Preparation of Aminated Sodium Lignosulfonate and Efficient Adsorption of Methyl Blue Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
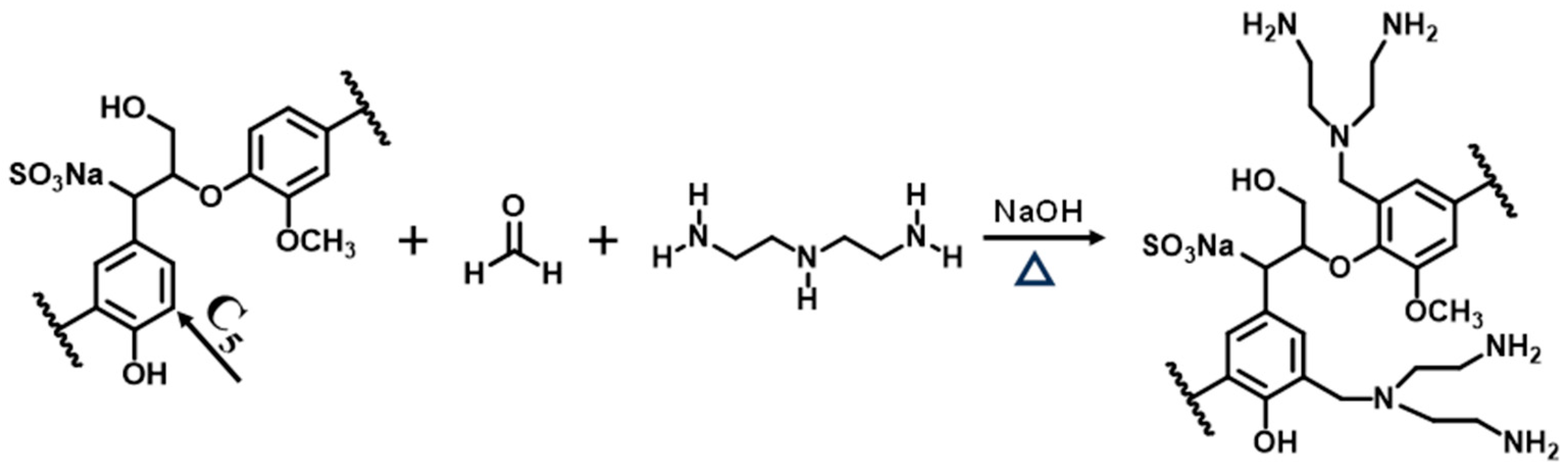
2.2. Synthesis of AELS
2.3. Characterization
2.4. Adsorption Experiment
3. Results and Discussion
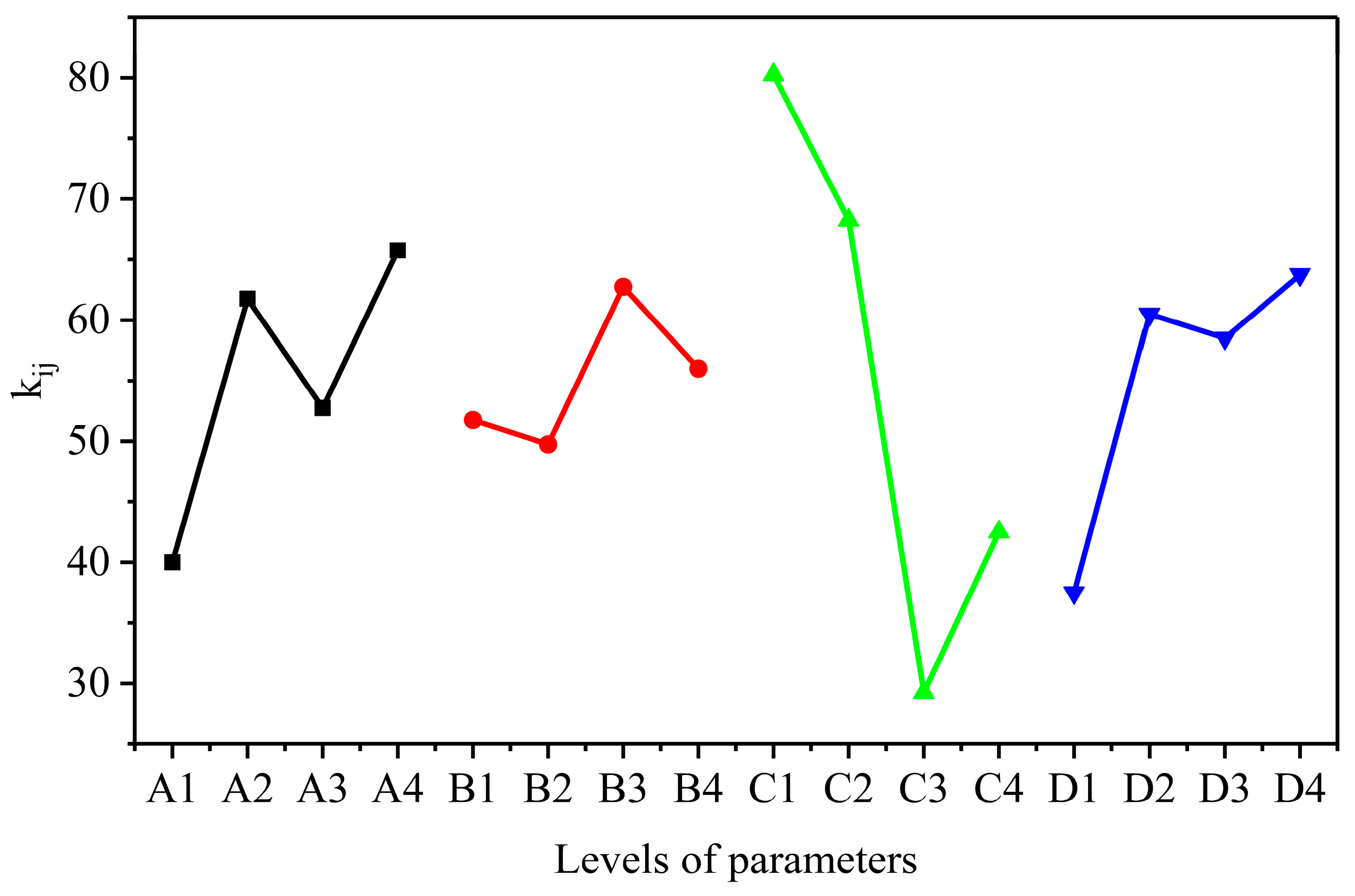
3.1. Optimization of the Synthetic Conditions of AELS
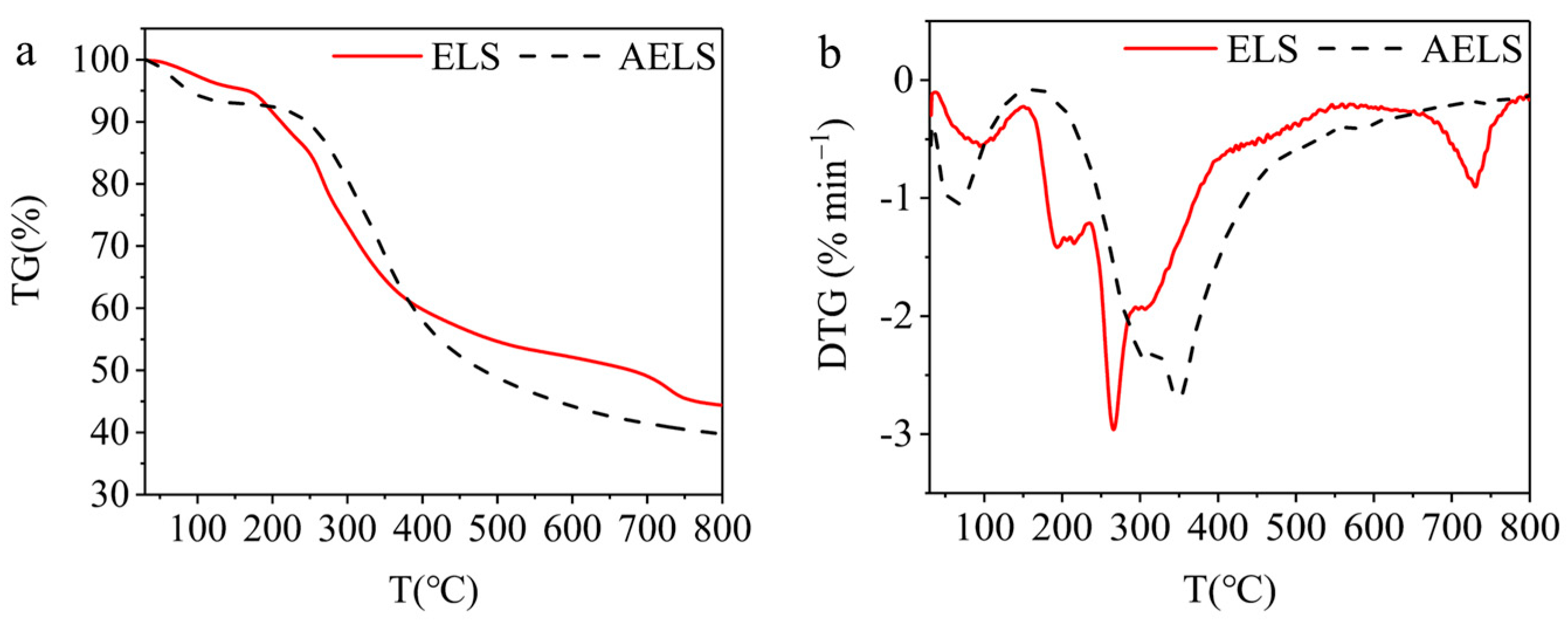
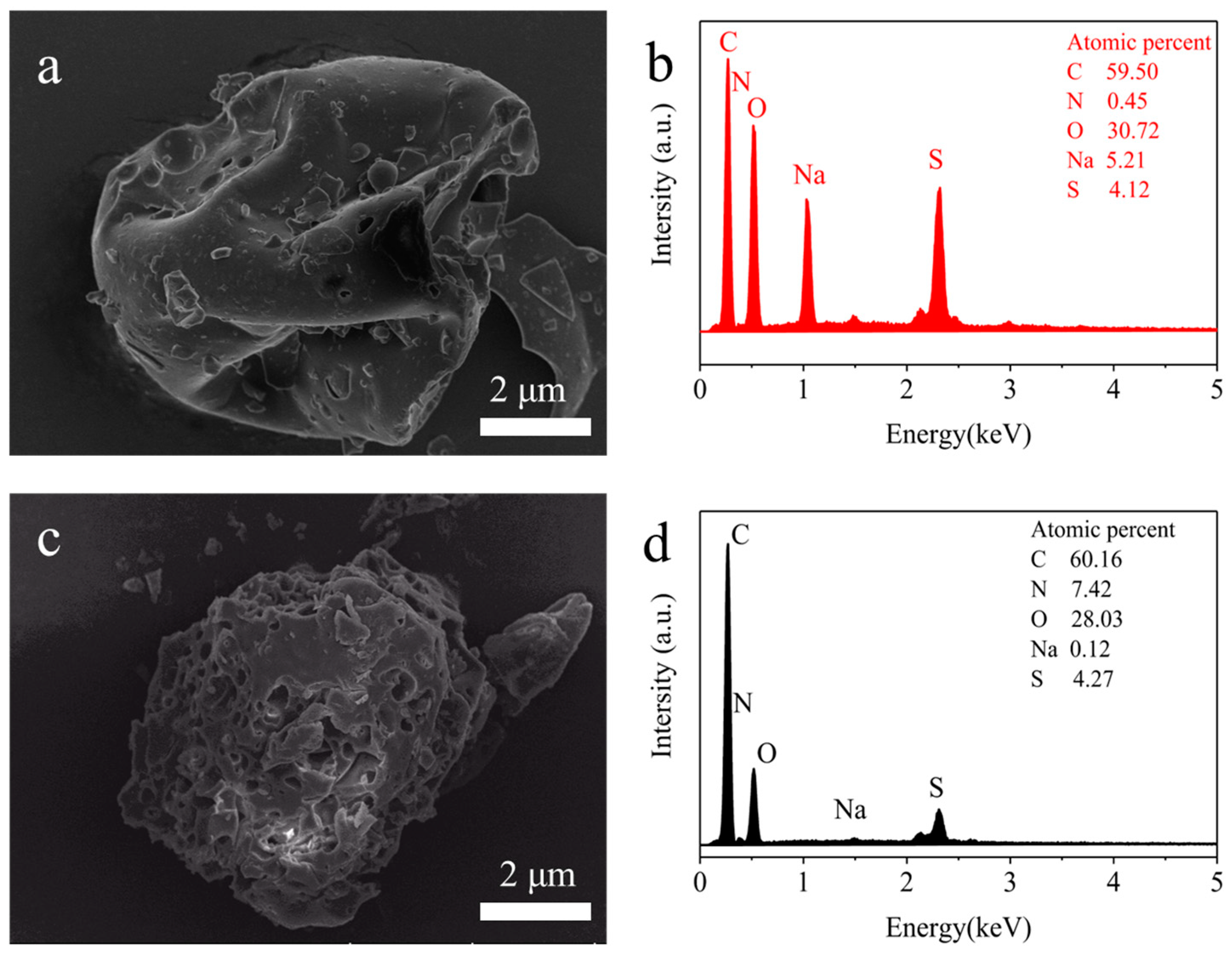
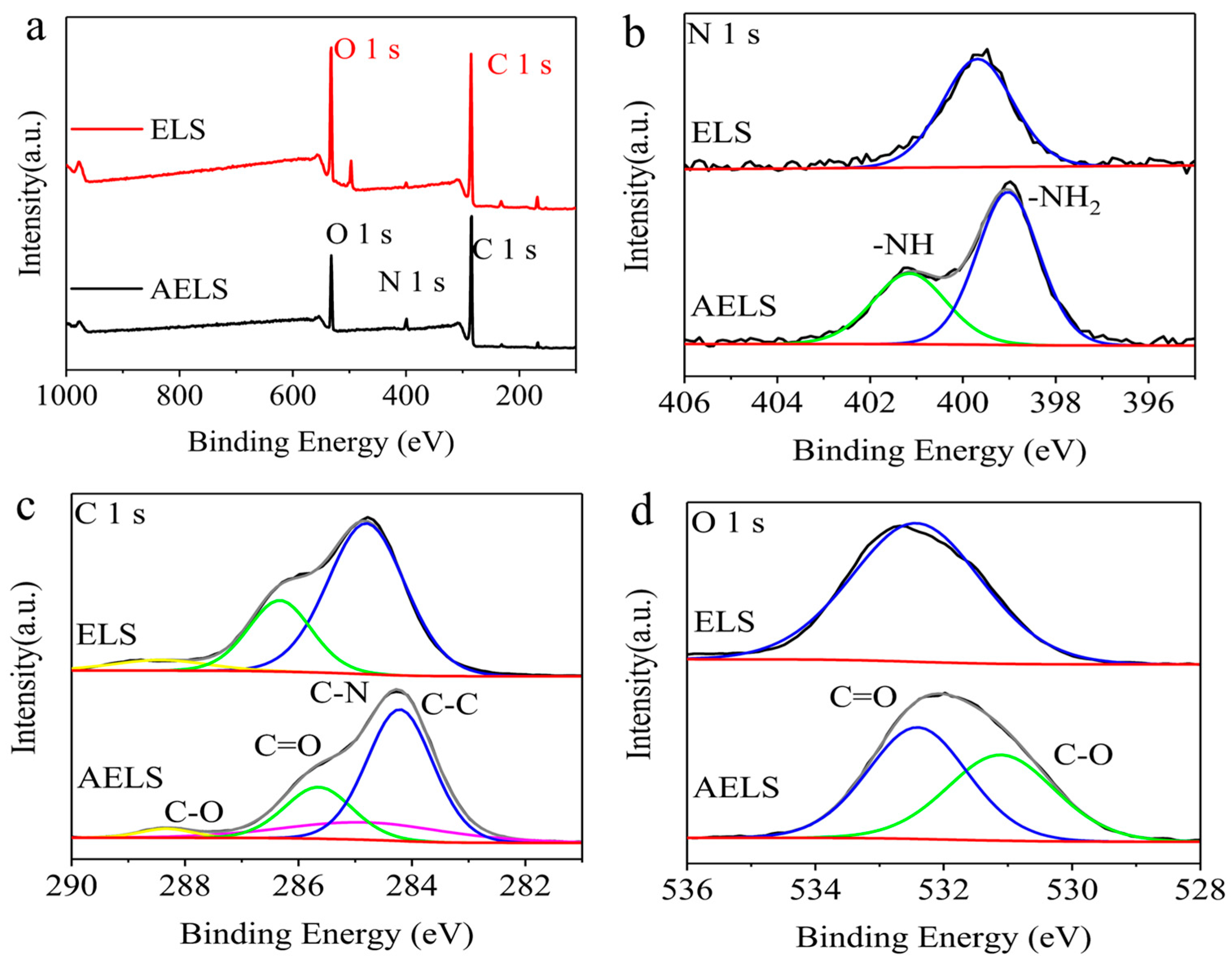
3.2. Characterization of AELS
3.3. Results of Adsorption Experiment
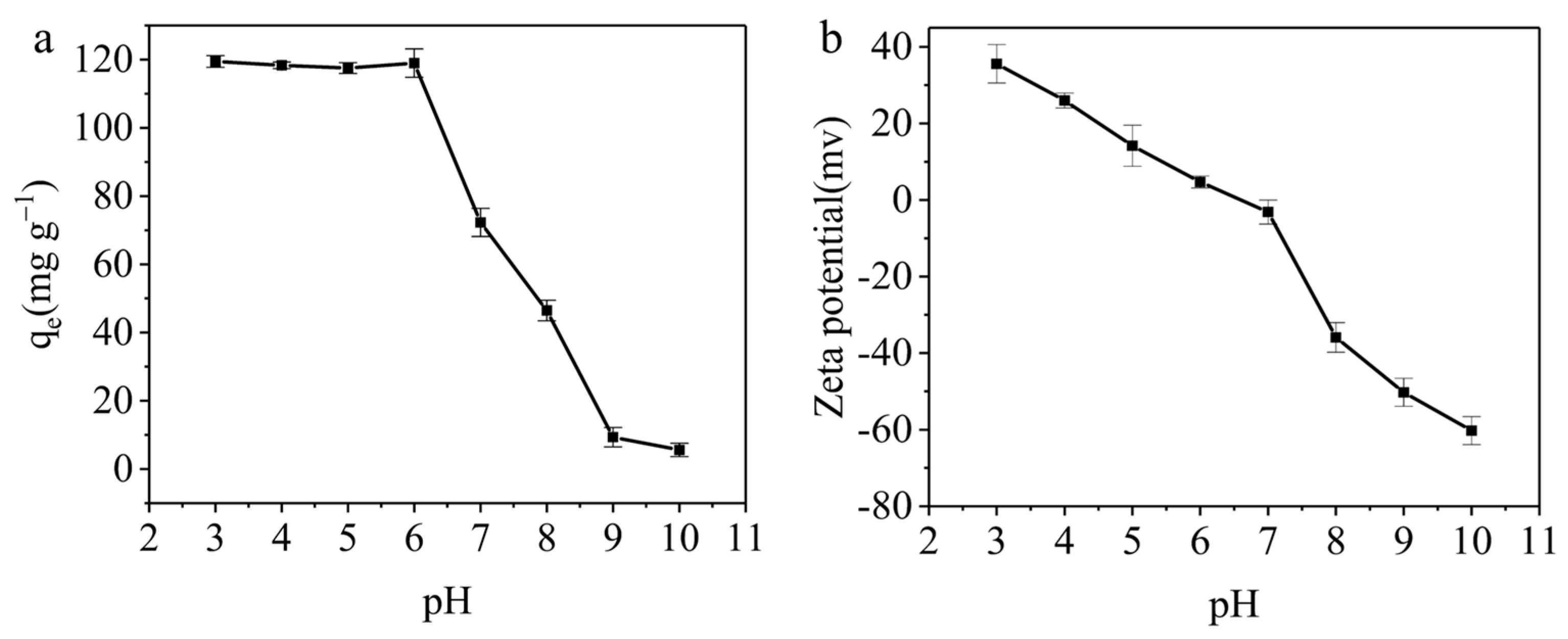
3.3.1. The Effect of pH on the Adsorption Capacity
3.3.2. The Effect of Adsorbent Dosage on the Adsorption Capacity and Removal Rate
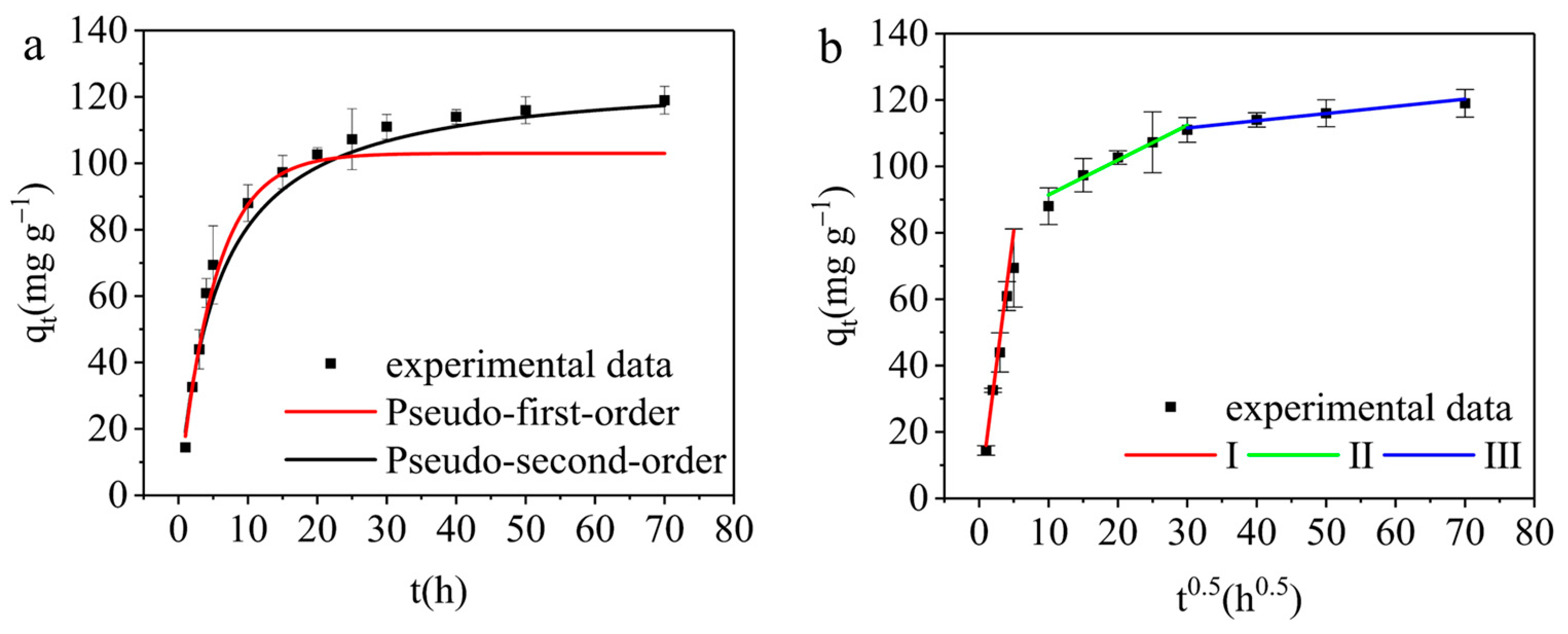
3.3.3. Adsorption Kinetics
3.3.4. Adsorption Isotherm
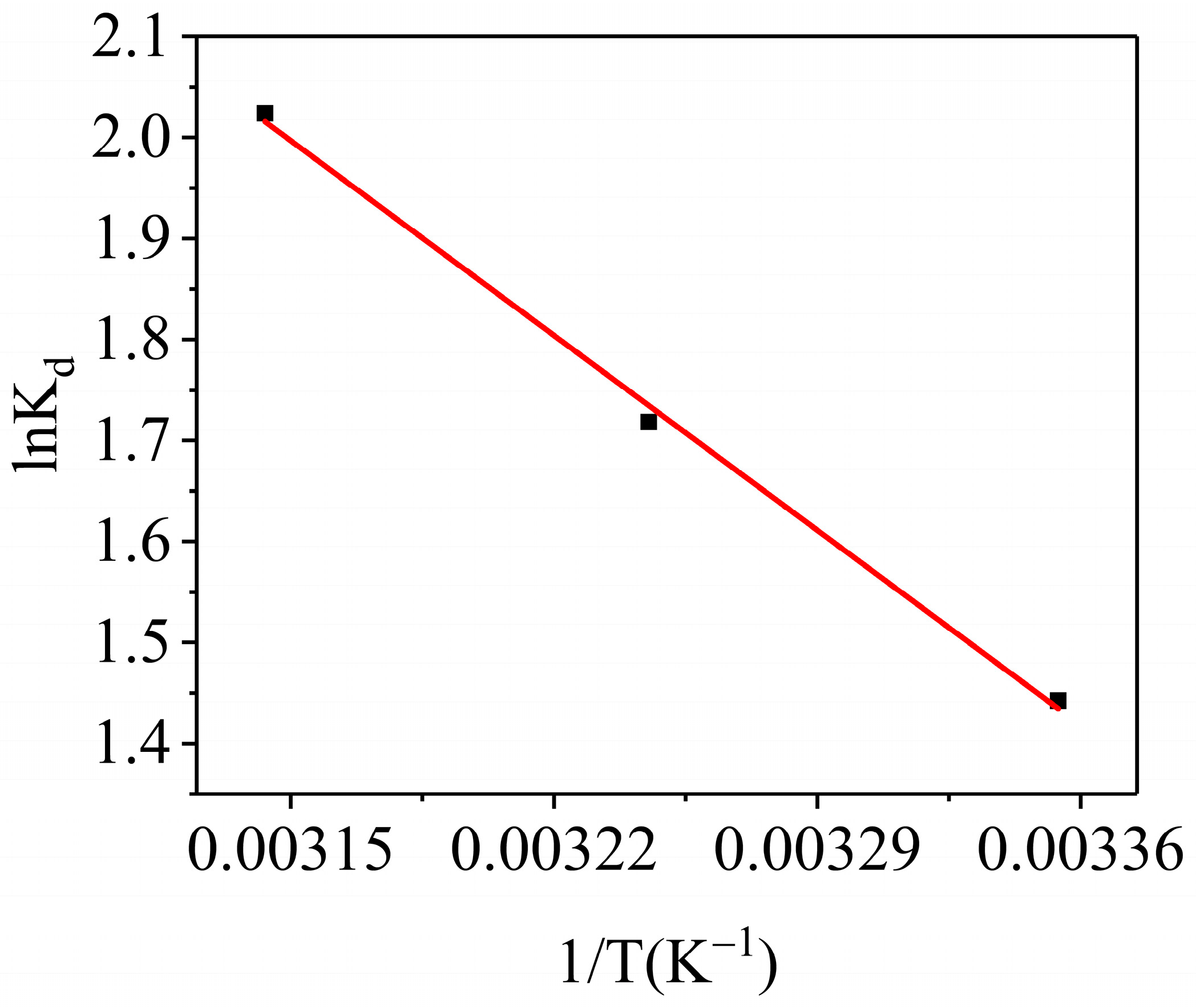
3.3.5. Adsorption Thermodynamics
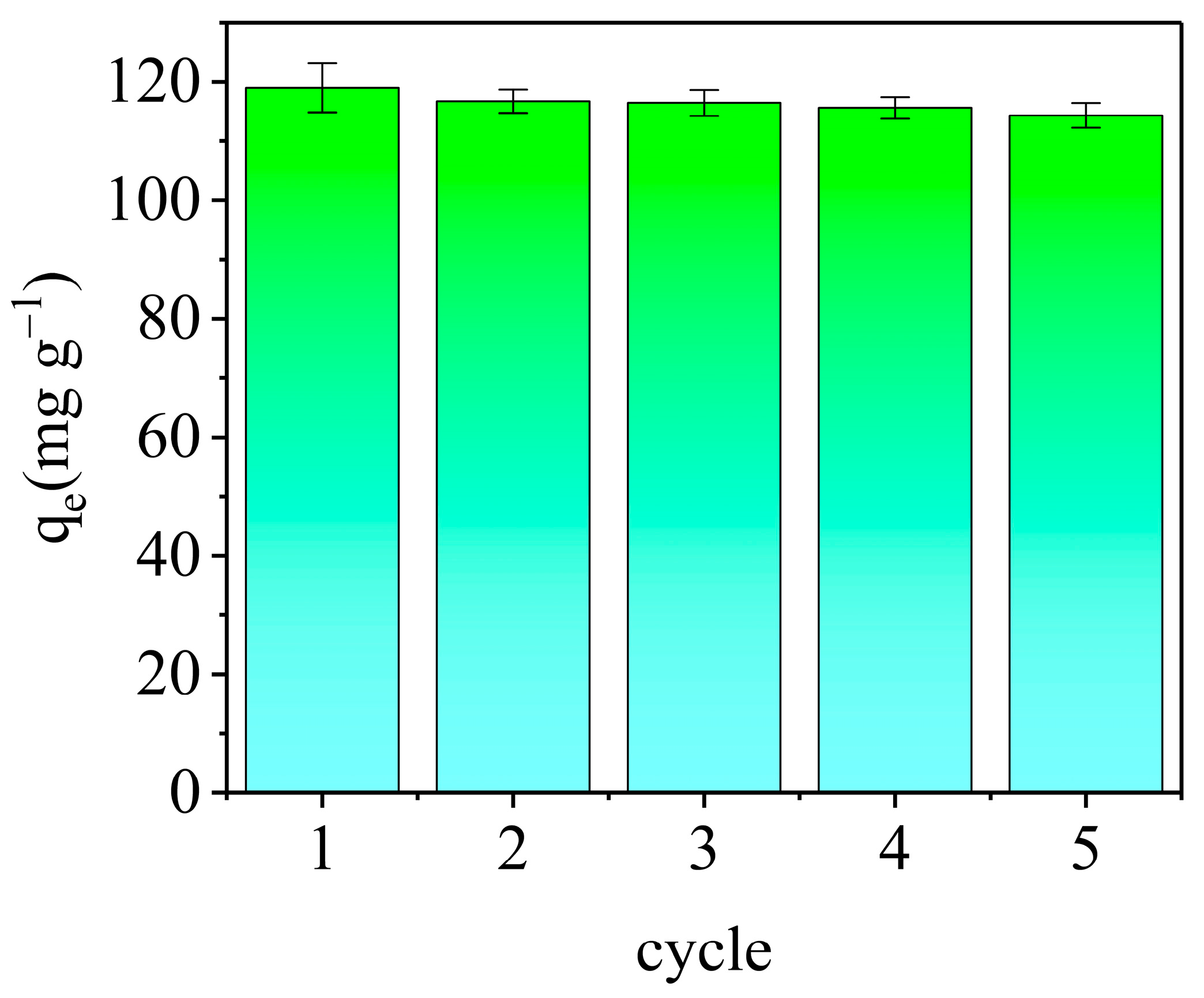
3.3.6. Regeneration and Reusability of AELS
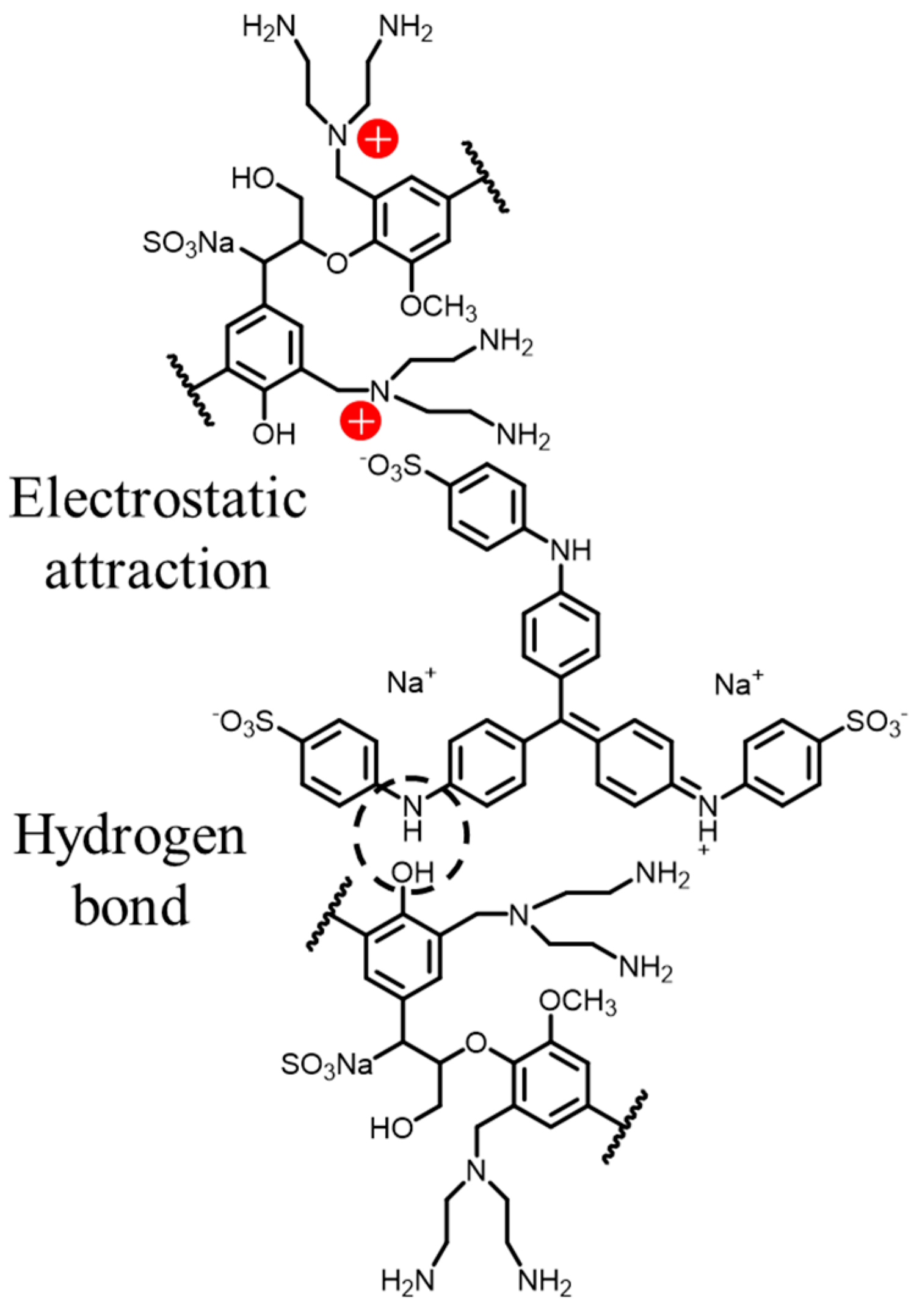
3.3.7. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghedjemis, A.; Ayeche, R.; Kebaili, M.; Benouadah, A.; Gil, L.F. Application of natural hydroxyapatite in the treatment of polluted water: Utilization of dromedary bone as bioadsorbent. Int. J. Appl. Ceram. Technol. 2022, 19, 2124–2134. [Google Scholar] [CrossRef]
- Mon, M.; Bruno, R.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Metal–organic framework technologies for water remediation: Towards a sustainable ecosystem. J. Mater. Chem. A 2018, 6, 4912–4947. [Google Scholar] [CrossRef]
- Das, T.R.; Patra, S.; Madhuri, R.; Sharma, P.K. Bismuth oxide decorated graphene oxide nanocomposites synthesized via sonochemical assisted hydrothermal method for adsorption of cationic organic dyes. J. Colloid Interface Sci. 2018, 509, 82–93. [Google Scholar] [CrossRef]
- Carneiro, P.A.; Umbuzeiro, G.A.; Oliveira, D.P.; Zanoni, M.V.B. Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. J. Hazard. Mater. 2010, 174, 694–699. [Google Scholar] [CrossRef]
- Sharma, P.; Hussain, N.; Borah, D.J.; Das, M.R. Kinetics and Adsorption Behavior of the Methyl Blue at the Graphene Oxide/Reduced Graphene Oxide Nanosheet–Water Interface: A Comparative Study. J. Chem. Eng. Data. 2013, 58, 3477–3488. [Google Scholar] [CrossRef]
- Dâas, A.; Hamdaoui, O. Extraction of anionic dye from aqueous solutions by emulsion liquid membrane. J. Hazard. Mater. 2010, 178, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Rajan, P.S. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Crespo-Monteiro, N.; Cazier, A.; Vocanson, F.; Lefkir, Y.; Reynaud, S.; Michalon, J.Y.; Kämpfe, T.; Destouches, N.; Jourlin, Y. Microstructuring of Mesoporous Titania Films Loaded with Silver Salts to Enhance the Photocatalytic Degradation of Methyl Blue under Visible Light. Nanomaterials 2017, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhao, H.N.; Chen, S.J.; Long, F.X.; Huang, B.; Yang, B.Y.; Pan, X.J. A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater. Chem. Eng. J. 2019, 356, 69–80. [Google Scholar] [CrossRef]
- Fathi, M.R.; Asfaram, A.; Farhangi, A. Removal of Direct Red 23 from aqueous solution using corn stalks: Isotherms, kinetics and thermodynamic studies. Spectrochim. Acta A 2015, 135, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Malarvizhi, R.; Ho, Y.S. The influence of pH and the structure of the dye molecules on adsorption isotherm modeling using activated carbon. Desalination 2010, 264, 97–101. [Google Scholar] [CrossRef]
- Shao, Y.M.; Wang, X.; Kang, Y.; Shu, Y.H.; Sun, Q.Q.; Li, L.S. Application of Mn/MCM-41 as an adsorbent to remove methyl blue from aqueous solution. J. Colloid Interface Sci. 2014, 429, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Shang, S.M.; Hu, E.L.; Yuen, C.W.M.; Jiang, S.X. Konjac glucomannan/graphene oxide hydrogel with enhanced dyes adsorption capability for methyl blue and methyl orange. Appl. Surf. Sci. 2015, 357, 866–872. [Google Scholar] [CrossRef]
- Hao, X.L.; Liu, H.; Zhang, G.S.; Zou, H.; Zhang, Y.B.; Zhou, M.M.; Gu, Y.C. Magnetic field assisted adsorption of methyl blue onto organo-bentonite. Appl. Clay Sci. 2012, 55, 177–180. [Google Scholar] [CrossRef]
- Zhu, H.L.; Luo, W.; Ciesielski, P.N.; Fang, Z.Q.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L.B. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Lora1, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Dawy, M.; Shabaka, A.A.; Nada, A.M.A. Molecular structure and dielectric properties of some treated lignins. Polym. Degrad. Stab. 1998, 62, 455–462. [Google Scholar] [CrossRef]
- Du, X.Y.; Li, J.B.; Lindström, M.E. Modification of industrial softwood kraft lignin using Mannich reaction with and without phenolation pretreatment. Ind. Crops Prod. 2014, 52, 729–735. [Google Scholar] [CrossRef]
- Yao, S.H.; Zhang, J.J.; Shen, D.K.; Xiao, R.; Gu, S.; Zhao, M.; Liang, J.Y. Removal of Pb (II) from water by the activated carbon modified by nitric acid under microwave heating. J. Colloid Interface Sci. 2016, 463, 118–127. [Google Scholar] [CrossRef]
- Shah, M.M.; Grover, T.A.; Barr, D.P.; Aust, S.D. On the mechanism of inhibition of the veratryl alcohol oxidase activity of lignin peroxidase H2 by EDTA. J. Biol. Chem. 1992, 267, 21564–21569. [Google Scholar] [CrossRef]
- Suppiah, K.; Teh, P.L.; Husseinsyah, S.; Rahman, R. Properties and characterization of carboxymethyl cellulose/halloysite nanotube bio-nanocomposite films: Effect of sodium dodecyl sulfate. Polym. Bull. 2018, 76, 365–386. [Google Scholar] [CrossRef]
- Meng, X.Z.; Scheidemantle, B.; Li, M.; Wang, Y.Y.; Zhao, X.H.; Toro-Gonzalez, M.; Singh, P.; Pu, Y.Q.; Wyman, C.E.; Ozcan, S.; et al. Synthesis, Characterization, and Utilization of a Lignin-Based Adsorbent for Effective Removal of Azo Dye from Aqueous Solution. ACS Omega 2020, 5, 2865–2877. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhu, L.L.; Wang, X.H.; Zheng, W.R.; Hao, C.; Jiang, C.L.; Wu, J.B. Synthesis of aminated calcium lignosulfonate and its adsorption properties for azo dyes. J. Ind. Eng. Chem. 2018, 61, 321–330. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhang, Y.K.; Hao, C.; Dai, X.H.; Zhou, Z.L.; Si, N.C. Ultrasonic-assisted synthesis of aminated lignin by a Mannich reaction and its decolorizing properties for anionic azo-dyes. RSC Adv. 2014, 4, 8156–28164. [Google Scholar] [CrossRef]
- Zhang, B.P.; Zhang, H.; Wang, Y.; Fang, S.Y. Adsorption behavior and mechanism of amine/quaternary ammonium lignin on tungsten. Int. J. Biol. Macromol. 2022, 216, 882–890. [Google Scholar] [CrossRef]
- Pan, H.; Sun, G.; Zhao, T. Synthesis and characterization of aminated lignin. Int. J. Biol. Macromol. 2013, 59, 221–226. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Almeida Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): Study of adsorption isotherms and kinetic models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Le, G.T.T.; Chanlek, N.; Manyam, J.; Opaprakasit, P.; Grisdanurak, N.; Sreearunothai, P. Insight into the ultrasonication of graphene oxide with strong changes in its properties and performance for adsorption applications. Chem. Eng. J. 2019, 373, 1212–1222. [Google Scholar] [CrossRef]
- Farias, R.S.; Buarque, H.L.D.; Cruz, M.R.; Cardoso, L.M.F.; Gondim, T.D.; Paulo, V.R. Adsorption of Congo red dye from aqueous solution onto amino-functionalized silica gel. Eng. Sanit. Ambient. 2018, 23, 1053–1060. [Google Scholar] [CrossRef]
- Silva, M.M.F.; Oliveira, M.M.; Avelino, M.C.; Fonseca, M.G.; Almeida, R.K.S.; Silva, E.C. Adsorption of an industrial anionic dye by modified-KSF-montmorillonite: Evaluation of the kinetic. thermodynamic and equilibrium data. Chem. Eng. J. 2012, 203, 259–268. [Google Scholar] [CrossRef]
- Tseng, R.L.; Wu, F.C.; Juang, R.S. Characteristics and applications of the Lagergren’s first-order equation for adsorption kinetics. J. Taiwan Inst. Chem Eng. 2010, 41, 661–669. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A.; Abdi, J.; Hayati, B.; Shekarchi, A.A. Bio-based magnetic metal-organic framework nanocomposite: Ultrasound-assisted synthesis and pollutant (heavy metal and dye) removal from aqueous media. Appl. Surf. Sci. 2019, 480, 288–299. [Google Scholar] [CrossRef]
- Azimi, E.B.; Badiei, A.; Ghasemi, J.B. Efficient removal of malachite green from wastewater by using boron-doped mesoporous carbon nitride. Appl. Surf. Sci. 2019, 469, 236–245. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Nandi, D.; Parameswaranpillai, J.; Lee, J.; Shivanna, J.M.; Nithya, R.; Siengchin, S. Adsorption of Cationic Dye onto ZSM-5 Zeolite-Based Bio Membrane: Characterizations, Kinetics and Adsorption Isotherm. J. Polym. Environ. 2022, 30, 3279–3292. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Cai, W.Q.; Tan, L.J.; Yu, J.G.; Jaroniec, M.; Liu, X.Q.; Cheng, B.; Verpoort, F. Synthesis of amino-functionalized mesoporous alumina with enhanced affinity towards Cr (VI) and CO2. Chem. Eng. J. 2014, 239, 207–215. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Pejčić, M.; Pašti, I.; Lazarević-Pašti, T. Spent Coffee Grounds as an Adsorbent for Malathion and Chlorpyrifos—Kinetics, Thermodynamics, and Eco-Neurotoxicity. Foods 2023, 12, 2397. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Yuan, G.Z.; Zhu, Y.F.; Yu, S.J. Carbon dots crosslinked chitosan/cellulose sponge capture of methyl blue by adsorption process. Luminescence 2021, 36, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Raji, F.; Pakizeh, M. Study of Hg (II) species removal from aqueous solution using hybrid ZnCl2 -MCM-41 adsorbent. Appl. Surf. Sci. 2013, 282, 415–424. [Google Scholar] [CrossRef]
- Bera, A.; Kumar, T.; Ojha, K.; Mandal, A. Adsorption of surfactants on sand surface in enhanced oil recovery: Isotherms, kinetics and thermodynamic studies. Appl. Surf. Sci. 2013, 284, 87–99. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Das, P. Optimization of crystal violet dye removal using novel soil-silver nanocomposite as nanoadsorbent using response surface methodology. J. Environ. Chem. Eng. 2014, 2, 708–714. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y.; Dunda, M. Adsorption of chromium (VI) on pomace-An olive oil industry waste: Batch and column studies. J. Hazard. Mater. 2006, 138, 142–151. [Google Scholar] [CrossRef]
- Li, Z.C.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Netto, M.S.; Oliveira, M.L.S.; Seliem, M.K.; Dotto, G.L.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Luo, P.; Zhao, Y.F.; Zhang, B.; Liu, J.D.; Yang, Y.; Liu, J.F. Study on the adsorption of Neutral Red from aqueous solution onto halloysite nanotubes. Water Res. 2010, 44, 1489–1497. [Google Scholar] [CrossRef]
- Özcan, A.S.; Erdem, B.; Özcan, A. Adsorption of Acid Blue 193 from aqueous solutions onto Na–bentonite and DTMA–bentonite. J. Colloid Interface Sci. 2004, 280, 44–54. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, Y.Y.; Xue, J.M.; Zhu, F.H.; Zhong, Z.P.; Liu, R.J. An innovative alcohol-solution combustion-calcination process for the fabrication of NiFe2O4 nanorods and their adsorption characteristics of methyl blue in aqueous solution. Mater. Express 2021, 8, 095003. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, Q.R.; Chi, H.J.; Li, M.L.; Lv, Y.F.; Feng, S.J.; Zhu, R.Y.; Li, K. Methyl blue adsorption properties and bacteriostatic activities of Mg-Al layer oxides via a facile preparation method. Appl. Clay Sci. 2018, 163, 119–128. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Q.; Zhao, X.S.; Che, Y.J.; Liu, H.C.; Zuo, W.; Zhang, Y.M. The methyl blue adsorption performance and mechanism of NaX zeolite synthesized from Huadian oil shale ash. J. Taiwan Inst. Chem. Eng. 2023, 147, 104904. [Google Scholar] [CrossRef]
- Sari, A.; Mendil, D.; Tuzena, M.; Soylak, M. Biosorption of palladium (II) from aqueous solution by moss (Racomitrium lanuginosum) biomass: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2009, 162, 874–879. [Google Scholar] [CrossRef] [PubMed]



| Dynamic Model | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | Intra-Particle Diffusion | |||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | ||||||
| R2 | 0.9852 | R2 | 0.9930 | R2 | 0.9754 | 0.9324 | 0.9279 | |
| Parameters | k1 | 0.19 ± 0.0085 | k2 | 0.0014 ± 0.0004 | kp | 16.17 ± 1.28 | 1.05 ± 0.14 | 0.22 ± 0.034 |
| qe RMSE | 103.00 ± 0.004 2.03 | qe RMSE | 126.94 ± 0.003 1.39 | C | −0.22 ± 2.51 | 80.94 ± 2.98 | 105.06 ± 1.43 | |
| T/K | 298 | 308 | 318 |
|---|---|---|---|
| Langmuir | |||
| qmax (mg g−1) | 149.08 ± 1.30 | 172.14 ± 1.23 | 183.69 ± 2.67 |
| KL (L mg−1) | 1.74 ± 0.022 | 0.69 ± 0.050 | 0.59 ± 0.08 |
| RL | 0.0019–0.054 | 0.005–0.13 | 0.006–0.15 |
| R2 | 0.9992 | 0.9961 | 0.9850 |
| RMSE | 0.51 | 2.77 | 5.93 |
| Freundlich | |||
| KF (L mg−1) | 44.66 ± 2.091 | 87.21 ± 13.88 | 87.61 ± 13.42 |
| n | 3.75 ± 0.22 | 6.96 ± 1.74 | 6.36 ± 1.40 |
| R2 | 0.9194 | 0.7823 | 0.8240 |
| RMSE | 5.04 | 20.62 | 20.28 |
| Temkin | |||
| b (J mol−1) | 128.46 ± 7.06 | 120.20 ± 15.39 | 106.36 ± 11.15 |
| KT (L mg−1) | 31.69 ± 2.66 | 41.24 ± 39.30 | 26.21 ± 19.14 |
| R2 | 0.9766 | 0.8850 | 0.9199 |
| RMSE | 2.71 | 14.98 | 13.68 |
| Adsorbent | Kinetic Model | Isotherm Model | qmax (mg g−1) | Reference |
|---|---|---|---|---|
| Mn/MCM-41 | Pseudo-second-order | Freundlich Dubinin–Radushkevich | 45.38 | [14] |
| KGM/GO hydrogel | Pseudo-second-order | Freundlich | 133.67 | [15] |
| Organo bentonite | Pseudo-second-order | Freundlich | 98.15 | [16] |
| Magnetic NiFe2O4 nanorods | Pseudo-second-order | Temkin | 97.73 | [50] |
| Mg3Al LDH | Pseudo-second-order | Langmuir | 98.77 | [51] |
| AELS | Pseudo-second-order | Langmuir | 153.42 | This work |
| Thermodynamic Parameter | ΔH0 (kJ mol−1) | ΔS0 (J mol−1 K−1) | ΔG0 (kJ mol−1) | ||
|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | |||
| 22.91 | 88.76 | −3.55 | −4.44 | −5.33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, L.-Z.; Guo, C.-F.; Gong, Z.-X.; Xu, H.; Yang, X.-J.; Wang, Y.-X.; Luo, X.-P. Preparation of Aminated Sodium Lignosulfonate and Efficient Adsorption of Methyl Blue Dye. Materials 2024, 17, 1046. https://doi.org/10.3390/ma17051046
Huo L-Z, Guo C-F, Gong Z-X, Xu H, Yang X-J, Wang Y-X, Luo X-P. Preparation of Aminated Sodium Lignosulfonate and Efficient Adsorption of Methyl Blue Dye. Materials. 2024; 17(5):1046. https://doi.org/10.3390/ma17051046
Chicago/Turabian StyleHuo, Li-Zhu, Chao-Fei Guo, Zhu-Xiang Gong, Hao Xu, Xue-Juan Yang, Yu-Xuan Wang, and Xi-Ping Luo. 2024. "Preparation of Aminated Sodium Lignosulfonate and Efficient Adsorption of Methyl Blue Dye" Materials 17, no. 5: 1046. https://doi.org/10.3390/ma17051046
APA StyleHuo, L.-Z., Guo, C.-F., Gong, Z.-X., Xu, H., Yang, X.-J., Wang, Y.-X., & Luo, X.-P. (2024). Preparation of Aminated Sodium Lignosulfonate and Efficient Adsorption of Methyl Blue Dye. Materials, 17(5), 1046. https://doi.org/10.3390/ma17051046






