Correlation between the Chemical Structure of (Meth)Acrylic Monomers and the Properties of Powder Clear Coatings Based on the Polyacrylate Resins
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
- 2-hydroxyethyl methacrylate (HEMA) (Sigma Aldrich, Darmstadt, Germany);
- Methyl methacrylate (MMA) (Sigma Aldrich, Darmstadt, Germany);
- n-butyl acrylate (nBA) (Sigma Aldrich, Darmstadt, Germany);
- tert-butyl acrylate (tBA) (Sigma Aldrich, Darmstadt, Germany);
- Dodecyl acrylate (DA) (Sigma Aldrich, Darmstadt, Germany);
- Ethyl acrylate (EA) (Sigma Aldrich, Darmstadt, Germany);
- Ethyl methacrylate (EMA) (Sigma Aldrich, Darmstadt, Germany);
- Benzyl acrylate (BAZ) (Sigma Aldrich, Darmstadt, Germany);
- Free radical initiator of polymerization: azobisisobutyronitrile (AIBN) (Sigma Aldrich, Darmstadt, Germany).
- Vestanat®B 1358/100 (Evonic Degussa, Marl, Germany);
- Degassing agent: benzoin (Sigma Aldrich, Darmstadt, Germany);
- Flow control agent: Byk 368P (Byk-Chemie, Wesel, Germany).
2.2. Synthesis of Polyacrylate Resins
2.3. Preparation of Powder Clear Coatings Based on Synthesized of Polyacrylate Resins
3. Measurements
3.1. Gel Permeation Chromatography (GPC)
3.2. Viscosity
3.3. Differential Scanning Calorimetry (DSC)
3.4. 1H-NMR Spectroscopy
3.5. Polymerization Test
- The coating is matt and soft.
- The coating is matt and can be scratched with a nail.
- Slight loss of gloss.
- No noticeable changes.
- The polymerization test was performed twice for each coating.
3.6. Flowability
3.7. Thickness and Gloss
3.8. Roughness
3.9. Adhesion to the Steel Surface
3.10. Hardness
3.11. Scratch Resistance
3.12. Water Contact Angle (WCA)
3.13. Impact Resistance
3.14. Cupping
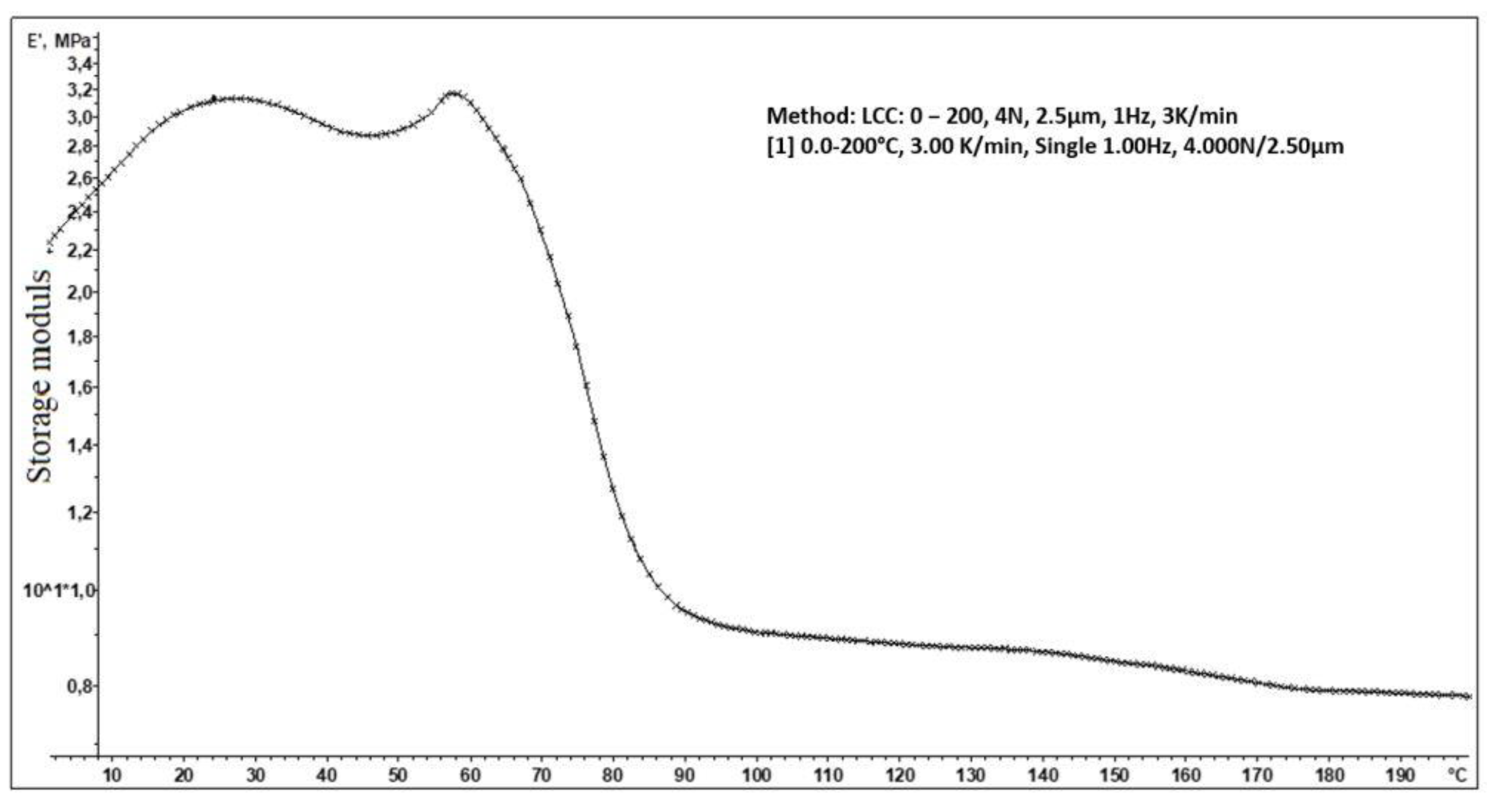
3.15. Dynamic Mechanical Analysis (DMA)
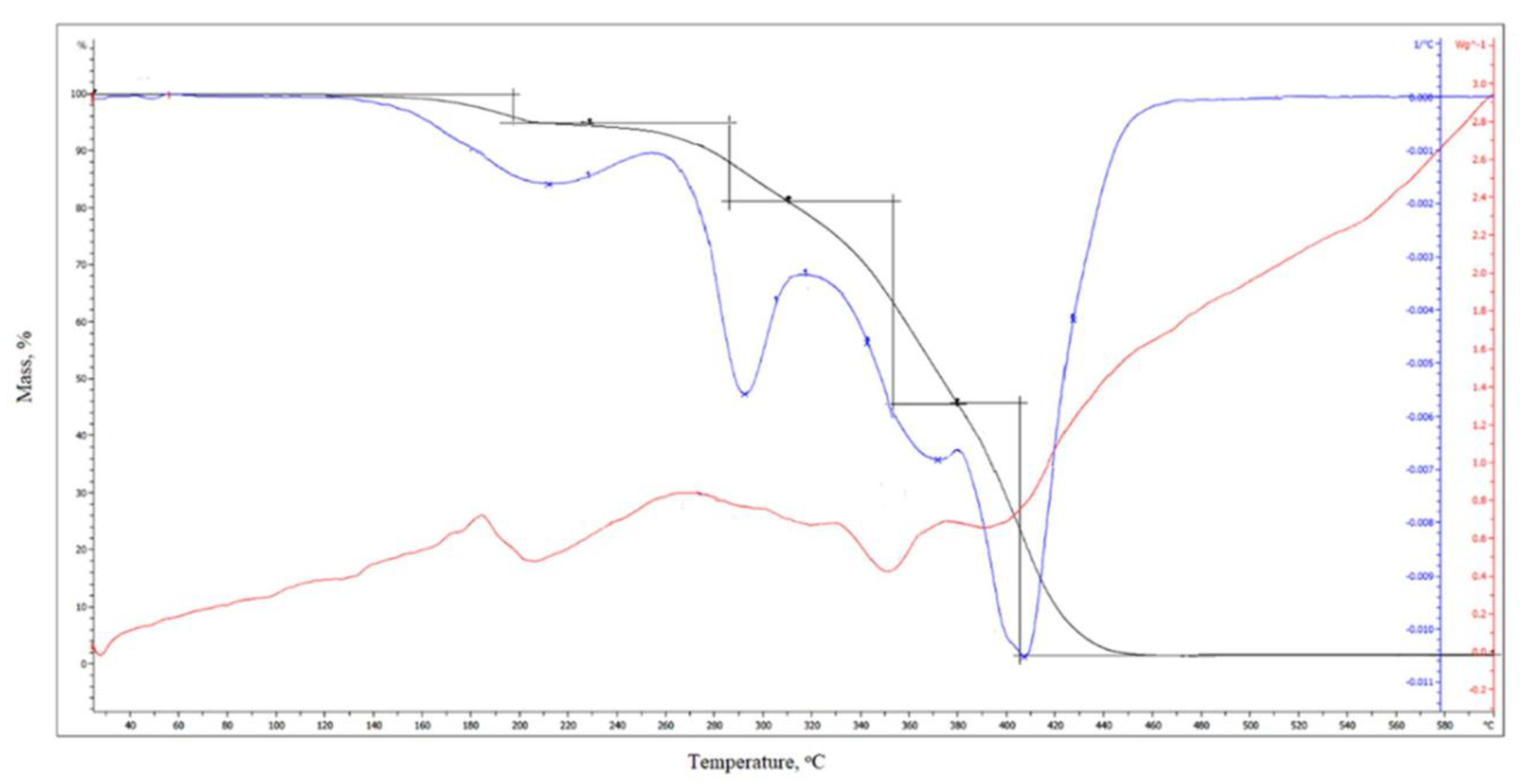
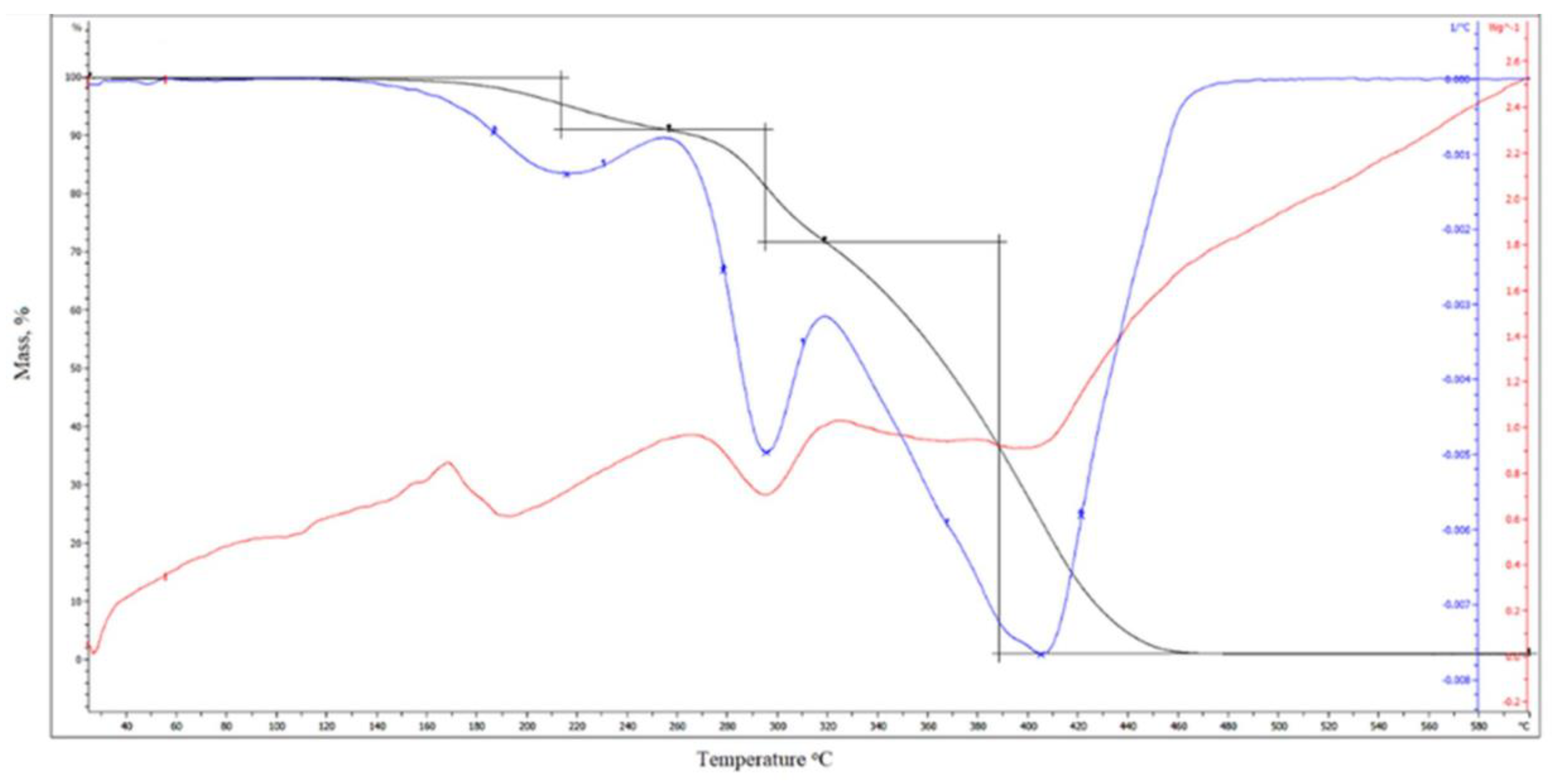
3.16. Thermogravimetric Analysis (TGA)
4. Results and Discussion
4.1. Choice of (Meth)Acrylic Monomers
4.2. Characterization of poly(meth)acrylate Resins
4.3. The Crosslinking Process and Properties of Powder Coatings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, F.N.; Nichols, M.E.; Pappas, S.P. Organic Coatings, Science and Technology; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Directive 2004/42/EC of the European Parliament and of the Council of April 21, 2004 on the Limitation of Emissions of Volatile Organic Compounds Due to the Use of Organic Solvents in Certain Paints and Varnishes and Vehicle Refinishing Products, and Amending Directive 1999/13/EC, Document 32004L0042. Available online: https://eur-lex.europa.eu/eli/dir/2004/42/oj (accessed on 4 February 2024).
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy, Document 32000L0060. Available online: https://eur-lex.europa.eu/eli/dir/2000/60/oj (accessed on 4 February 2024).
- Emmanouil, S. Powder Coatings Chemistry and Technology; Vincentz Network GmbH: Hanover, Germany, 2014. [Google Scholar]
- Available online: https://ecoline.com.pl/ (accessed on 18 January 2024).
- Pilch-Pitera, B. Farby i Lakiery Proszkowe: Otrzymywanie, Formowanie, Nanoszenie i Ocena Właściwości; Oficyna Wydawnicza Politechniki Rzeszowskiej: Rzeszów, Poland, 2015. [Google Scholar]
- Li, C.; Johansson, M.; Buisjen, B. Limonene-derived polycarbonates as biobased UV-curable (powder) coating resins. Prog. Org. Coat. 2021, 151, 106073. [Google Scholar] [CrossRef]
- Yang, M.S.; Huang, J.; Noël, J.J. A Mechanistic Study on the Anti-Corrosive Performance of Zinc-Rich Polyester/TGIC Powder Coatings. Processes 2022, 10, 1853. [Google Scholar] [CrossRef]
- Ayrilmis, N. A review on electrostatic powder coatings for the furniture industry. Int. J. Adhes. Adhes. 2022, 113, 103062. [Google Scholar] [CrossRef]
- Malshe, V.C.; Waghoo, G. Weathering study of epoxy paint. Prog. Org. Coat. 2004, 51, 267. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, W.; Fan, J.; Ren, F.; Xu, C. Synthesis and characterization of carboxyl group-containing acrylic resin for powder coatings. Prog. Org. Coat. 2008, 62, 179–182. [Google Scholar] [CrossRef]
- Czachor-Jadacka, D.; Pilch-Pitera, B.; Byczynski, Ł.; Kisiel, M.; Zioło, A. Hydrophobic polyurethane powder clear coatings with lower curing temperature: Study on the synthesis of new blocked polyisocyanates. Prog. Org. Coat. 2021, 159, 106402. [Google Scholar] [CrossRef]
- Fernández-Álvarez, M.; Velasco, F.; Bautista, A. Epoxy powder coatings hot mixed with nanoparticles to improve their abrasive wear. Wear 2020, 448, 203211. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, M. Advances in cationic photopolymerization. Pure Appl. Chem. 2012, 84, 2089. [Google Scholar] [CrossRef]
- Okada, K.; Yamaguchi, K.; Takeda, H. Acrylic/polyester hybrid powder coating system having excellent weather durability. Prog. Org. Coat. 1998, 34, 169. [Google Scholar] [CrossRef]
- Hughes, A.E.; Johnston, P.; Simons, T.J. Chapter 9—Self-healing coatings. In Recent Advances in Smart Self-Healing Polymers and Composites, 2nd ed.; Woodhead Publishing: Oxford, UK, 2022; pp. 217–270. [Google Scholar] [CrossRef]
- Tong, J.; Xie, S.; Miao, J.T.; Luo, J.; Liu, R. Preparation of UV-cured polyurethane-urea acrylate coatings with high hardness and toughness. Prog. Org. Coat. 2024, 186, 107969. [Google Scholar] [CrossRef]
- Wang, K.S.; Cheng, Y.W.; Lin, H.Y.; Chen, M.H.; Yeh, S.C.; Huang, Y.C.; Wu, C.H.; Jeng, R.J.; Liu, T.Y. Synthesis of dendritic urethane acrylates for fabricating a robust honeycomb-like structure acting for SERS detection. Prog. Org. Coat. 2023, 184, 107840. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z.; Wang, M.; Zhang, Y.; Lu, X. UV-curable epoxy acrylate composite coatings with high flexibility, super-hydrophobicity, wear-resistance and self-healing property. Prog. Org. Coat. 2023, 182, 107649. [Google Scholar] [CrossRef]
- Qualicoat. Specifications for a Quality Label for Liquid and Powder Organic Coatings on Aluminium for Architectural Applications, 16th ed.; Qualicoat: Zurich, Switzerland, 2019. [Google Scholar]
- PN-EN ISO 8130-11; Coating Powders—Part 11: Inclined-Plane Flow Test. ISO: Geneva, Switzerland, 2019.
- PN-EN ISO 2813; Paints and Varnishes—Determination of Specular Gloss of Non-Metallic Paint Films at 20 Degrees, 60 Degrees and 85 Degrees. ISO: Geneva, Switzerland, 2014.
- PN-EN ISO 2808; Paints and Varnishes—Determination of Film Thickness. ISO: Geneva, Switzerland, 2019.
- PN-EN ISO 12085; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Part 2: Terms, Definitions and Surface Texture Parameters. ISO: Geneva, Switzerland, 2018.
- PN-EN ISO 2409; Paints and Varnishes—Cross-Cut Test. ISO: Geneva, Switzerland, 2013.
- PN-EN ISO 1522; Paints and Varnishes—Pendulum Damping Test. ISO: Geneva, Switzerland, 2011.
- PN-EN ISO 1518; Paints and Varnishes—Scratch Hardness Tests—Part 1: Constant-Loading Method. ISO: Geneva, Switzerland, 2016.
- PN-EN ISO 19403-6:2020-08; Paints and Varnishes—Wetting—Part 3: Determination of Surface Tension of Liquids by the Hanging Drop Method. ISO: Geneva, Switzerland, 2020.
- PN-EN ISO 6272-1; Paints and Varnishes—Rapid-Deformation (Impact Resistance) Tests—Part 1: Falling-Weight Test, Large-Area Indenter. ISO: Geneva, Switzerland, 2011.
- PN-EN ISO 1520; Paints and Varnishes—Cupping Test. ISO: Geneva, Switzerland, 2019.
- Ali, U.; Juhanni, K.; Karim, A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Takács, E.; Wojnárovits, L. Comparison of the reactivity of acrylate and methacrylate monomers. Radiat. Phys. Chem. 1995, 46, 1007–1010. [Google Scholar] [CrossRef]
- Ibrahim, S.; Lotfy, S. Properties of butyl acrylate polymers synthesized by radiation and miniemulsion polymerization techniques as flexible coating for packaging materials. J. Vinyl Addit. Technol. 2020, 27, 1. [Google Scholar] [CrossRef]
- Xingjiang, W.; Hong, R.; Meng, J.; Cheng, R.; Zhu, Z.; Wu, G.; Li, Q.; Wang, C.F.; Chen, S. Hydrophobic Poly(tert-butylacrylate)PhotonicCrystalstowardsRobust Energy-Saving Performance. Angew. Int. Ed. 2019, 58, 13556–13564. [Google Scholar] [CrossRef]
- Martinez, G.; Sanchez-Chaves, M.; Madruga, E.L.; Fernandez-Monreal, C. Monomer reactivity ratios and microstructural analysis of 2-hydroxyethyl methacrylate–t-butyl acrylate copolymers. Polymer 2000, 41, 6021–6026. [Google Scholar] [CrossRef]
- Saindane, P.; Jagtap, R.N. RAFT copolymerization of amphiphilic poly (ethyl acrylate-b-acrylic acid) as wetting and dispersing agents for water borne coating. Prog. Org. Coat. 2015, 79, 106–114. [Google Scholar] [CrossRef]
- Hamlic, D.; Rodic, P.; Poberznik, M.; Jerb, M.; Kovec, J.; Milosev, I. The Effect of the Methyl and Ethyl Group of the Acrylate Precursor in Hybrid Silane Coatings Used for Corrosion Protection of Aluminium Alloy 7075-T6. Coatings 2020, 10, 172. [Google Scholar] [CrossRef]
- Fang, C.; Zhou, F.; Zhu, X. The application research of benzyl methacrylate (BzMA) in acrylate latex pressure sensitive adhesives. Int. J. Adhes. Adhes. 2021, 107, 102861. [Google Scholar] [CrossRef]
- Qiang, R.; Fanghong, G.; Dongliang, Z.; Xiaoxiao, S.; Jianbo, F.; Bibiao, J. Blends of high density polyethylene with branched poly(styrene-co-dodecyl acrylate): Rheological, mechanical, and thermal properties. Appl. Polym. Sci. 2008, 109, 1618–1624. [Google Scholar] [CrossRef]
- Riemer, S.; Prevost, S.; Dzionara, M.; Appavou, M.S.; Schweins, R.; Gradzielski, M. Aggregation behaviour of hydrophobically modified polyacrylate—Variation of alkyl chain length. Polymer 2015, 70, 194–206. [Google Scholar] [CrossRef]
- Dang, W.; Zhang, D.; Ha, Z.; Wang, J.; Xia, Y.; Chen, X.; Lei, L.; Shi, S. Emulsion polymerization of super-hydrophobic acrylate enabled by a novel “ferrying” strategy for developing waterborne coatings with reduced surface tension and glossiness. Prog. Org. Coat. 2024, 186, 108073. [Google Scholar] [CrossRef]
- Popescu, D.; Hoogenboom, R.; Keul, H.; Moeller, M. Hydroxy functional acrylate and methacrylate monomers prepared via lipase—Catalyzed transacylation reactions. J. Mol. Catal. B Enzym. 2010, 62, 80–89. [Google Scholar] [CrossRef]
- Pilch-Pitera, B.; Czachor, D.; Kowalczyk, K.; Pavlova, E.; Wojturski, J.; Florczak, Ł.; Byczyński, Ł. Conductive polyurethane-based powder clear coatings modified with carbon nanotubes. Prog. Org. Coat. 2019, 137, 105367. [Google Scholar] [CrossRef]
- Soroush, M.; Grady, M.C. Chapter 1—Polymers, Polymerization Reactions, and Computational Quantum Chemistry. Comput. Quantum Chem. 2019, 1, 16. [Google Scholar] [CrossRef]
- Massoumi, B.; Jaymand, M. Chemical and electrochemical grafting of polythiophene onto poly(methyl methacrylate), and its electrospun nanofibers with gelatin Ramis. J. Mater. Sci. Mater. Electron 2016, 12, 27. [Google Scholar] [CrossRef]
- Pham, Q.T.; Petiaud, R.; Llauro-Darricades, M.F.; Waton, H. Proton & Carbon NMR Spectra of Polymers; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Babac, C.; Utkan, G.; David, G.; Simionescu, B.; Piskin, E. Production of nanoparticles of methyl methacrylate and butyl methacrylate copolymers by microemulsion polymerization in the presence of maleic acid terminated poly(N-acetylethylenimine) macromonomers as cosurfactant. Eur. Polym. J. 2004, 40, 1947–1952. [Google Scholar] [CrossRef]
- Chiantore, O.; Lazzari, M. Characterization of Acrylic Resins. Int. J. Polym. Anal. Charact. 1996, 2, 395–408. [Google Scholar] [CrossRef]
- Kardar, P.; Ebrahimi, M.; Bastani, S. Curing behaviour and mechanical properties of pigmented UV-curable epoxy acrylate coatings. Pigment Resin Technol. 2014, 43, 177–184. [Google Scholar] [CrossRef]
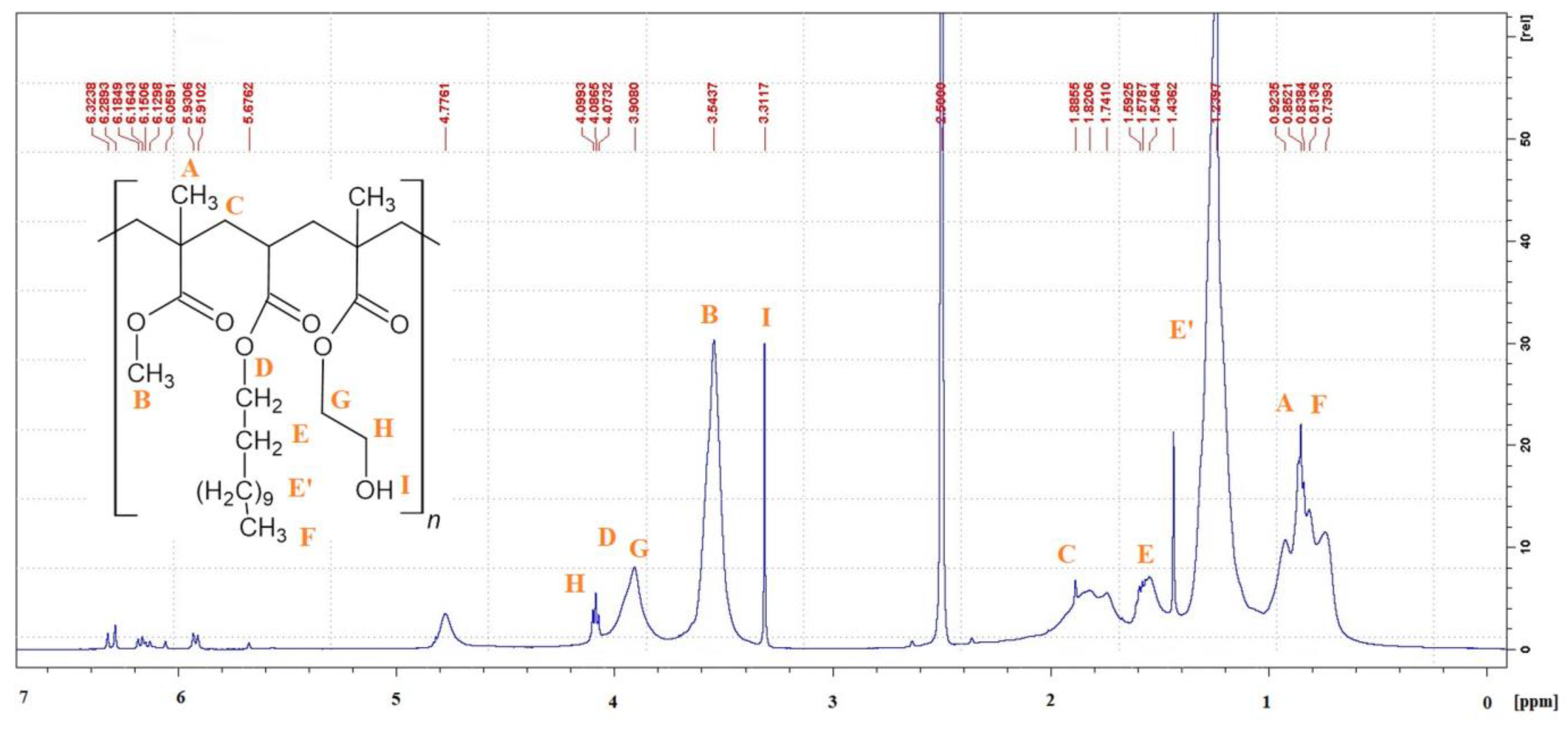

| Resin Symbol | 2-hydroxyethyl methacrylate (HEMA) | methyl methacrylate (MMA) | n-butyl acrylate (nBA) | tert-butyl acrylate (tBA) | ethyl acrylate (EA) | ethyl methacrylate (EMA) | benzyl acrylate (BAZ) | dodecyl acrylate (DA) |
|---|---|---|---|---|---|---|---|---|
| HEMA/6MMA/nBA | 1 | 6 | 1 | - | - | - | - | - |
| HEMA/6MMA/tBA | 1 | 6 | - | 1 | - | - | - | - |
| HEMA/6MMA/EA | 1 | 6 | - | - | 1 | - | - | - |
| HEMA/6MMA/EMA | 1 | 6 | - | - | - | 1 | - | - |
| HEMA/6MMA/BAZ | 1 | 6 | - | - | - | - | 1 | - |
| HEMA/6MMA/DA | 1 | 6 | - | - | - | - | - | 1 |
| HEMA/6MMA/0.5nBA/0.5DA | 1 | 6 | 0.5 | - | - | - | - | 0.5 |
| Name of Monomer | Symbol | Chemical Structure | Resin Properties | Reference |
|---|---|---|---|---|
| methyl methacrylate | MMA | 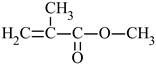 |
| [32,33] |
| n-butyl acrylate | nBA | 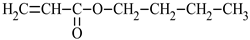 |
| [34] |
| tert-butyl acrylate | tBA | 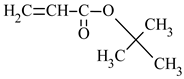 |
| [35,36] |
| ethyl acrylate | EA |  |
| [37] |
| ethyl methacrylate | EMA |  |
| [38] |
| benzyl acrylate | BAZ | 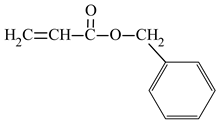 |
| [39] |
| dodecyl acrylate | DA | 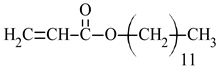 |
| [40,41] |
| 2-hydroxyethyl methacrylate | HEMA |  |
| [42] |
| Mass Unit | Average Molecular Mass Number (Mn) | Average Molecular Mass Weight (Mw) | Molecular Mass Z-Average (Mz) | |
|---|---|---|---|---|
| Resin Symbol | [Da] | [Da] | [Da] | |
| HEMA/6MMA/nBA | 8860 | 53,610 | 22,760 | |
| HEMA/6MMA/tBA | 9820 | 55,500 | 18,520 | |
| HEMA/6MMA/EA | 8240 | 34,930 | 15,030 | |
| HEMA/6MMA/EMA | 7420 | 17,360 | 36,270 | |
| HEMA/6MMA/BAZ | 7540 | 13,910 | 21,180 | |
| HEMA/6MMA/DA | 6670 | 39,410 | 23,550 | |
| HEMA/6MMA/0.5nBA/0.5DA | 8020 | 53,320 | 21,700 | |
| Resin Symbol | Glass Transition Temperature (Tg) [°C] | Viscosity [Pa*s] |
|---|---|---|
| HEMA/6MMA/nBA | 54.03 | 23.85 |
| HEMA/6MMA/tBA | 78.29 | 22.20 |
| HEMA/6MMA/EA | 54.19 | 19.00 |
| HEMA/6MMA/EMA | 52.93 | 24.30 |
| HEMA/6MMA/BAZ | 42.62 | 36.15 |
| HEMA/6MMA/DA | 39.40 | 17.25 |
| HEMA/6MMA/0.5nBA/0.5DA | 50.31 | 20.73 |
| Physical or Mechanical Parameter | Powder Coating Symbol | ||||||
|---|---|---|---|---|---|---|---|
| L_HEMA/6MMA/nBA | L_HEMA/6MMA/tBA | L_HEMA/6MMA/EA | L_HEMA/6MMA/EMA | L_HEMA/6MMA/BAZ | L_HEMA/6MMA/DA | L_HEMA/6MMA/0.5nBA.0.5DA | |
| Flowability [cm] | 1.30 | 0.90 | 0.95 | 0.75 | 2.20 | 5.00 | 4.40 |
| Roughness: Ra Rz | 0.69/2.74 | 1.79/8.73 | 2.88/14.12 | 6.33/11.73 | 1.42/6.79 | 1.09/5.11 | 0.42/2.05 |
| Gloss 60 °C [GU] | 83.45 | 34.73 | 34.24 | 11.73 | 63.12 | 64.62 | 79.63 |
| Adhesion to the steel substrate [0—good 5—bad] | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| Relative hardness [-] | 0.54 | 0.56 | 0.55 | 0.51 | 0.67 | 0.33 | 0.50 |
| Scratch resistance [g] | 500 | 450 | 250 | 250 | 550 | 300 | 550 |
| Contact angle [deg] | 85.40 | 83.93 | 87.91 | 83.91 | 87.02 | 93.14 | 93.53 |
| Impact resistance [J/cm2] | 15 | 10 | 15 | 10 | 20 | 25 | 30 |
| Cupping [mm] | 5.43 | 4.37 | 5.12 | 5.07 | 9.96 | 11.18 | 13.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pojnar, K.; Pilch-Pitera, B. Correlation between the Chemical Structure of (Meth)Acrylic Monomers and the Properties of Powder Clear Coatings Based on the Polyacrylate Resins. Materials 2024, 17, 1655. https://doi.org/10.3390/ma17071655
Pojnar K, Pilch-Pitera B. Correlation between the Chemical Structure of (Meth)Acrylic Monomers and the Properties of Powder Clear Coatings Based on the Polyacrylate Resins. Materials. 2024; 17(7):1655. https://doi.org/10.3390/ma17071655
Chicago/Turabian StylePojnar, Katarzyna, and Barbara Pilch-Pitera. 2024. "Correlation between the Chemical Structure of (Meth)Acrylic Monomers and the Properties of Powder Clear Coatings Based on the Polyacrylate Resins" Materials 17, no. 7: 1655. https://doi.org/10.3390/ma17071655
APA StylePojnar, K., & Pilch-Pitera, B. (2024). Correlation between the Chemical Structure of (Meth)Acrylic Monomers and the Properties of Powder Clear Coatings Based on the Polyacrylate Resins. Materials, 17(7), 1655. https://doi.org/10.3390/ma17071655






