Phase Formation during the Synthesis of the MAB Phase from Mo-Al-B Mixtures in the Thermal Explosion Mode
Abstract
1. Introduction
2. Materials and Methods
- -
- A rotary ball mill (BM). Mixing was performed for 8 h at a jar rotational speed of 100 rpm;
- -
3. Results and Discussion
- -
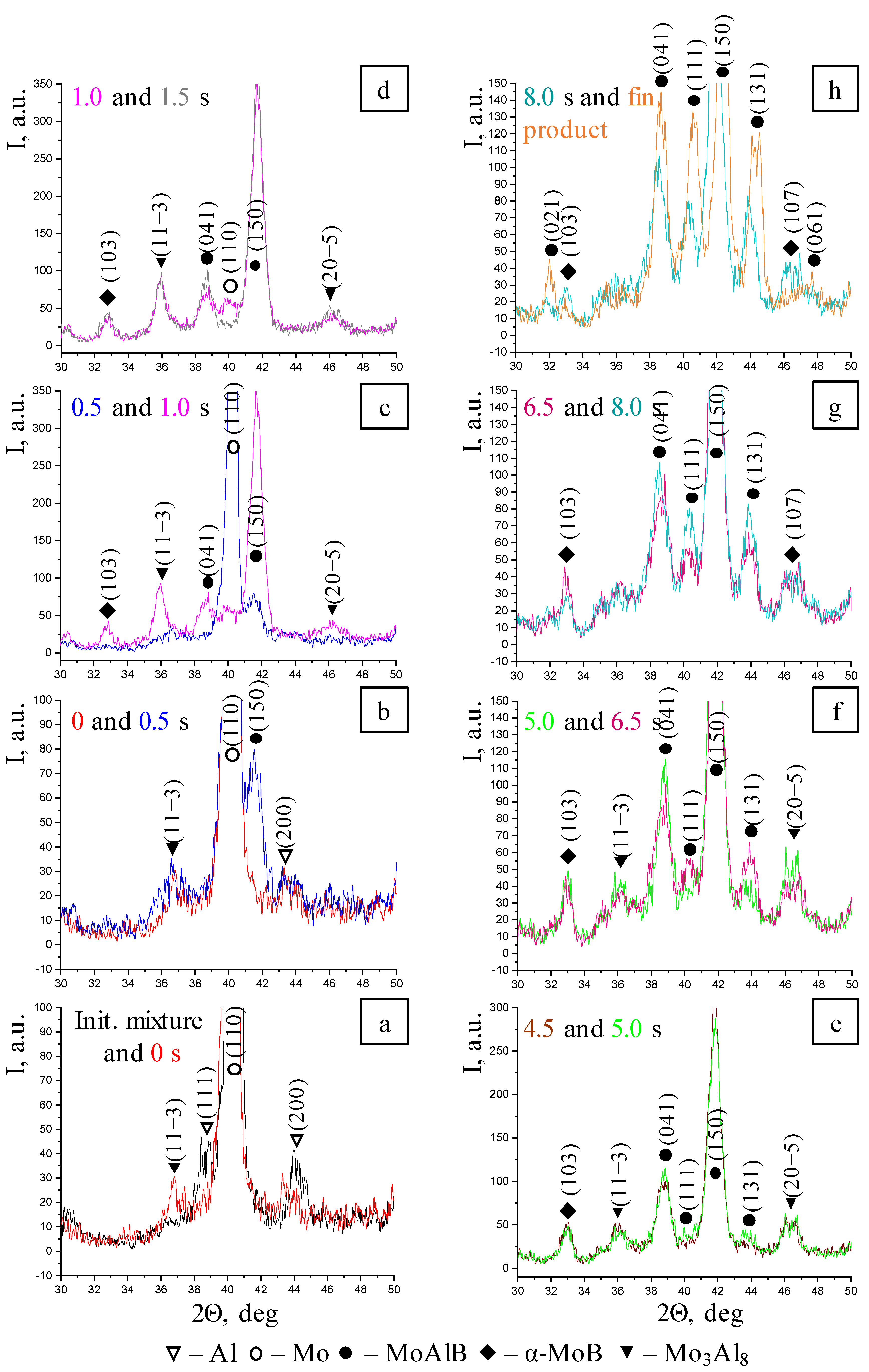
- Melting of Al and formation of the Mo3Al8 intermetallic compound, which subsequently reacts;
- -
- Emergence of the MoAlB phase, as indicated by the highest-intensity (150) reflection;
- -
- Formation of the α-MoB impurity phase after Mo3Al8 and MoAlB and its presence until the end of phase formation;
- -
- After interaction starts, the peaks belonging to Al are present in the time interval of 0.5–1.0 s, while the diffraction lines corresponding to molybdenum remain for 1.5 s;
- -
- All phase changes occur within 8 s, while the intermediate stage (“window”) lasts approximately 3 s.
- -
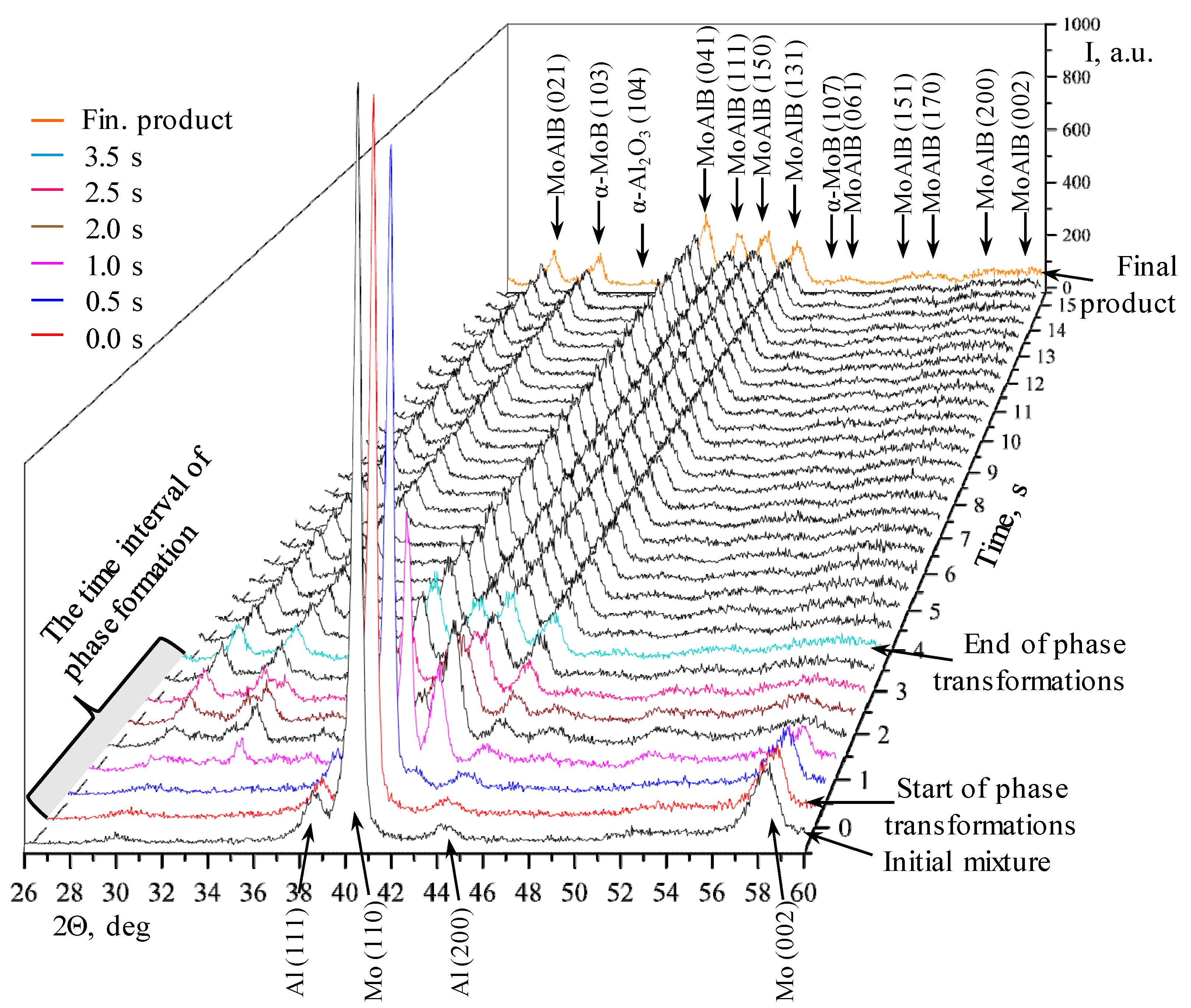
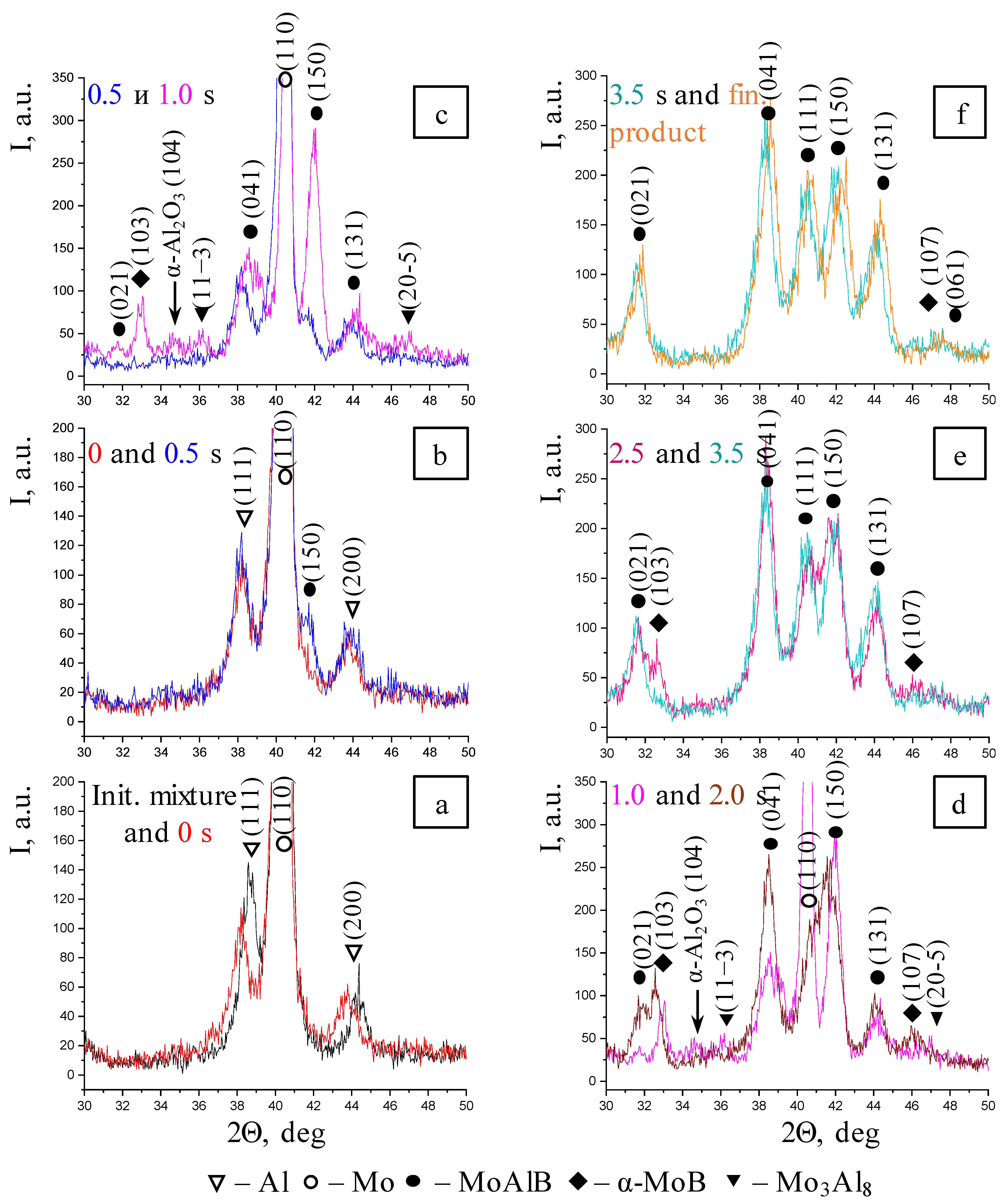
- Gas-phase mass transfer of volatile MoO3 and B2O2 oxides to the surface of B and Mo particles with the formation of the intermediate MoB phase, and in parallel with this, a solid-phase reaction between the initial components with the formation of the primary MoAlB phase corresponding (as in the case of the BM mixture) to the highest-intensity (150) reflection;
- -
- Formation of all the major diffraction lines of the MAB phase within 2.5 s;
- -
- Simultaneous formation of the MoB and Mo3Al8 impurity phases; the intermetallic compound disappears at the post-reaction stage;
- -
- After the interaction begins, the peaks belonging to Al are present in the time interval of 0.5–1.0 s; the lines corresponding to molybdenum are observed in the XRD pattern for 2.0 s;
- -
- All the phase transformations occur within 3.5 s.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spear, K.E.; Liao, P.K. The B-Mo (Boron-Molybdenum) system. Bull. Alloy Phase Diagr. 1988, 9, 457–466. [Google Scholar] [CrossRef]
- Carlson, O.N. The Al-B (Aluminum-Boron) System. Bull. Alloy Phase Diagr. 1990, 11, 560–566. [Google Scholar] [CrossRef]
- Saunders, N. The Al-Mo system (Aluminum-Molybdenum). J. Phase Equilib. 1997, 18, 370–378. [Google Scholar] [CrossRef]
- Kota, S.; Sokol, M.; Barsoum, M.W. A progress report on the MAB phases: Atomically laminated, ternary transition metal borides. Int. Mater. Rev. 2020, 65, 226–255. [Google Scholar] [CrossRef]
- Sun, Z.M. Progress in research and development on MAX phases: A family of layered ternary compounds. Int. Mater. Rev. 2011, 56, 143–166. [Google Scholar] [CrossRef]
- Halla, F.; Thury, W. Über boride von molybdän und wolfram. Z. Anorg. Allg. Chem. 1942, 3, 229–2375. [Google Scholar] [CrossRef]
- Jeitschko, W. Die kristallstruktur von MoAlB. Monatshefte Chem. 1966, 97, 1472–1476. [Google Scholar] [CrossRef]
- Kota, S.; Zapata-Solvas, E.; Ly, A.; Lu, J.; Elkassabany, O.; Huon, A.; Lee, W.E.; Hultman, L.; May, S.J.; Barsoum, M.W. Synthesis and characterization of an alumina forming nanolaminated boride: MoAlB. Sci. Rep. 2016, 6, 26475. [Google Scholar] [CrossRef]
- Bai, Y.; Qi, X.; Duff, A.; Li, N.; Kong, F.; He, X.; Wang, R.; Lee, W.E. Density functional theory insights into ternary layered boride MoAlB. Acta Mater. 2017, 132, 69–81. [Google Scholar] [CrossRef]
- Kota, S.; Agne, M.; Zapata-Solvas, E.; Dezellus, O.; Lopez, D.; Gardiola, B.; Radovic, M.; Barsoum, M.W. Elastic properties, thermal stability, and thermodynamic parameters of MoAlB. Phys. Rev. B 2017, 95, 144108. [Google Scholar] [CrossRef]
- Kota, S.; Zapata-Solvas, E.; Chen, Y.; Radovic, W.; Lee, W.E.; Barsoum, M.W. Isothermal and cyclic oxidation of MoAlB in air from 1100 °C to 1400 °C. J. Electrochem. Soc. 2017, 164, C930–C938. [Google Scholar] [CrossRef]
- Shi, O.; Xu, L.; Jiang, A.; Xu, Q.; Xiao, Y.; Zhu, D.; Grasso, S.; Hu, C. Synthesis and oxidation resistance of MoAlB single crystals. Ceram. Int. 2019, 45, 2446–2450. [Google Scholar] [CrossRef]
- Potanin, A.Y.; Bashkirov, E.A.; Pogozhev, Y.S.; Rupasov, S.I.; Levashov, E.A. Synthesis, structure and properties of MAB phase MoAlB ceramics produced by combination of SHS and HP techniques. J. Eur. Ceram. Soc. 2022, 42, 6379–6390. [Google Scholar] [CrossRef]
- Xu, L.; Shi, O.; Liu, C.; Zhu, D.; Grasso, S.; Hu, C. Synthesis, microstructure and properties of MoAlB ceramics. Ceram. Int. 2018, 44, 13396–13401. [Google Scholar] [CrossRef]
- Okada, S.; Iizumi, K.; Kudaka, K.; Kudou, K.; Miyamoto, M.; Yu, Y.; Lundström, T. Single crystal growth of (MoxCr1−x)AlB and (MoxW1−x)AlB by metal Al solutions and properties of the crystals. J. Solid State Chem. 1997, 133, 36–43. [Google Scholar] [CrossRef]
- Liu, C.; Hou, Z.; Jia, Q.; Liu, X.; Zhang, S. Low temperature synthesis of phase pure MoAlB powder in molten NaCl. Materials. 2020, 13, 785. [Google Scholar] [CrossRef]
- Su, X.; Dong, J.; Chu, L.; Sun, H.; Grasso, S.; Hu, C. Synthesis, microstructure and properties of MoAlB ceramics prepared by in situ reactive spark plasma sintering. Ceram. Int. 2020, 46, 15214–15221. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Wang, T.; Wang, C.; Xie, Z.; Wang, W.; Xin, T. Synthesis, microstructure and properties of MoAlB MAB phase films. Ceram. Int. 2023, 49, 23714–23720. [Google Scholar] [CrossRef]
- Borovinskaya, I.P.; Gromov, A.A.; Levashov, E.A.; Maksimov, Y.M.; Mukasyan, A.S.; Rogachev, A.S. Concise Encyclopedia of Self-Propagating High-Temperature Synthesis: History, Theory, Technology, and Products; Elsevier: Amsterdam, The Netherlands, 2017; 468p. [Google Scholar] [CrossRef]
- Levashov, E.A.; Mukasyan, A.S.; Rogachev, A.S.; Shtansky, D.V. Self-Propagating high-temperature synthesis of advanced materials and coatings. Int. Mater. Rev. 2017, 62, 203–239. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Rogachev, A.S. Discrete reaction waves: Gasless combustion of solid powder mixtures. Prog. Energy Combust. Sci. 2008, 34, 377–416. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Wang, X. Self-Propagating high temperature synthesis (SHS) of intermetallic compounds titanium and nickel aluminides. Mater. Manuf. Process. 1994, 9, 75–87. [Google Scholar] [CrossRef]
- Khoptiar, Y.; Gotman, I. Ti2AlC ternary carbide synthesized by thermal explosion. Mater. Lett. 2002, 57, 72–76. [Google Scholar] [CrossRef]
- Khoptiar, Y.; Gotman, I.; Gutmanas, E.Y. Pressure-assisted combustion synthesis of dense layered Ti3AlC2 and its mechanical properties. J. Am. Ceram. Soc. 2005, 88, 28–33. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Xiao, W.; Zhang, L.; Pu, Y.; Guo, S. Rapid synthesis of Ti2AlN ceramic via thermal explosion. Mater. Lett. 2015, 149, 5–7. [Google Scholar] [CrossRef]
- Liang, B.; Dai, Z.; Zhang, W.; Li, Q.; Niu, D.; Jiao, M.; Yang, L.; Guan, X. Rapid synthesis of MoAlB ceramic via thermal explosion. J. Mater. Res. Technol. 2021, 14, 2954–2961. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Dai, Z.; Zhu, D.; Liang, B.; Zhang, W.; Liu, Y.; Zhang, R.; Zhang, J.; Feng, X.; et al. Preparation of Fe2AlB2 material via thermal explosion induced by spark plasma sintering. J. Asian Ceram. Soc. 2022, 10, 262–269. [Google Scholar] [CrossRef]
- Merz, J.; Richardson, P.; Cuskelly, D. Formation of Mn2AlB2 by induction-assisted self-propagating high-temperature synthesis. Open Ceram. 2021, 8, 100190. [Google Scholar] [CrossRef]
- Liang, B.; Feng, X.; Zhang, W.; Zhang, J.; Yang, L. Preparation of high-content MoAlB by thermal explosion from Mo/Al/B2O3 system. J. Mater. Res. Technol. 2022, 18, 2077–2082. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Borovinskaya, I.P.; Khomenko, I.O.; Mukasyan, A.S.; Ponomarev, V.I.; Rogachev, A.S.; Shkiro, V.M. Dynamics of phase formation during SHS. Ann. Chim. 1995, 20, 123–138. [Google Scholar]
- Khomenko, I.O.; Ponomarev, V.I.; Borovinskaya, I.P. Peculiarities of the time-resolved x-ray diffraction applied to the study of phase-forming processes in an SHS wave. Int. J. Self-Propag. High-Temp. Synth. 1994, 3, 117–121. [Google Scholar]
- Woracek, R.; Santisteban, J.; Fedrigo, A.; Strobl, M. Diffraction in neutron imaging—A review. Nucl. Instrum. Methods Phys. Res. Sect. A 2018, 878, 141–158. [Google Scholar] [CrossRef]
- Kardjilov, N.; Manke, I.; Woracek, R.; Hilger, A.; Banhart, J. Advances in neutron imaging. Mater. Today 2018, 21, 652–672. [Google Scholar] [CrossRef]
- Huang, S.; Luo, S.; Qin, L.; Shu, D.; Sun, B.; Lunt, A.; Korsunsky, A.; Mi, J. 3D local atomic structure evolution in a solidifying Al-0.4Sc dilute alloy melt revealed in operando by synchrotron X-ray total scattering and modelling. Scr. Mater. 2022, 211, 114484. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, Z.; Guo, B.; Li, W.; Mi, J. Determining the Critical Fracture Stress of Al Dendrites near the Melting Point via Synchrotron X-ray Imaging. Acta Metall. Sin. 2023, 36, 857–864. [Google Scholar] [CrossRef]
- Qin, L.; Du, W.; Cipiccia, S.; Bodey, A.J.; Rau, C.; Mi, J. Synchrotron X-ray operando study and multiphysics modelling of the solidification dynamics of intermetallic phases under electromagnetic pulses. Acta Mater. 2024, 265, 119593. [Google Scholar] [CrossRef]
- Merz, J.; Cuskelly, D.; Gregg, A.; Studer, A.; Richardson, P. On the complex synthesis reaction mechanisms of the MAB phases: High-speed in-situ neutron diffraction and ex-situ X-ray diffraction studies of MoAlB. Ceram. Int. 2023, 49, 38789–38802. [Google Scholar] [CrossRef]
- Hendaoui, A.; Vrel, D.; Amara, A.; Langlois, P.; Andasmas, M.; Guerioune, M. Synthesis of high-purity polycrystalline MAX phases in Ti–Al–C system through mechanically activated self-propagating high-temperature synthesis. J. Eur. Ceram. Soc. 2010, 30, 1049–1057. [Google Scholar] [CrossRef]
- Riley, D.P.; Kisi, E.H.; Phelan, D. SHS of Ti3SiC2: Ignition temperature depression by mechanical activation. J. Eur. Ceram. Soc. 2006, 26, 1051–1058. [Google Scholar] [CrossRef]
- Potanin, A.Y.; Loginov, P.A.; Levashov, E.A.; Pogozhev, Y.S.; Patsera, E.I.; Kochetov, N.A. Effect of mechanical activation on Ti3AlC2 MAX phase formation under self-propagating high-temperature synthesis. Eurasian Chem. Technol. J. 2015, 17, 233–242. [Google Scholar] [CrossRef]
- Potanin, A.Y.; Bashkirov, E.A.; Levashov, E.A.; Loginov, P.A.; Berezin, M.A.; Kovalev, D.Y. Nucleation and growth of the Fe2AlB2 MAB phase in the combustion wave of mechanically activated Fe–Al–B reaction mixtures. Ceram. Int. 2023, 49, 37849–37860. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, B.; Wang, X.; Ye, W.; Li, R.; Chen, X.; Wang, J.; Zhang, G. Preparation of fine-grained MoAlB with preferable mechanical properties and oxidation resistance. Int. J. Refract. Met. Hard Mater. 2020, 93, 105345. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Ivanov, E.; Boldyrev, V.V. The science and technology of mechanical alloying. Mater. Sci. Eng. A 2001, 304, 151–158. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Boldyreva, E.V.; Boldyrev, V.V. Role of mixing and milling in mechanochemical synthesis (Review). Russ. J. Inorg. Chem. 2021, 66, 433–453. [Google Scholar] [CrossRef]
- Levashov, E.A.; Kurbatkina, V.V.; Rogachev, A.S.; Kochetov, N.A. Mechanoactivation of SHS systems and processes. Int. J. Self-Propag. High-Temp Synth. 2007, 16, 46–50. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Rogachev, A.S.; Aruna, S.T. Combustion synthesis in nanostructured reactive systems. Adv. Powder Technol. 2015, 26, 954–976. [Google Scholar] [CrossRef]
- Kovalev, D.Y.; Ponomarev, V.I. Time-resolved X-Ray diffraction in SHS research and related areas: An Overview. Int. J. Self-Propag. High-Temp Synth. 2019, 28, 114–123. [Google Scholar] [CrossRef]
- Shelekhov, E.V.; Sviridova, T.A. Programs for X-ray analysis of polycrystals. Metal Sci. Heat Treat. 2000, 42, 309–313. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Kovalev, D.Y.; Kochetov, N.A.; Ponomarev, V.I.; Mukasyan, A.S. Effect of mechanical activation on thermal explosion in Ni-Al mixtures. Int. J. Self-Propag. High-Temp. Synth. 2010, 19, 120–125. [Google Scholar] [CrossRef]
- Gaffet, E.; Charlot, F.; Klein, D.; Bernard, F.; Niepce, J.C. Mechanically activated SHS reaction in the Fe-Al system: In situ time resolved diffraction using synchrotron radiation. Mater. Sci. Forum. 1998, 269–272, 379–384. [Google Scholar] [CrossRef]
- Kovalev, D.Y.; Potanin, A.Y.; Levashov, E.A.; Shkodich, N.F. Phase formation dynamics upon thermal explosion synthesis of magnesium diboride. Ceram. Int. 2016, 42, 2951–2959. [Google Scholar] [CrossRef]
- Levashov, E.A.; Pogozhev, Y.S.; Potanin, A.Y.; Kochetov, N.A.; Kovalev, D.Y.; Shvyndina, N.V.; Sviridova, T.A. Self-propagating high-temperature synthesis of advanced ceramics in the Mo-Si-B system: Kinetics and mechanism of combustion and structure formation. Ceram. Int. 2014, 40, 6541–6552. [Google Scholar] [CrossRef]
- Eremina, E.N.; Kurbatkina, V.V.; Levashov, E.A.; Rogachev, A.S.; Kochetov, N.A. Obtaining the composite MoB material by means of force SHS compacting with preliminary mechanical activation of Mo–10%B mixture. Chem. Sustain. Dev. 2005, 13, 197–204. [Google Scholar]
- Babkin, S.B.; Bloshenko, V.N.; Borovinskaya, I.P. Mechanism of mass transfer with combustion of the SHS-system Mo+B. Combust. Explos. Shock Waves 1991, 27, 333–338. [Google Scholar] [CrossRef]
- Egishyan, A.V.; Manukyan, K.V.; Harutyunyan, A.B.; Kharatyan, S.L. Influence of molybdenum and boron oxides on combustion in the Mo–B gasless system. Int. J. Self-Propag. High-Temp Synth. 2006, 15, 33–40. [Google Scholar]

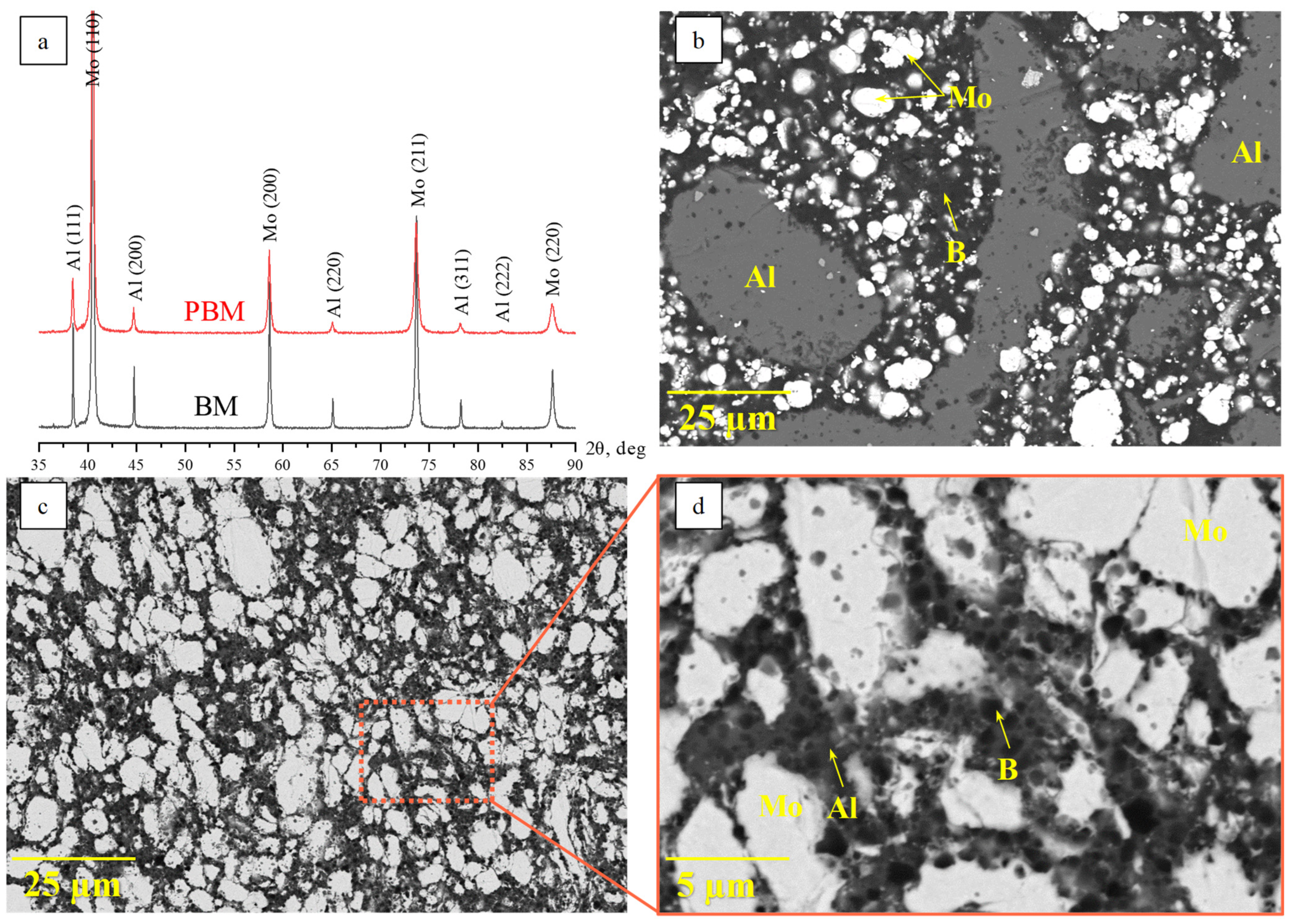
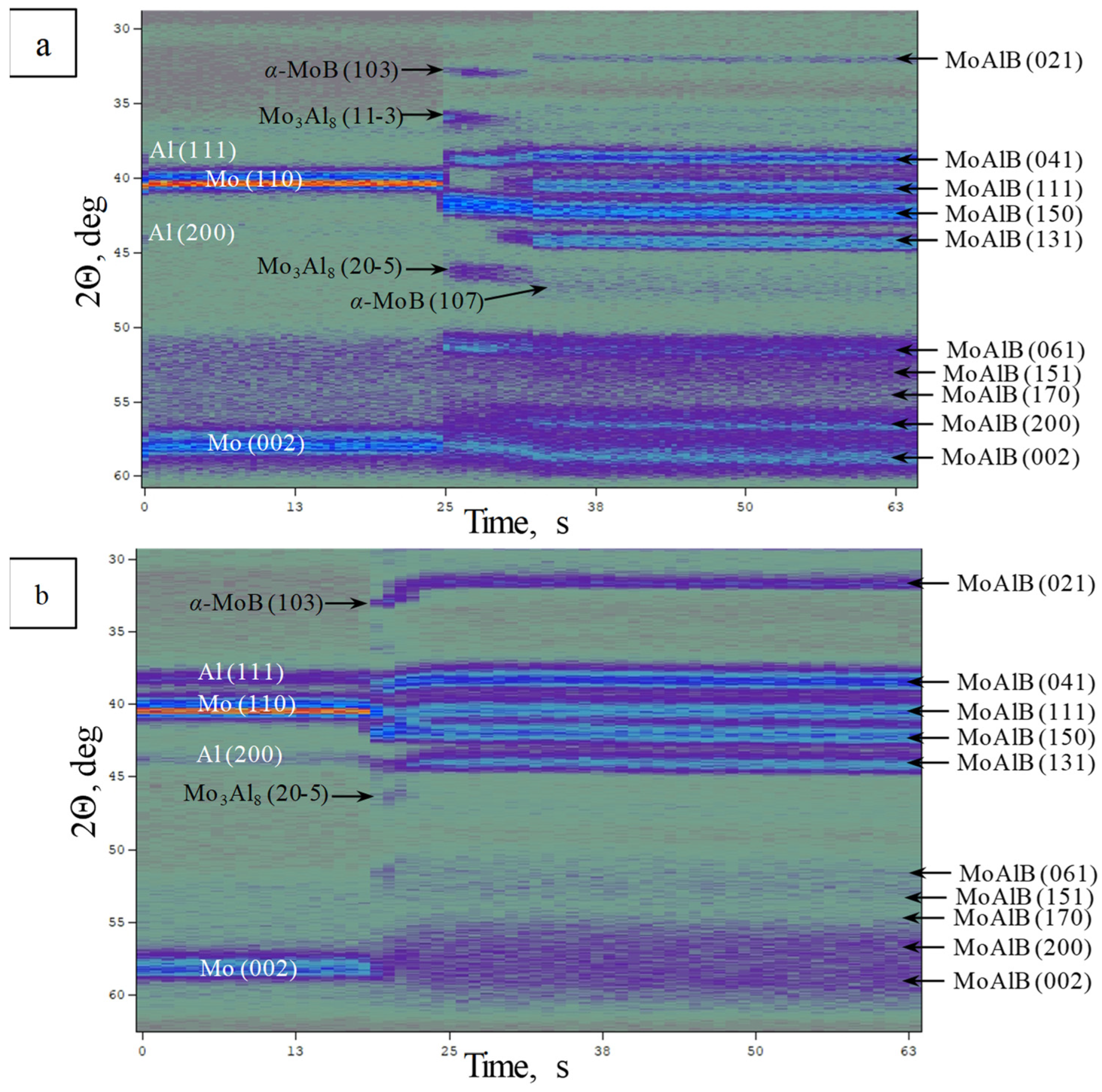
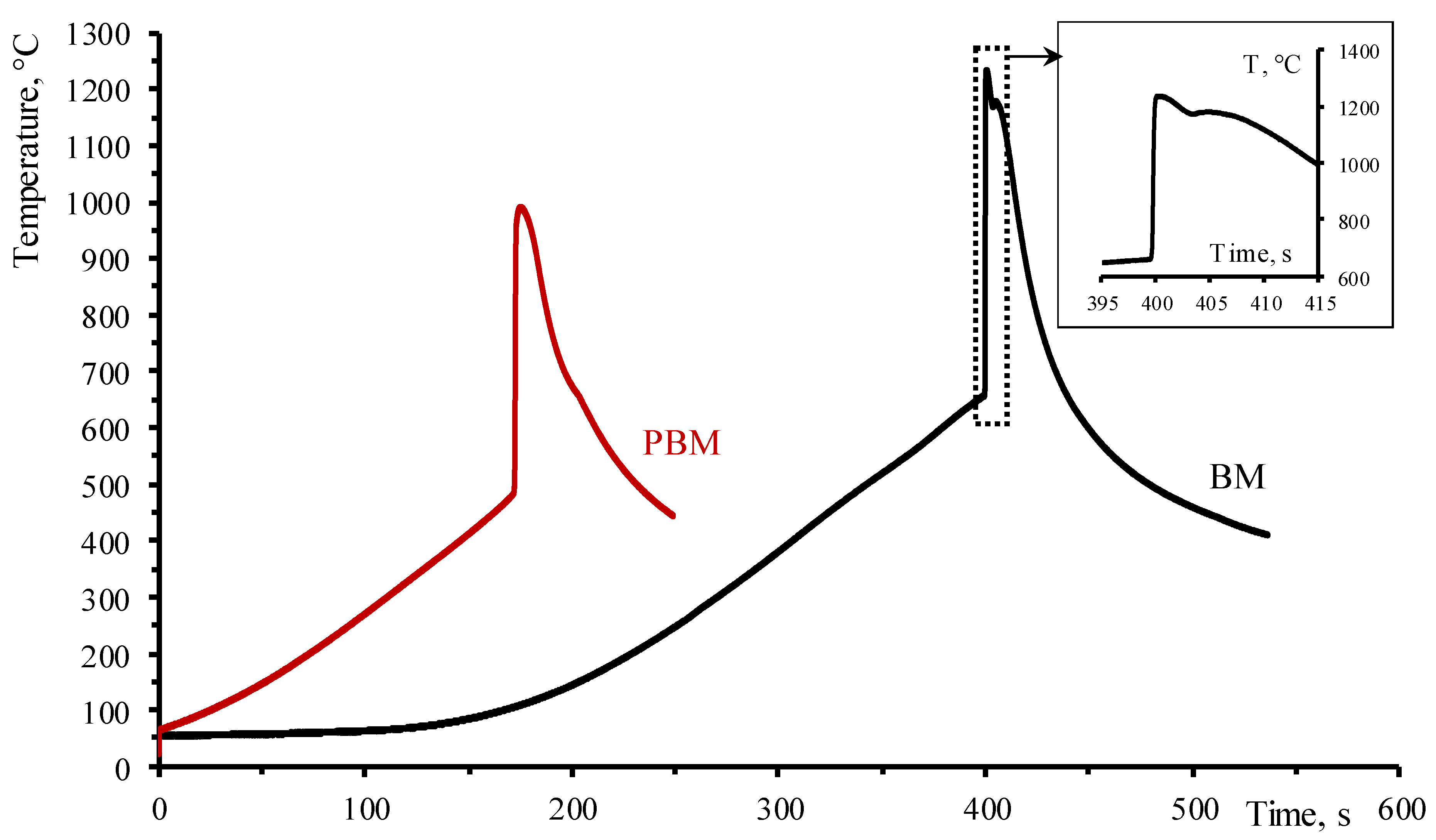
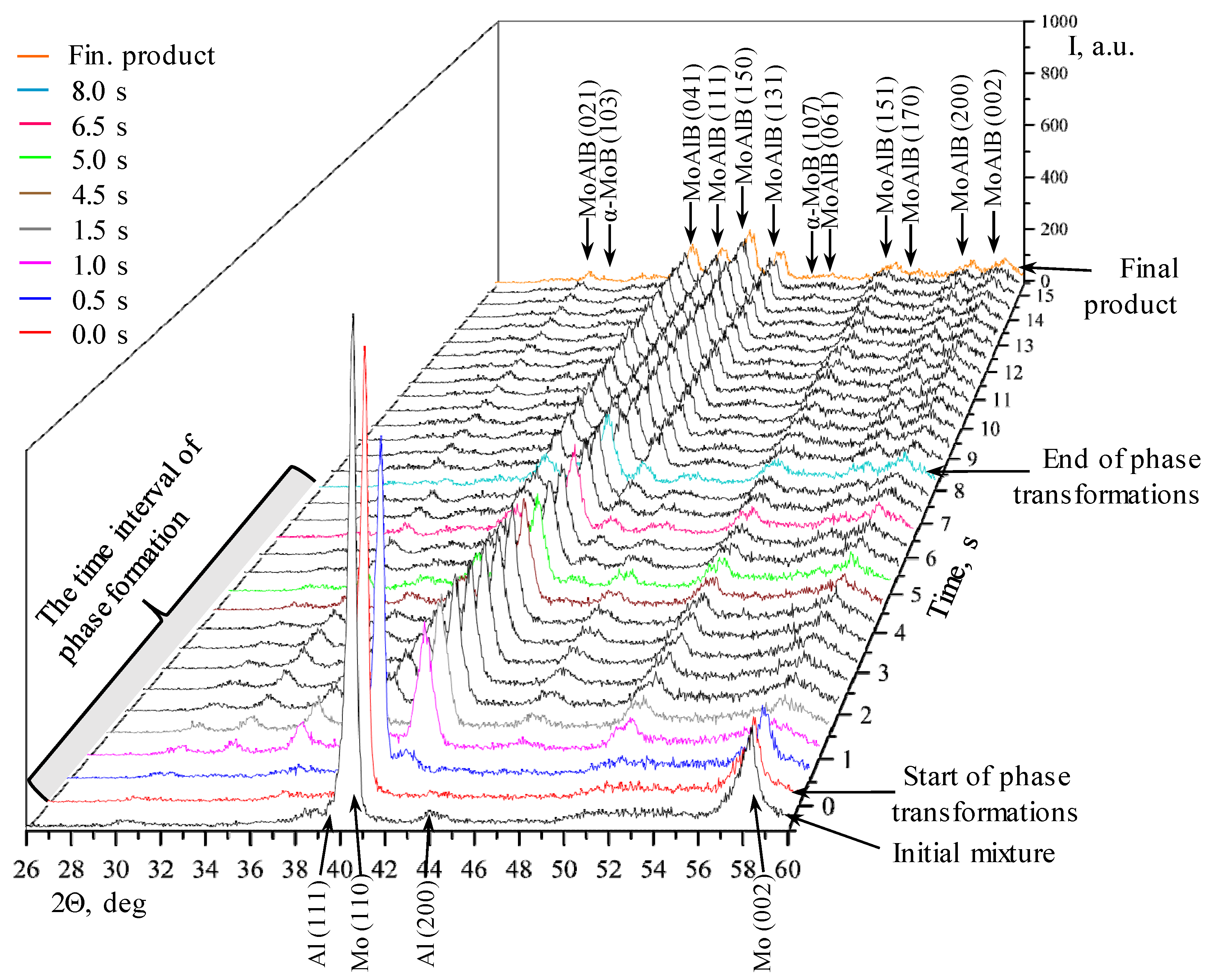

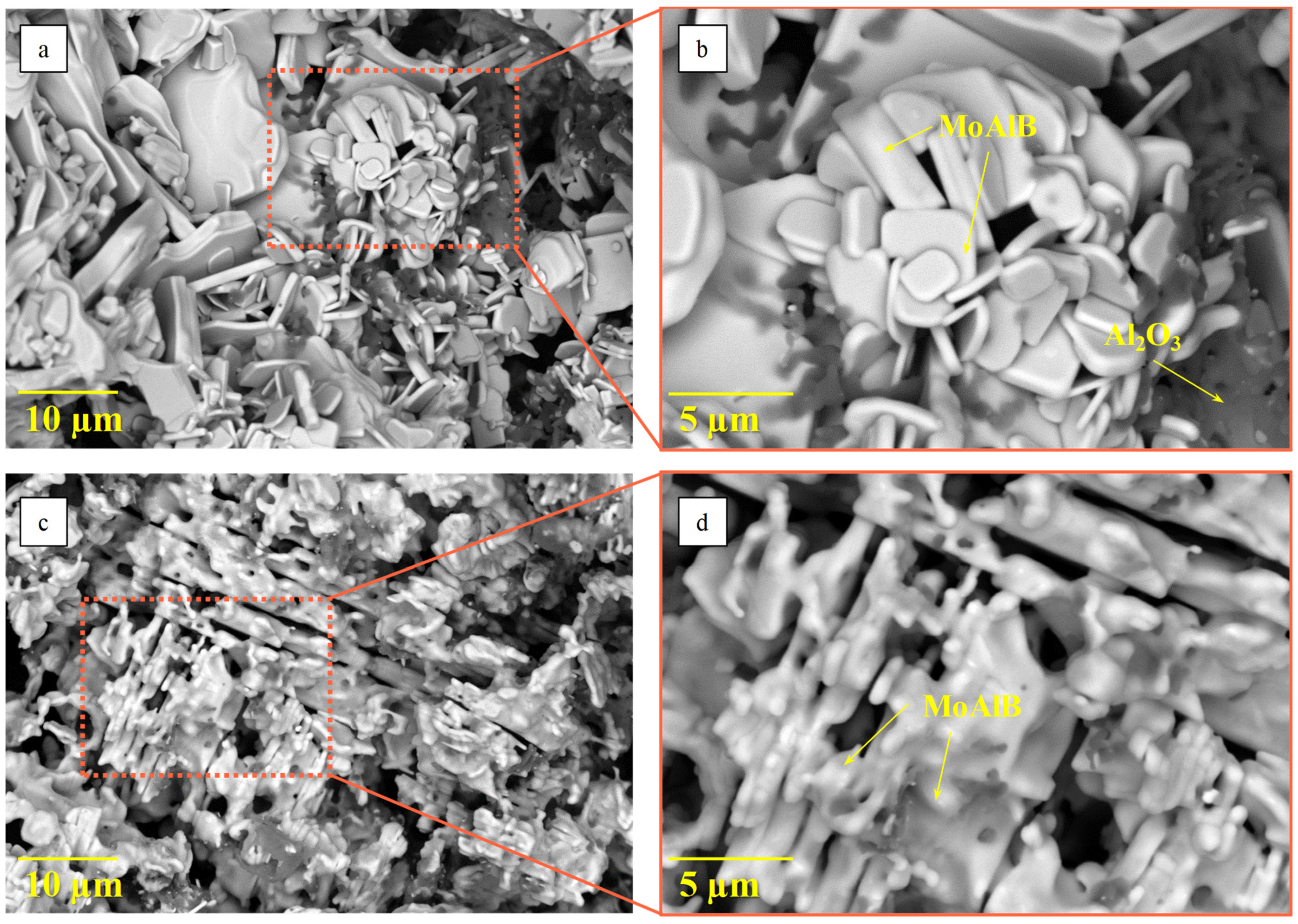
| Phase | MoAlB | α-MoB | β-MoB | α-Al2O3 | ||
| Space group | Cmcm | I41/amd | Cmcm | R-3c | ||
| Crystal system | Orthorhombic | Tetragonal | Orthorhombic | Hexagonal | ||
| Phase parameters | Wt., % | Unit cell parameters, Å | Unit cell volume, Å3 | Wt., % | Wt., % | Wt., % |
| SHS products from BM mixture | 92 | a = 3.211 | 139.22 | 5 | 1 | 2 |
| b = 13.977 | ||||||
| c = 3.102 | ||||||
| SHS products from PBM mixture | 94 | a = 3.213 | 139.41 | 3 | - | 3 |
| b = 13.979 | ||||||
| c = 3.104 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potanin, A.Y.; Bashkirov, E.A.; Kovalev, D.Y.; Sviridova, T.A.; Levashov, E.A. Phase Formation during the Synthesis of the MAB Phase from Mo-Al-B Mixtures in the Thermal Explosion Mode. Materials 2024, 17, 1025. https://doi.org/10.3390/ma17051025
Potanin AY, Bashkirov EA, Kovalev DY, Sviridova TA, Levashov EA. Phase Formation during the Synthesis of the MAB Phase from Mo-Al-B Mixtures in the Thermal Explosion Mode. Materials. 2024; 17(5):1025. https://doi.org/10.3390/ma17051025
Chicago/Turabian StylePotanin, Artem Yu., Evgeny A. Bashkirov, Dmitry Yu. Kovalev, Tatiana A. Sviridova, and Evgeny A. Levashov. 2024. "Phase Formation during the Synthesis of the MAB Phase from Mo-Al-B Mixtures in the Thermal Explosion Mode" Materials 17, no. 5: 1025. https://doi.org/10.3390/ma17051025
APA StylePotanin, A. Y., Bashkirov, E. A., Kovalev, D. Y., Sviridova, T. A., & Levashov, E. A. (2024). Phase Formation during the Synthesis of the MAB Phase from Mo-Al-B Mixtures in the Thermal Explosion Mode. Materials, 17(5), 1025. https://doi.org/10.3390/ma17051025







