3.2. Tensile Test
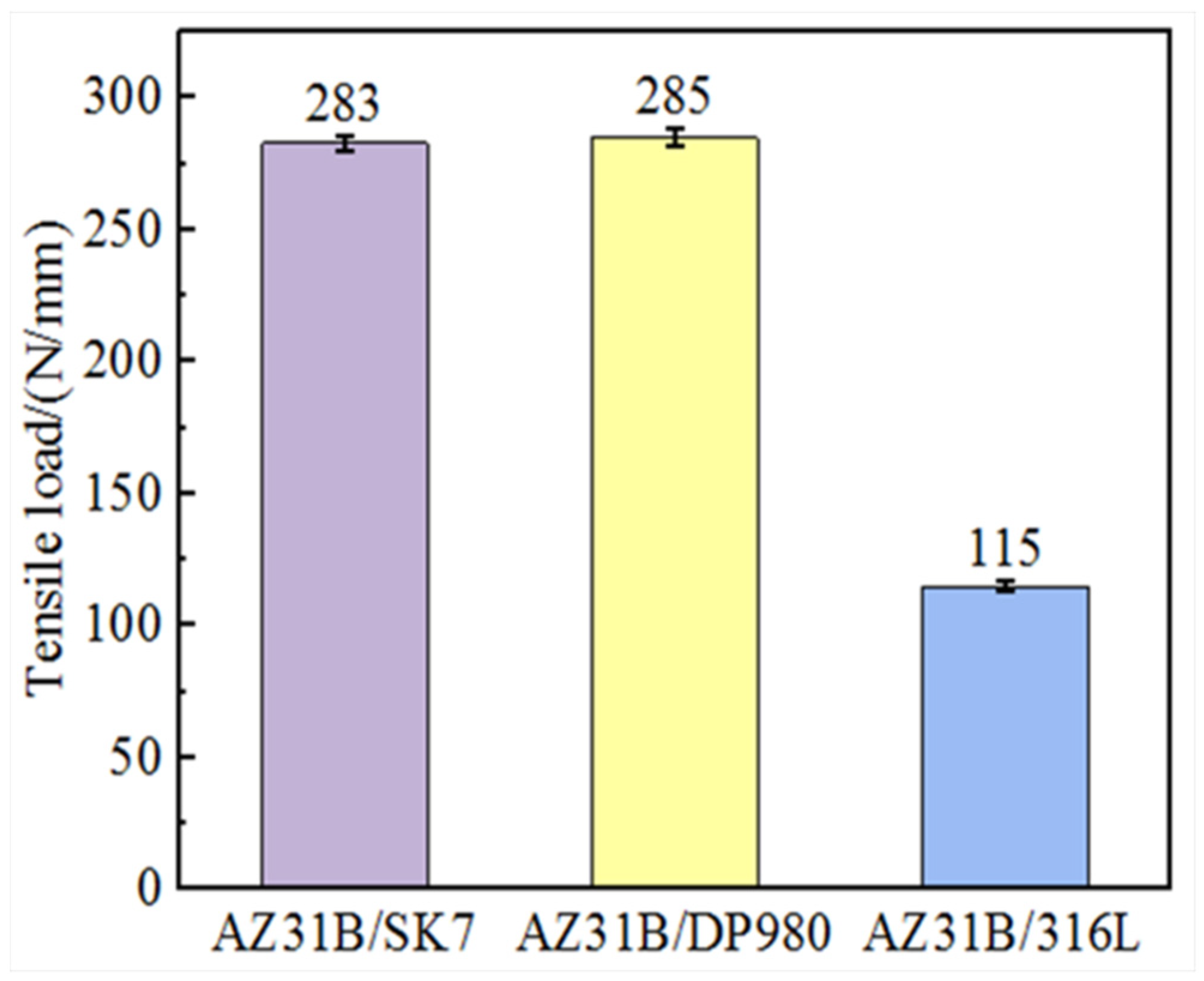
The tensile load of AZ31B/steel (SK7, DP980, and 316L) joints was tested under identical process parameters to compare the influence of different elements on the mechanical properties of the joint, and the results are presented in
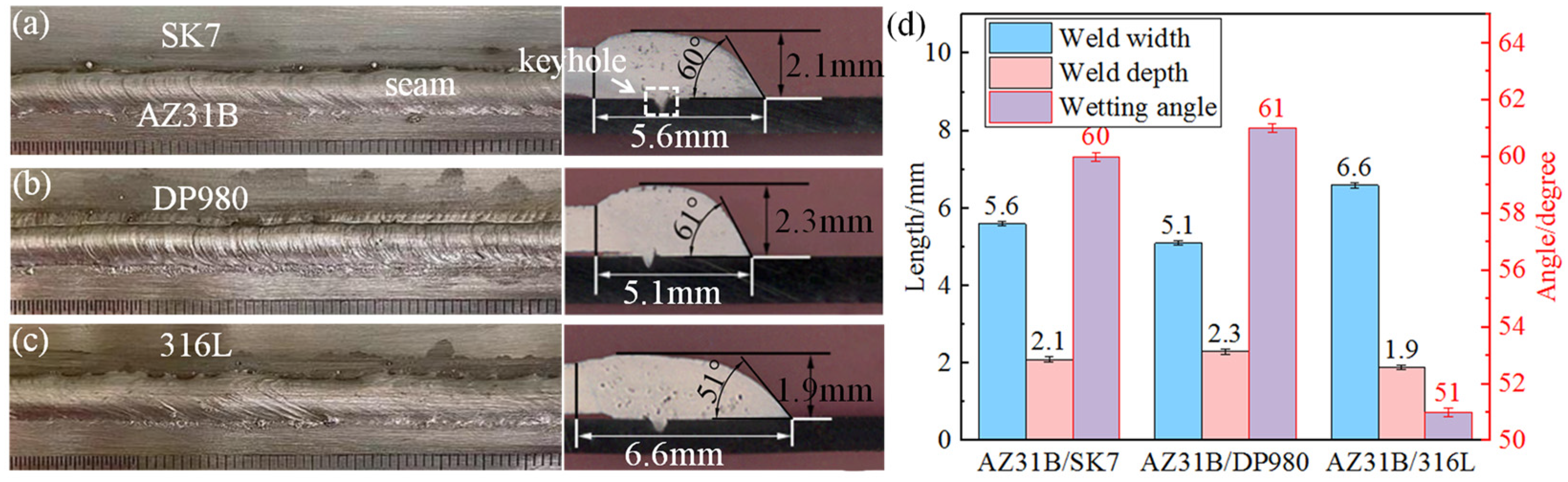
Figure 3. It was evident that the tensile load of AZ31B/SK7 was slightly lower at 283 N/mm compared to that of AZ31B/DP980, which achieved a value of 285 N/mm. Conversely, the AZ31B/316L exhibited the smallest tensile load of only 115 N/mm, but it demonstrated a larger interface bonding area and a smaller wetting angle in
Figure 2c. As Mg and Fe did not interact with each other, the wettability of the melt would be a key factor that determined the bonding state [
22] between the FZ and the steel. The results showed that the joint with the best wettability had the worst mechanical properties, so the joint properties of the Mg/steel were not solely determined by the spreading and wetting behavior. Previous studies have highlighted that the interface strength resulted from a synergistic interplay of various factors, including a reduced wetting angle, obtaining a continuous interface layer at the micro and nano scales, and a larger bonding area at the interface, all contributing to enhanced joint strength [
23]. Therefore, the differences in mechanical properties of the three joints may be attributed to the differences in the interface structure and interface microstructure, which were verified by subsequent experiments.
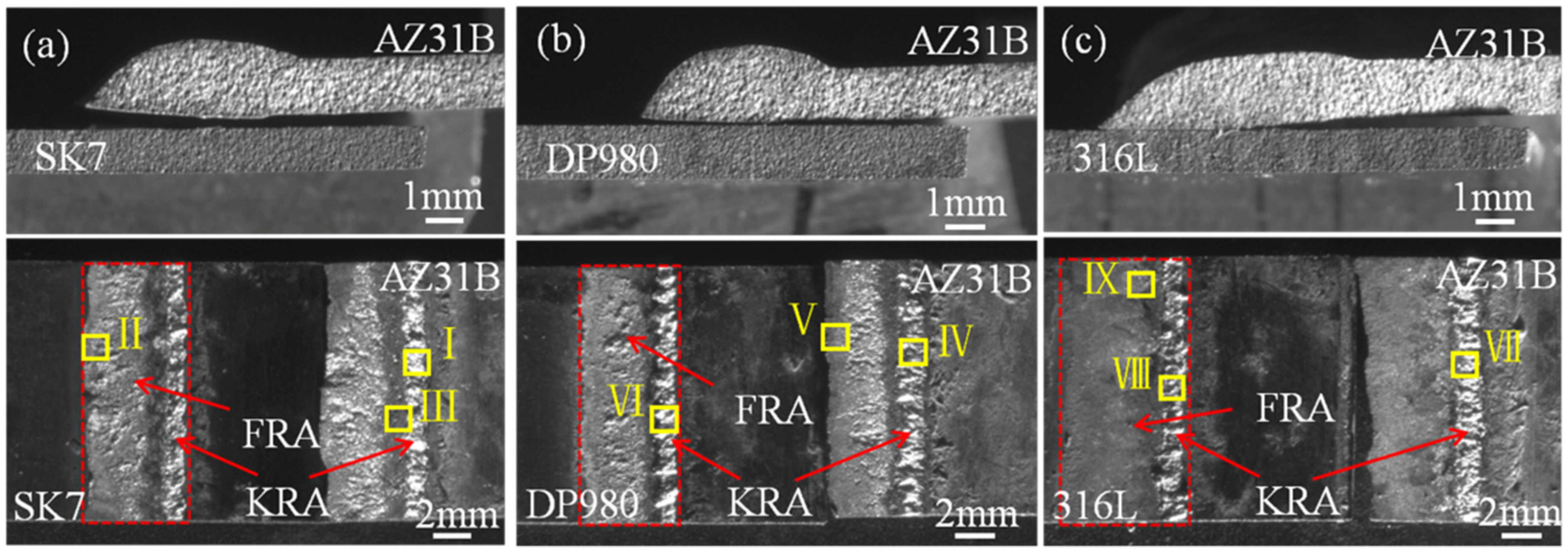
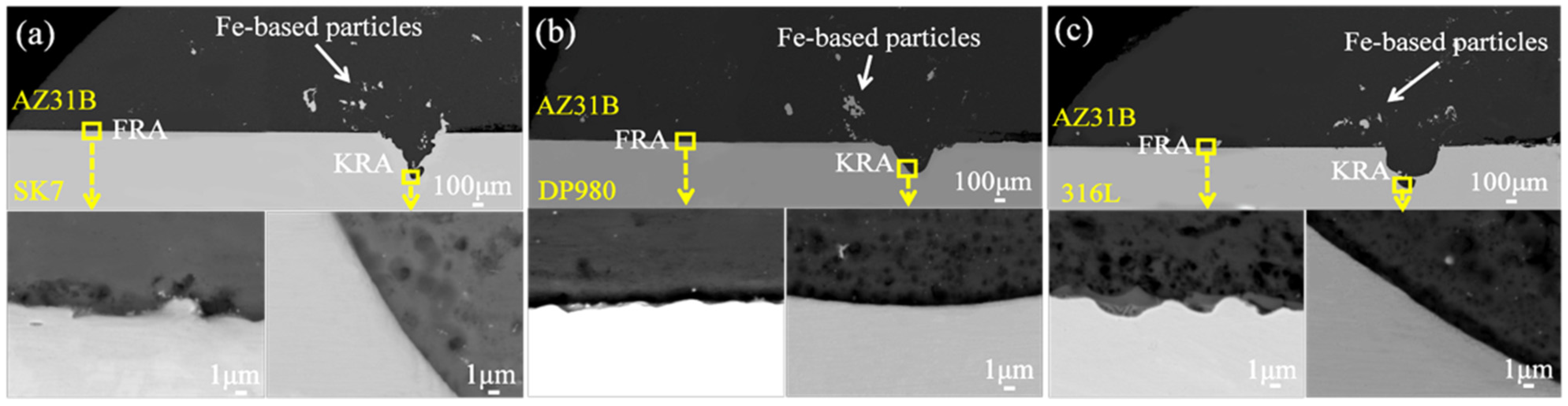
The fracture modes of these three joints were all along the bonding interface between the Mg alloy and steel. And the fracture path and fracture surface are shown in
Figure 4. It is noteworthy that the fracture surfaces exhibited distinct metallurgical regions (red boxes), which aligned with previous findings and were closely associated with the thermal gradient distribution within the joint [
23]. According to the thermal gradient distribution, the joint could be divided into two areas, namely the weld front reaction area (FRA) and the keyhole reaction area (KRA), respectively.
The locations in different areas marked by the yellow boxes in
Figure 4 underwent microscopic magnification as presented in
Figure 5 and quantitative point analysis results which are shown in
Table 3,
Table 4 and
Table 5. I–IX in
Figure 4 correspond to I–IX in
Figure 5. Based on binary phase diagrams of Al-Mg, Al-Fe, and Al-Mn [
24,
25,
26], Fe
3Al IMCs were both formed at the KRA of AZ31B/SK7 and AZ31B/DP980, while Al
11Mn
4 IMCs were observed at the KRA of the AZ31B/DP980 with a higher Mn content. These findings have been previously reported [
27,
28] and further validated through micro XRD analysis conducted during this study. It was suggested that fracture along Mg/Fe may be related to the thickness of the interface layer. Different from the fracture morphology of the other two joints (
Figure 5b,e), the fracture surface of the FRA of AZ31B/316L (
Figure 5e) was smoother. Based on the quantitative point analysis results, it can be inferred that the brittle eutectic structure consisting of Mg
17Al
12 + (Mg) and Mg
17Al
12 IMCs (
Table 5) may account for the low strength and brittle fracture behavior.
3.3. Microstructure
The microstructure of these different types of joints and the Mg/Fe interfaces at both FRA and KRA are depicted in
Figure 6. The weld exhibited massive nano-scale Fe-based particles in the Mg/Fe interface of the three joints. The dispersed Fe-based particles can be attributed to the overflow of molten metal from the keyhole during pulsed laser welding, which, due to insufficient time for complete expulsion during the cooling and solidification, resulted in their retention within the weld. However, a multi-layer structure was observed at the FRA of the AZ31B/316L joint and no obvious interface layer was observed in the FRA and KRA of the AZ31B/SK7 and AZ31B/DP980 joints.
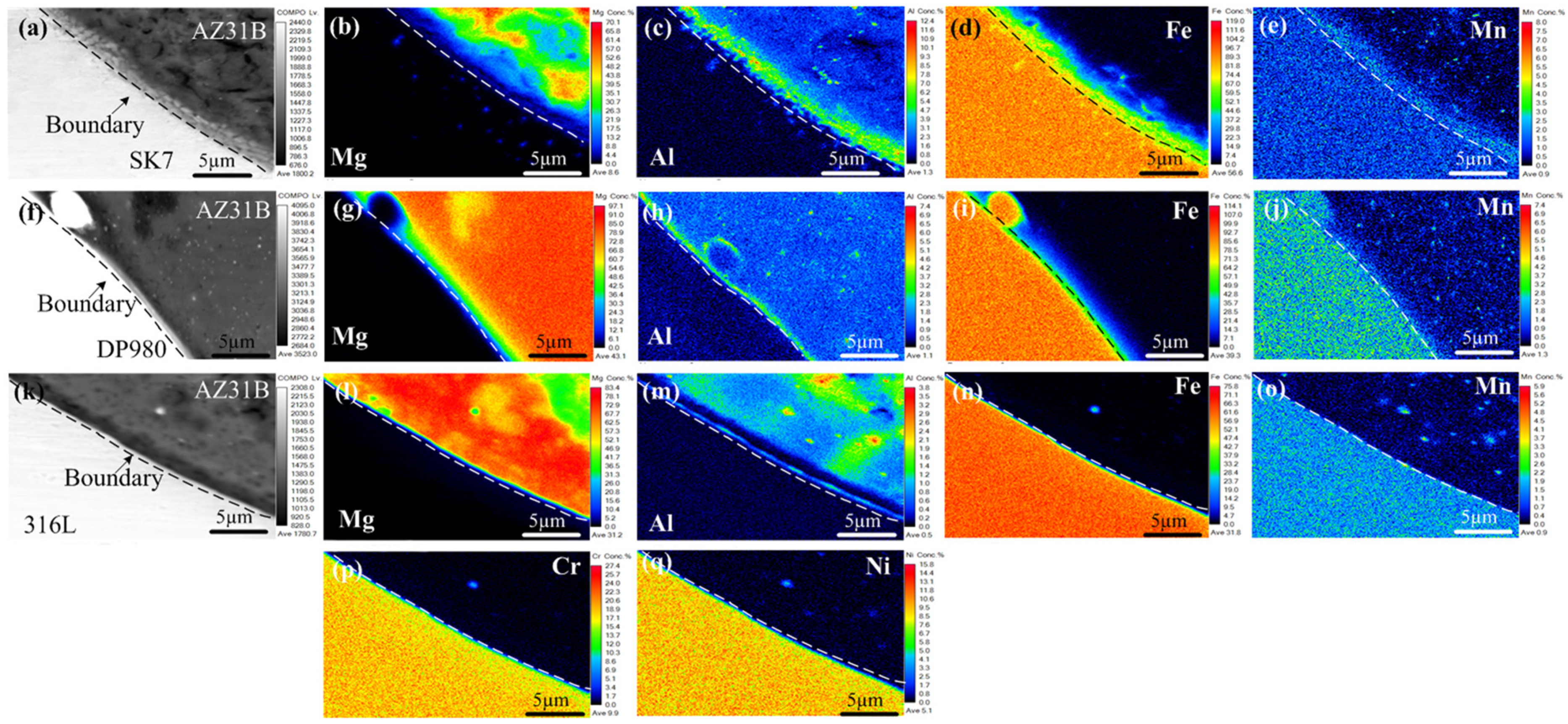
The KRA of the three joints in
Figure 6 was subjected to EPMA mapping analysis to further investigate the distribution of elements and chemical composition within the interface layer, as depicted in
Figure 7. Notably, a nanometer-scale thickness interface layer with Al element segregation (
Figure 7c,h,m) was observed at the Mg/Fe interface for all three joints examined. This was similar to the interface layer formation observed during laser welding-brazing [
29], where Fe-Al IMCs formed due to segregation and reaction between Al and Fe elements. The Mg/Fe interface of the three joint keyholes exhibited an uneven granular distribution of Mn, and Al had a similar distribution with Mn. The results showed that new compounds or solid solutions at the nanoscale may be formed. All of this meant that metallurgical bonding was achieved between the Mg and steel, which had a positive effect on the enhancement of the mechanical properties of the joint. As shown in
Figure 7p,q, no noticeable enrichment of Cr and Ni was observed at the keyhole interface in the AZ31B/316L, suggesting that these two alloying elements did not directly participate in the reaction at the interface.
The line analysis results of the Mg/Fe interfaces at the keyhole regions of three types of joints are illustrated in
Figure 8. The Al contents exhibited an initial increase followed by a decrease, attributed to segregation at the interface. Similarly, the Mn contents displayed a distribution trend consistent with that of the Al at the interface, confirming the formation of an Al–Mn phase in the keyhole region. Furthermore, the variations in Cr and Ni contents in the AZ31B/316L were found to be in agreement with those observed for Fe. The thickness of the interface layer can be determined by observing the variation trend of each constituent element. For the AZ31B/SK7 joint, the interface layer at the keyhole exhibited a thickness of 0.9 μm, while for the AZ31B/DP980 and AZ31B/316L joints, it measured 1.5 μm and only 0.6 μm, respectively. Within a certain range, an increased compound thickness at the interface was more advantageous for joint performance [
30].
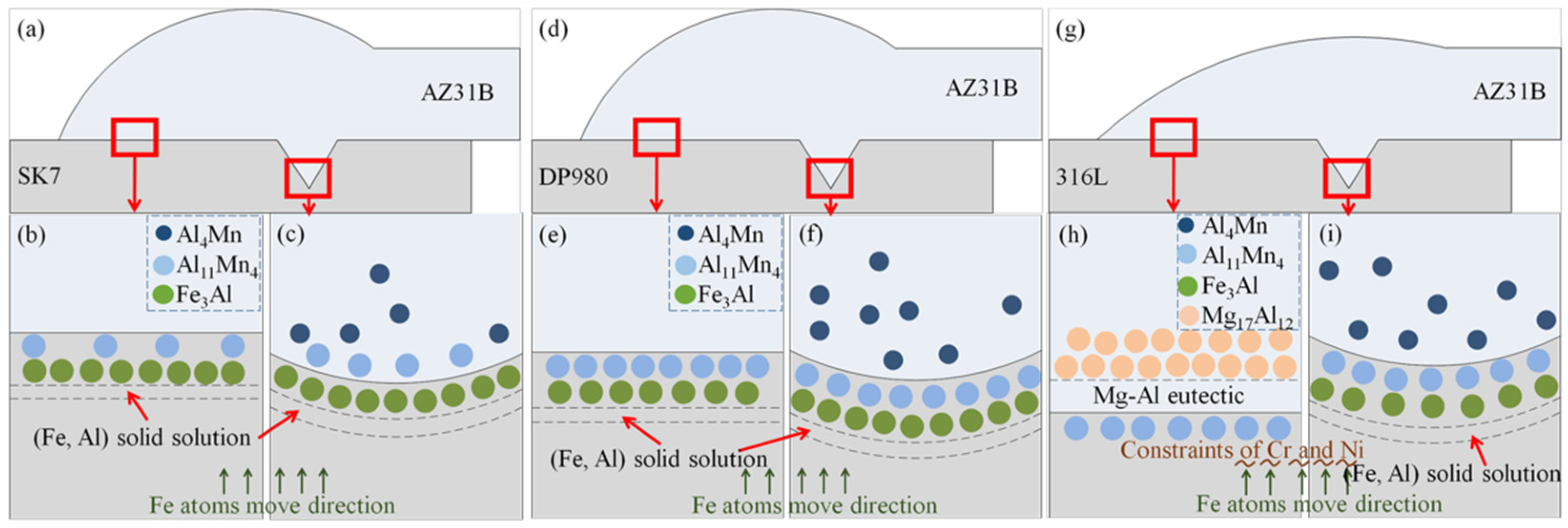
In order to gain further insights into its composition and structure, quantitative point analysis using EPMA was performed at various positions within the keyholes of three joints, and the corresponding results are presented in
Table 6,
Table 7 and
Table 8. The elemental composition proportions indicated that there was an enrichment of Mn and Al at the interface in points 1A, 1C, and 1D (
Table 6), which aligned with the findings from line analysis. According to the Fe–Al binary phase diagram [
25] and Al-Mn binary phase diagram [
26], it can be inferred that an (Fe) solid solution, Fe
3Al, Al
11Mn
4, may form when Al from AZ31B combined with Fe or Mn from SK7. Additionally, Al-Mn compounds were preferentially formed over Al-Fe compounds according to the mixing enthalpy relationship [
31], as evidenced by their respective mixing enthalpy values (Al-Ni < Al-Mn < Al-Fe < Al-Cr < Al-Mg < 0 J/mol). The compound Al
11Mn
4 exhibited two distinct crystal structures: high-temperature Al
11Mn
4 (HT-Al
11Mn
4), which belonged to the orthorhombic system, and low-temperature Al
11Mn
4 (LT-Al
11Mn
4), which belonged to the triclinic system. At a temperature of 1002 °C, Al reacted with Mn to form a high-temperature phase known as HT-Al
11Mn
4. Upon cooling, this phase underwent a transformation into a low-temperature phase called LT-Al
11Mn
4 at 910 °C. The reaction can be represented by the following formulas:
The LT-Al11Mn4 compound and the granular μ(Al4Mn) compound were formed, which was the source of the granular Al-Mn phase near the Mg/Fe interface on the Mg side. Consequently, a layered LT-Al11Mn4 was generated in the interface layer. The atomic radius of Fe is 126 pm, while that of Mn is 127 pm, and their atomic radii exhibit comparable magnitudes. Therefore, there was a high likelihood of Fe atoms substituting some Mn atoms, leading to the formation of an LT-Al11(Mn, Fe)4 IMCs-based solid solution. Point 1D was situated in the interface layer near the weld where it was probable that Fe3Al existed based on elemental composition and analysis of the binary phase diagram.
The points 2A and 2B located at the keyhole of AZ31B/DP980 were both situated at the interface layer, where Al from AZ31B may react with Mn to form an Al-Mn phase or with Fe to form a Fe-Al phase. According to the content ratio (
Table 7), it was plausible that the interface layer at room temperature could consist of Fe
3Al, (Fe) solid solution, or LT-Al
11Mn
4.
The position of point 3A was located at the keyhole of the AZ31B/316L, positioned right on the periphery of the steel spatter within the weld. The ratios of Mn to Fe and Al to Mg were found to be higher compared to those in the original base metal, indicating an enrichment phenomenon of alloy elements at the Mg/Fe interface, thereby suggesting a potential formation of a new phase. From the quantitative results as presented in
Table 8, it can be inferred that both the (Fe) solid solution Fe
3Al and LT-Al
11Mn
4 might coexist in this region.
In summary, Al and Mn elements were found to be enriched at the Mg/Fe interface in the KRA of all three joints. The interfacial compounds consisted of a phase containing Al-Mn particles, as well as Al-Mn and Al-Fe IMCs. Compared to the AZ31B/SK7 joint with a low Mn content, the AZ31B/DP980 joint with a high Mn content exhibited a thicker layer of interfacial compound (
Figure 8). However, the contribution of Al-Mn compounds to the mechanical properties of the joints was not found to be significant (
Figure 3). The interfacial compound thickness of the AZ31B/316L was smaller compared to the other joints, while the load-bearing capacity of the AZ31B/316L was weaker than that of the other joints (
Figure 8). Conversely, Li et al. [
32] observed strong metallurgical bonding between Mg/Fe interfaces under TEM examination but found that it generated only a nanoscale interface layer. Therefore, it can be concluded that differences in mechanical properties between these three joints were primarily caused by the difference in the FRA of the weld.
The mapping analysis results at the Mg/Fe interface in front of the three joint welds are presented in
Figure 9. Similar to the phenomenon observed at the keyhole interface (
Figure 7), enrichment of Al and Mn was also observed at this interface. However, the μ(Al
4Mn) particle phase was formed but less. Notably, the microstructure of the Mg/Fe interface in front of the AZ31B/316L differed significantly from that of the other two types of joints, as it exhibited a composite structure with multiple layers. Two forms of Al enrichment were observed at the Mg/Fe interface, namely a layered structure and a network structure (
Figure 9k). To further ascertain the composition of the interface layer, element composition analysis was conducted at various positions in the FRA in
Figure 9k, as shown in
Table 9. The main constituents at the interface were found to be Mg and Al, with a significantly higher Al content compared to the AZ31B base metal. Referring to the Mg–Al binary phase diagram [
24], it was observed that the interface layer adjacent to the Mg side primarily consisted of Mg
17Al
12 IMCs, while the interface layer near the steel side comprised a eutectic mixture of [Mg
17Al
12 + α-Mg]. These brittle and thick layers of compounds played a crucial role in contributing to the inferior mechanical properties exhibited by the joint.
To confirm the phase composition of the interface layer, micro XRD analysis was conducted on the fracture surfaces of three joints. The corresponding results are presented in
Figure 10. The fracture surfaces of AZ31B/SK7 and AZ31B/DP980 joints not only detected Fe and Mg, but also confirmed the presence of Fe
3Al and A
11Mn
4, indicating that both joint fractures indeed generated the aforementioned Fe–Al and Al–Mn phases at the interface in addition to the residual Mg. Moreover, on the fracture surface of AZ31B/316L joint, significant amounts of Mg
17Al
12 compound were detected, indicating that these phases were indeed formed at the FRA during the welding process.