Influence of Zn Addition on the Aging Precipitate Behavior and Mechanical Properties of Al-Cu-Li Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results
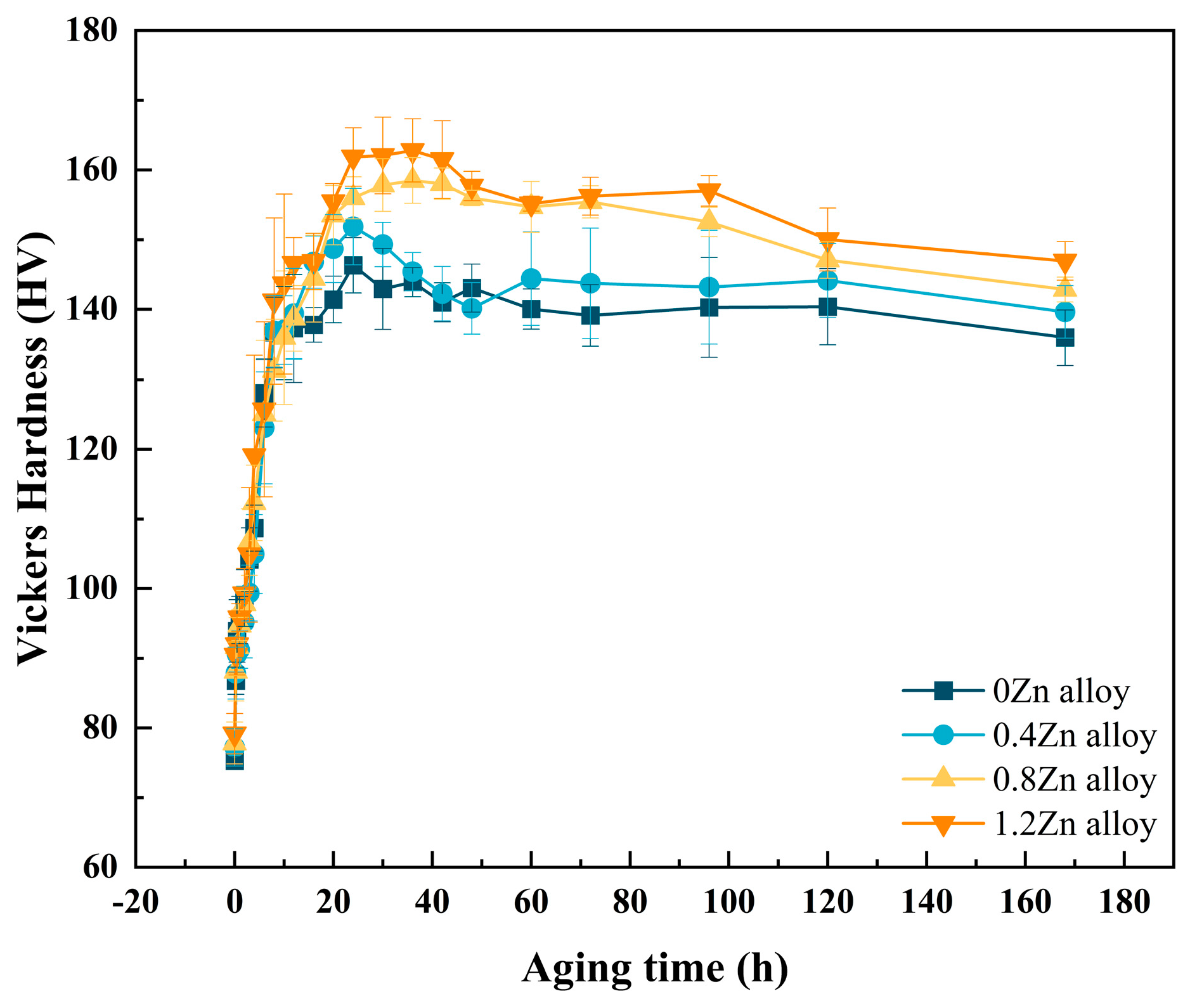
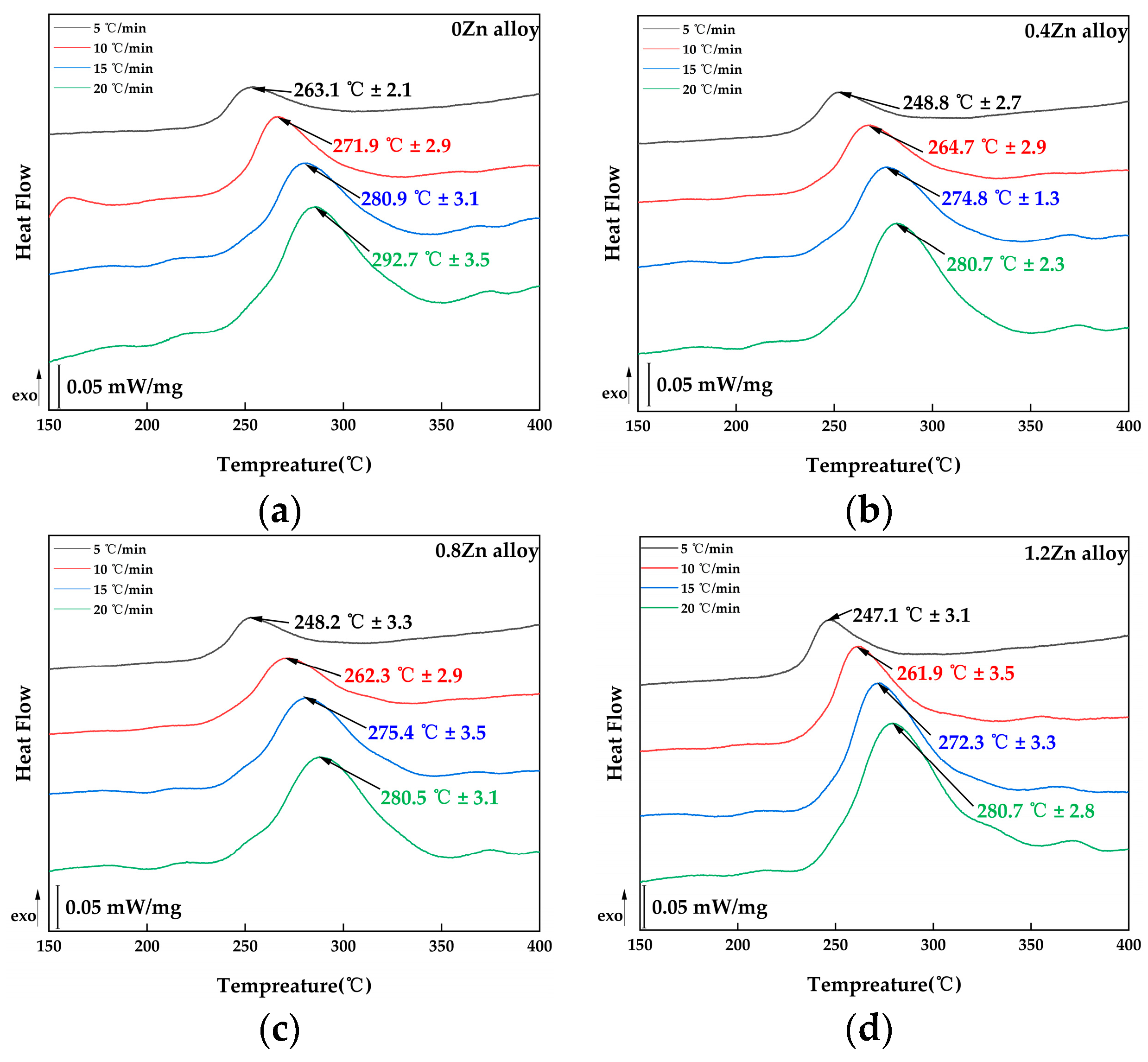
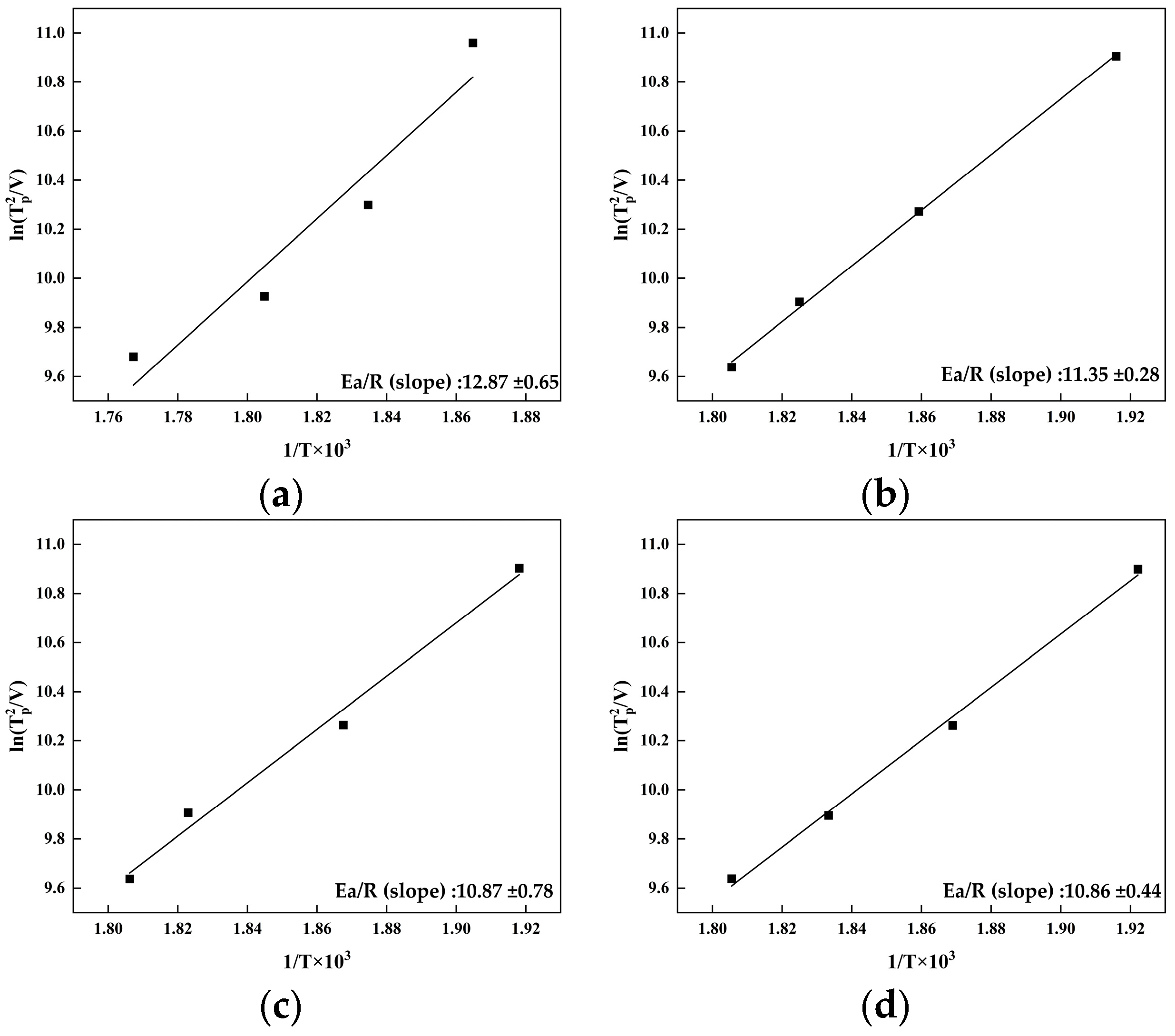
3.1. Artificial Age-Hardening Behavior
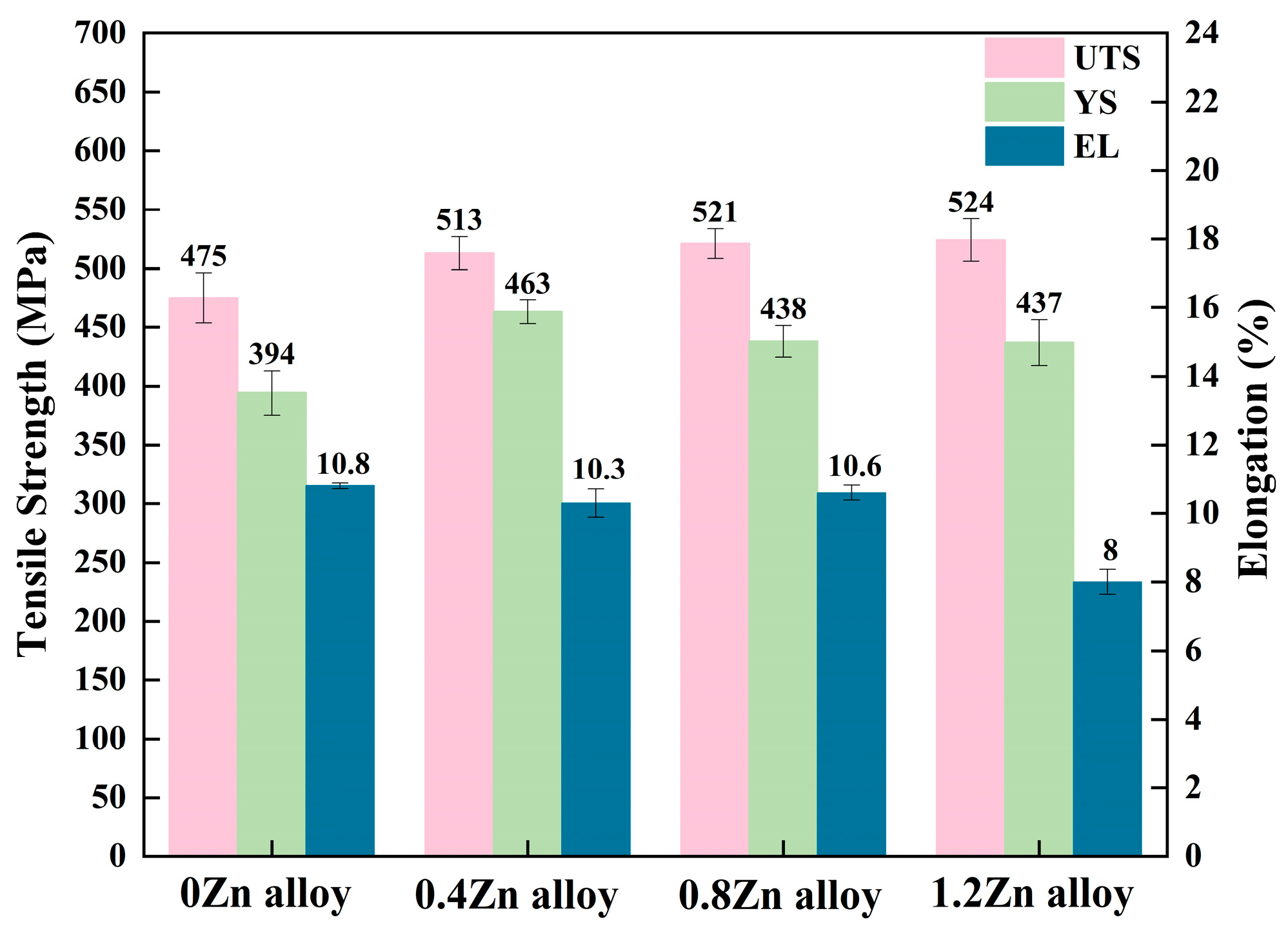
3.2. Mechanical Properties under Peak Aging Temper
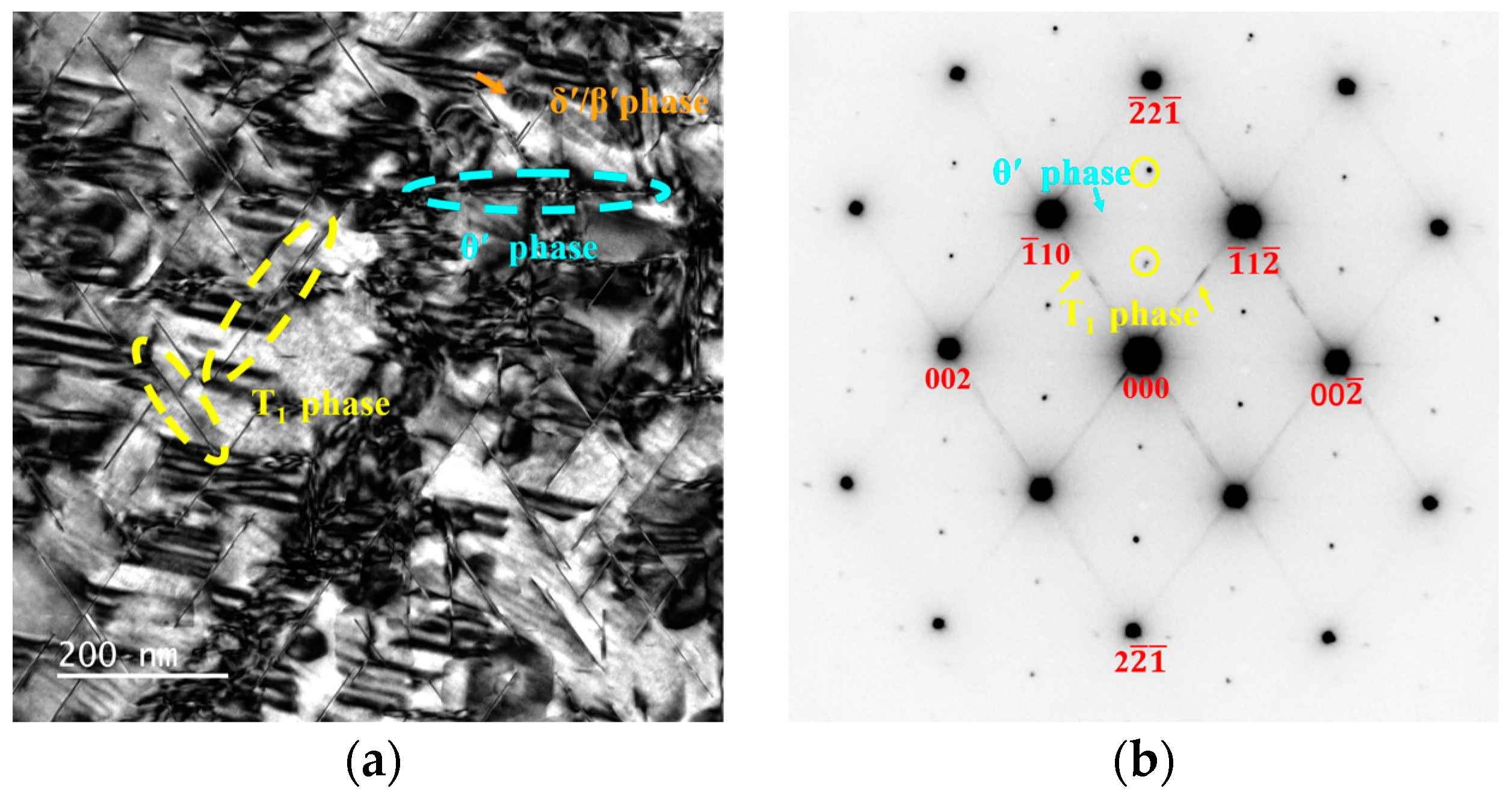
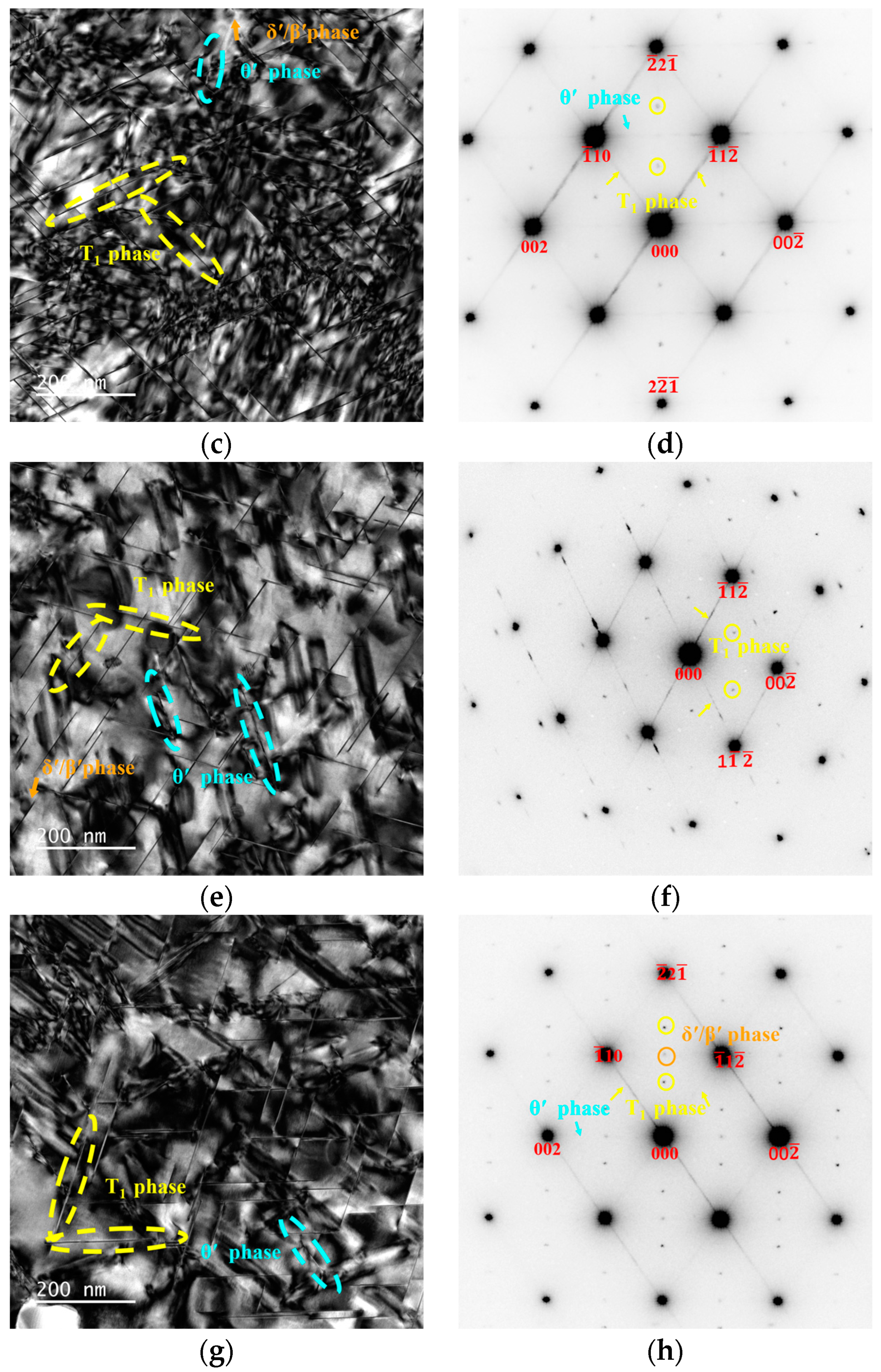
3.3. Precipitation at the Peak-Aged Temper
4. Discussion
4.1. Effect of Zn on the Age-Hardening Precipitates
4.2. Effect of Zn on the Mechanical Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rioja, R.J.; Liu, J. The Evolution of Al-Li Base Products for Aerospace and Space Applications. Metall. Mater. Trans. A 2012, 43, 3325–3337. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminum alloys. Mater. Des. (1980–2015) 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Polmear, I.J. Recent Developments in Light Alloys. Mater. Trans. JIM 1996, 37, 12–31. [Google Scholar] [CrossRef]
- Gable, B.M.; Zhu, A.W.; Csontos, A.A.; Starke, E.A., Jr. The role of plastic deformation on the competitive microstructural evolution and mechanical properties of a novel Al–Li–Cu–X alloy. J. Light Met. 2001, 1, 1–14. [Google Scholar] [CrossRef]
- Gumbmann, E.; De Geuser, F.; Sigli, C.; Deschamps, A. Influence of Mg, Ag and Zn minor solute additions on the precipitation kinetics and strengthening of an Al-Cu-Li alloy. Acta Mater. 2017, 133, 172–185. [Google Scholar] [CrossRef]
- Van Smaalen, S.; Meetsma, A.; De Boer, J.; Bronsveld, P. Refinement of the crystal structure of hexagonal Al2CuLi. J Solid State Chem. 1990, 85, 293–298. [Google Scholar] [CrossRef]
- Lv, K.; Zhu, C.; Zheng, J.; Wang, X.; Chen, B. Precipitation of T1 phase in 2198 Al–Li alloy studied by atomic-resolution HAADF-STEM. J. Mater. Res. 2019, 34, 3535–3544. [Google Scholar] [CrossRef]
- Gumbmann, E.; Lefebvre, W.; De Geuser, F.; Sigli, C.; Deschamps, A. The effect of minor solute additions on the precipitation path of an Al-Cu-Li alloy. Acta Mater. 2016, 115, 104–114. [Google Scholar] [CrossRef]
- Gilmore, D.L.; Starke, E.A. Trace element effects on precipitation processes and mechanical properties in an Al-Cu-Li alloy. Metall. Mater. Trans. A 1997, 28, 1399–1415. [Google Scholar] [CrossRef]
- Huang, B.P.; Zheng, Z.Q. Independent and combined roles of trace Mg and Ag additions in properties precipitation process and precipitation kinetics of Al–Cu–Li–(Mg)–(Ag)–Zr–Ti alloys. Acta Mater. 1998, 46, 4381–4393. [Google Scholar] [CrossRef]
- Gault, B.; De Geuser, F.; Bourgeois, L.; Gabble, B.M.; Ringer, S.P.; Muddle, B.C. Atom probe tomography and transmission electron microscopy characterisation of precipitation in an Al–Cu–Li–Mg–Ag alloy. Ultramicroscopy 2011, 111, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Murayama, M.; Hono, K. Role of Ag and Mg on precipitation of T1 phase in an Al-Cu-Li-Mg-Ag alloy. Scr. Mater. 2001, 44, 701–706. [Google Scholar] [CrossRef]
- Xu, X.; Wu, G.; Zhang, L.; Tong, X.; Zhang, X.; Sun, J.; Li, L.; Xiong, X. Effects of heat treatment and pre-stretching on the mechanical properties and microstructure evolution of extruded 2050 Al–Cu–Li alloy. Mater. Sci. Eng. A 2022, 845, 143236. [Google Scholar] [CrossRef]
- Li, J.F.; Ye, Z.H.; Liu, D.Y.; Chen, Y.L.; Zhang, X.H.; Xu, X.Z.; Zheng, Z.Q. Influence of Pre-deformation on Aging Precipitation Behavior of Three Al–Cu–Li Alloys. Acta Metall. Sin. Engl. 2017, 30, 133–145. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Wang, H. Significant roles of minor Mg addition in microstructural evolution and mechanical properties of cast Al–3Li–2Cu-0.15Zr alloy. J. Alloys Compd. 2020, 857, 157600. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Wu, G.; Zhang, X.; Rong, M.; Wang, C. Microstructural characteristics and mechanical properties of extruded Al-4Cu-1Li-0.4Mg-0.1Zr-xZn alloy. Mater. Sci. Eng. A 2019, 743, 223–232. [Google Scholar] [CrossRef]
- Sun, J.; Wu, G.; Zhang, L.; Zhang, X.; Liu, L.; Zhang, J. Microstructure characteristics of an ultra-high strength extruded Al-4.7Cu1Li-0.5Mg-0.1Zr1Zn alloy during heat treatment. J. Alloys Compd. 2020, 813, 152216. [Google Scholar] [CrossRef]
- Zhu, X.H.; Zheng, Z.Q.; Zhong, S. Effect of Mg and Zn on microstructure and tensile properties of 2099 alloy. J. Nonferr. Met. 2010, 20, 1861–1867. [Google Scholar]
- Kumar, K.; Brown, S.; Pickens, J. Microstructural evolution during aging of an Al-Cu-Li-Ag-Mg-Zr alloy. Acta Mater. 1996, 44, 1899–1915. [Google Scholar] [CrossRef]
- Xu, X.; Wu, G.; Zhang, L.; Tong, X.; Qi, F.; Guo, Y.; Li, L.; Xiong, X.; Wang, C. Regulation of precipitation behavior among T1, S′, and θ′ phases in Al–Cu–Li-(Mg–Ag) alloys by optimizing Ag/Mg ratios. Mater. Sci. Eng. A 2023, 876, 145158. [Google Scholar] [CrossRef]
- Tiryakioğlu, M.; Robinson, J.S.; Salazar-Guapuriche, M.A.; Zhao, Y.Y.; Eason, P.D. Hardness–strength relationships in the aluminum alloy 7010. Mater. Sci. Eng. A 2015, 631, 196–200. [Google Scholar] [CrossRef]
- Mao, B.P.; Yan, X.D.; Shen, J. Precipitation behavior of T1 phase during thermo-mechanical treatment of 2197 Al-Li alloy. J. Nonferr. Met. 2015, 25, 2366–2371. [Google Scholar]
- Quist, W.E.; Narayanan, G.H. 8—Aluminum-Lithium Alloys. Treatise Mater. Sci. Technol. 1989, 31, 219–254. [Google Scholar]
- Hirosawa, S.; Sato, T.; Kamio, A.; Flower, H. Classification of the role of microalloying elements in phase decomposition of Al based alloys. Acta Mater. 2000, 48, 1797–1806. [Google Scholar] [CrossRef]
- Niessen, A.K.; de Boer, F.R.; Boom, R.; de Châtel, P.F.; Mattens, W.C.M.; Miedema, A.R. Model predictions for the enthalpy of formation of transition metal alloys II. Calphad 1983, 7, 51–70. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, G.; Zhang, L.; Zhang, X.; Shi, C.; Sun, J. Effect of Zn on precipitation evolution and mechanical properties of a high strength cast Al-Li-Cu alloy. Mater. Charact. 2020, 160, 110089. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. Calorim. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Morris, B. The components of the Wired Spanning Forest are recurrent. Probab. Theory Relat. Fields 2003, 125, 259–265. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.; Zhang, Z. Theoretical and experimental study of precipitation and coarsening kinetics of θ′ phase in Al–Cu alloy. Vacuum 2021, 189, 110263. [Google Scholar] [CrossRef]
- Gaber, A.; Matsuda, K.; Ali, A.M.; Zou, Y.; Ikeno, S. DSC and HRTEM investigation of the precipitates in Al–1.0%Mg2 Si–0.5%Ag alloy. Mater. Sci. Technol. 2004, 20, 1627–1631. [Google Scholar] [CrossRef]
- Huang, J.C.; Ardell, A.J. Crystal structure and stability of T 1, precipitates in aged Al–Li–Cu alloys. Mater. Sci. Technol. 1987, 3, 176–188. [Google Scholar] [CrossRef]
- Rodgers, B.I.; Prangnell, P.B. Quantification of the influence of increased pre-stretching on microstructure-strength relationships in the Al–Cu–Li alloy AA2195. Acta Mater. 2016, 108, 55–67. [Google Scholar] [CrossRef]


| Alloy | Cu | Li | Mg | Zn | Mn | Zr | Fe | Al |
|---|---|---|---|---|---|---|---|---|
| 0Zn | 3.54 | 0.81 | 0.42 | — | 0.38 | 0.10 | <0.01 | Bal. |
| 0.4Zn | 3.62 | 0.96 | 0.40 | 0.42 | 0.41 | 0.12 | 0.01 | Bal. |
| 0.8Zn | 3.63 | 0.86 | 0.40 | 0.79 | 0.39 | 0.12 | <0.01 | Bal. |
| 1.2Zn | 3.56 | 0.90 | 0.42 | 1.22 | 0.40 | 0.11 | <0.01 | Bal. |
| Alloys | Area Number Density (pcs/μm2) | Diameter (nm) |
|---|---|---|
| 0Zn | 97.0 ± 3.1 | 83.55 ± 4.4 |
| 0.4Zn | 101.6 ± 2.4 | 101.20 ± 5.0 |
| 0.8Zn | 114.8 ± 2.1 | 98.05 ± 5.7 |
| 1.2Zn | 115.6 ± 3.9 | 118.67 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Yan, L.; Li, X.; Zhang, Y.; Li, Z.; Wen, K.; Liu, H.; Xiong, B. Influence of Zn Addition on the Aging Precipitate Behavior and Mechanical Properties of Al-Cu-Li Alloy. Materials 2024, 17, 1562. https://doi.org/10.3390/ma17071562
Wang M, Yan L, Li X, Zhang Y, Li Z, Wen K, Liu H, Xiong B. Influence of Zn Addition on the Aging Precipitate Behavior and Mechanical Properties of Al-Cu-Li Alloy. Materials. 2024; 17(7):1562. https://doi.org/10.3390/ma17071562
Chicago/Turabian StyleWang, Meiqi, Lizhen Yan, Xiwu Li, Yongan Zhang, Zhihui Li, Kai Wen, Hongwei Liu, and Baiqing Xiong. 2024. "Influence of Zn Addition on the Aging Precipitate Behavior and Mechanical Properties of Al-Cu-Li Alloy" Materials 17, no. 7: 1562. https://doi.org/10.3390/ma17071562
APA StyleWang, M., Yan, L., Li, X., Zhang, Y., Li, Z., Wen, K., Liu, H., & Xiong, B. (2024). Influence of Zn Addition on the Aging Precipitate Behavior and Mechanical Properties of Al-Cu-Li Alloy. Materials, 17(7), 1562. https://doi.org/10.3390/ma17071562






