Influence of Calcareous Deposits on Hydrogen Embrittlement Susceptibility of Q460 Steel
Abstract
1. Introduction
2. Experimental
2.1. Material and Solution
2.2. Calcareous Deposition Experiments
2.3. SSRT
3. Results and Discussion
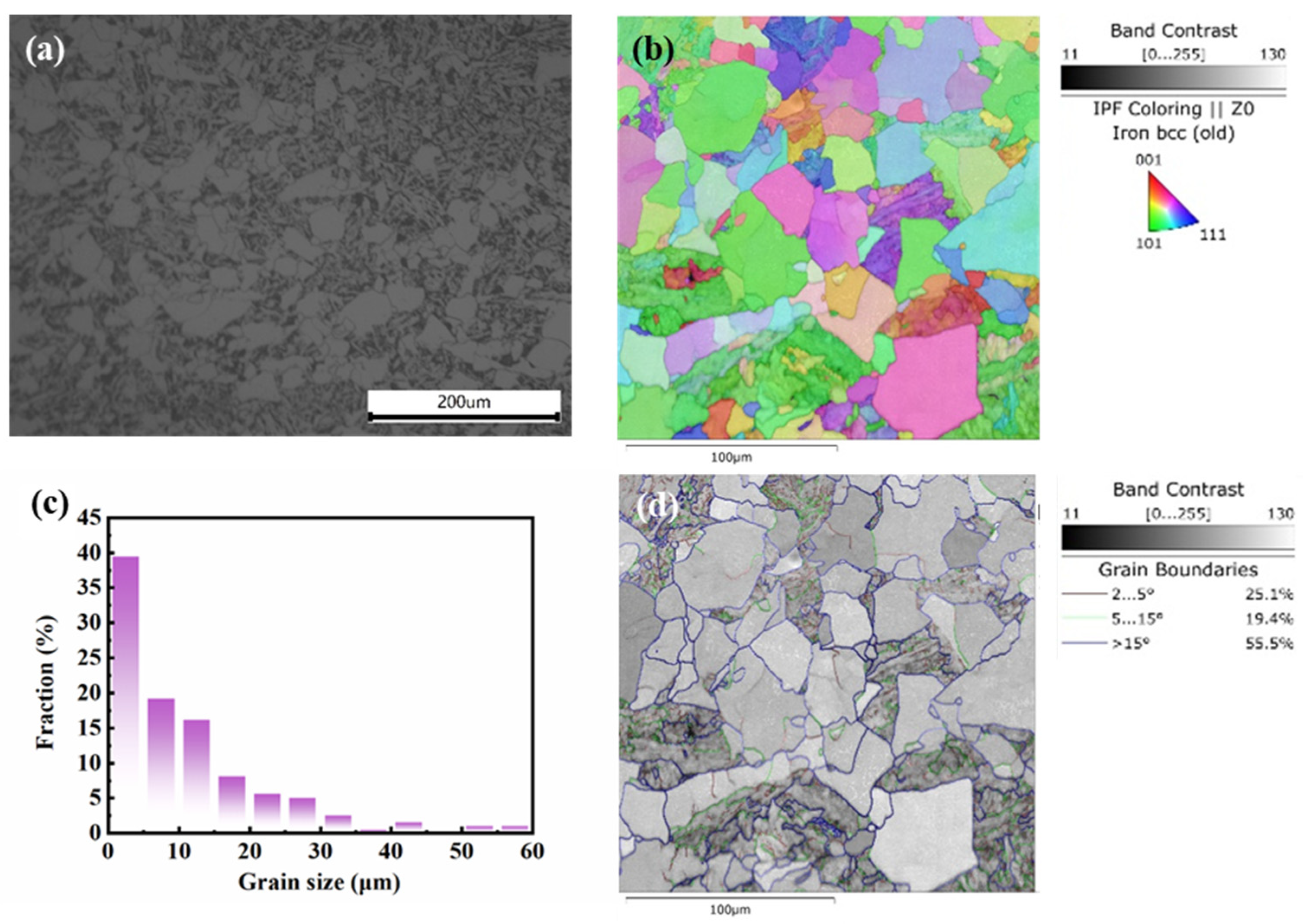
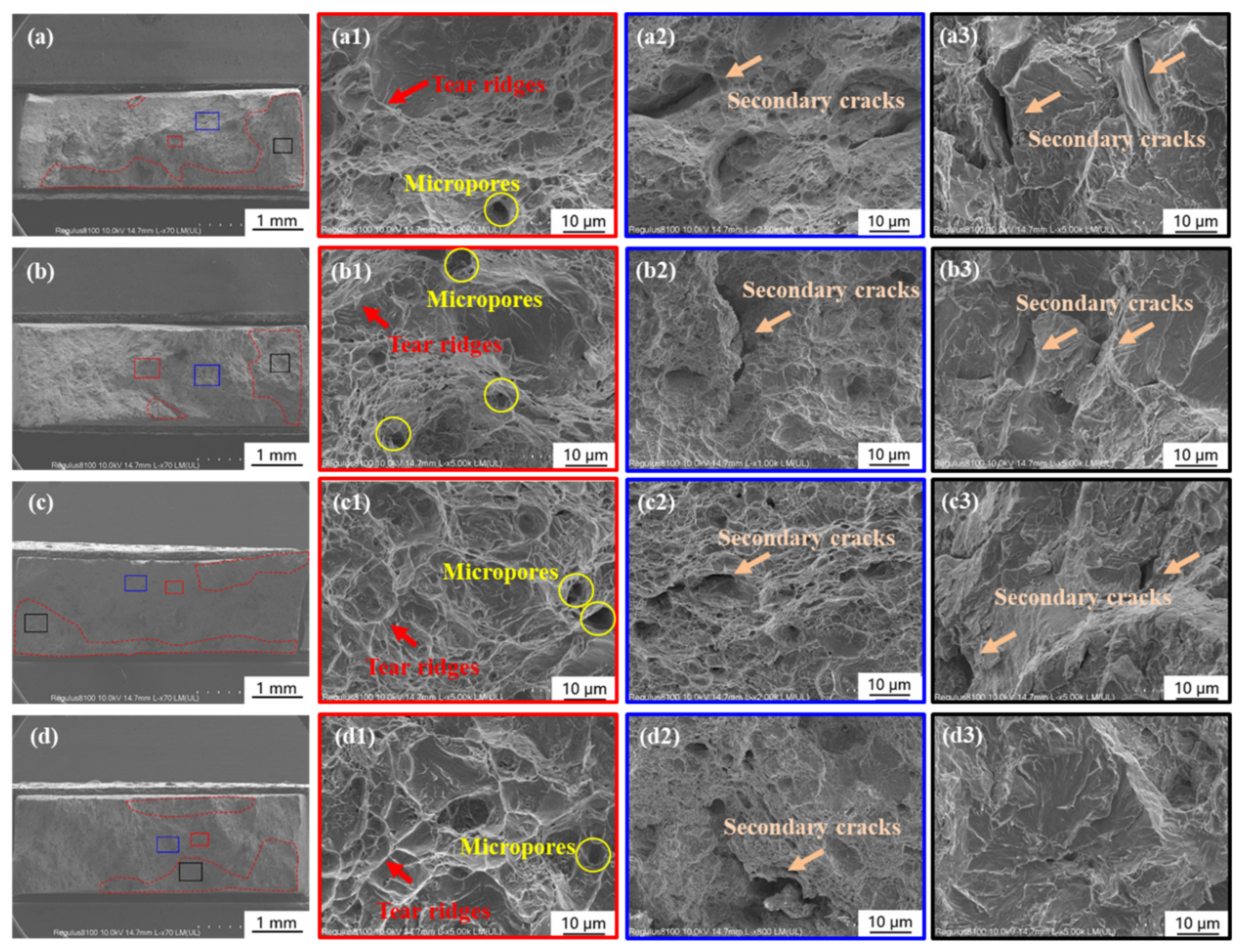
3.1. Microstructure Characterization
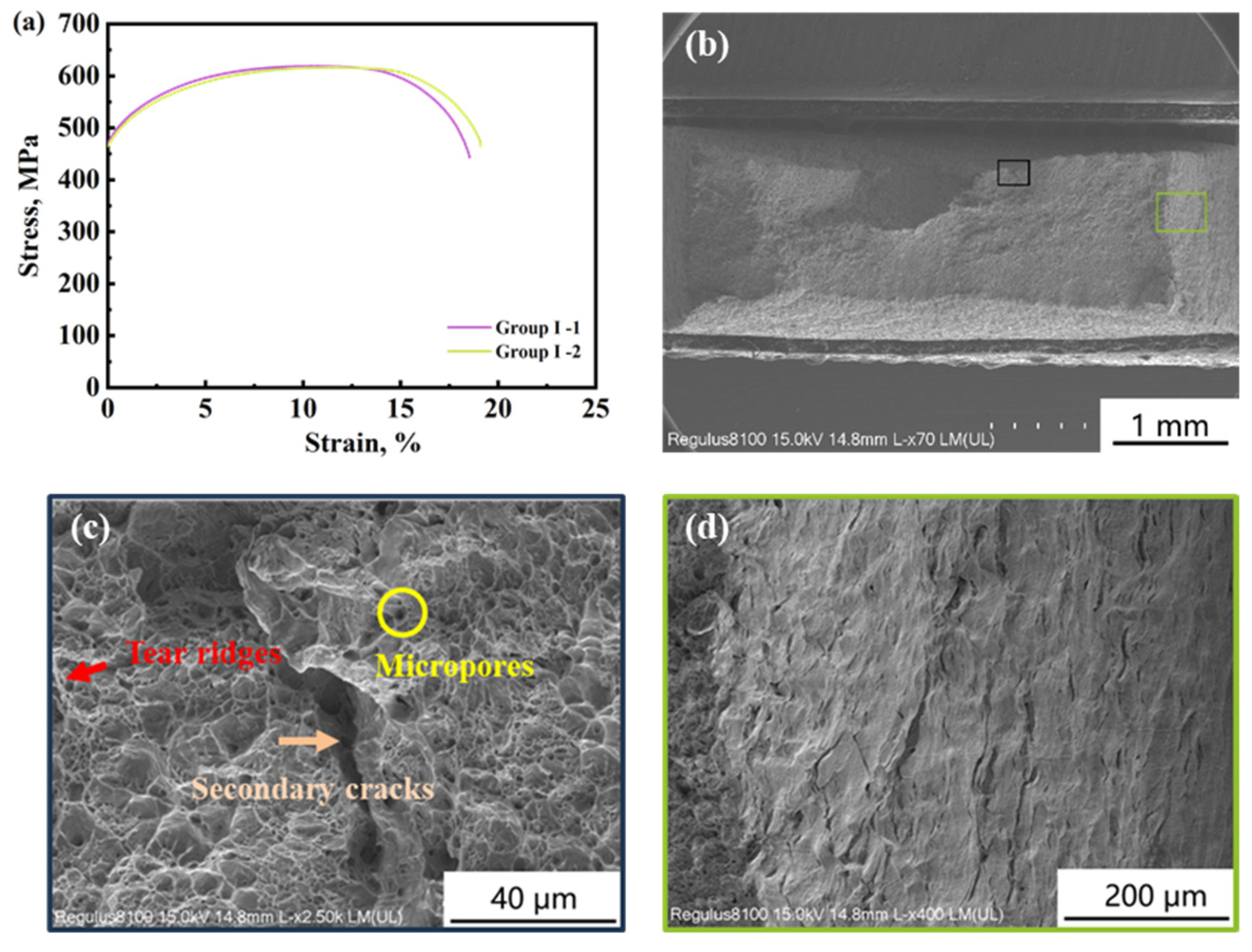
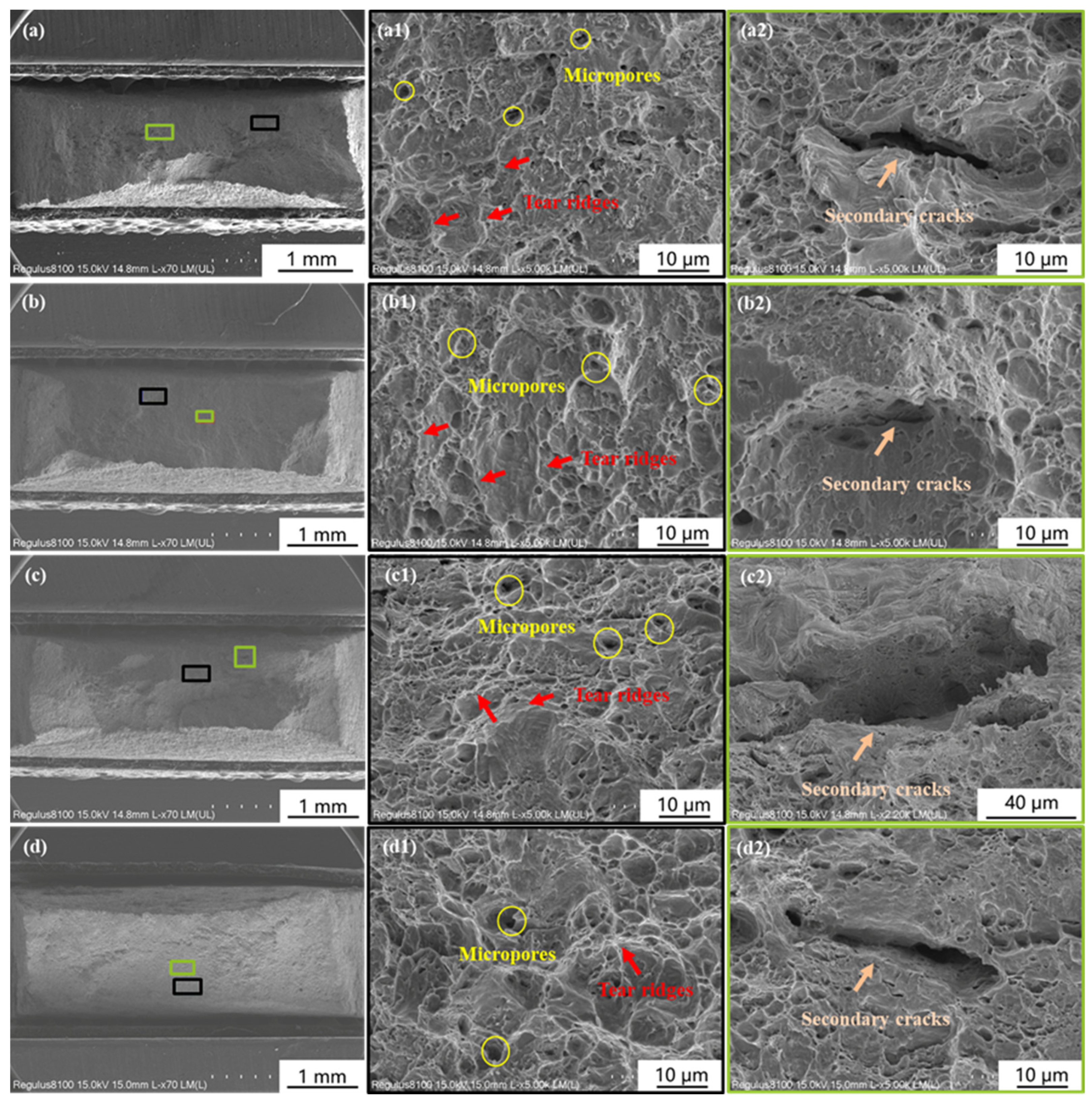
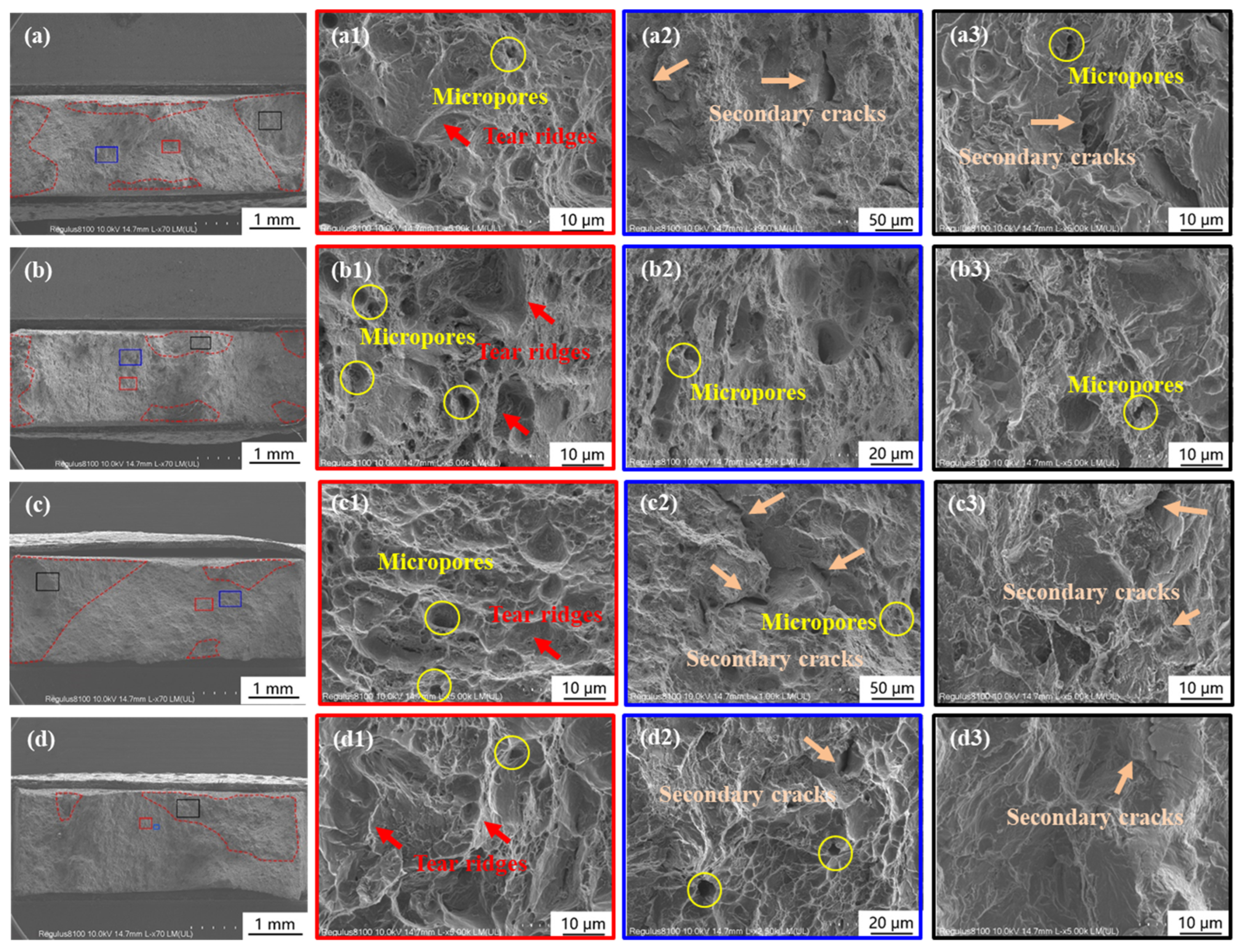
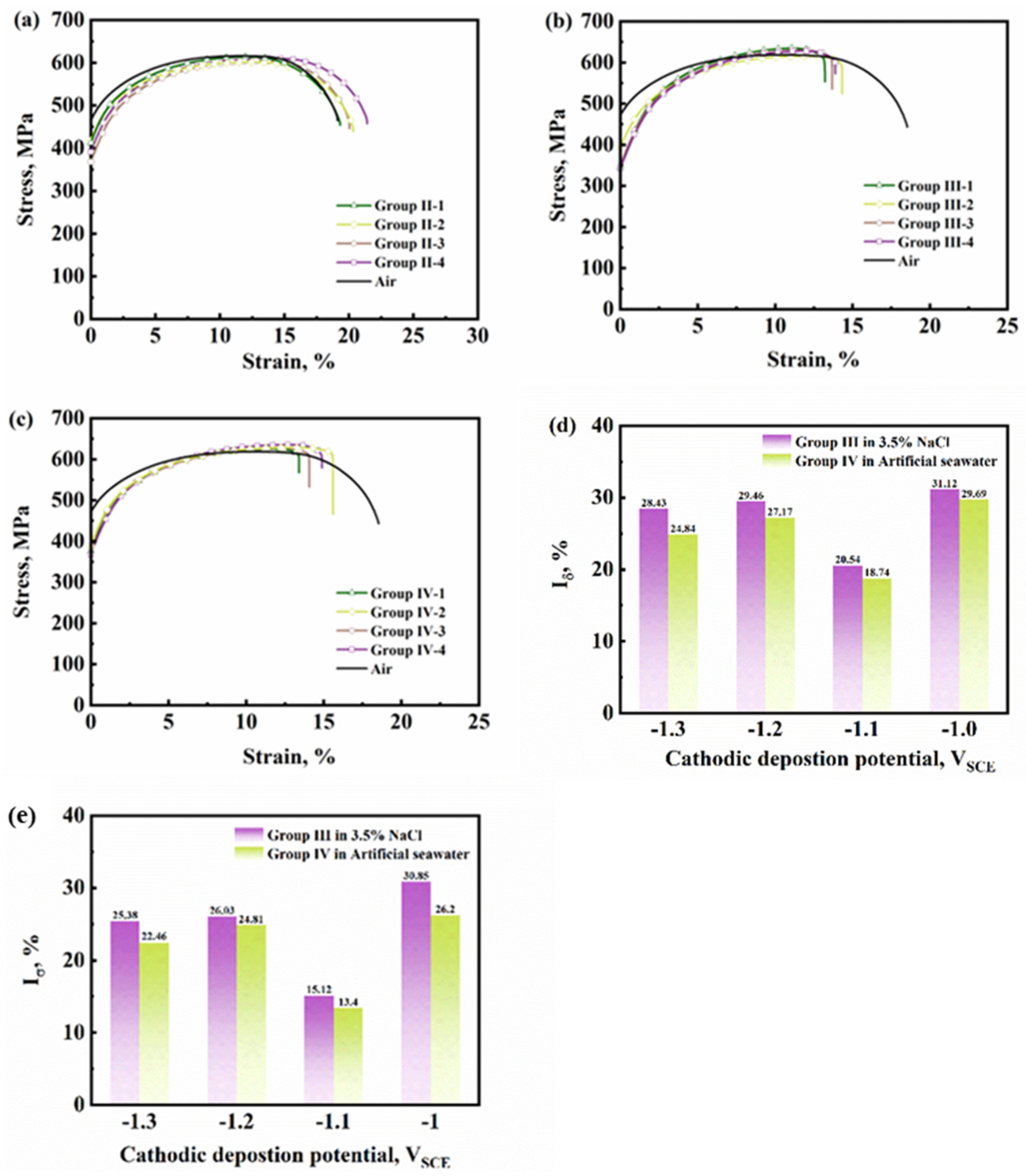
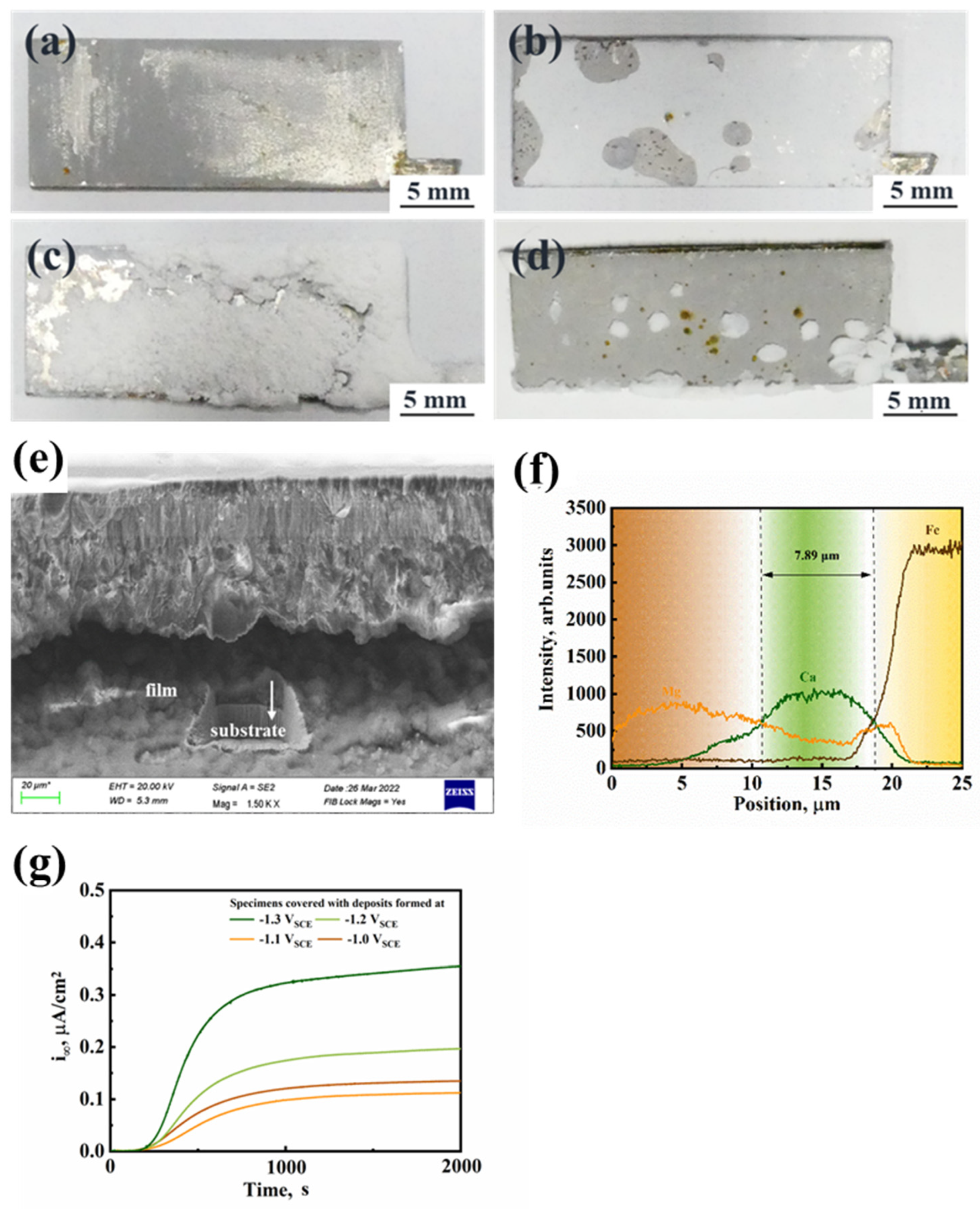
3.2. Influence of Calcareous Deposits on HE Behavior
4. Conclusions
- When performing SSRT in 3.5% NaCl solution and artificial seawater with hydrogen charging, Q460 steel had the smallest HE susceptibility when covered with the calcareous deposits formed under −1.1 VSCE. This kind of deposit had a thin calcium-rich inner layer and a condensed magnesium-rich outer layer, which can inhibit hydrogen entry more significantly than the deposits formed under the other three cathodic potentials.
- A sustained deposition reaction during SSRT in artificial seawater can decrease the HE susceptibility of Q460 steel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woollin, P. Hydrogen embrittlements stress corrosion cracking of superduplex stainless steel. In Proceedings of the CORROSION 2001, Houston, TX, USA, 13 March 2001; p. 01018. [Google Scholar]
- Simoni, L.; Caselani, J.Q.; Ramos, L.B.; Schroeder, R.M.; Malfatti, C.d.F. The influence of calcareous deposits on hydrogen uptake and embrittlement of API 5CT P110 steel. Corros. Sci. 2017, 118, 178–189. [Google Scholar] [CrossRef]
- Thodla, R.; Chandra, A.; Li, X.; Taylor, C.D.; Ke, H.; Sridhar, N. Hydrogen embrittlement of 718 under cathodic polarization. Corros. Sci. 2020, 165, 108361. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Li, T.; Zhao, Y.; Deng, Q.; Wang, Y.; Jiang, W. Comparison of hydrogen embrittlement susceptibility of three cathodic protected subsea pipeline steels from a point of view of hydrogen permeation. Corros. Sci. 2018, 131, 104–115. [Google Scholar] [CrossRef]
- Cho, S.; Kim, G.-I.; Ko, S.-J.; Yoo, J.-S.; Jung, Y.-S.; Yoo, Y.-H.; Kim, J.-G. Comparison of Hydrogen Embrittlement Susceptibility of Different Types of Advanced High-Strength Steels. Materials 2022, 15, 3406. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.; Morizot, A.P. Calcareous scales formed by cathodic protection—An assessment of characteristics and kinetics. J. Cryst. Growth 2002, 243, 490–502. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Yang, D.; Kunte, H.J.; De Marco, R.; Wang, X. Investigation of the calcareous deposits formation controlled by interfacial pH and its effect on the hydrogen entry into AISI 4135 steel in seawater. Int. J. Hydrogen Energy 2021, 46, 5824–5841. [Google Scholar] [CrossRef]
- Smith, W.R.; Paul, S. Natural Deposit Coatings on Steel during Cathodic Protection and Hydrogen Ingress. Coatings 2015, 5, 816–829. [Google Scholar] [CrossRef]
- Xiong, X.L.; Yang, H.C.; Zhang, N.; Niu, T. The combined effect of cathodic potential and calcareous deposits on hydrogen evolution and permeation of Q460 steel. Int. J. Hydrogen Energy 2024. Under review. [Google Scholar]
- Gao, Z.; Liu, X.; Wen, L.; Kang, W. Effect of calcareous sediments on hydrogen evolution potential of 16Mn steel in seawater. Int. J. Electrochem. Sci. 2016, 11, 3007–3023. [Google Scholar] [CrossRef]
- Okstad, T.; Rannestad, Ø.; Johnsen, R.; Nisancioglu, K. Significance of Hydrogen Evolution during Cathodic Protection of Carbon Steel in Seawater. Corrosion 2007, 63, 857–865. [Google Scholar] [CrossRef]
- Tian, H.; Xin, J.; Li, Y.; Wang, X.; Cui, Z. Combined effect of cathodic potential and sulfur species on calcareous deposition, hydrogen permeation, and hydrogen embrittlement of a low carbon bainite steel in artificial seawater. Corros. Sci. 2019, 158, 108089. [Google Scholar] [CrossRef]
- Zucchi, F.; Grassi, V.; Monticelli, C.; Trabanelli, G. Hydrogen embrittlement of duplex stainless steel under cathodic protection in acidic artificial sea water in the presence of sulphide ions. Corros. Sci. 2006, 48, 522–530. [Google Scholar] [CrossRef]
- ASTM-D1141-98; Standard Practice for the Preparation of Substitute Ocean Water. Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2004.
- ASTM E8/E8M-22; Standard Test Methods for Tension Testing of Metallic Materials. American Society for Testing Materials: West Conshohocken, PA, USA, 2022.
- Zhao, W.; Zhang, T.; Zhao, Y.; Sun, J.; Wang, Y. Hydrogen permeation and embrittlement susceptibility of X80 welded joint under high-pressure coal gas environment. Corros. Sci. 2016, 111, 84–97. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Du, C.; Wang, H.; Li, C.; Li, X. Effect of cathodic potentials on the SCC behavior of E690 steel in simulated seawater. Mater. Sci. Eng. A 2015, 642, 22–31. [Google Scholar] [CrossRef]
- Delafosse, D.; Magnin, T. Hydrogen induced plasticity in stress corrosion cracking of engineering systems. Eng. Fract. Mech. 2001, 68, 693–729. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, X.Z.; Du, C.W.; Li, J.K.; Li, X.G. Effect of hydrogen-induced plasticity on the stress corrosion cracking of X70 pipeline steel in simulated soil environments. Mater. Sci. Eng. A 2016, 658, 348–354. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Tsuzaki, K.; Raabe, D. Hydrogen-assisted failure in a twinning-induced plasticity steel studied under in situ hydrogen charging by electron channeling contrast imaging. Acta Mater. 2013, 61, 4607–4618. [Google Scholar] [CrossRef]
- Miresmaeili, R.; Liu, L.; Kanayama, H. A possible explanation for the contradictory results of hydrogen effects on macroscopic deformation. Int. J. Press. Vessel. Pip. 2012, 99–100, 34–43. [Google Scholar] [CrossRef]
- Venezuela, J.; Zhou, Q.; Liu, Q.; Zhang, M.; Atrens, A. Hydrogen Trapping in Some Automotive Martensitic Advanced High-Strength Steels. Adv. Eng. Mater. 2018, 20, 1700468. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, Q.; Zhou, Q.; Venezuela, J.; Zhang, M. Evaluation of automobile service performance using laboratory testing. Mater. Sci. Technol. 2018, 34, 1893–1909. [Google Scholar] [CrossRef]
- Atrens, A.; Venezuela, J.; Liu, Q.; Zhou, Q.; Verbeken, K.; Tapia-Bastidas, C.; Gray, E.; Christien, F.; Wolski, K. Electrochemical and Mechanical Aspects of Hydrogen Embrittlement Evaluation of Martensitic Steels. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 201–225. [Google Scholar]
- Ou, K.C.; Wu, J.K. Effect of calcareous deposits formation on the hydrogen absorption of steel. Mater. Chem. Phys. 1997, 48, 52–55. [Google Scholar] [CrossRef]
- Xing, Y.; Sun, Y.; Wang, X.; Wang, Z.; Du, Y.; Lu, M.; Qiao, L.; Zhang, L. Effect of surface calcareous deposits on hydrogen uptake of X80 steel under strong cathodic current. Int. J. Hydrogen Energy 2021, 46, 4555–4566. [Google Scholar] [CrossRef]

| Composition | Concentration, mol/L |
|---|---|
| NaCl | 0.42 |
| MgCl2 | 5.46 × 10−2 |
| Na2SO4 | 2.80 × 10−2 |
| CaCl2 | 1.05 × 10−2 |
| KCl | 9.30 × 10−3 |
| NaHCO3 | 2.80 × 10−2 |
| Group | Cathodic Deposition | SSRT Environmental Condition |
|---|---|---|
| I | Not proceed | air |
| II | Proceed | air |
| III | Proceed | 3.5% NaCl, hydrogen charging with 0.5 mA cm−2 |
| IV | Proceed | Artificial seawater, hydrogen charging with 0.5 mA cm−2 |
| Group | Cathodic Deposition Potential, VSCE | YS, MPa | UTS, MPa | , % | , % |
|---|---|---|---|---|---|
| I | - | 470.52 | 617.97 | 22.66 | - |
| - | 464.73 | 615.33 | 21.94 | - | |
| II | −1.0 | 411.83 | 614.00 | 22.95 | - |
| −1.1 | 417.94 | 601.41 | 23.58 | - | |
| −1.2 | 367.93 | 604.36 | 23.25 | - | |
| −1.3 | 390.61 | 610.09 | 24.68 | - | |
| III | −1.0 | 345.05 | 634.58 | 15.36 | 31.12 |
| −1.1 | 393.58 | 615.17 | 17.72 | 20.54 | |
| −1.2 | 348.61 | 629.83 | 15.73 | 29.46 | |
| −1.3 | 342.28 | 628.17 | 15.96 | 28.43 | |
| IV | −1.0 | 375.95 | 625.17 | 15.68 | 29.69 |
| −1.1 | 388.62 | 628.67 | 18.12 | 18.74 | |
| −1.2 | 382.68 | 627.08 | 16.24 | 27.17 | |
| −1.3 | 366.84 | 635.67 | 16.76 | 24.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Yang, H.; Chen, T.; Zhang, N.; Niu, T. Influence of Calcareous Deposits on Hydrogen Embrittlement Susceptibility of Q460 Steel. Materials 2024, 17, 1110. https://doi.org/10.3390/ma17051110
Xiong X, Yang H, Chen T, Zhang N, Niu T. Influence of Calcareous Deposits on Hydrogen Embrittlement Susceptibility of Q460 Steel. Materials. 2024; 17(5):1110. https://doi.org/10.3390/ma17051110
Chicago/Turabian StyleXiong, Xilin, Haichun Yang, Tongqian Chen, Na Zhang, and Tong Niu. 2024. "Influence of Calcareous Deposits on Hydrogen Embrittlement Susceptibility of Q460 Steel" Materials 17, no. 5: 1110. https://doi.org/10.3390/ma17051110
APA StyleXiong, X., Yang, H., Chen, T., Zhang, N., & Niu, T. (2024). Influence of Calcareous Deposits on Hydrogen Embrittlement Susceptibility of Q460 Steel. Materials, 17(5), 1110. https://doi.org/10.3390/ma17051110






