Research Enhancing Acidic Mine Wastewater Purification: Innovations in Red Mud–Loess
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Flexible Wall Penetration Test
- (1)
- Sample Preparation: A cylindrical mold, 5 cm tall and 5 cm wide, was cleaned, dried, and coated with Vaseline.
- (2)
- Saturation: Using a peristaltic pump, distilled water was progressively transferred into the soil column in a bottom-up arrangement until the liquid level exceeded the sample by 3 cm. This process indicates specimen saturation.
- (3)
- Measurement: The GeotestTK2000 flexible wall permeameter was employed following ASTM D5084-10 standards [42]. Osmotic pressure was 100 kPa, confining pressure was 110 kPa, and the hydraulic gradient was set to 200 for efficiency due to the sample’s firm strength and low permeability. The test involved vacuuming for 8 h at −100 kPa, introducing degassed water into the vacuum saturation cylinder, removing the saturated sample, placing it in the pressure chamber, injecting water, sealing it, and applying confining and osmotic pressure. After a three-day penetration test with hourly water production measurements, the instrument was turned off, and the sample was removed. The permeability coefficient is mathematically represented by Equation (1) based on test results.where ks is the saturated sample permeability coefficient (cm/s); i is the seepage hydraulic gradient; Q is the cumulative seepage flow rate (cm3); A is the cross-sectional area of the seepage sample (cm2); and t is the time (s).
2.3. Shear Test
2.4. Microscopic Testing and Characterization
2.4.1. X-ray Diffraction Analysis (XRD)
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.3. Scanning Electron Microscope-Energy Dispersive X-ray Spectroscopy (SEM-EDS)
2.5. Methods
2.5.1. Penetration Test
2.5.2. Shear Test
2.5.3. Microscopic Testing and Characterization
3. Results and Discussion
3.1. Permeability Characteristics of Red Mud–Loess Engineered Barrier
3.1.1. Effect of Quartz Sand Content
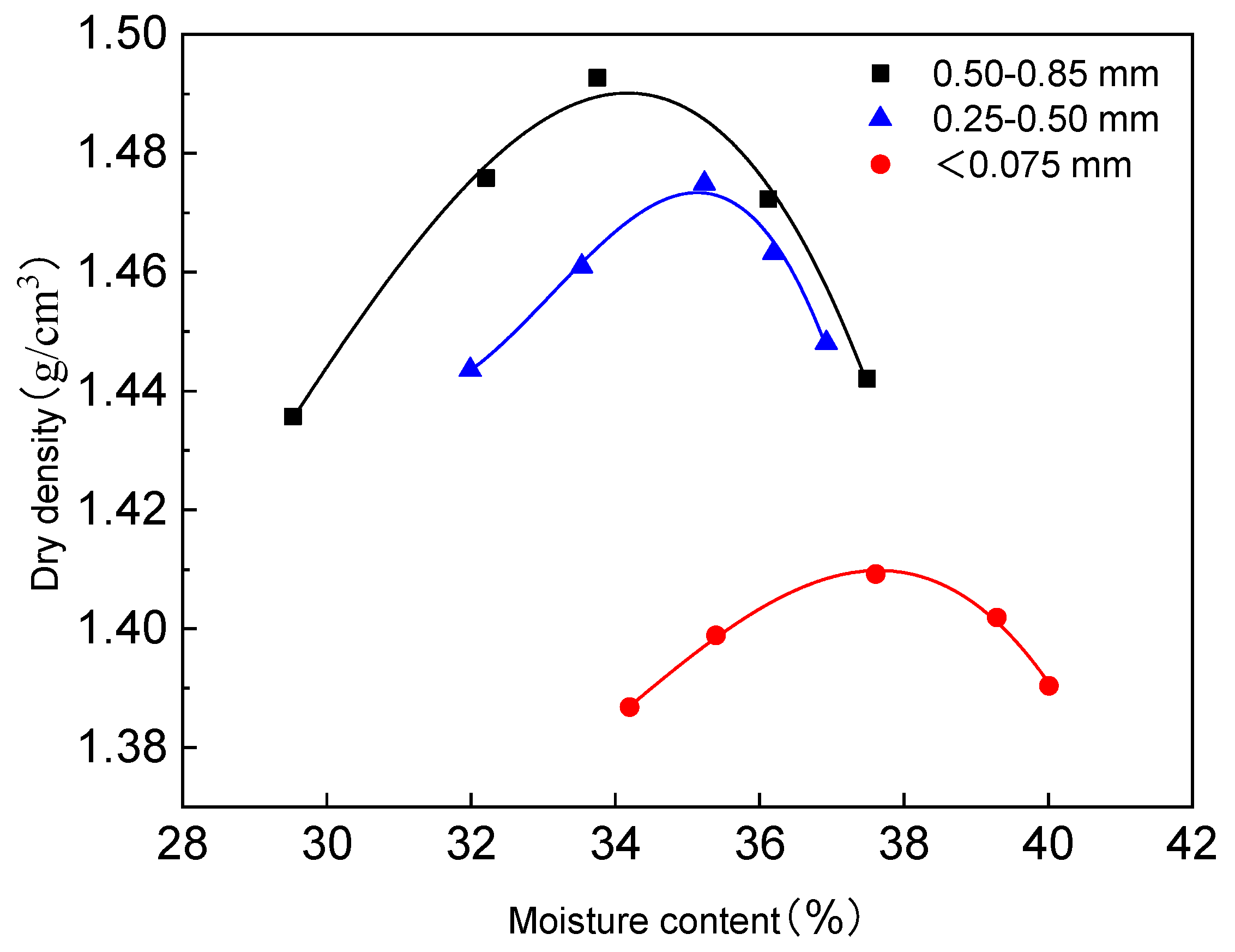
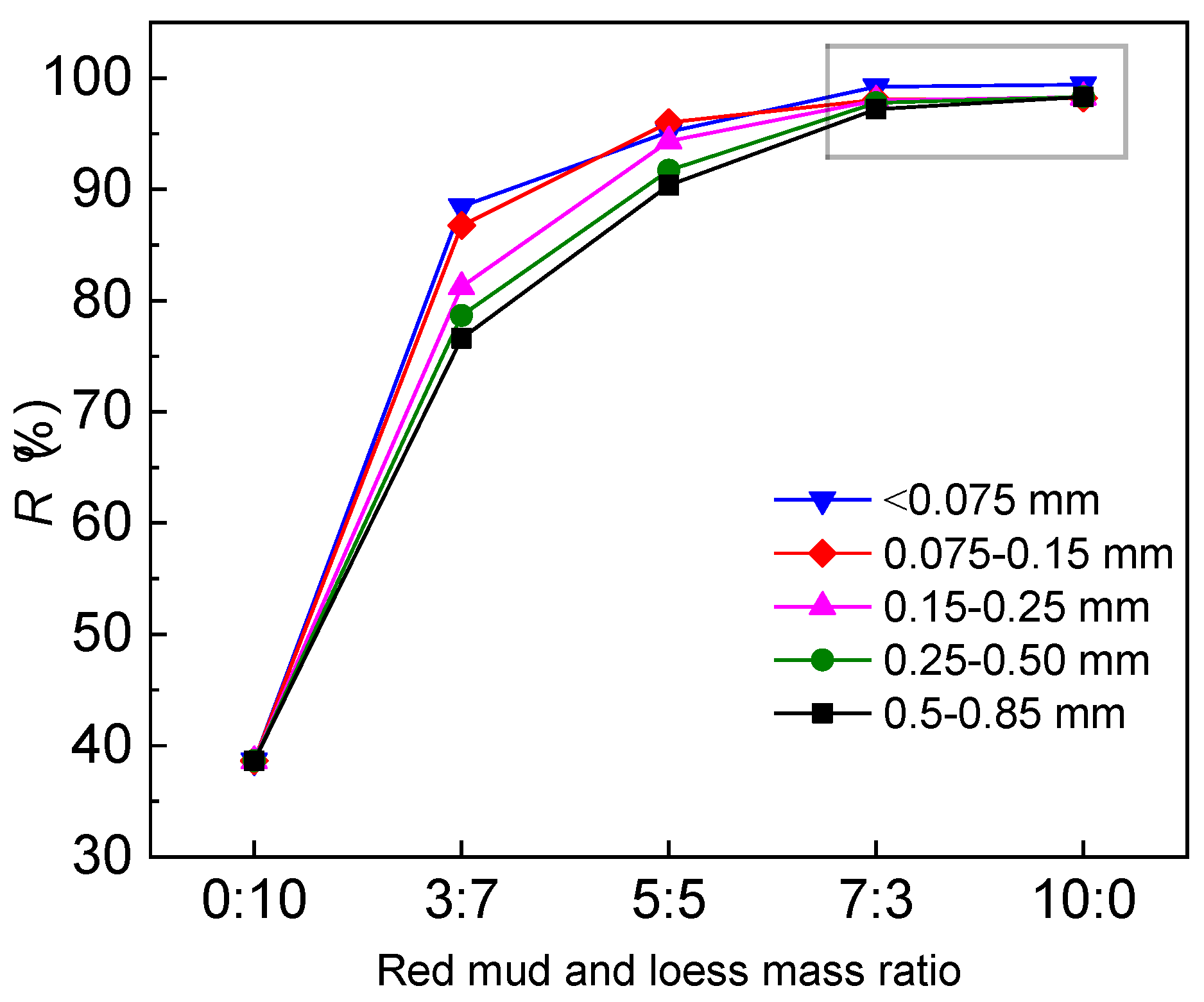
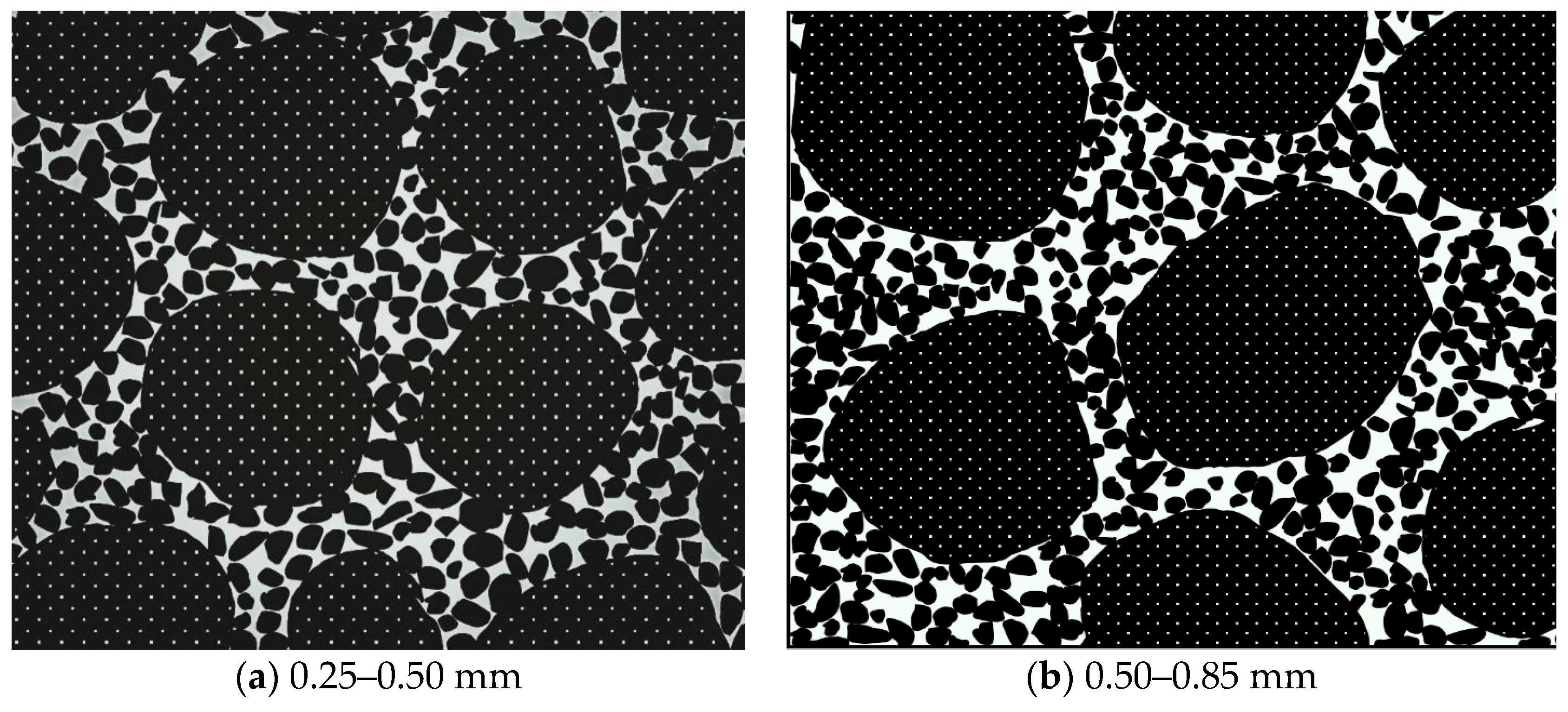
3.1.2. Particle Size Impact
- (1)
- Optimal Mass Ratio for Coarse-Grained Red Mud–Loess Mixtures
- (2)
- Permeability Characteristics of Coarse-Grained Red Mud–Loess Mixed Materials
3.2. Shear Strength Properties of Red Mud–Loess Engineered Barrier
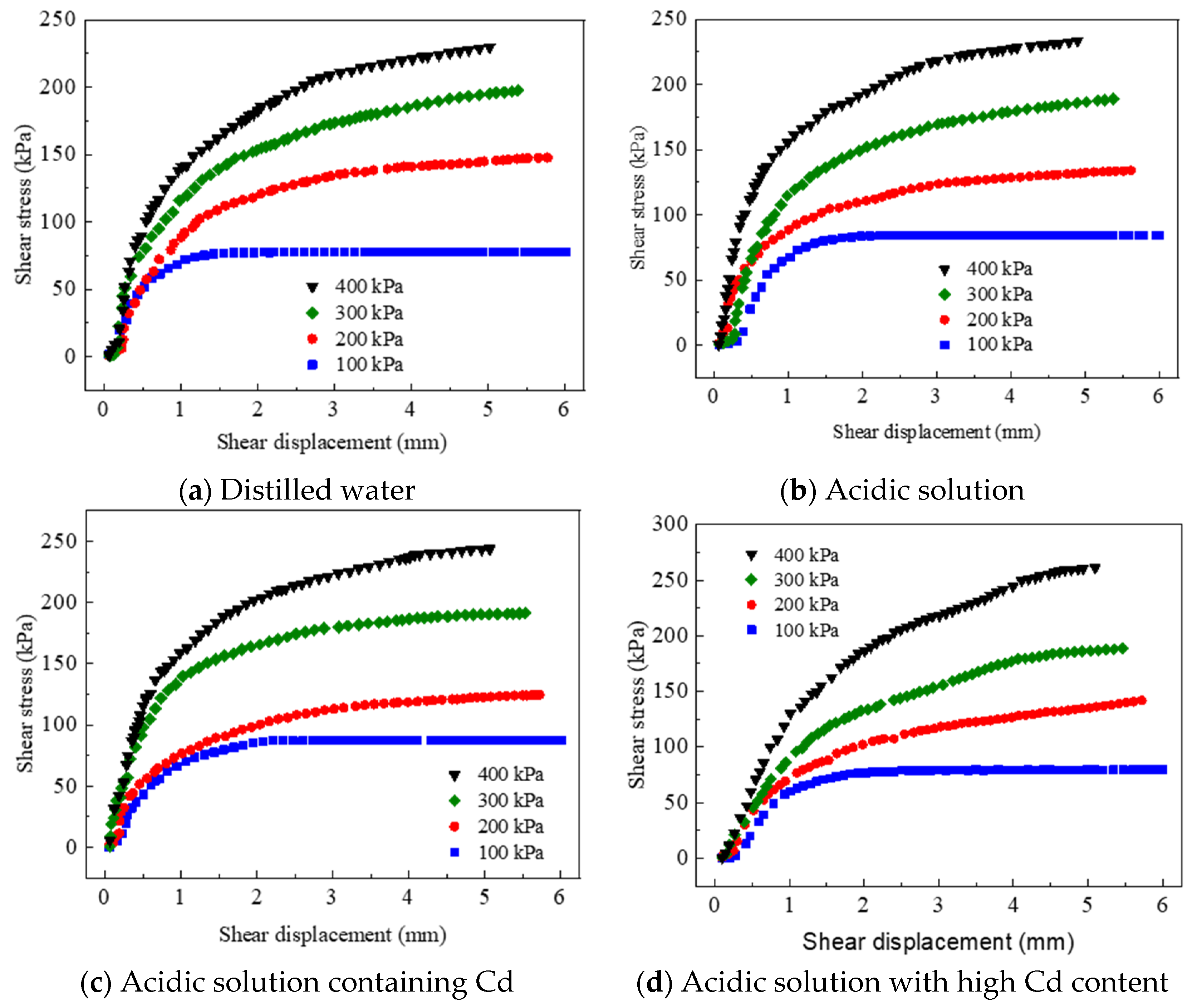
3.2.1. Shear Stress Behavior of Red Mud–Loess (7:3) in Different Pore Conditions
- (1)
- The Theoretical Adsorption Capacity of Coarse-Grained Red Mud–Loess Mixed Material (7:3):
- (2)
- Shear stress–shear displacement curve of red mud–loess mixed material (7:3)
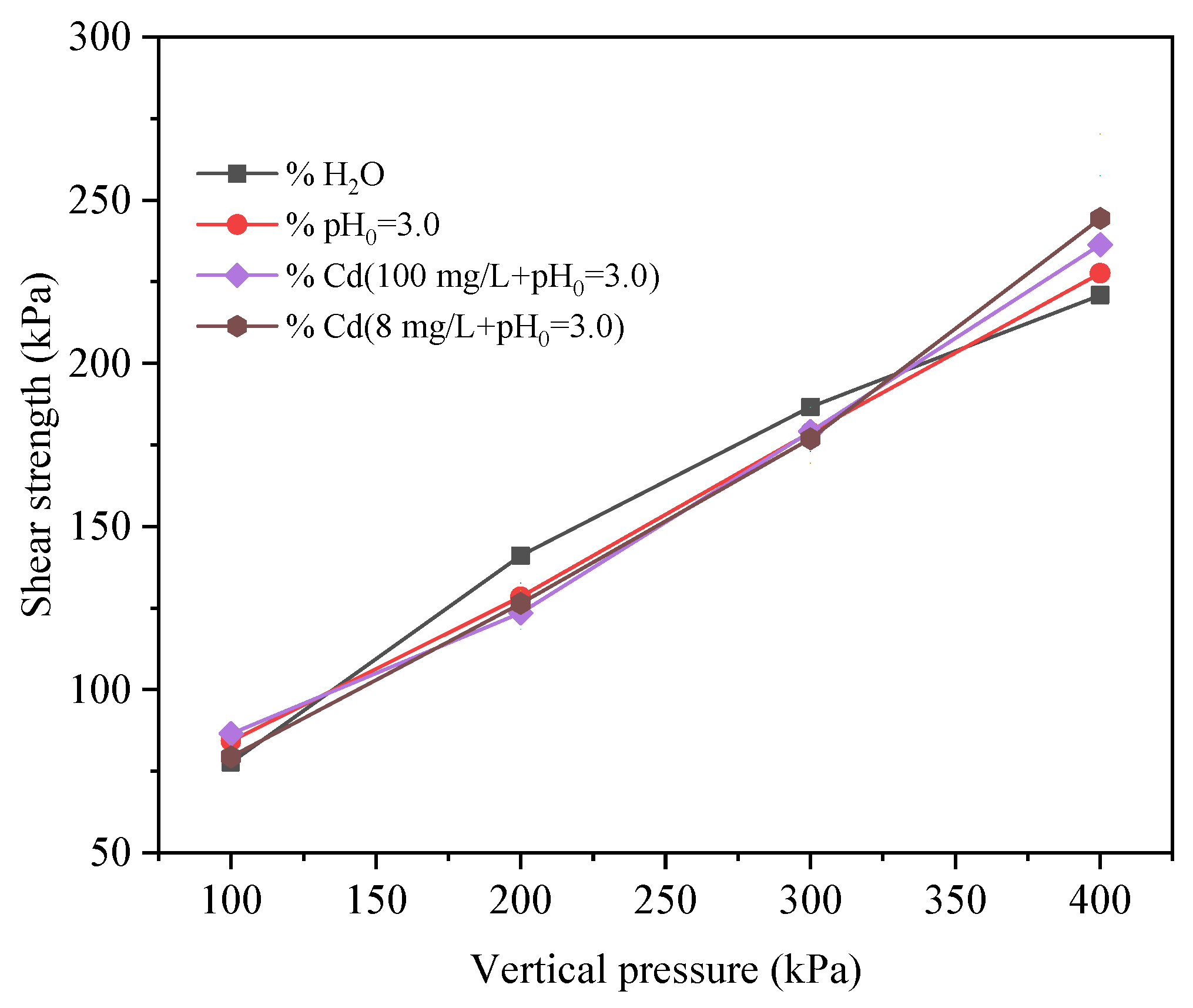
3.2.2. Changes in Shear Strength Parameters
3.3. Research on the Environmental Safety Characteristics of Solidified Soil
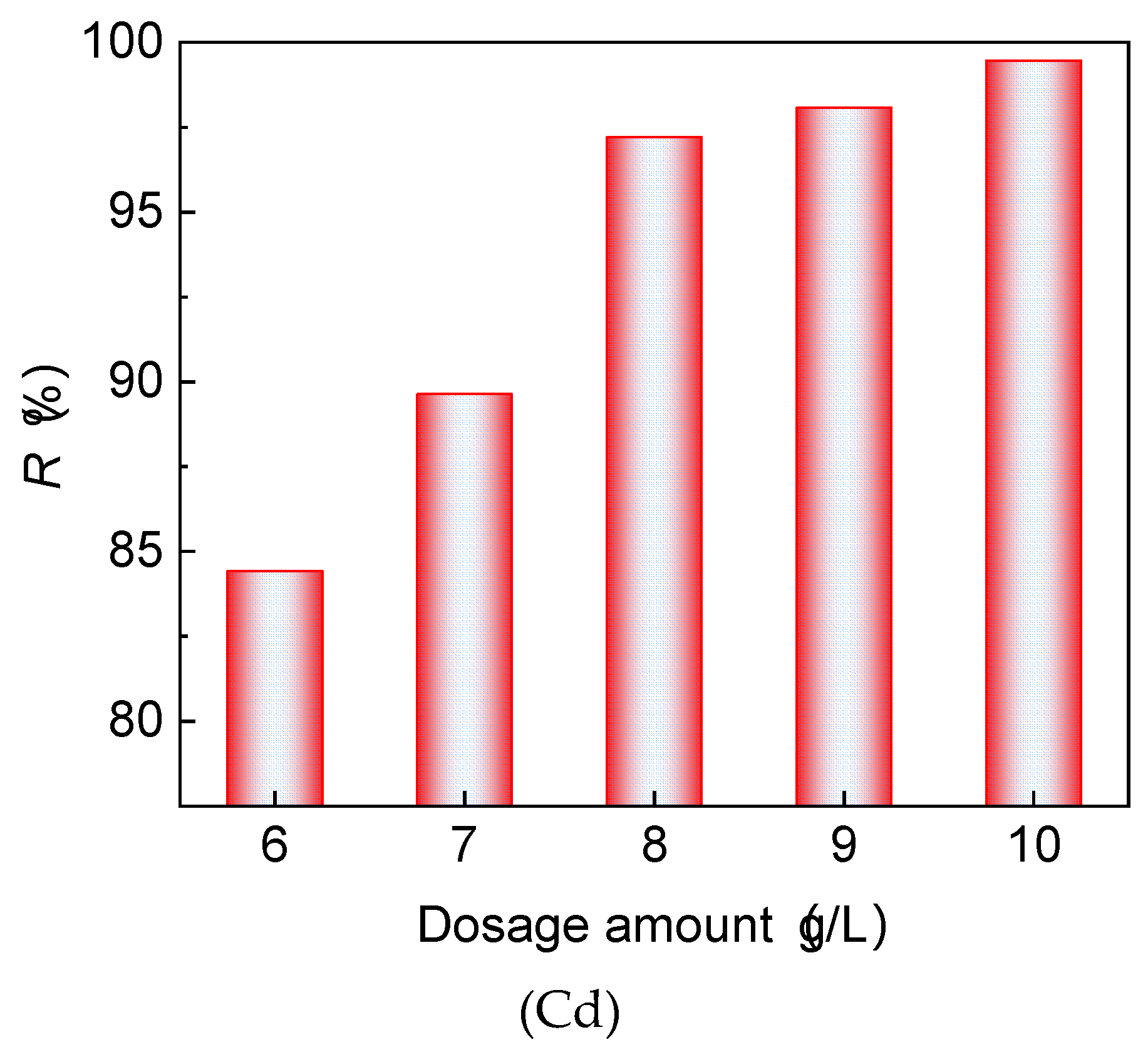
3.3.1. Acidity and Alkalinity Study in Cement-Solidification Cadmium-Contaminated Soil with Solid Waste
3.3.2. Cadmium Leaching Toxicity and Speciation in Cement-Solidified Soil with Solid Waste
3.4. Microscopic Analysis of Stabilization and Solidification (S/S) of High-Concentration Heavy Metal-Contaminated Soil
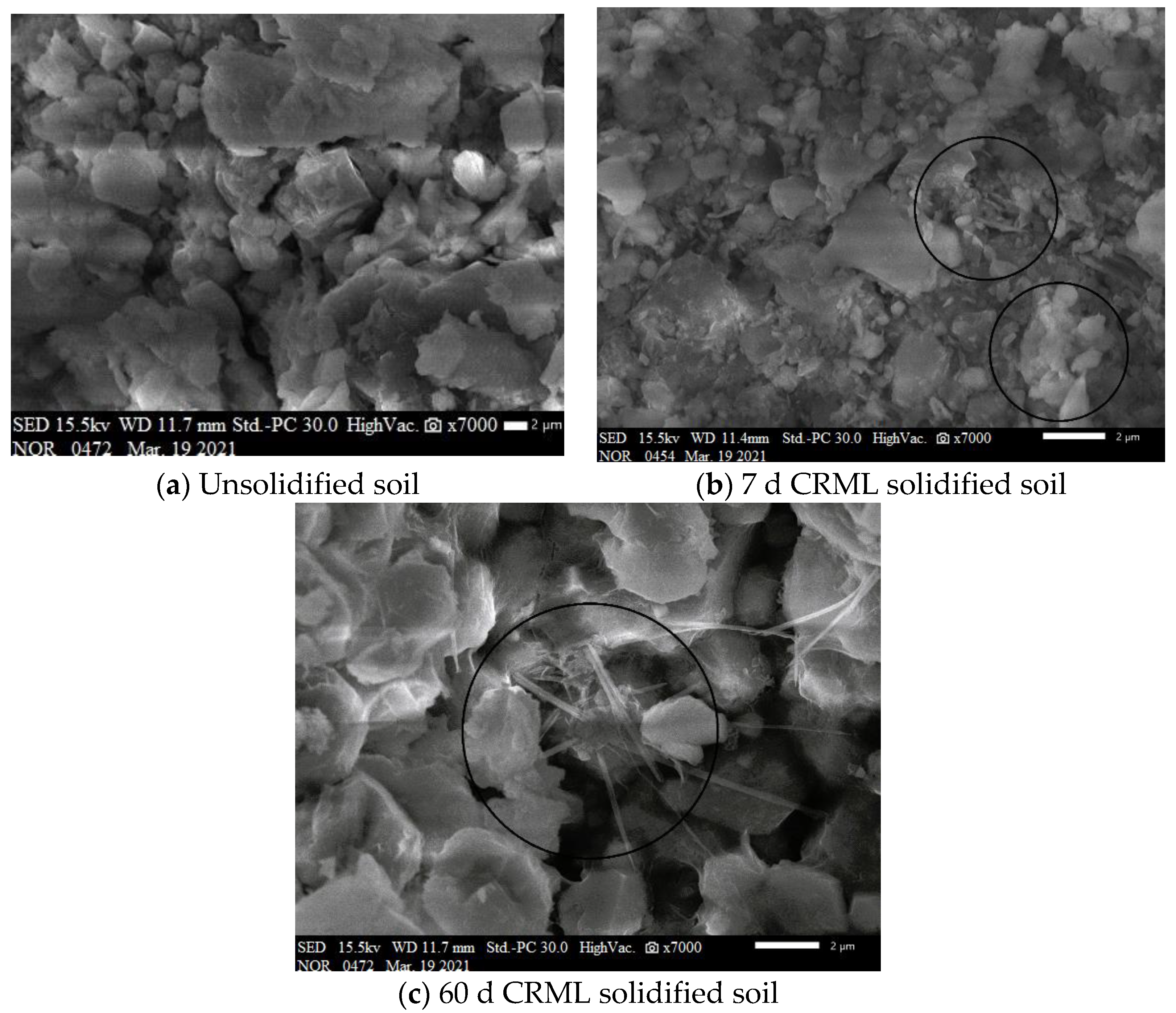
3.4.1. Comparison of Microscopic Morphology in Unsolidified and Solidified Cd-Contaminated Soil
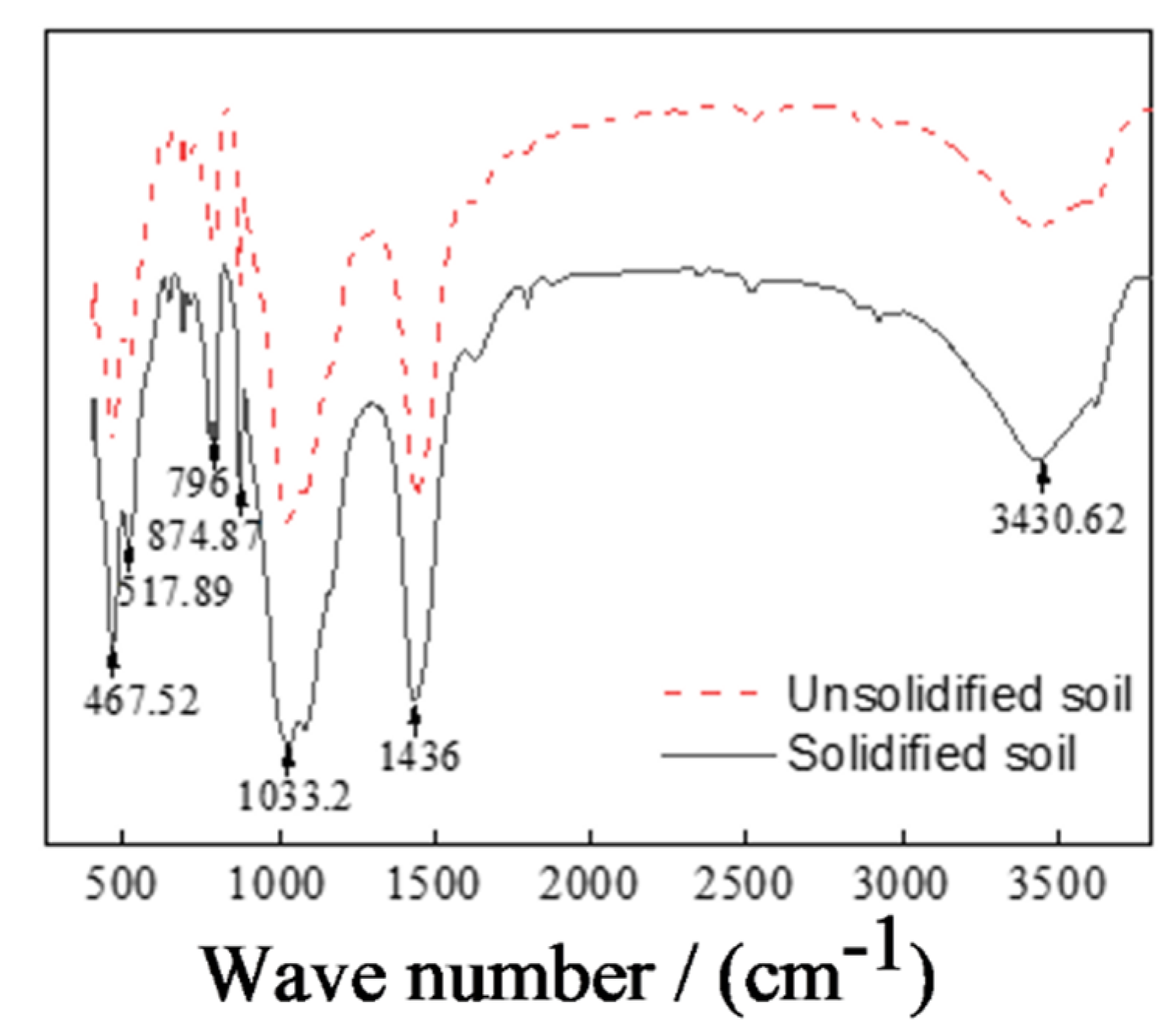
3.4.2. Functional Group Comparison in Unsolidified and CRML-Solidified Cadmium-Contaminated Soil
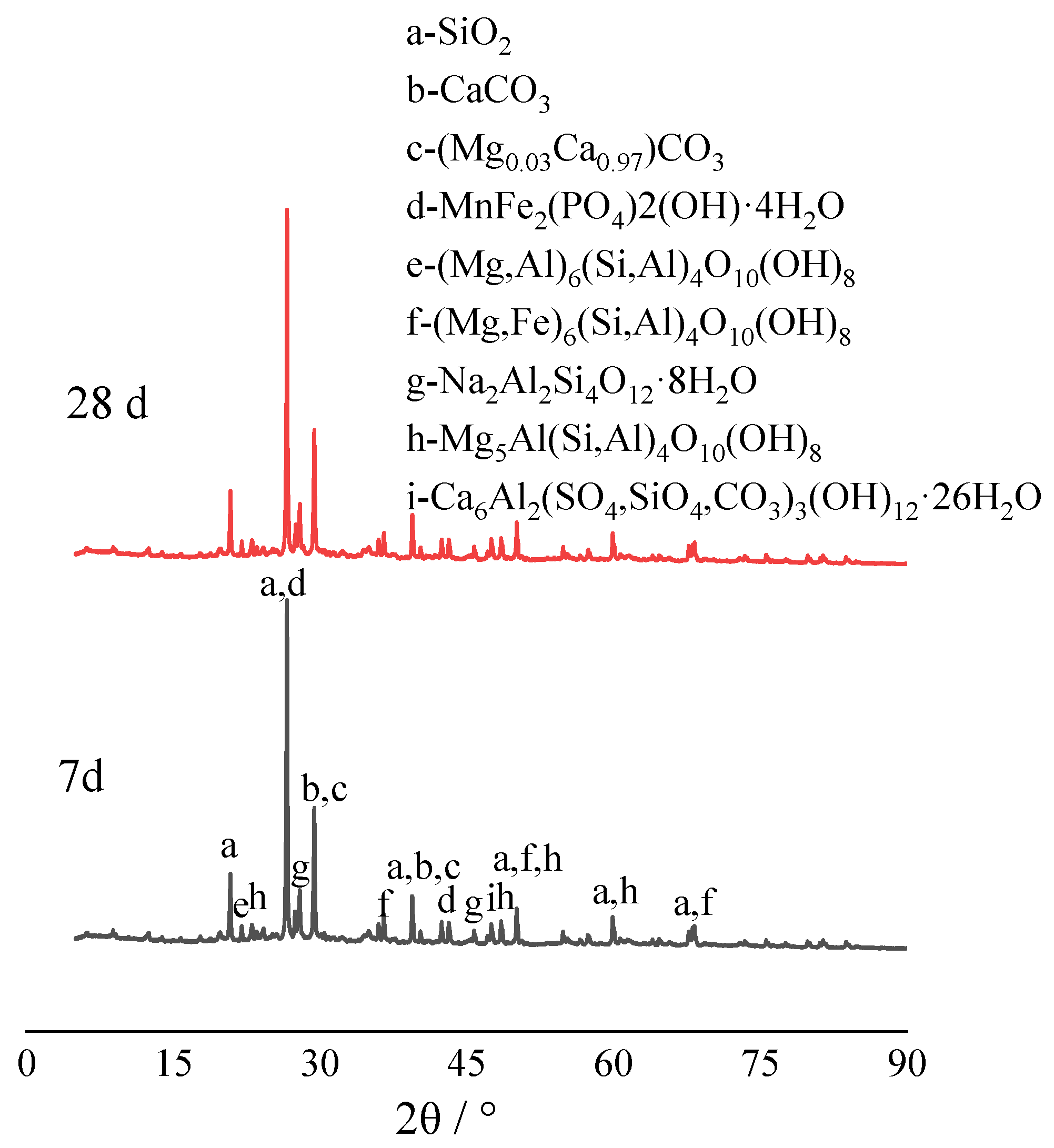
3.4.3. Mineral Composition Analysis of Solidified Cadmium-Contaminated Soil over Time
4. Conclusions
- (1)
- Quartz sand amount influences fine-grained red mud–loess mixed material (7:3) permeability. A quartz sand content of less than 80% meets the permeability standards for red mud–loess barriers. The durability of the planned barrier diminishes when the composition of the red mud–loess mixture (7:3) deteriorates. Red mud and loess with particle sizes from 0.50 to 0.85 mm in a 7:3 mass ratio generate a 28% moisture-rich mixed material. With a dry density of 1.04 g/cm3, the red mud–loess mixture (7:3) satisfies the permeability standards for a barrier active medium, with a permeability coefficient of 3.39 × 10−4 cm/s.
- (2)
- Loess has a strong ability to neutralize acidic solutions, which is particularly effective in removing heavy metals from wastewater. A mass ratio of 7:3 (red mud to loess) is found to be efficient in the removal of Cd, while also elevating the acidity of acidic wastewater to comply with groundwater guidelines. The mixture controls the high alkalinity of red mud, enhancing its utility in engineering applications such as barriers for acidic wastewater treatment. This study suggests that the quartz sand content significantly affects the permeability of the fine-grained red mud–loess mixtures, with an optimal particle size range identified for maintaining effective permeability in engineered barriers.
- (3)
- Red mud–loess mixed materials (7:3) were tested in four pore solutions: distilled water, acidic solution, Cd-containing acidic solution, and high Cd-containing acidic solution. These materials initially exhibited an increase in behavior followed by gradual stabilization. Moreover, using a mass ratio of 7:3 for red mud to loess proved effective in removing Cd. The observed trend indicated strain hardening. Cd-loaded red mud–loess mixed material (7:3) demonstrated increased cohesiveness and a lower friction angle with higher target ion loading. However, as pollution increased, the cohesiveness of the Cd-loaded red mud–loess mixed materials decreased, despite an increase in the internal friction angle. Interestingly, after loading Cd, the red mud–loess mixed material (7:3) exhibited somewhat greater shear strength than uncontaminated samples.
- (4)
- The red mud–loess mixed material includes Cd acidic solutions (7:3) and the pore solution is acidic. With distilled water as the pore solution and red mud–loess mixed material (7:3), H+ and target ions disrupt particle aggregation. The clay component in the red mud–loess mixed material (7:3) lacks cement because carbonates degrade. The red mud–loess mixed material (7:3) becomes more irregular and rough owing to H+ corrosion in the acidic solution and Cd adsorption products adhering.
- (5)
- SEM analysis of the CRML-solidified soil at 7 and 60 days reveals irregular blocks and granules with needle-rod-shaped crystals, which enhance soil structure and strength over time. CRML-solidified cadmium-contaminated soil undergoes significant alterations in functional groups (-OH and Si-O), indicating the presence of adsorbed water, crystallization water, and mineral formations. The curing agent triggers reactions that enhance soil reactivity, leading to the formation of amorphous silica, calcium carbonate, and cadmium carbonate. XRD analysis of CRML-treated cadmium-contaminated soil demonstrates consistent phase composition between 7 and 28 days. Additionally, the curing agent facilitates the liberation of active groups, thereby boosting soil reactivity. These findings contribute to the understanding of soil remediation and the efficient utilization of solid waste resources in environmental protection. This study’s innovative approach using red mud and loess as PRB active media demonstrates its potential for cleansing acidic wastewater in mining areas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szram, E.; Kondracka, M.; Fabiańska, M.; Marynowski, L. Soil degradation caused by post-mining and post-metallurgical waste-detection using gas chromatography-mass spectrometry (GC-MS) and electrical resistivity tomography (ERT). In Proceedings of the 25th EGU General Assembly (EGU-5477), Vienna, Austria, 23–28 April 2023. [Google Scholar]
- Priatmadi, B.J. Reduction in acidity and heavy metal concentrations of acid mine drainage with organic matter and coal fly ash treatments in two different reclaimedmining soil. J. Degrad. Min. Lands Manag. 2023, 10, 4379–4387. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. National Soil Pollution Survey Bulletin of the Ministry of Land and Resources of the People’s Republic of China; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2014.
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Khoeurn, K.; Sakaguchi, A.; Tomiyama, S.; Igarashi, T. Long-term acid generation and heavy metal leaching from the tailings of Shimokawa mine, Hokkaido, Japan: Column study under natural condition. J. Geochem. Explor. 2019, 201, 1–12. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Zhang, Z.; Zhang, Y. Review: Acid mine drainage (AMD) in abandoned coal mines of Shanxi, China. Water 2021, 13, 8. [Google Scholar] [CrossRef]
- Johnsond, B.; Hallbergk, B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Galán, M.; Baena-Moreno, F.M.; Vázquez, S.; Arroyo-Torralvo, F.; Vilches, L.F.; Zhang, Z. Remediation of acid mine drainage. Environ. Chem. Lett. 2019, 17, 1529–1538. [Google Scholar] [CrossRef]
- Taoliang, W. Measures and suggestions for water pollution control in Yangquan Coal Mine Laojiao. Shanxi Water Conserv. 2017, 33, 31–32+34. (In Chinese) [Google Scholar]
- Mhlongo, S.E.; Amponsah-Dacosta, F. A review of problems and solutions of abandoned mines in South Africa. Int. J.Min. Reclam. Environ. 2015, 30, 279–294. [Google Scholar] [CrossRef]
- Rezaie, B.; Anderson, A. Sustainable resolutions for environmental threat of the acid mine drainage. Sci. Total Environ. 2020, 717, 137211. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, R.T.; Acree, S.D.; Ross, R.R.; Puls, R.W.; Lee, T.R.; Woods, L.L. Fifteen-year assessment of a permeable reactive barrier for treatment of chromate and trichloroethylene in groundwater. Sci. Total Environ. 2014, 468, 186–194. [Google Scholar] [CrossRef]
- Powell, R.M.; Blowes, D.W.; Gillham, R.W.; Schultz, D.; Sivavec, T.; Puls, R.W.; Vogan, J.L.; Powell, P.D.; Landis, R. Permeable reactive barrier technologies for contaminant remediation. US EPA 1998, 600, 1–94. [Google Scholar]
- Suo, C.; Fang, P.; Cao, H.; Cao, J.; Liu, K.; Dong, X. Influence and microscopic mechanism of the solid waste-mixture on solidification of Cu2+-contaminated soil. Constr. Build. Mater. 2021, 305, 124651. [Google Scholar] [CrossRef]
- Chen, M.; Qian, L.; Yan, J. Principle, Design and Application of Groundwater Permeable Reactive Wall Repair Technology; Science Press: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Hou, D.; Al-Tabbaa, A.; Luo, J. Assessing effects of site characteristics on remediation secondary life cycle impact with a generalised framework. J. Environ. Plan. Manag. 2014, 57, 1083–1100. [Google Scholar] [CrossRef]
- Levchuk, I.; Bhatnagar, A.; Sillanpää, M. Overview of technologies for removal of methyl tert-butyl ether (MTBE) from water. Sci. Total Environ. 2014, 476–477, 415–433. [Google Scholar] [CrossRef]
- Lindsey, B.D.; Ayotte, J.D.; Jurgens, B.C.; Desimone, L.A. Using groundwater age distributions to understand changes in methyl tert-butyl ether (MtBE) concentrations in ambient groundwater, northeastern United States. Sci. Total Environ. 2017, 579, 579–587. [Google Scholar] [CrossRef]
- Mohebali, S. Degradation of methyl t-butyl ether (MTBE) by photochemical process in nanocrystalline TiO2 slurry: Mechanism, by-products and carbonate ion effect. J. Environ. Chem. Eng. 2013, 1, 1070–1078. [Google Scholar] [CrossRef]
- O’hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ “Iron Wall” for remediation of VOCs. Ground Water 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Benner, S.G.; Blowes, D.W.; Gould, W.D.; Herbert, R.B.; Ptacek, C.J. Geochemistry of a permeable reactive barrier for metals and acid mine drainage. Environ. Sci. Technol. 1999, 33, 2793–2799. [Google Scholar] [CrossRef]
- Benner, S.G.; Blowes, D.W.; Ptacek, C.J. A full-scale porous reactive wall for prevention of acid mine drainage. Groundw. Monit. Remediat. 1997, 17, 99–107. [Google Scholar] [CrossRef]
- Conca, J.L.; Wright, J. An Apatite II permeable reactive barrier to remediate groundwater containing Zn, Pb and Cd. Appl. Geochem. 2006, 21, 2188–2200. [Google Scholar] [CrossRef]
- Groudev, S.; Spasova, I.; Nicolova, M. Acid mine drainage cleanup in a uranium deposit by means of a passive treatment system. Physicochem. Probl. Miner. Process. 2007, 41, 265–274. [Google Scholar]
- Gibert, O.; Cortina, J.L.; de Pablo, J.; Ayora, C. Performance of a field-scale permeable reactive barrier based on organic substrate and zero-valent iron for in situ remediation of acid mine drainage. Environ. Sci. Pollut. Res. Int. 2013, 20, 7854–7862. [Google Scholar] [CrossRef]
- Calero, M.; Hernáinz, F.; Blázquez, G.; Tenorio, G.; Martín-Lara, M.A. Study of Cr (III) biosorption in a fixed-bed column. J. Hazard. Mater. 2009, 171, 886–893. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.; Xie, M.; Dong, X. Removal of Mn (II) from Acidic Wastewater on the Red Mud–Loess Mixture: Fixed–Bed Column Tests. IOP Conf. Ser. Earth Environ. Sci. 2022, 1087, 012078. [Google Scholar] [CrossRef]
- Ozdemir, O.; Turan, M.; Turan, A.Z.; Faki, A.; Engin, A.B. Feasibility analysis of color removal from textile dyeing wastewater in a fixed-bed column system by surfactant-modified zeolite (SMZ). J. Hazard. Mater. 2009, 166, 647–654. [Google Scholar] [CrossRef] [PubMed]
- García-Mateos, F.J.; Ruiz-Rosas, R.; Marqués, M.D.; Cotoruelo, L.M.; Rodríguez-Mirasol, J.; Cordero, T. Removal of paracetamol on biomass-derived activated carbon: Modeling the fixed bed breakthrough curves using batch adsorption experiments. Chem. Eng. J. 2015, 279, 18–30. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X. Applications of red mud as an environmental remediation material: A review. J. Hazard. Mater. 2021, 408, 124420. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Xu, Z.; Ma, H.; Guo, Y. Mechanism study on manganese (II) removal from acid mine wastewater using red mud and its application to a lab-scale column. J. Clean. Prod. 2020, 253, 119955. [Google Scholar] [CrossRef]
- He, Y.-F.; Zhang, L.; Wang, R.-M.; Li, H.-R.; Wang, Y. Loess clay based copolymer for removing Pb (II) ions. J. Hazard. Mater. 2012, 227, 334–340. [Google Scholar] [CrossRef]
- Hu, W.; Cheng, W.C.; Wen, S. Investigating the effect of degree of compaction, initial water content, and electric field intensity on electrokinetic remediation of an artificially Cu-and Pb-contaminated loess. Acta Geotech. 2023, 18, 937–949. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, Z.; Ruan, M. Characteristics of sulfate-reducing bacteria and organic bactericides and their potential to mitigate pollution caused by coal gangue acidification. Environ. Technol. Innov. 2020, 20, 101142. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Sanz, J.L.; Bijmans, M.F.; Stams, A.J. Sulfate reduction at low pH to remediate acid mine drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef]
- Leiva, E.; Tapia, C.; Rodríguez, C. Removal of Mn(II) from acidic wastewaters using graphene oxide-ZnO nanocomposites. Molecules 2021, 26, 2713. [Google Scholar] [CrossRef]
- Wang, F.; Li, L. Sorption and Desorption of Cd2+ by Size Fractions of Micro-Aggregates from a Paddy Soil. Environ. Sci. 2006, 27, 590–593. [Google Scholar]
- Doula, M.K. Removal of Mn2+ ions from drinking water by using Clinoptilolite and a Clinoptilolite-Fe oxide system. Water Res. 2006, 40, 3167–3176. [Google Scholar] [CrossRef]
- Neculita, C.M.; Rosa, E. A review of the implications and challenges of manganese removal from mine drainage. Chemosphere 2019, 214, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Salih, W.; Yu, W.; Dong, X.; Hao, W. Study on stress-strain-resistivity and microscopic mechanism of red mud waste modified by desulphurization gypsum-fly ash under drying-wetting cycles. Constr. Build. Mater. 2020, 249, 118772. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.; Yu, L.; Zhang, X.; Duan, W.; Park, J.; Dong, X. Removal Characteristics and Mechanism of Mn (II) from Acidic Wastewater Using Red Mud-Loess Mixture. Pol. J. Environ. Stud. 2022, 31, 5781–5792. [Google Scholar] [CrossRef]
- ASTM D5084-10; StandardTest Methods for Measurement of Hydraulic Conductivity of Saturated Porous Materials Using a Flexible Wall Permeameter. ASTM Standards: West Conshohocken, PA, USA, 2016.
- GB/T 50123-2019; Standard for Geotechnical Testing Methods. Ministry of Housing and Urban-Rural Development of the People’s Republic of China. State Administration for Market Regulation: Beijing, China, 2019.
- Zhang, Y.; Jin, F.; Shen, Z.; Wang, F.; Lynch, R.; Al-Tabbaa, A. Adsorption of methyl tert-butyl ether (MTBE) onto ZSM-5 zeolite: Fixed-bed column tests, breakthrough curve modelling and regeneration. Chemosphere 2019, 220, 422–431. [Google Scholar] [CrossRef]
- Hu, M.; Cui, X.; Wang, X.; Liu, H.; Du, W. Experimental study on the effect of fine particles on the permeability of calcareous sand. Rock Soil Mech. 2019, 40, 2925–2930. [Google Scholar]
- Ferreira, L.M.; Coelho, C.A.; Reis, P.N. Numerical simulations of the low-velocity impact response of semicylindrical woven composite shells. Materials 2023, 16, 3442. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lyu, Q.; Tong, T.; Sun, K.; Lin, L.-C.; Tang, C.Y.; Yang, F.; Guiver, M.D.; Quan, X.; Dong, Y. Robust ultrathin nanoporous MOF membrane with intra-crystalline defects for fast water transport. Nat. Commun. 2022, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Sharma, A.; Jung, J.P. Advanced 3D Through-Si-Via and Solder Bumping Technology: A Review. Materials 2023, 16, 7652. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The usability of sorbents in restoring enzymatic activity in soils polluted with petroleum-derived products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Cheng, W.-C.; Hu, W.; Rahman, M.M. Changes in air and liquid permeability properties of loess due to the effect of lead contamination. Front. Earth Sci. 2023, 11, 1165685. [Google Scholar] [CrossRef]
- Ding, Y.; Rao, Y.; Ni, Q.; Xu, W.; Liu, D.; Zhang, H. Effects of gradation and void ratio on the coefficient of permeability of coarse-grained soil. Hydrogeol. Eng. Geol. 2019, 46, 108–116. [Google Scholar]
- Khalaf, A.A.; Kopecskó, K. Utilizing residual red mud by geopolymerization process–From an expansive soil to a rock-like material. Clean. Eng. Technol. 2023, 14, 100647. [Google Scholar] [CrossRef]
- Nizioł, M.; Jankowski-Mihułowicz, P.; Węglarski, M. The Influence of the Washing Process on the Impedance of Textronic Radio Frequency Identification Transponder Antennas. Materials 2023, 16, 4639. [Google Scholar] [CrossRef]
- Chen, X.; Feng, W.; Wen, H.; Duan, W.; Suo, C.; Xie, M.; Dong, X. Study on the Mechanical and Leaching Characteristics of Permeable Reactive Barrier Waste Solidified by Cement-Based Materials. Materials 2021, 14, 6985. [Google Scholar] [CrossRef]





| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | TiO2 | Na2O | MnO | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Red mud | 20.17 | 24.34 | 9.40 | 18.26 | 0.64 | 1.26 | 3.56 | 9.61 | 0.03 | 0.47 |
| Loess | 58.88 | 11.75 | 4.54 | 7.98 | 2.18 | 2.05 | 0.60 | 1.70 | 0.07 | 0.03 |
| Red Mud–Loess Mass Ratio | Particle Size (mm) | pH0 | Dosage (g/L) | Contact Time (min) | Initial Concentration (mg/L) | Temperature (°C) |
|---|---|---|---|---|---|---|
| 0:10 | <0.075 | 3.0 | 8 (Cd) | 600 | 100 | 25 |
| 3:7 | 0.075–0.15 | |||||
| 5:5 | 0.15–0.25 | |||||
| 7:3 | 0.25–0.50 | |||||
| 10:0 | 0.50–0.85 |
| Sample Size (cm) | Quartz Sand Content (%) | Particle Size (mm) | Initial Moisture Content (%) |
|---|---|---|---|
| 5 × 10 | 0 | <0.075 | 30 |
| 70 | <0.075 | 8 | |
| 80 | <0.075 | 5 | |
| 0 | 0.25–0.50 | 29 | |
| 0 | 0.50–0.85 | 28 |
| Red Mud–Loess Mass Ratio | Particle Size (mm) | pH0 | Dosage (g/L) Cd | Contact Time (min) | Initial Concentration (mg/L) | Temperature (°C) |
|---|---|---|---|---|---|---|
| 7:3 | 0.50–0.85 | 3.0 | 6 | 600 | 100 | 25 |
| 7 | ||||||
| 8 | ||||||
| 9 | ||||||
| 10 |
| Sample No | Sample Size (mm) | Vertical Pressure (kPa) | Pore Water Category |
|---|---|---|---|
| 1 | 61.8 × 20 | 100 | Distilled water (H2O) |
| 2 | 200 | Acidic solution (pH0 = 3.0) | |
| 3 | 300 | Acidic solution containing Cd C(Cd) = 100 mg/L, pH0 = 3.0 | |
| 4 | 400 | Acidic solution with high Cd content m(Cd) = 6 mg/g, pH0 = 3.0 |
| Pore Solution | c (kPa) | φ (°) | tan φ | R2 |
|---|---|---|---|---|
| Distilled water (H2O) | 37.80 | 25.42 | 0.4752 | 0.9719 |
| Acidic solution (pH0 = 3.0) | 34.75 | 25.65 | 0.4803 | 0.9989 |
| Acidic solution containing Cd C(Cd) = 100 mg/L, pH0 = 3.0 | 28.05 | 27.37 | 0.5176 | 0.9735 |
| Acidic solution with high Cd content m(Cd) = 6 mg/g, pH0 = 3.0 | 20.55 | 28.59 | 0.5451 | 0.9885 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, W.T.; Xiao, Z.; Dong, X. Research Enhancing Acidic Mine Wastewater Purification: Innovations in Red Mud–Loess. Materials 2024, 17, 2050. https://doi.org/10.3390/ma17092050
Salih WT, Xiao Z, Dong X. Research Enhancing Acidic Mine Wastewater Purification: Innovations in Red Mud–Loess. Materials. 2024; 17(9):2050. https://doi.org/10.3390/ma17092050
Chicago/Turabian StyleSalih, Wdah. T., Zean Xiao, and Xiaoqiang Dong. 2024. "Research Enhancing Acidic Mine Wastewater Purification: Innovations in Red Mud–Loess" Materials 17, no. 9: 2050. https://doi.org/10.3390/ma17092050
APA StyleSalih, W. T., Xiao, Z., & Dong, X. (2024). Research Enhancing Acidic Mine Wastewater Purification: Innovations in Red Mud–Loess. Materials, 17(9), 2050. https://doi.org/10.3390/ma17092050





