Rapid Synthesis of Noble Metal Colloids by Plasma–Liquid Interactions
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Noble Metal Colloids
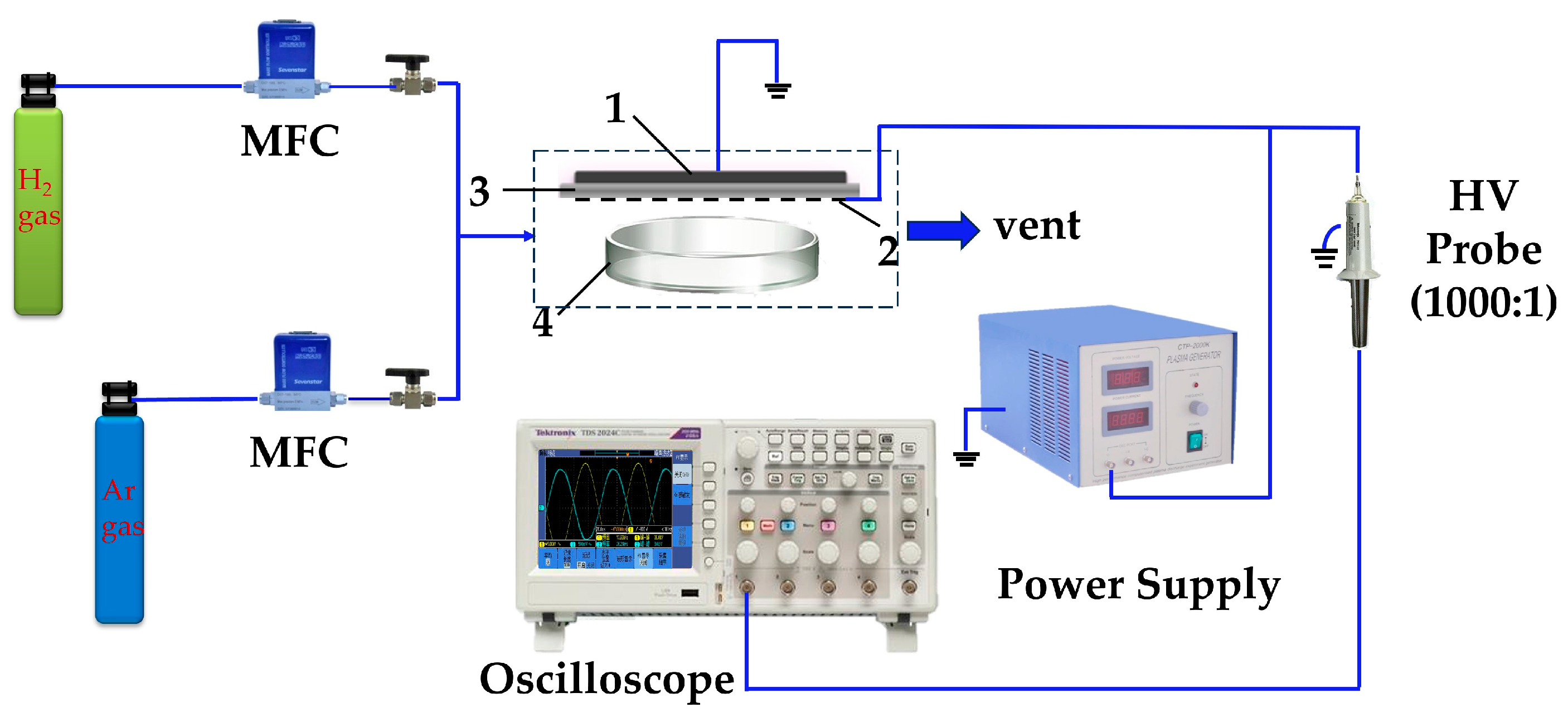
2.2.1. Surface Dielectric Barrier Discharge (DBD) Reactor
2.2.2. Direct Preparation of Au Colloid-P, Pt Colloid-P, and Pd Colloid-P with Plasma
2.2.3. Preparation of Au Colloid-PA and Pd Colloid-PA with Plasma-Activated Solution
2.3. Characterisation
3. Results and Discussion
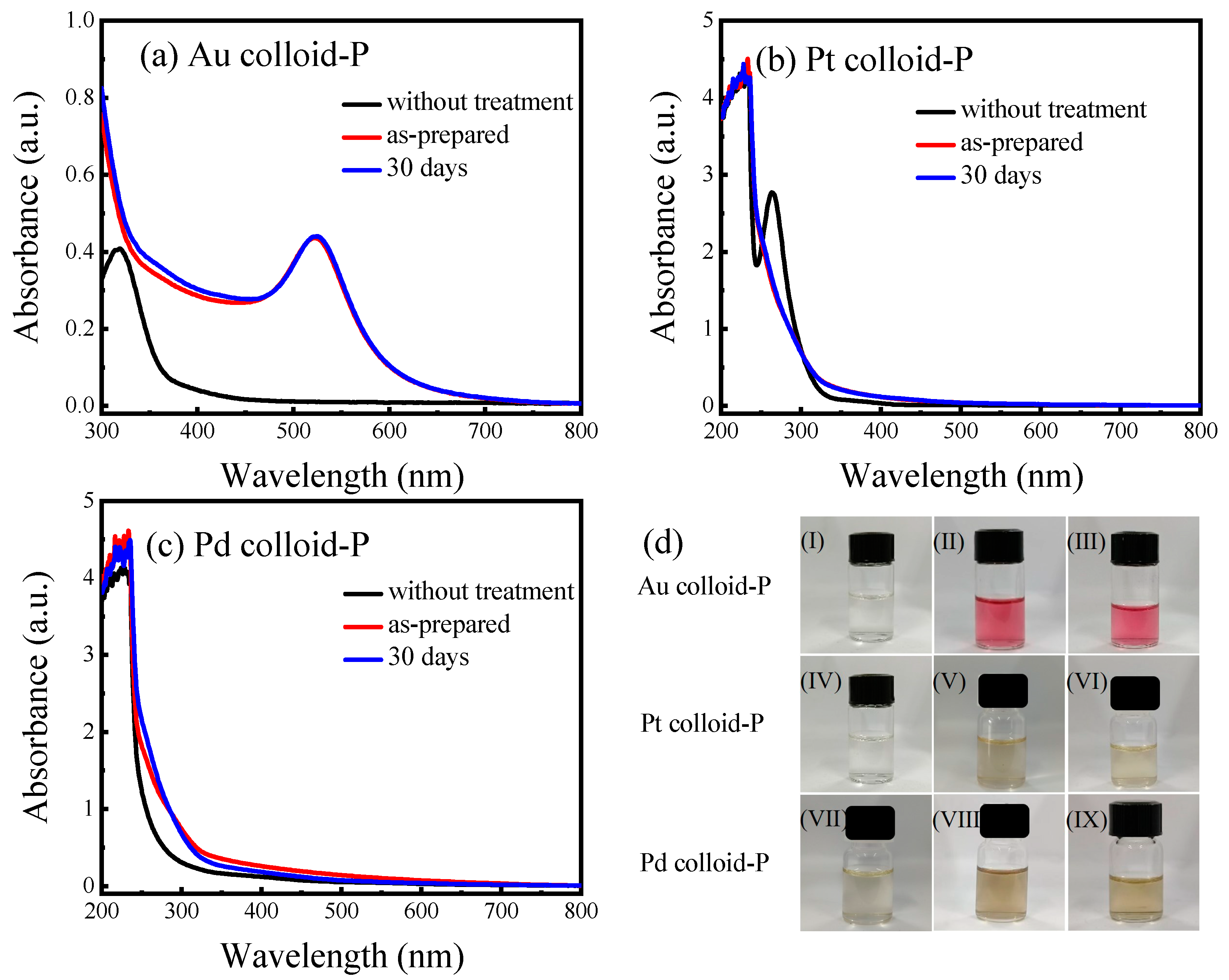
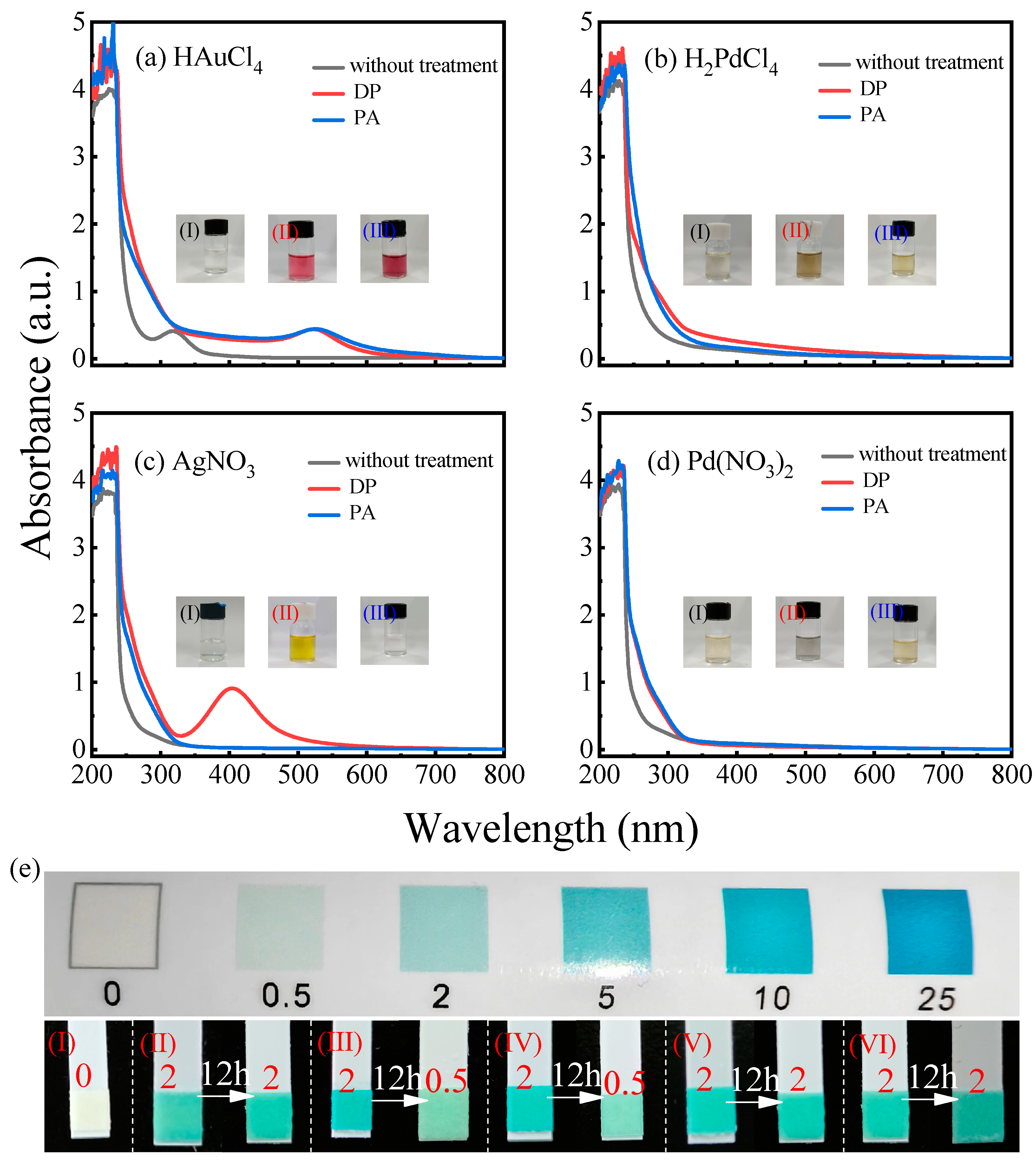
3.1. Light-Absorbing Characteristics and Stability of Metal Colloids Prepared with Plasma
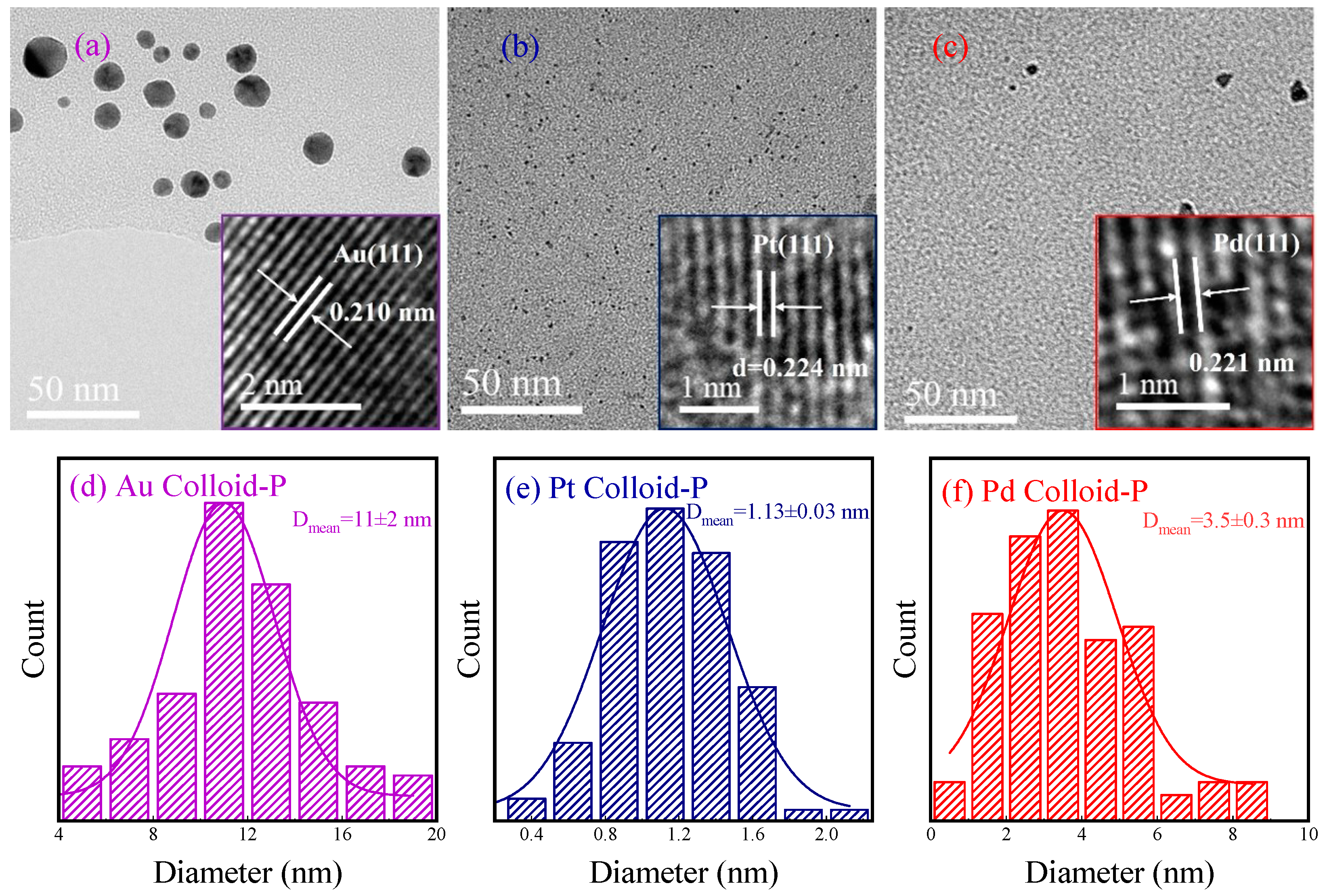
3.2. Morphology and Particle Size of Metal Colloids Prepared with Plasma
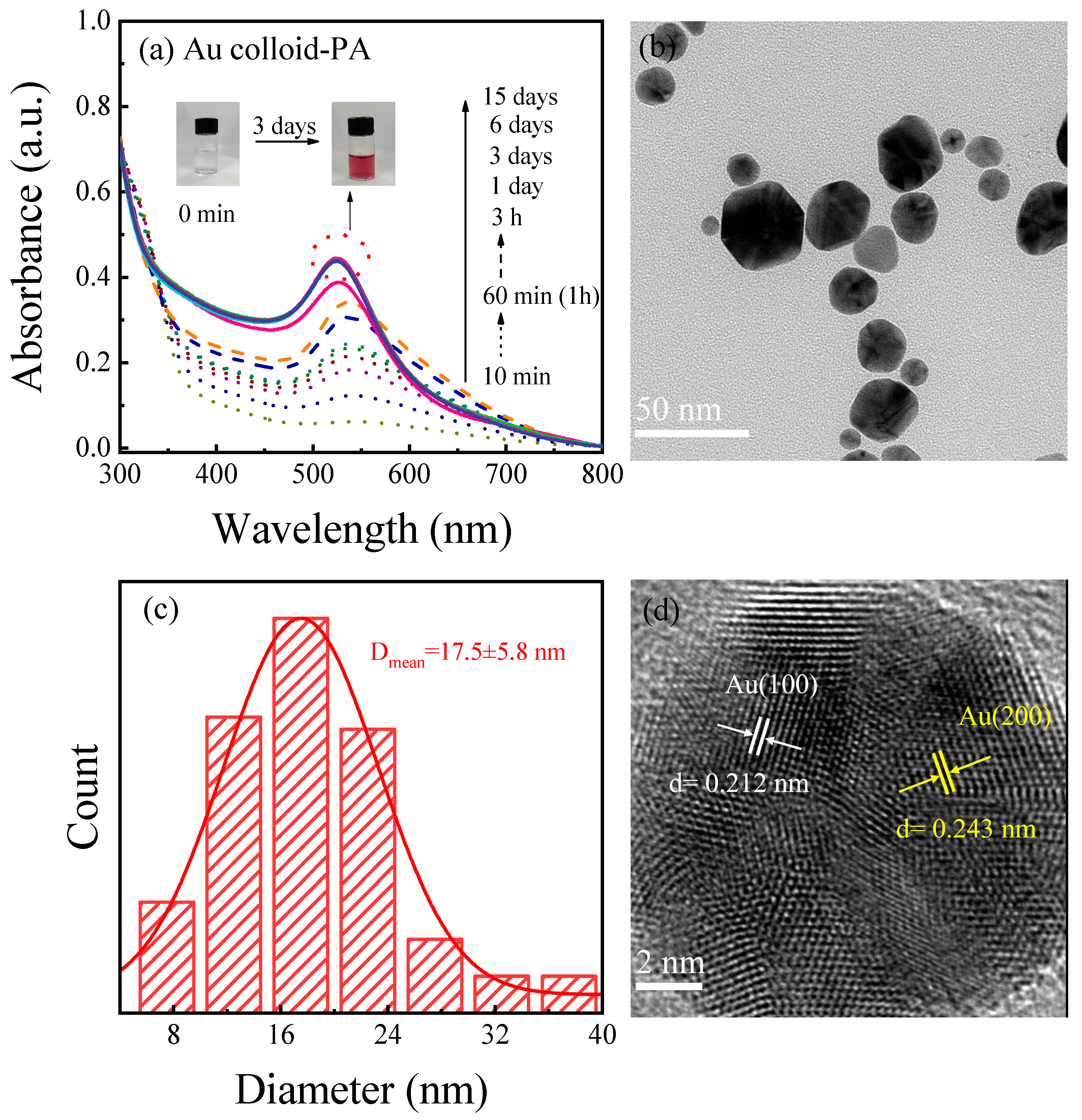
3.3. Light Absorption Properties and Morphology of Au Colloid-PA Prepared with Plasma-Activated Solution
3.4. Mechanism of Plasma Preparation of Metal Colloids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, Z.; Al-Thabaiti, S.A.; Rafiquee, M.Z.A. Cu-based tri-metallic nanoparticles with noble metals (Ag, Pd, and Ir) and their catalytic activities for hydrogen generation. Int. J. Hydrogen Energy 2021, 46, 39754–39767. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, T.Y.; Bootharaju, M.S.; Kim, J.H.; Chung, D.Y.; Hyeon, T. Noble Metal-Based Multimetallic Nanoparticles for Electrocatalytic Applications. Adv. Sci. 2021, 9, 2104054. [Google Scholar] [CrossRef]
- Quinson, J. Colloidal surfactant-free syntheses of precious metal nanoparticles for electrocatalysis. Curr. Opin. Electrochem. 2022, 34, 100977. [Google Scholar] [CrossRef]
- Longato, A.; Vanzan, M.; Colusso, E.; Corni, S.; Martucci, A. Enhancing Tungsten Oxide Gasochromism with Noble Metal Nanoparticles: The Importance of the Interface. Small 2022, 19, 2205522. [Google Scholar] [CrossRef]
- Konwar, D.; Basumatary, P.; Lee, U.; Yoon, Y.S. P-doped SnFe nanocubes decorated with PdFe alloy nanoparticles for ethanol fuel cells. J. Mater. Chem. A 2021, 9, 10685–10694. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm. 2015, 496, 159–172. [Google Scholar] [CrossRef]
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Quinson, J.; Bucher, J.; Simonsen, S.B.; Kuhn, L.T.; Kunz, S.; Arenz, M. Monovalent Alkali Cations: Simple and Eco-Friendly Stabilizers for Surfactant-Free Precious Metal Nanoparticle Colloids. ACS Sustain. Chem. Eng. 2019, 7, 13680–13686. [Google Scholar] [CrossRef]
- Sivaraman, S.K.; Kumar, S.; Santhanam, V. Monodisperse sub-10nm gold nanoparticles by reversing the order of addition in Turkevich method—The role of chloroauric acid. J. Colloid Interface Sci. 2011, 361, 543–547. [Google Scholar] [CrossRef]
- Iqbal, M.; Usanase, G.; Oulmi, K.; Aberkane, F.; Bendaikha, T.; Fessi, H.; Zine, N.; Agusti, G.; Errachid, E.-S.; Elaissari, A. Preparation of gold nanoparticles and determination of their particles size via different methods. Mater. Res. Bull. 2016, 79, 97–104. [Google Scholar] [CrossRef]
- Britto Hurtado, R.; Cortez-Valadez, M.; Aragon-Guajardo, J.R.; Cruz-Rivera, J.J.; Martínez-Suárez, F.; Flores-Acosta, M. One-step synthesis of reduced graphene oxide/gold nanoparticles under ambient conditions. Arab. J. Chem. 2020, 13, 1633–1640. [Google Scholar] [CrossRef]
- Hossain, M.M.; Robinson Junior, N.A.; Mok, Y.S.; Wu, S. Investigation of silver nanoparticle synthesis with various nonthermal plasma reactor configurations. Arab. J. Chem. 2023, 16, 105174. [Google Scholar] [CrossRef]
- Seitkalieva, M.M.; Samoylenko, D.E.; Lotsman, K.A.; Rodygin, K.S.; Ananikov, V.P. Metal nanoparticles in ionic liquids: Synthesis and catalytic applications. Coord. Chem. Rev. 2021, 445, 213982. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Das, D.; Borges e Soares, G.A.; Chakrabarti, P.; Ai, Z.; Chopra, H.; Hasan, M.A.; Cavalu, S. Novel Green Approaches for the Preparation of Gold Nanoparticles and Their Promising Potential in Oncology. Processes 2022, 10, 426. [Google Scholar] [CrossRef]
- Zhang, T.; Ouyang, B.; Zhang, X.; Xia, G.; Wang, N.; Ou, H.; Ma, L.; Mao, P.; Ostrikov, K.; Di, L.; et al. Plasma-enabled synthesis of Pd/GO rich in oxygen-containing groups and defects for highly efficient 4-nitrophenol reduction. Appl. Surf. Sci. 2022, 597, 153727. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, J.; Zhang, T.; Zhu, A.; Xia, G.; Zhang, X.; Di, L. Plasma synthesis of graphite oxide supported PdNi catalysts with enhanced catalytic activity and stability for 4-nitrophenol reduction. Catal. Today 2023, 418, 114069. [Google Scholar] [CrossRef]
- Hua, Y.; Zhao, L.; Zhao, Q.; Xia, G.; Zhang, X.; Di, L. Cold Plasma for Preparation of Pd/graphene Catalysts toward 4-nitrophenol Reduction: Insight into Plasma Treatment. Mod. Low Temp. Plasma 2023, 1, 7. [Google Scholar] [CrossRef]
- Zhang, J.; Hua, Y.; Li, H.; Zhang, X.; Shi, C.; Li, Y.; Di, L.; Wang, Z. Phase reconstruction of Co3O4 with enriched oxygen vacancies induced by cold plasma for boosting methanol-to-formate electro-oxidation. Chem. Eng. J. 2023, 478, 147288. [Google Scholar] [CrossRef]
- Di, L.; Fu, Z.; Dong, M.; Zhu, A.; Xia, G.; Zhang, X. Cold plasma-prepared Ru-based catalysts for boosting plasma-catalytic CO2 methanation. Chem. Eng. Sci. 2023, 280, 119056. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Motyka-Pomagruk, A.; Cyganowski, P.; Babinska, W.; Terefinko, D.; Jamroz, P.; Lojkowska, E.; Pohl, P.; Sledz, W. Antibacterial Activity of Fructose-Stabilized Silver Nanoparticles Produced by Direct Current Atmospheric Pressure Glow Discharge towards Quarantine Pests. Nanomaterials 2018, 8, 751. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, X.; Rider, A.E.; Furman, S.A.; Ostrikov, K. Fast, energy-efficient synthesis of luminescent carbon quantum dots. Green Chem. 2014, 16, 2566–2570. [Google Scholar] [CrossRef]
- Burakov, V.; Kiris, V.; Nedelko, M.; Tarasenka, N.; Nevar, A.; Tarasenko, N. Plasmas in and in contact with liquid for synthesis and surface engineering of carbon and silicon nanoparticles. J. Phys. D Appl. Phys. 2018, 51, 484001. [Google Scholar] [CrossRef]
- Dvořák, P.; Talába, M.; Obrusník, A.; Kratzer, J.; Dědina, J. Concentration of atomic hydrogen in a dielectric barrier discharge measured by two-photon absorption fluorescence. Plasma Sources Sci. Technol. 2017, 26, 085002. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Fatoba, O.O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V.; et al. A critical review on ozone and co-species, generation and reaction mechanisms in plasma induced by dielectric barrier discharge technologies for wastewater remediation. J. Environ. Chem. Eng. 2021, 9, 105758. [Google Scholar] [CrossRef]
- Vanraes, P.; Bogaerts, A. The essential role of the plasma sheath in plasma–liquid interaction and its applications—A perspective. J. Appl. Phys. 2021, 129, 220901. [Google Scholar] [CrossRef]
- Ramos, S.V.; Cisquini, P.; Nascimento, R.C., Jr.; Franco, A.R., Jr.; Vieira, E.A. Morphological changes and kinetic assessment of Cu2O powder reduction by non-thermal hydrogen plasma. J. Mater. Res. Technol. 2021, 11, 328–341. [Google Scholar] [CrossRef]
- Morales-Lara, F.; Abdelkader-Fernández, V.K.; Melguizo, M.; Turco, A.; Mazzotta, E.; Domingo-García, M.; López-Garzón, F.J.; Pérez-Mendoza, M. Ultra-small metal nanoparticles supported on carbon nanotubes through surface chelation and hydrogen plasma reduction for methanol electro-oxidation. J. Mater. Chem. A 2019, 7, 24502–24514. [Google Scholar] [CrossRef]
- Sabat, K.C. Production of Nickel by Cold Hydrogen Plasma: Role of Active Oxygen. Plasma Chem. Plasma Process. 2022, 42, 833–853. [Google Scholar] [CrossRef]
- Hühn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Correction to Selected Standard Protocols for the Synthesis, Phase Transfer, and Characterization of Inorganic Colloidal Nanoparticles. Chem. Mater. 2021, 33, 4830. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, Ł.; Młotek, M.; Krawczyk, K. Hydrogen production from ethanol using dielectric barrier discharge. Energy 2019, 174, 261–268. [Google Scholar] [CrossRef]
- Levko, D.; Shchedrin, A.; Chernyak, V.; Olszewski, S.; Nedybaliuk, O. Plasma kinetics in ethanol/water/air mixture in a ‘tornado’-type electrical discharge. J. Phys. D Appl. Phys. 2011, 44, 145206. [Google Scholar] [CrossRef]
- De Vos, C.; Baneton, J.; Witzke, M.; Dille, J.; Godet, S.; Gordon, M.J.; Sankaran, R.M.; Reniers, F. A comparative study of the reduction of silver and gold salts in water by a cathodic microplasma electrode. J. Phys. D Appl. Phys. 2017, 50, 105206. [Google Scholar] [CrossRef]
- Adamovich, I.; Agarwal, S.; Ahedo, E.; Alves, L.L.; Baalrud, S.; Babaeva, N.; Bogaerts, A.; Bourdon, A.; Bruggeman, P.J.; Canal, C.; et al. The 2022 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2022, 55, 373001. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Gorbanev, Y.; Dewilde, S.; Smits, E.; Bogaerts, A. Reduction of Human Glioblastoma Spheroids Using Cold Atmospheric Plasma: The Combined Effect of Short- and Long-Lived Reactive Species. Cancers 2018, 10, 394. [Google Scholar] [CrossRef]
- Xu, C.; Chaudhuri, S.; Held, J.; Andaraarachchi, H.P.; Schatz, G.C.; Kortshagen, U.R. Silver Nanoparticle Synthesis in Glycerol by Low-Pressure Plasma-Driven Electrolysis: The Roles of Free Electrons and Photons. J. Phys. Chem. Lett. 2023, 14, 9960–9968. [Google Scholar] [CrossRef]
- He, X.; Lin, J.; He, B.; Xu, L.; Li, J.; Chen, Q.; Yue, G.; Xiong, Q.; Liu, Q.H. The formation pathways of aqueous hydrogen peroxide in a plasma-liquid system with liquid as the cathode. Plasma Sources Sci. Technol. 2018, 27, 085010. [Google Scholar] [CrossRef]
- Bjelajac, A.; Phillipe, A.-M.; Guillot, J.; Fleming, Y.; Chemin, J.-B.; Choquet, P.; Bulou, S. Gold nanoparticles synthesis and immobilization by atmospheric pressure DBD plasma torch method. Nanoscale Adv. 2023, 5, 2573–2582. [Google Scholar] [CrossRef]
- Sauvageau, J.F.; Turgeon, S.; Chevallier, P.; Fortin, M.A. Colloidal Suspensions of Platinum Group Metal Nanoparticles (Pt, Pd, Rh) Synthesized by Dielectric Barrier Discharge Plasma (DBD). Part. Part. Syst. Charact. 2018, 35, 1700365. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, D.; Cai, Y.; Ji, X.; Xie, R.; Yang, W. Tuning the size of gold nanoparticles in the citrate reduction by chloride ions. Nanoscale 2012, 4, 5071–5076. [Google Scholar] [CrossRef]
- Darwish, M.; Mafla-Gonzalez, C.; Kolenovic, B.; Deremer, A.; Centeno, D.; Liu, T.; Kim, D.-Y.; Cattabiani, T.; Drwiega, T.J.; Kumar, I.; et al. Rapid synthesis of metal nanoparticles using low-temperature, low-pressure argon plasma chemistry and self-assembly. Green Chem. 2022, 24, 8142–8154. [Google Scholar] [CrossRef]
- Quinson, J.; Neumann, S.; Wannmacher, T.; Kacenauskaite, L.; Inaba, M.; Bucher, J.; Bizzotto, F.; Simonsen, S.B.; Theil Kuhn, L.; Bujak, D.; et al. Colloids for Catalysts: A Concept for the Preparation of Superior Catalysts of Industrial Relevance. Angew. Chem. Int. Ed. 2018, 57, 12338–12341. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Q.; Liu, Q.; Ostrikov, K. Visualization of gold nanoparticles formation in DC plasma-liquid systems. Plasma Sci. Technol. 2021, 23, 075504. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Chen, Q.; Ostrikov, K. Recent advances towards aqueous hydrogen peroxide formation in a direct current plasma–liquid system. High Volt. 2022, 7, 405–419. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Xu, L.; Wang, X.; Chen, Q.; Ostrikov, K. The Ag+ Reduction Process in a Plasma Electrochemical System Tuned by the pH Value. J. Electrochem. Soc. 2021, 168, 123508. [Google Scholar] [CrossRef]
- Gong, X.; Ma, Y.; Lin, J.; He, X.; Long, Z.; Chen, Q.; Liu, H. Tuning the Formation Process of Silver Nanoparticles in a Plasma Electrochemical System by Additives. J. Electrochem. Soc. 2018, 165, E540–E545. [Google Scholar] [CrossRef]
- Lu, P.; Kim, D.-W.; Park, D.-W. Simple reactor for the synthesis of silver nanoparticles with the assistance of ethanol by gas–liquid discharge plasma. Plasma Sci. Technol. 2019, 21, 044005. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Li, H.; Hua, Y.; Zhang, X.; Di, L. Rapid Synthesis of Noble Metal Colloids by Plasma–Liquid Interactions. Materials 2024, 17, 987. https://doi.org/10.3390/ma17050987
Pang Y, Li H, Hua Y, Zhang X, Di L. Rapid Synthesis of Noble Metal Colloids by Plasma–Liquid Interactions. Materials. 2024; 17(5):987. https://doi.org/10.3390/ma17050987
Chicago/Turabian StylePang, Yuanwen, Hong Li, Yue Hua, Xiuling Zhang, and Lanbo Di. 2024. "Rapid Synthesis of Noble Metal Colloids by Plasma–Liquid Interactions" Materials 17, no. 5: 987. https://doi.org/10.3390/ma17050987
APA StylePang, Y., Li, H., Hua, Y., Zhang, X., & Di, L. (2024). Rapid Synthesis of Noble Metal Colloids by Plasma–Liquid Interactions. Materials, 17(5), 987. https://doi.org/10.3390/ma17050987






