Electromagnetic Field Drives the Bioelectrocatalysis of γ-Fe2O3-Coated Shewanella putrefaciens CN32 to Boost Extracellular Electron Transfer
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials and Chemicals
2.2. Cultivation of Heterotrophic Bacteria
2.3. Application of Magnetic Fields and Data Acquisition
2.4. Bacterial Characterization and Pretreatment
2.5. Electrochemical Testing of the S. putrefaciens CN32-Magnetic Field Coupling System
3. Results and Discussion
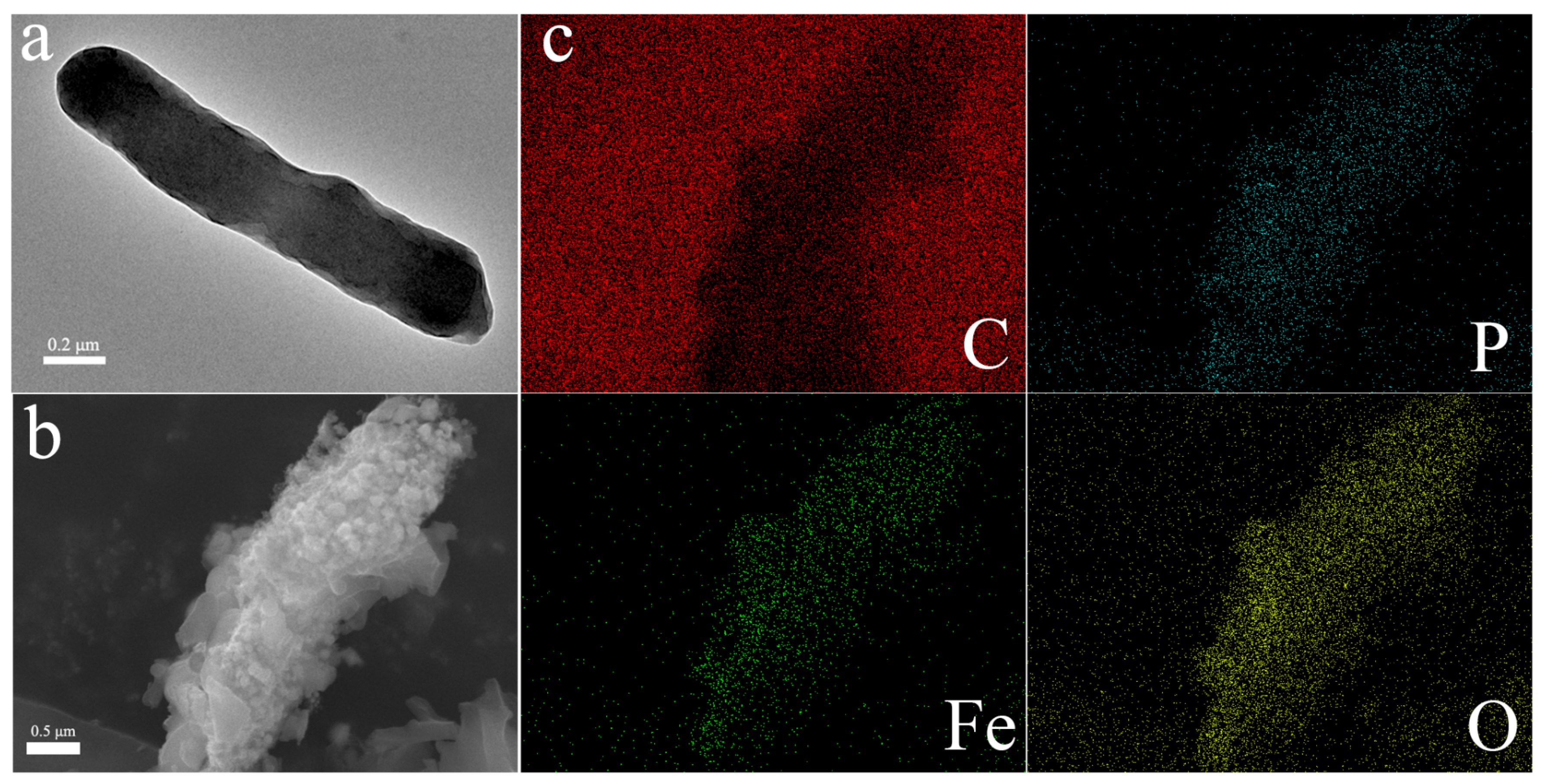
3.1. Assembly of CN32@γ-Fe2O3
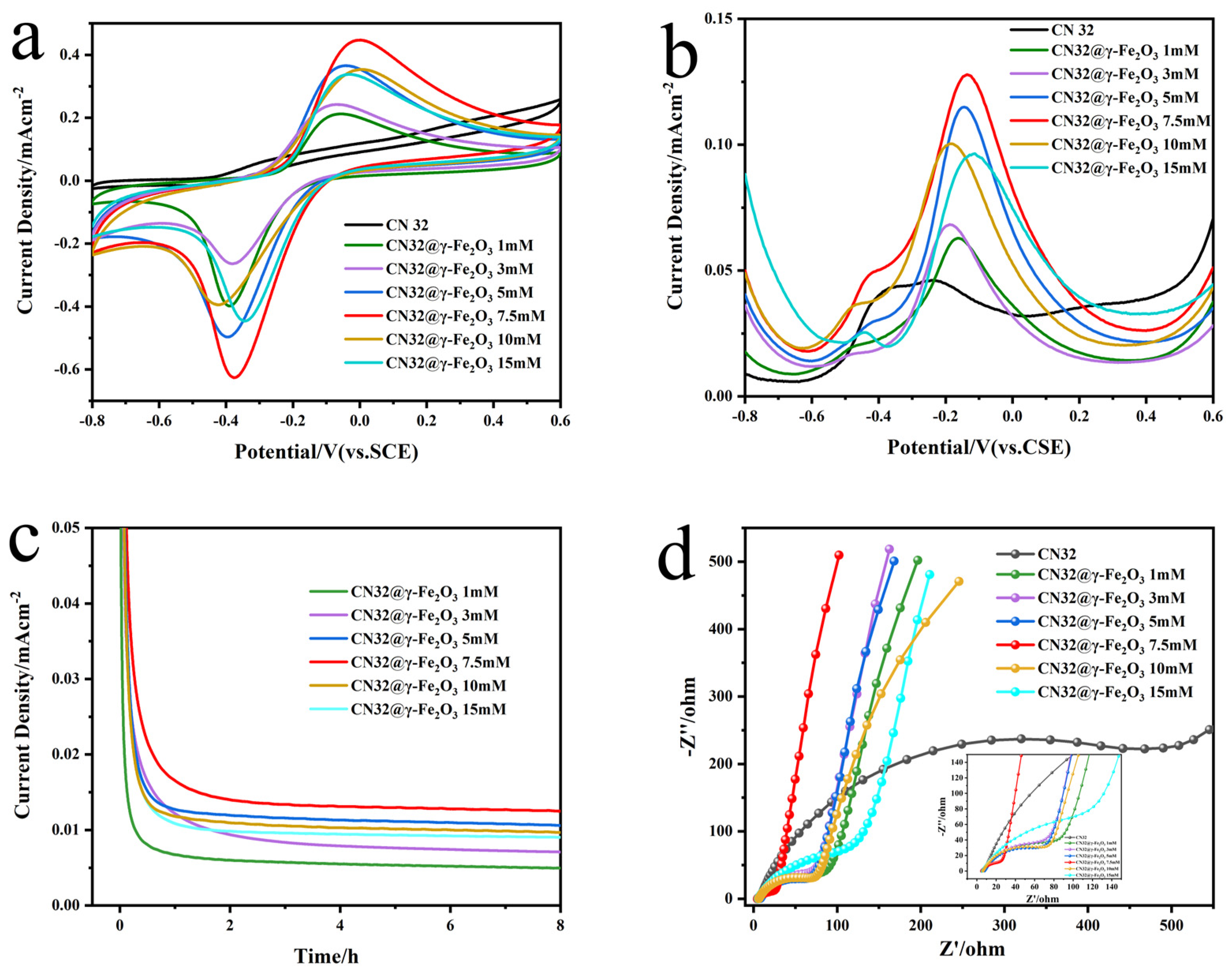
3.2. Exploration of the Optimal Coating Amount of γ-Fe2O3 MNPs
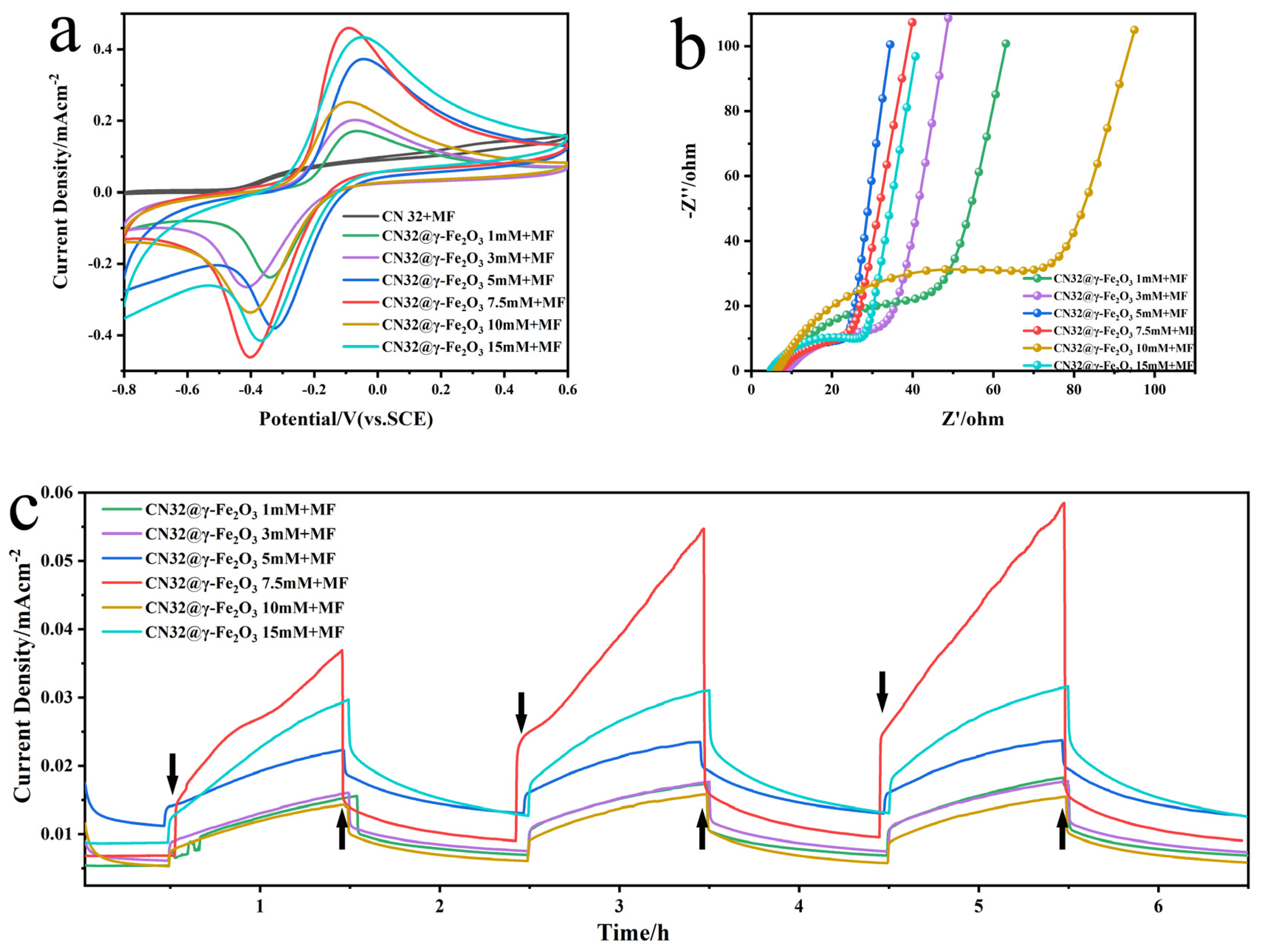
3.3. Effect of EMF on Functionalized CN32@γ-Fe2O3
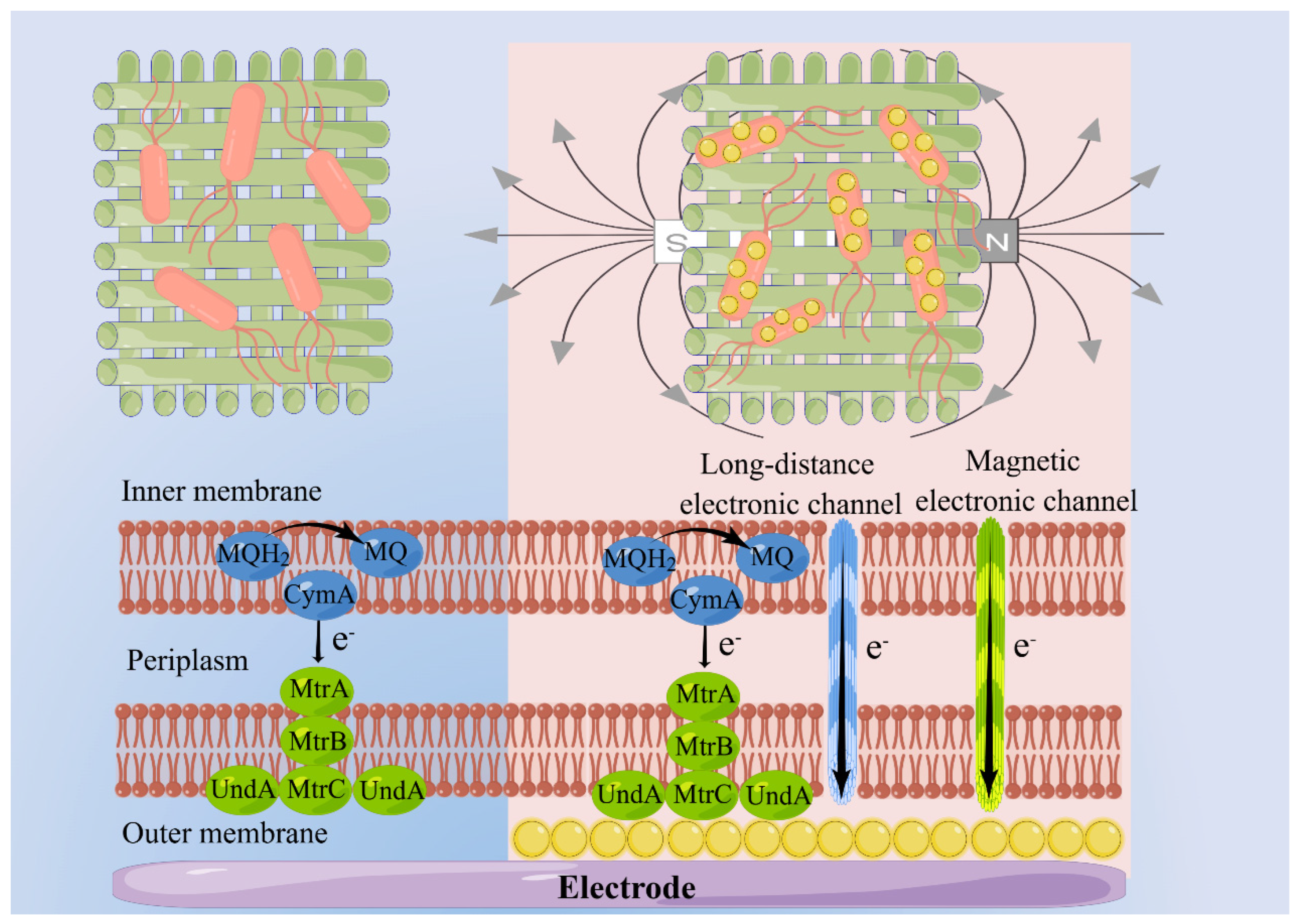
3.4. The Mechanism of MNPs and EMF Synergistically Enhance the DET Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Bao, S.-J.; Li, C.M. Electrocatalysis in microbial fuel cells—From electrode material to direct electrochemistry. Energy Environ. Sci. 2010, 3, 544–553. [Google Scholar] [CrossRef]
- Houghton, J.; Santoro, C.; Soavi, F.; Serov, A.; Ieropoulos, I.; Arbizzani, C.; Atanassov, P. Supercapacitive microbial fuel cell: Characterization and analysis for improved charge storage/delivery performance. Bioresour. Technol. 2016, 218, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-W.; Choi, S.-J.; Cha, J.-H.; Lee, T.-H.; Kim, C.-W. Microbial community dynamics and electron transfer of a biocathode in microbial fuel cells. Korean J. Chem. Eng. 2010, 27, 1513–1520. [Google Scholar] [CrossRef]
- Lovley, D.R. Powering microbes with electricity: Direct electron transfer from electrodes to microbes. Environ. Microbiol. Rep. 2010, 3, 27–35. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Chen, X.; Yuan, X.; Li, N.; He, W.; Feng, Y. Tailoring spatial structure of electroactive biofilm for enhanced activity and direct electron transfer on iron phthalocyanine modified anode in microbial fuel cells. Biosens. Bioelectron. 2021, 191, 113410. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Qiao, Y.; Wu, Z.-Y.; Wu, X.-S.; Xie, J.-L.; Yu, S.-H.; Guo, J.; Li, C.M. Tailoring Unique Mesopores of Hierarchically Porous Structures for Fast Direct Electrochemistry in Microbial Fuel Cells. Adv. Energy Mater. 2016, 6, 1501535. [Google Scholar] [CrossRef]
- Wu, X.; Qiao, Y.; Shi, Z.; Tang, W.; Li, C.M. Hierarchically Porous N-Doped Carbon Nanotubes/Reduced Graphene Oxide Composite for Promoting Flavin-Based Interfacial Electron Transfer in Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2018, 10, 11671–11677. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Qiao, Y.; Li, C.M. Boosting Microbial Electrocatalytic Kinetics for High Power Density: Insights into Synthetic Biology and Advanced Nanoscience. Electrochem. Energy Rev. 2018, 1, 567–598. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Sun, J.; Zhang, L.; Jiao, J.; Wang, Q.; Liu, S. Nanomaterials Facilitating Microbial Extracellular Electron Transfer at Interfaces. Adv. Mater. 2020, 33, 2004051. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Lyu, Q.; Wang, H.; Ji, X.; Yan, Z.; Chen, F.; Dahlgren, R.A.; Zhang, M. Modular configurations of living biomaterials incorporating nano-based artificial mediators and synthetic biology to improve bioelectrocatalytic performance: A review. Sci. Total Environ. 2022, 824, 153857. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Hou, N.; Liu, X.-L.; Mu, Y. Advances in interfacial engineering for enhanced microbial extracellular electron transfer. Bioresour. Technol. 2022, 345, 126562. [Google Scholar] [CrossRef]
- Ganesh, I. Electrochemical conversion of carbon dioxide into renewable fuel chemicals—The role of nanomaterials and the commercialization. Renew. Sustain. Energy Rev. 2016, 59, 1269–1297. [Google Scholar] [CrossRef]
- Mouhib, M.; Antonucci, A.; Reggente, M.; Amirjani, A.; Gillen, A.J.; Boghossian, A.A. Enhancing bioelectricity generation in microbial fuel cells and biophotovoltaics using nanomaterials. Nano Res. 2019, 12, 2184–2199. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhang, Q.; Li, C. Microbial Fuel Cells: Nanomaterials Based on Anode and Their Application. Energy Technol-ger. 2020, 8, 2000206. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336. [Google Scholar] [CrossRef]
- Bian, R.; Jiang, Y.; Wang, Y.; Sun, J.K.; Hu, J.; Jiang, L.; Liu, H. Highly Boosted Microbial Extracellular Electron Transfer by Semiconductor Nanowire Array with Suitable Energy Level. Adv. Funct. Mater. 2018, 28, 1707408. [Google Scholar] [CrossRef]
- Wang, W.; You, S.; Gong, X.; Qi, D.; Chandran, B.K.; Bi, L.; Cui, F.; Chen, X. Bioinspired Nanosucker Array for Enhancing Bioelectricity Generation in Microbial Fuel Cells. Adv. Mater. 2016, 28, 270–275. [Google Scholar] [CrossRef] [PubMed]
- McCuskey, S.R.; Su, Y.; Leifert, D.; Moreland, A.S.; Bazan, G.C. Bazan, Living Bioelectrochemical Composites. Adv. Mater. 2020, 32, 1908178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Hui, S.; Jiang, L.P.; Zhu, J.J. Functional Nanomaterial-Modified Anodes in Microbial Fuel Cells: Advances and Perspectives. Chem. Eur. J. 2022, 29, e202202002. [Google Scholar] [CrossRef] [PubMed]
- Song, R.-B.; Wu, Y.; Lin, Z.-Q.; Xie, J.; Tan, C.H.; Loo, J.S.C.; Cao, B.; Zhang, J.-R.; Zhu, J.-J.; Zhang, Q. Living and Conducting: Coating Individual Bacterial Cells with In Situ Formed Polypyrrole. Angew. Chem. Int. Ed. 2017, 56, 10516–10520. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, N.; Sakimoto, K.K.; Herlihy, D.M.; Nguyen, S.C.; Alivisatos, A.P.; Harris, C.B.; Schwartzberg, A.; Yang, P. Spectroscopic elucidation of energy transfer in hybrid inorganic–biological organisms for solar-to-chemical production. Proc. Natl. Acad. Sci. USA 2016, 113, 11750–11755. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, K.K.; Wong, A.B.; Yang, P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 2016, 351, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, X.; Shi, Z.; Li, X.; Qian, S.; Sun, X.; Sun, W.; Guo, C.; Li, C.M. Photoactive Manganese Ferrite-Modified Bacterial Anode to Simultaneously Boost Both Mediated and Direct Electron Transfer Processes in Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2022, 10, 3355–3362. [Google Scholar] [CrossRef]
- Yang, C.; Aslan, H.; Zhang, P.; Zhu, S.; Xiao, Y.; Chen, L.; Khan, N.; Boesen, T.; Wang, Y.; Liu, Y.; et al. Carbon dots-fed Shewanella oneidensis MR-1 for bioelectricity enhancement. Nat. Commun. 2020, 11, 1379. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.C.; Zhao, Z.P.; Peng, L.L.; Shiu, H.Y.; Ding, M.N.; Song, F.; Guan, X.; Lee, C.K.; Huang, J.; Zhu, D.; et al. Silver nanoparticles boost charge-extraction efficiency in Shewanella microbial fuel cells. Science 2021, 373, 1336–1340. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Wang, Y.-Z.; Fang, Z.; Shi, Y.-T.; Cheng, Q.-W.; Chen, Y.-X.; Shi, W.; Yong, Y.-C. Single cell electron collectors for highly efficient wiring-up electronic abiotic/biotic interfaces. Nat. Commun. 2020, 11, 4087. [Google Scholar] [CrossRef]
- Leonel, A.G.; Mansur, A.A.P.; Mansur, H.S. Advanced Functional Nanostructures based on Magnetic Iron Oxide Nanomaterials for Water Remediation: A Review. Water Res. 2021, 190, 116693. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, K.A.; Huang, S.-J.; Wang, C.-T.; Kumar, S. Fundamental understanding of microbial fuel cell technology: Recent development and challenges. Chemosphere 2022, 288, 132446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Zhao, X.; Li, Y. Factors affecting the efficiency of a bioelectrochemical system: A review. RSC Adv. 2019, 9, 19748–19761. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Devi, P.; Kumar, P. Magnetic-field induced sustainable electrochemical energy harvesting and storage devices: Recent progress, opportunities, and future perspectives. Nano Energy 2021, 87, 106119. [Google Scholar] [CrossRef]
- Tong, Z.-H.; Yu, H.-Q.; Li, W.-W.; Wang, Y.-K.; Sun, M.; Liu, X.-W.; Sheng, G.-P. Application of a weak magnetic field to improve microbial fuel cell performance. Ecotoxicology 2015, 24, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Sheng, G.-P.; Liu, X.-W.; Cai, P.-J.; Sun, M.; Xiao, X.; Wang, Y.-K.; Tong, Z.-H.; Dong, F.; Yu, H.-Q. Impact of a static magnetic field on the electricity production of Shewanella-inoculated microbial fuel cells. Biosens. Bioelectron. 2011, 26, 3987–3992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Li, X.F.; Ren, Y.P.; Wang, X.H. Effect of static magnetic field on the performances of and anode biofilms in microbial fuel cells. RSC Adv. 2016, 6, 82301–82308. [Google Scholar] [CrossRef]
- Al-Mayyahi, R.B.; Park, S.-G.; Jadhav, D.A.; Hussien, M.; Mohamed, H.O.; Castaño, P.; Al-Qaradawi, S.Y.; Chae, K.-J. Unraveling the influence of magnetic field on microbial and electrogenic activities in bioelectrochemical systems: A comprehensive review. Fuel 2023, 331, 125889. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, B.; Wang, Q.; Sun, J.; Xie, G.; Ren, N.; Ren, Z.J.; Xing, D. Pulse electromagnetic fields enhance extracellular electron transfer in magnetic bioelectrochemical systems. Biotechnol. Biofuels 2017, 10, 238. [Google Scholar] [CrossRef]
- Bhagat, M.S.; Mungray, A.K.; Mungray, A.A. Comparative investigation of solenoid magnetic field direction on the performance of osmotic microbial fuel cell. Mater. Today Chem. 2022, 24, 100778. [Google Scholar] [CrossRef]
- Hou, X.; Huang, L.; Zhou, P. Synergetic interaction of magnetic field and loaded magnetite for enhanced acetate production in biocathode of microbial electrosynthesis system. Int. J. Hydrogen Energy 2021, 46, 7183–7194. [Google Scholar] [CrossRef]
- Kong, Q.; Shi, Q.; Guo, W.; Qi, X.; Zhao, Z.; Qin, M. Synergistic effect of zero-valent iron and static magnetic field on wastewater purification and bioelectricity generation in electroactive constructed wetlands. Bioresour. Technol. 2023, 385, 129417. [Google Scholar] [CrossRef] [PubMed]
- Madondo, N.I.; Rathilal, S.; Bakare, B.F.; Tetteh, E.K. Application of Magnetite-nanoparticles and Static Magnetic Field on a Microbial Fuel Cell in Anaerobic Digestion. Chem.—Asian J. 2023, 18, e202300256. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Sugimoto, T. Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemite particles. J Colloid Interface Sci. 2003, 265, 283–295. [Google Scholar] [CrossRef]
- Deng, X.; Dohmae, N.; Kaksonen, A.H.; Okamoto, A. Biogenic Iron Sulfide Nanoparticles to Enable Extracellular Electron Uptake in Sulfate-Reducing Bacteria. Angew. Chem. Int. Ed. 2020, 59, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Ha, P.T.; Moon, H.; Kim, B.H.; Ng, H.Y.; Chang, I.S. Determination of charge transfer resistance and capacitance of microbial fuel cell through a transient response analysis of cell voltage. Biosens. Bioelectron. 2010, 25, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.S.; Pandit, S.; Gurung, A.; Ashun, E.; Ko, S.-Y.; Oh, S.-E. Granular activated carbon assisted biocathode for effective electrotrophic denitrification in microbial fuel cells. Chemosphere 2024, 352, 141341. [Google Scholar] [CrossRef]
- Wu, X.; Zou, L.; Huang, Y.; Qiao, Y.; Long, Z.-E.; Liu, H.; Li, C.M. Shewanella putrefaciens CN32 outer membrane cytochromes MtrC and UndA reduce electron shuttles to produce electricity in microbial fuel cells. Enzym. Microb. Technol. 2018, 115, 23–28. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Shi, Z.; Wang, Z.; Wu, X. Electromagnetic Field Drives the Bioelectrocatalysis of γ-Fe2O3-Coated Shewanella putrefaciens CN32 to Boost Extracellular Electron Transfer. Materials 2024, 17, 1501. https://doi.org/10.3390/ma17071501
Wang X, Shi Z, Wang Z, Wu X. Electromagnetic Field Drives the Bioelectrocatalysis of γ-Fe2O3-Coated Shewanella putrefaciens CN32 to Boost Extracellular Electron Transfer. Materials. 2024; 17(7):1501. https://doi.org/10.3390/ma17071501
Chicago/Turabian StyleWang, Xiaohai, Zhuanzhuan Shi, Zhikai Wang, and Xiaoshuai Wu. 2024. "Electromagnetic Field Drives the Bioelectrocatalysis of γ-Fe2O3-Coated Shewanella putrefaciens CN32 to Boost Extracellular Electron Transfer" Materials 17, no. 7: 1501. https://doi.org/10.3390/ma17071501
APA StyleWang, X., Shi, Z., Wang, Z., & Wu, X. (2024). Electromagnetic Field Drives the Bioelectrocatalysis of γ-Fe2O3-Coated Shewanella putrefaciens CN32 to Boost Extracellular Electron Transfer. Materials, 17(7), 1501. https://doi.org/10.3390/ma17071501




