yu, H.; Shao, S.; Tu, J.; Zhu, X.; Yuan, X.; Wei, J.; Chen, L.; Ye, H.; Li, C. Carrier mobility enhancement on the H-terminated diamond surface. Diam. Relat. Mater. 2020, 104, 107750. [Google Scholar] [CrossRef]
- Hiroshi, K. High-current metal oxide semiconductor field-effect transistors on H-terminated diamond surfaces and their high-frequency operation. Jpn. J. Appl. Phys. 2012, 51, 090111. [Google Scholar]
- Sato, H.; Kasu, M. Maximum hole concentration for Hydrogen-terminated diamond surfaces with various surface orientations obtained by exposure to highly concentrated NO2. Diam. Relat. Mater. 2013, 31, 47–49. [Google Scholar] [CrossRef]
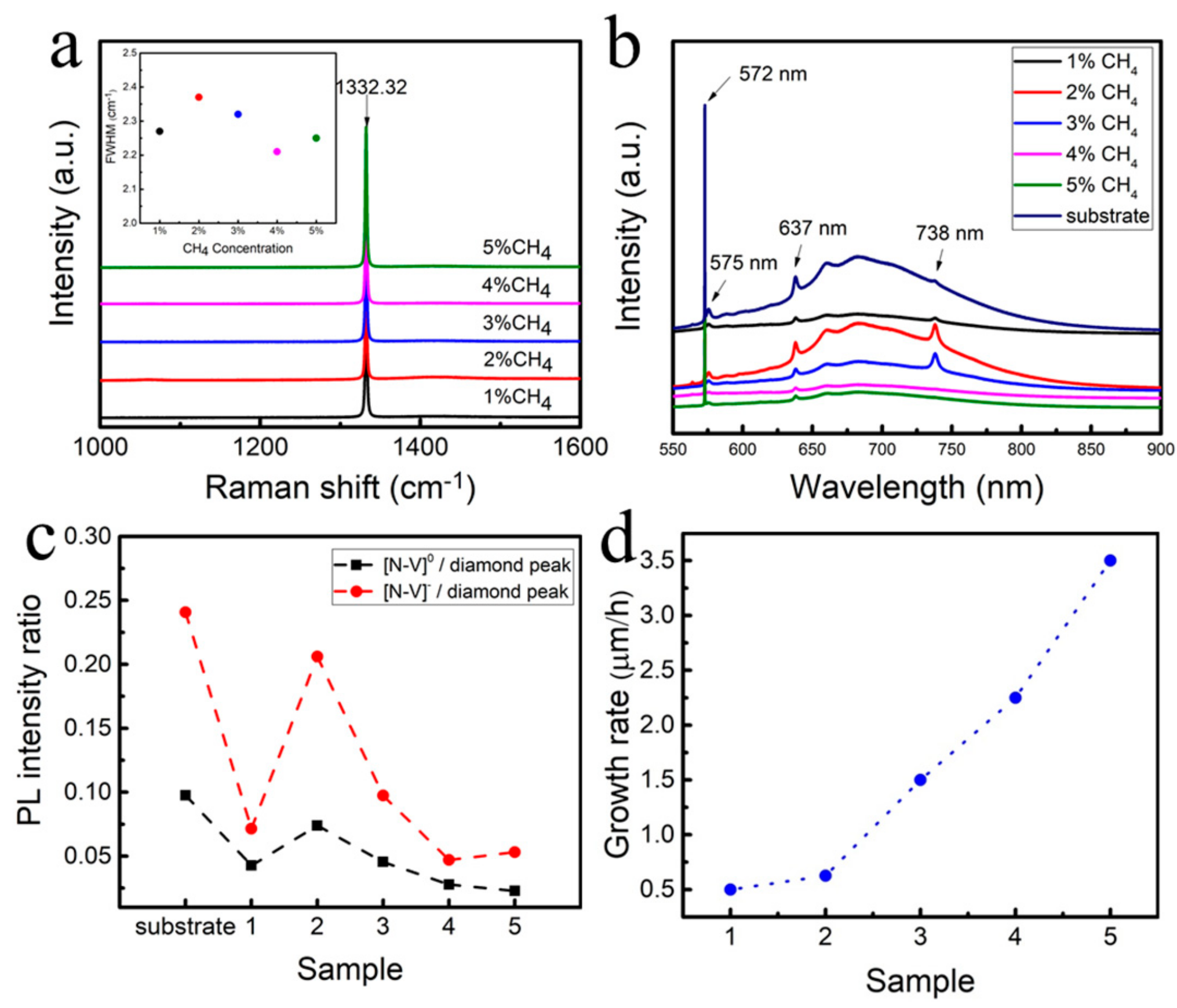
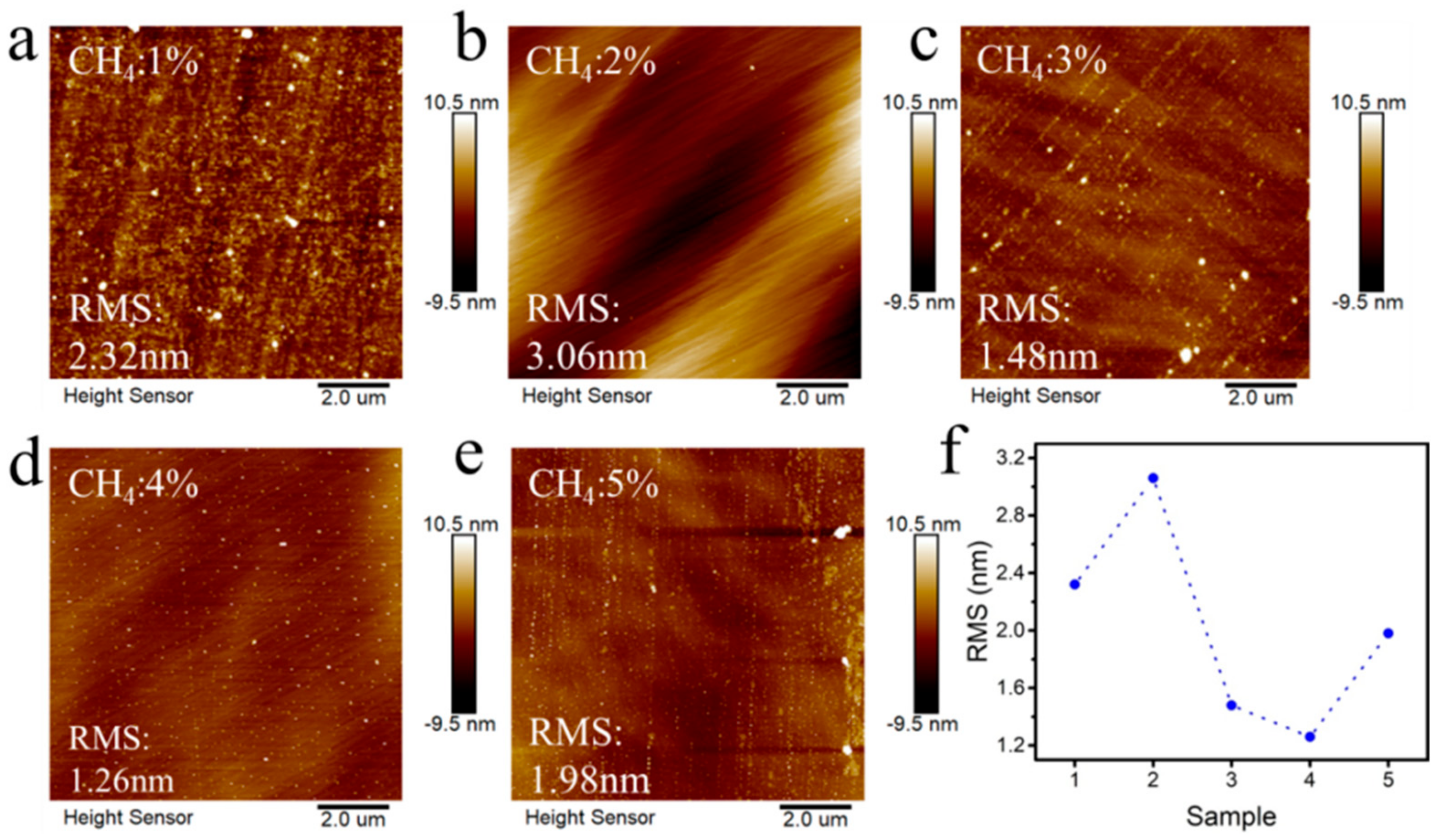

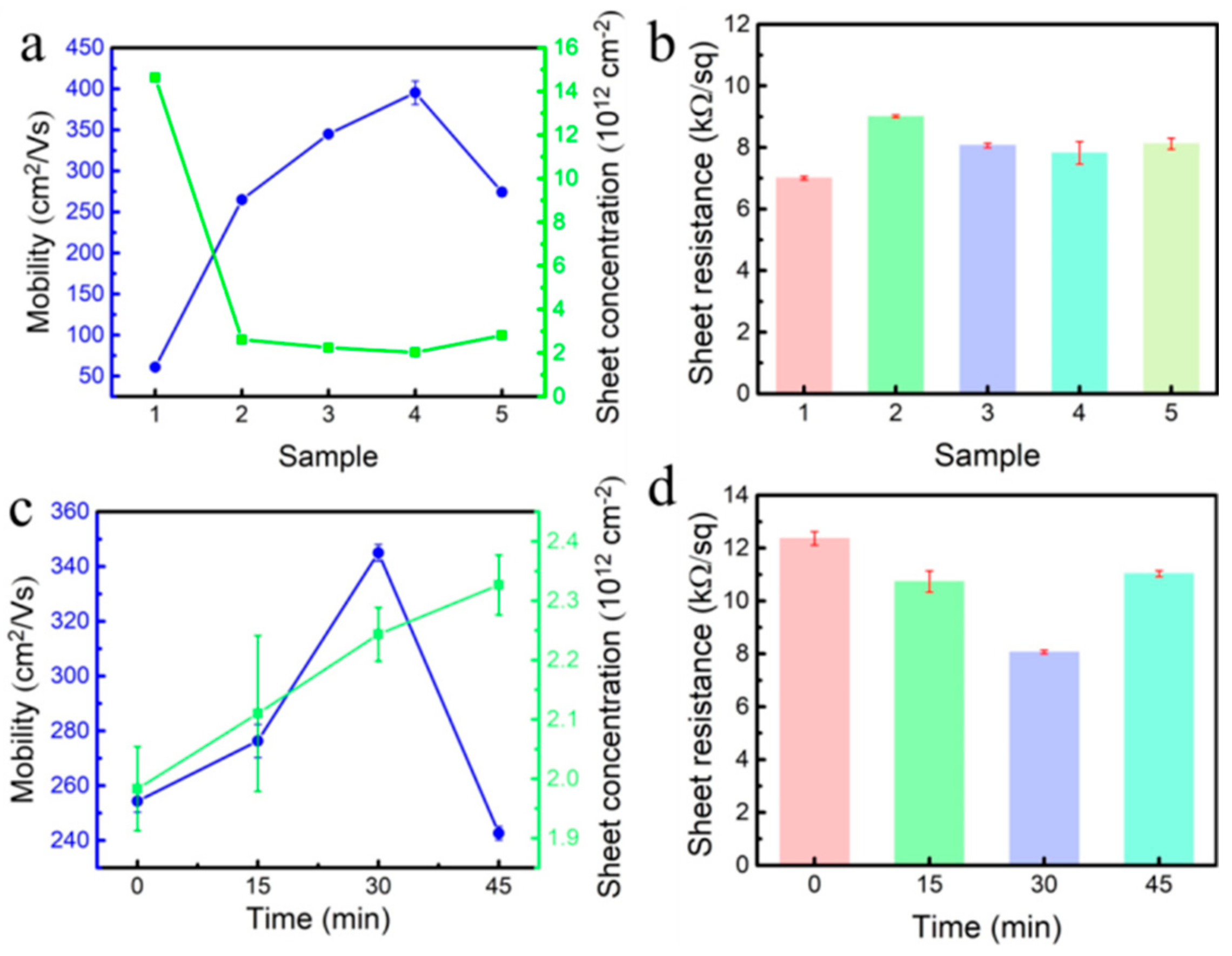
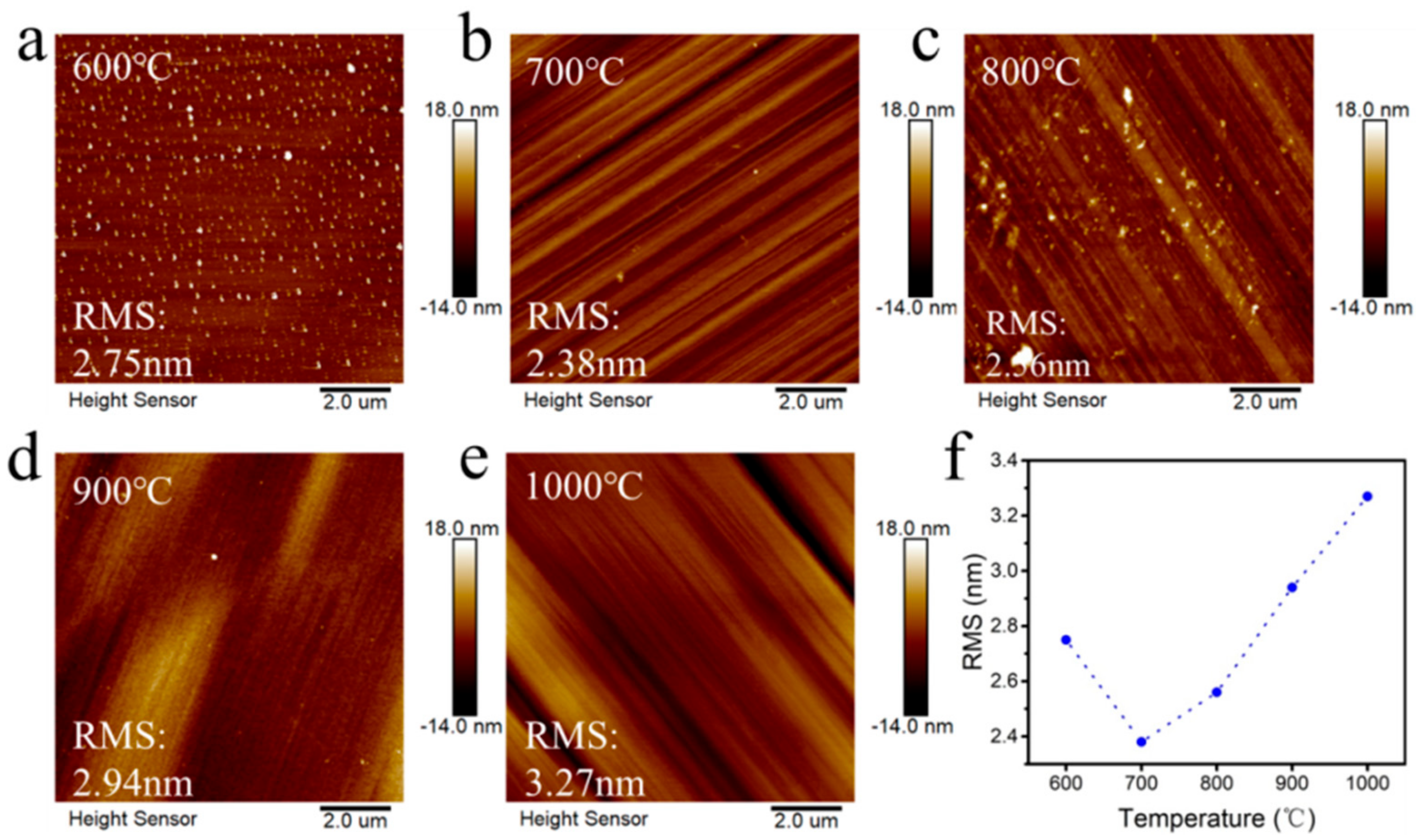
| Sample | CH4 Concentration/% | Temperature/°C | Power/W | Pressure/kPa | Duration/h |
|---|---|---|---|---|---|
| 1,2,3,4,5 | 1–5 | 950–980 | 4200–4600 | 13–15 | 8 |
| Temperature/°C | Power/W | Pressure/kPa | Duration/h |
|---|---|---|---|
| 600 | 2600 | 6.5 | 0.5 |
| 700 | 3300 | 9 | |
| 800 | 3800 | 11 | |
| 900 | 4500 | 12 | |
| 1000 | 5000 | 15 |
| Sample | CH4 Concentration/% | Growth Thickness (um) |
|---|---|---|
| 1 | 1 | 4 |
| 2 | 2 | 5 |
| 3 | 3 | 12 |
| 4 | 4 | 18 |
| 5 | 5 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yang, M.; Mu, Y.; Yang, C.; Jia, Z.; Liu, C.; Li, H.; Jiang, N.; Nishimura, K.; Guo, L.; et al. The Influence of Process Parameters on Hydrogen-Terminated Diamond and the Enhancement of Carrier Mobility. Materials 2025, 18, 112. https://doi.org/10.3390/ma18010112
Chen X, Yang M, Mu Y, Yang C, Jia Z, Liu C, Li H, Jiang N, Nishimura K, Guo L, et al. The Influence of Process Parameters on Hydrogen-Terminated Diamond and the Enhancement of Carrier Mobility. Materials. 2025; 18(1):112. https://doi.org/10.3390/ma18010112
Chicago/Turabian StyleChen, Xingqiao, Mingyang Yang, Yuanyuan Mu, Chengye Yang, Zhenglin Jia, Chaoping Liu, He Li, Nan Jiang, Kazuhito Nishimura, Liangchao Guo, and et al. 2025. "The Influence of Process Parameters on Hydrogen-Terminated Diamond and the Enhancement of Carrier Mobility" Materials 18, no. 1: 112. https://doi.org/10.3390/ma18010112
APA StyleChen, X., Yang, M., Mu, Y., Yang, C., Jia, Z., Liu, C., Li, H., Jiang, N., Nishimura, K., Guo, L., Chee, K. W. A., Yuan, Q., Li, X., & Song, H. (2025). The Influence of Process Parameters on Hydrogen-Terminated Diamond and the Enhancement of Carrier Mobility. Materials, 18(1), 112. https://doi.org/10.3390/ma18010112







