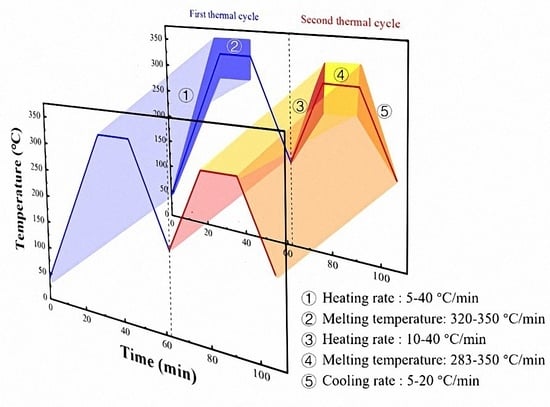
The Influence of Thermal Parameters on the Self-Nucleation Behavior of Polyphenylene Sulfide (PPS) during Secondary Thermoforming
Abstract
Highlights
- The influence of temperature parameters in two thermal cycles on the self-nucleation (SN) behavior was revealed.
- The heating rate and processing melt temperature during the first thermal cycle exhibited a synergistic effect on the SN behavior.
- Under appropriate conditions, both the first and second thermal cycles can generate SN behavior, but the mechanisms were different.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.2.1. Differential Scanning Calorimetry
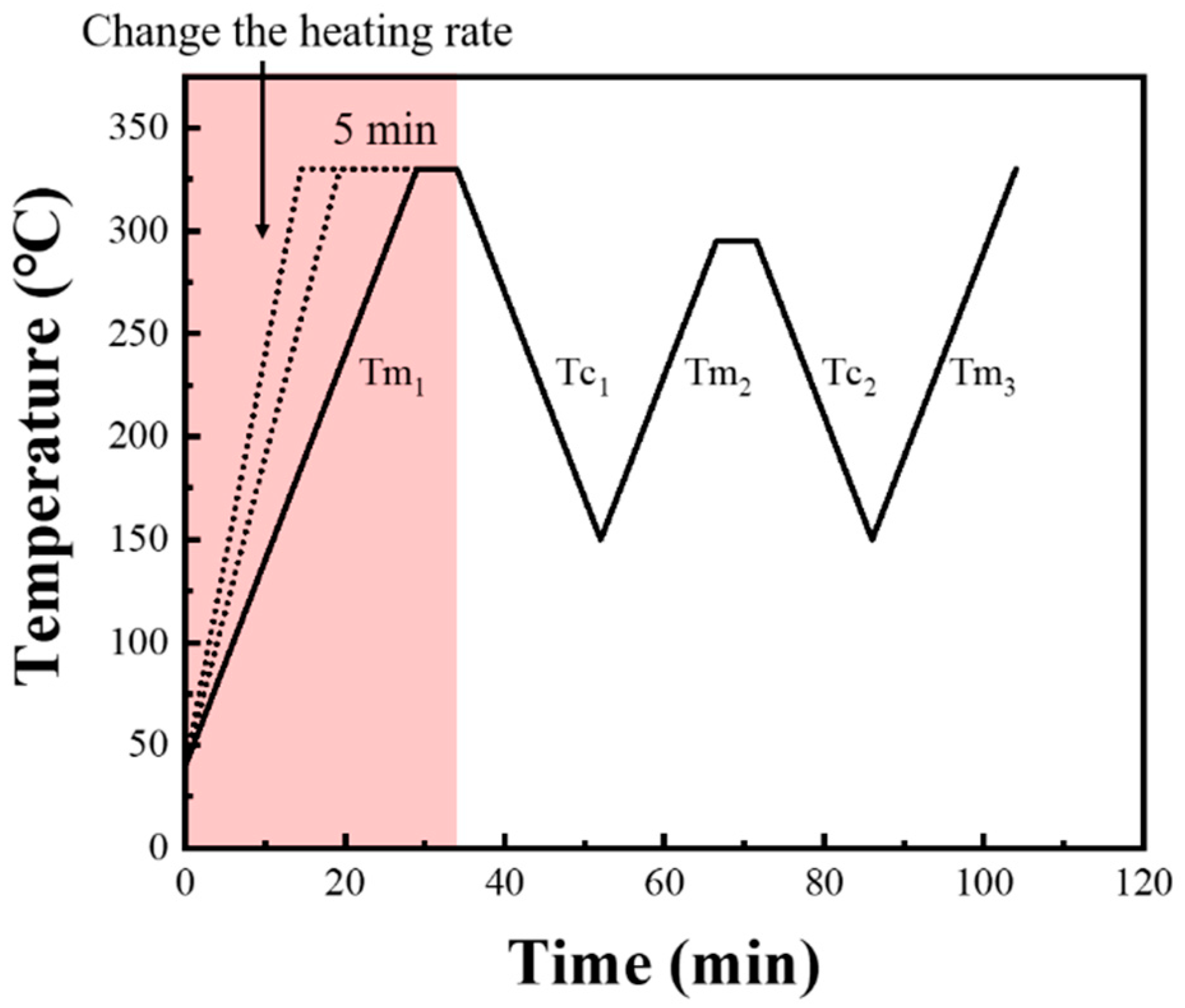
- (a)
- Due to the broad melting range of PPS, the melting peak occurs between 250 °C and 290 °C. To ensure melting completely and eliminated any thermal history effects during the first melting process, the sample was heated to the initial processing melt temperature (Ts1) at a specified heating rate (Hr1), and held for 5 min. Incomplete melting due to a too-low Ts1 would impact the consistency of the PPS melt during the first thermal cycle, ultimately affecting the investigation of PPS crystallization behavior.
- (b)
- The sample was then cooled to 150 °C at a designated cooling rate, completing its initial crystallization.
- (c)
- The crystallized sample was subsequently reheated to the processing melt temperature (Ts) at a specified heating rate (Hr2), and held for 5 min. Different Ts led to distinct molecular chain conformations in PPS, subsequently resulting in varied SN behaviors and crystallization behaviors.
- (d)
- The sample was cooled again to 150 °C at a specified cooling rate (Cr2) for its second crystallization.
- (e)
- Finally, the twice-crystallized sample was heated from 150 °C to 330 °C at a rate of 10 °C/min to analyze the crystalline structure forming in the second crystallization.
2.2.2. Polarized Optical Microscopy
3. Results and Discussion
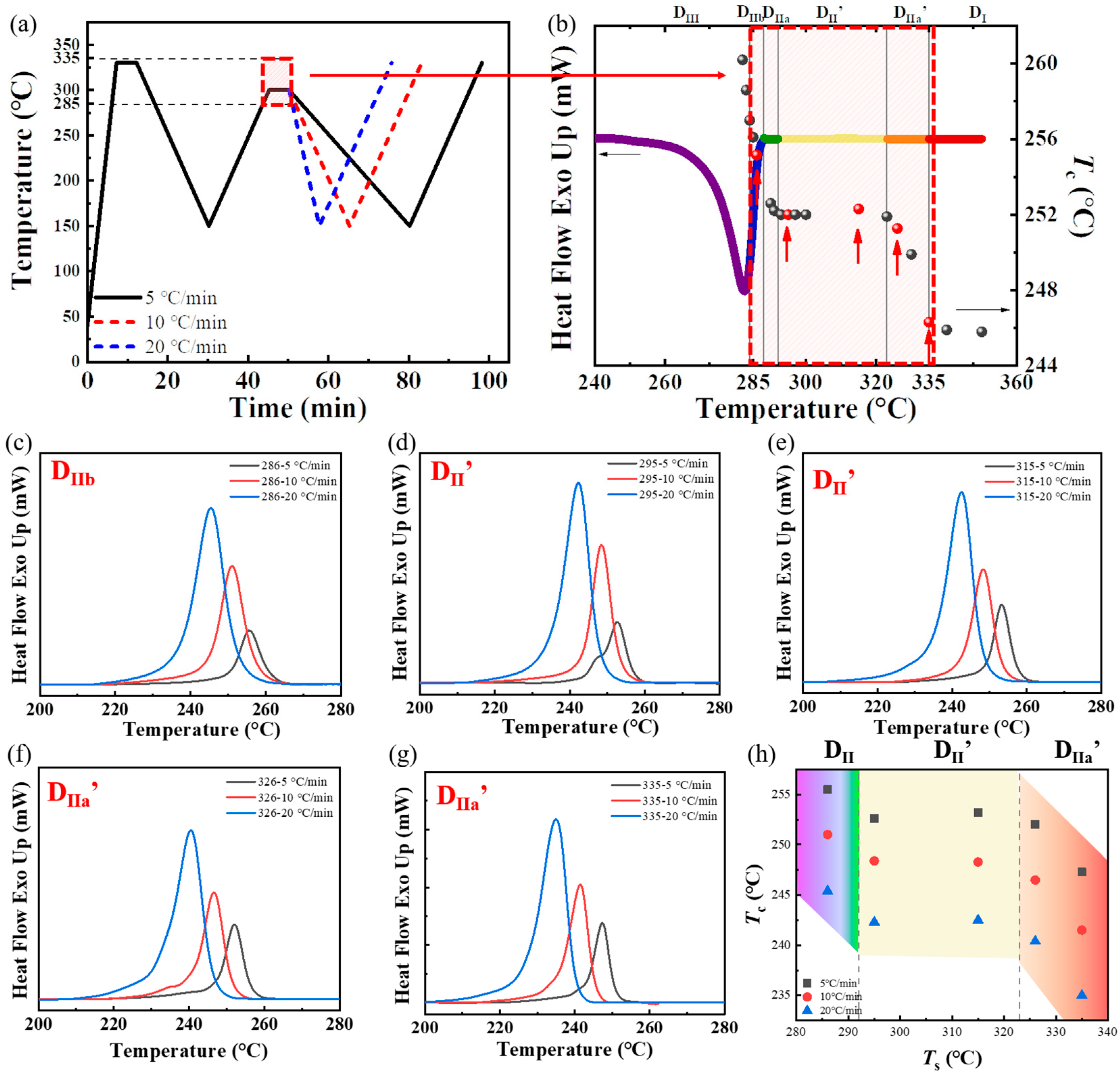
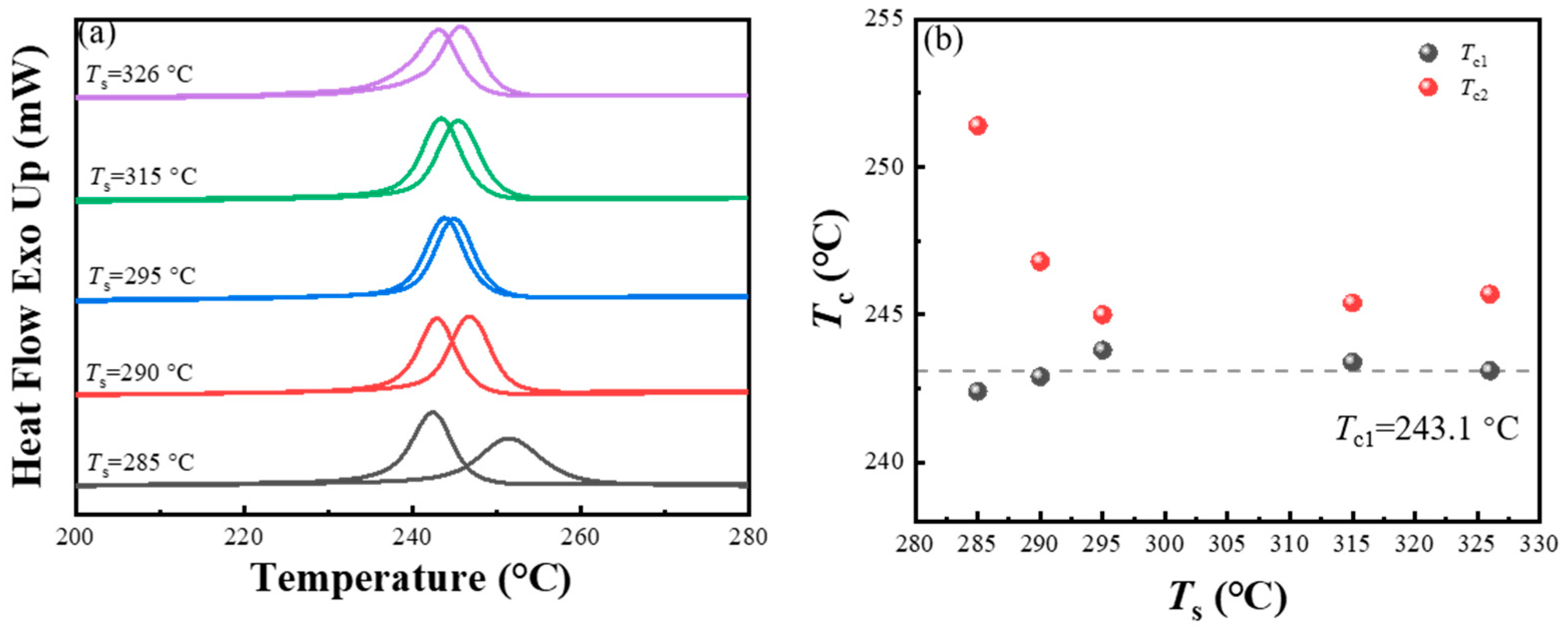
3.1. Effect of the Cooling Rate in the Second Thermal Cycle on the SN Behavior of PPS
3.2. Effect of the Heating Rate in the Second Thermal Cycle on the SN Behavior of PPS
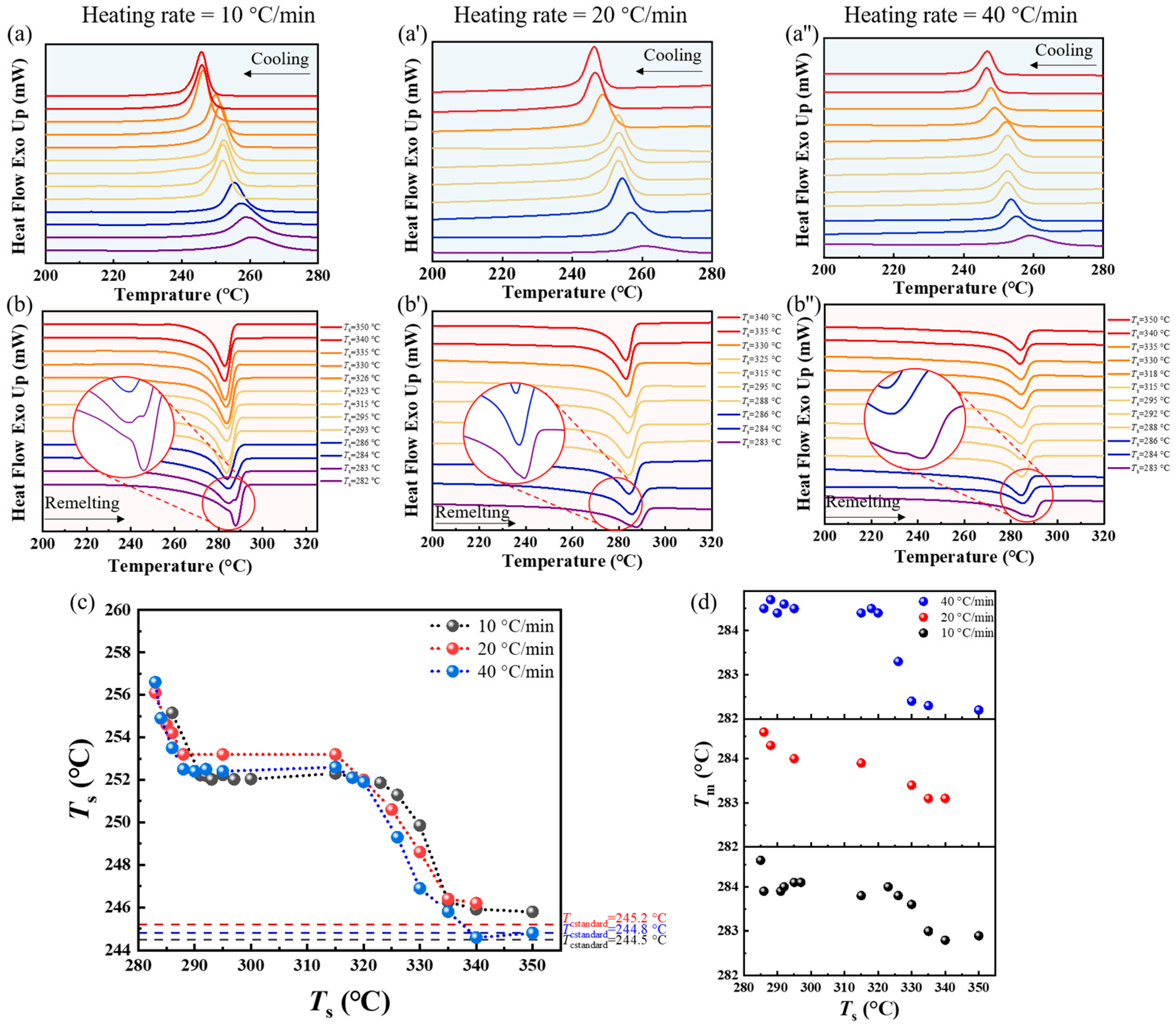
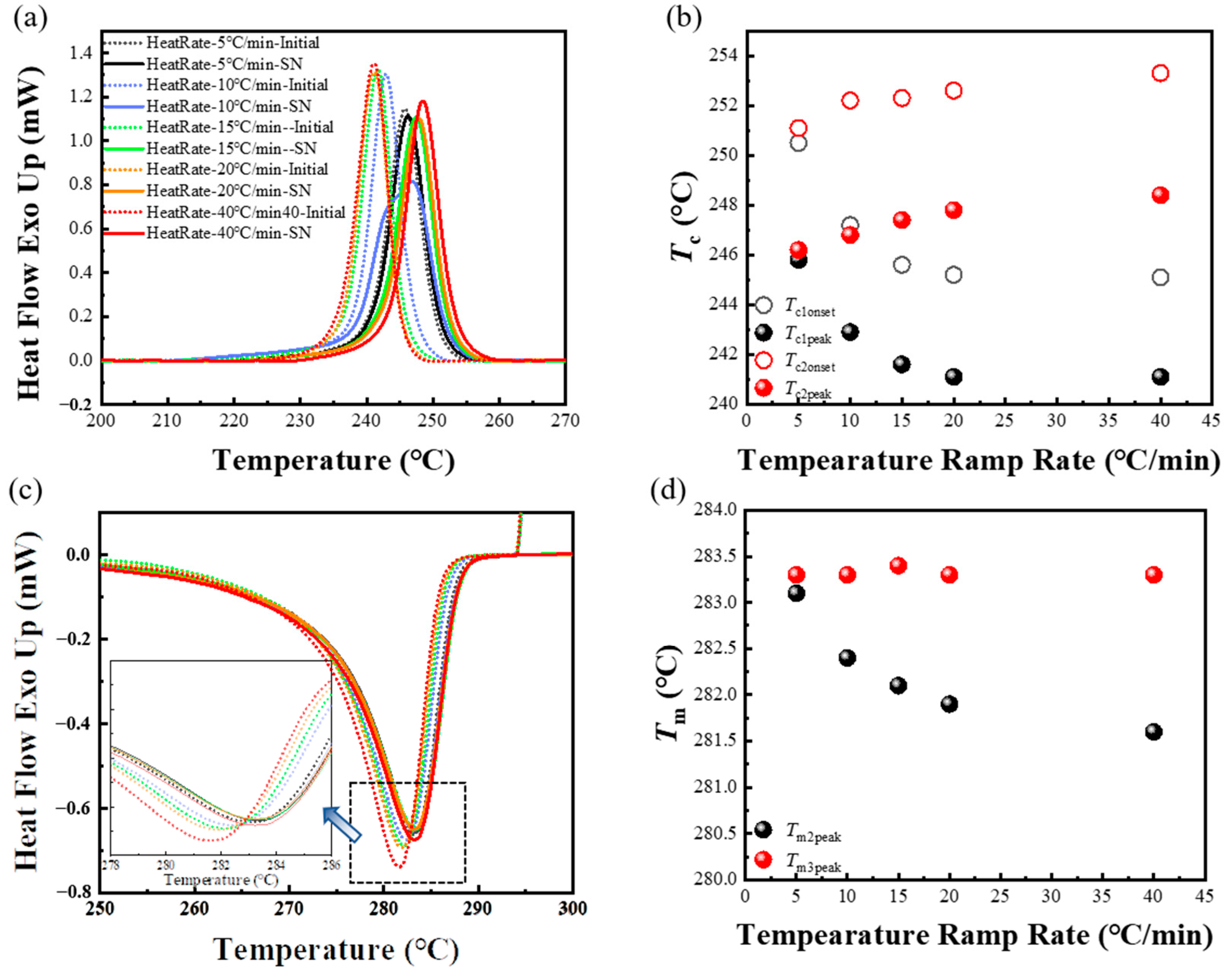
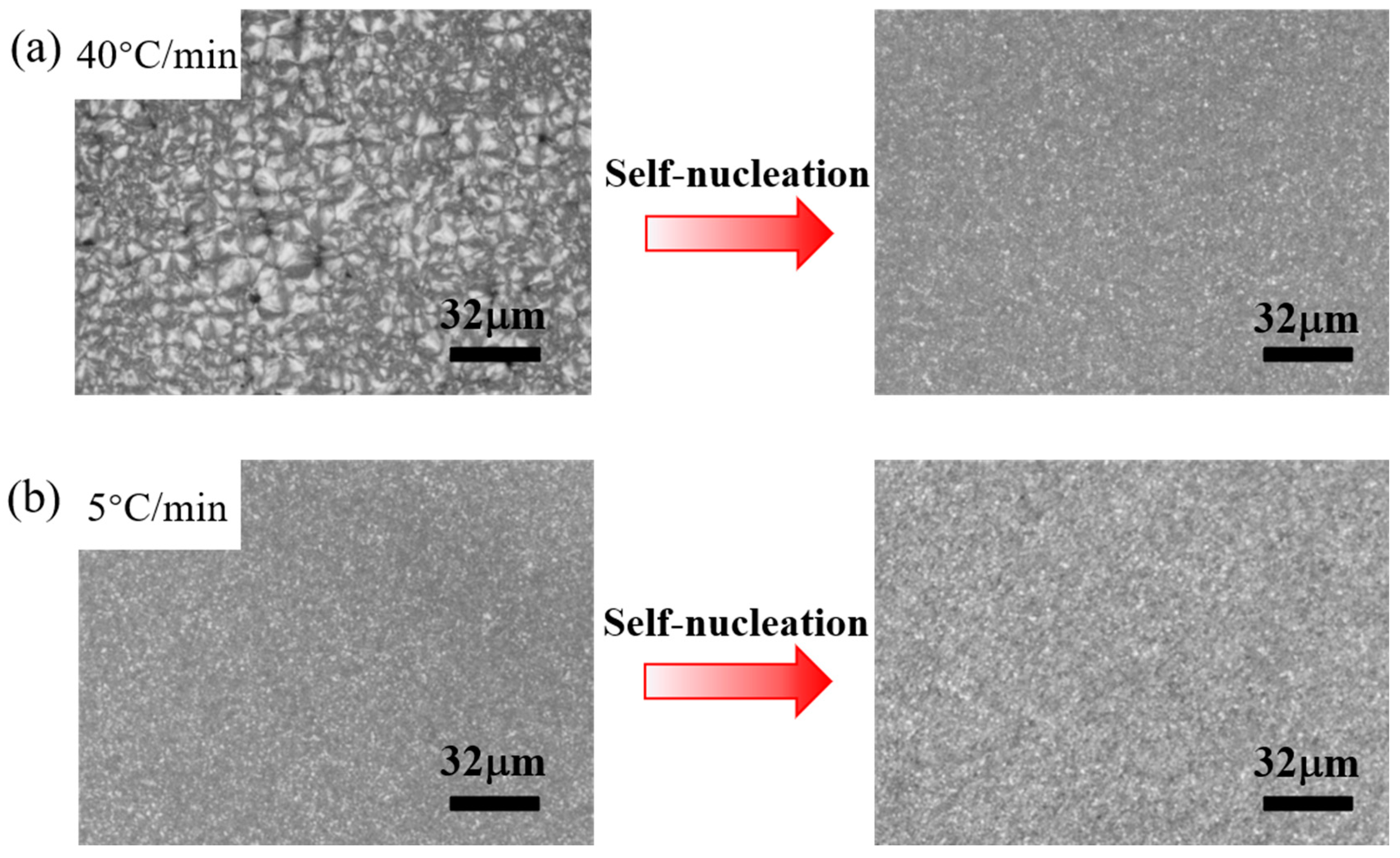
3.3. Effect of the Heating Rate in the First Thermal Cycle on the SN Behavior of PPS
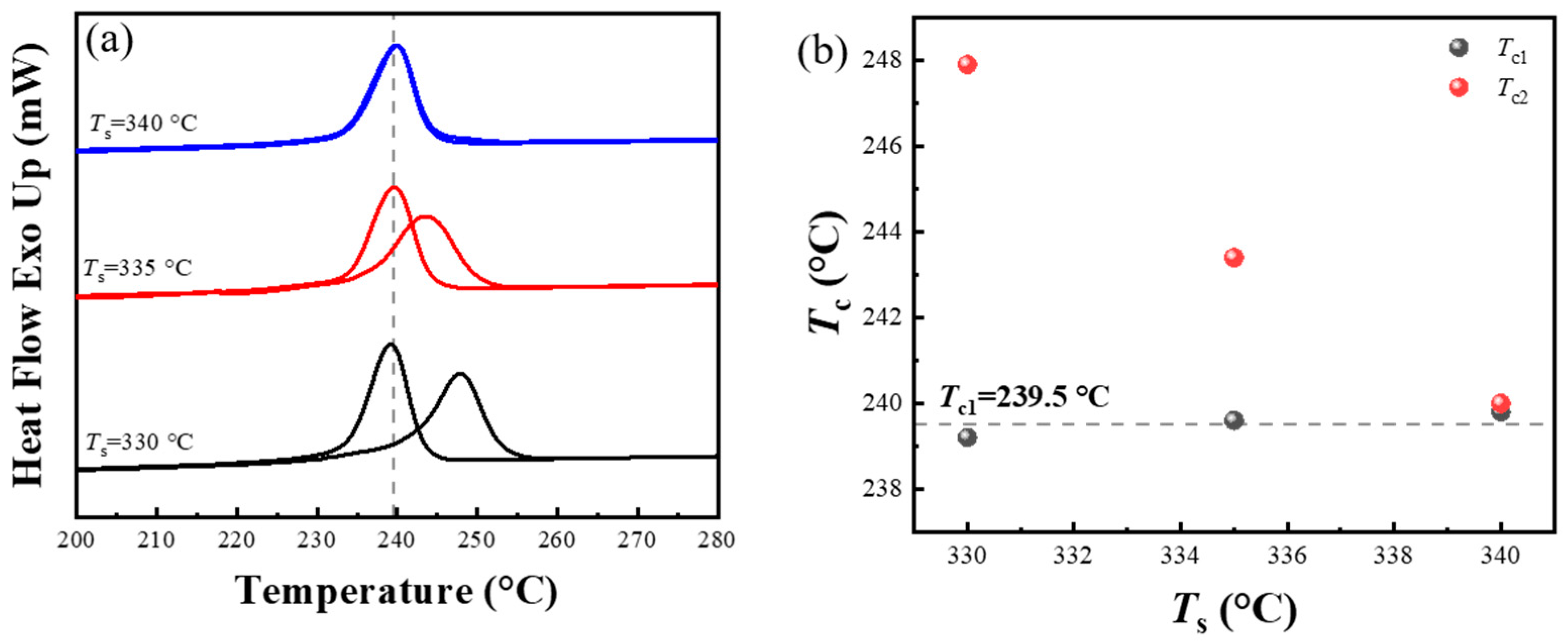
3.4. Effect of the Processing Melt Temperature in the First Thermal Cycle on the SN Behavior of PPS
4. Conclusions
- (1)
- While the cooling rate in the second thermal cycle changed the crystallization temperature of PPS, it did not influence the SN behavior of PPS, which was manifested as the same temperature range of the special SN domain, as well as the consistent change trend between the processing melt temperature and crystallization temperature in the second thermal cycle across different crystallization rates.
- (2)
- The heating rate of the second thermal cycle has a marginal influence on the perfection of the crystal. During the heating process of spherulites to melting, a slower heating rate led to significant annealing and thickening of lamellae, such as a rate less than 10 °C/min. As a result, the overall temperature range required for the formation of the specific Domain II’ in PPS was lower by approximately 4 °C.
- (3)
- When PPS undergoes the first thermal cycle, there is a synergistic effect between the heating rate and the processing melt temperature. At a slower heating rate or a lower processing melt temperature, such as a rate less than 10 °C/min or a first-cycle processing melt temperature below 330 °C, PPS displays SN behavior. The SN behavior observed in the first thermal cycle originates from crystals formed during the cold crystallization of PPS. These crystals are annealed, and the lamella thickening at a lower heating rate. Additionally, the defect excluded from the lamella might heighten the existing entanglement of the molecular chains. This results in PPS requiring a higher temperature to revert to the random coil conformation. In addition, even with a faster heating rate like 40 °C/min, if the processing melt temperature is too low and insufficient to disrupt the locally ordered regions of the PPS molecular chains, it will also lead to a strong melt memory effect. Therefore, controlling the SN behavior of PPS requires simultaneous control of these two factors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jagadeesh, P.; Rangappa, S.M.; Siengchin, S.; Puttegowda, M.; Thiagamani, S.M.K.; Rajeshkumar, G.; Kumar, M.H.; Oladijo, O.P.; Fiore, V.; Cuadrado, M.M.M. Sustainable recycling technologies for thermoplastic polymers and their composites: A review of the state of the art. Polym. Compos. 2022, 43, 5831–5862. [Google Scholar] [CrossRef]
- Stoeffler, K.; Andjelic, S.; Legros, N.; Roberge, J.; Schougaard, S.B. Polyphenylene sulfide (PPS) composites reinforced with recycled carbon fiber. Compos. Sci. Technol. 2013, 84, 65–71. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, H.B.; Ding, Z.R.; Li, N. Experimental and constitutive modelling studies of semicrystalline thermoplastics under solid-state stamp forming conditions. Polymer 2021, 228, 123939. [Google Scholar] [CrossRef]
- Özdemir, A.O.; Karatas, C.; Kocak, H.; Demiral, M. Investigation of earing defect and thickness distribution in stamp forming of continuous glass fiber reinforced polypropylene composite laminates. J. Thermoplast. Compos. Mater. 2023, 36, 2154–2168. [Google Scholar] [CrossRef]
- Sitohang, R.D.R.; Grouve, W.J.B.; Warnet, L.L.; Akkerman, R. Effect of in-plane fiber waviness defects on the compressive properties of quasi-isotropic thermoplastic composites. Compos. Struct. 2021, 272, 114166. [Google Scholar] [CrossRef]
- Chen, J.P.; Fu, K.K.; Li, Y. Understanding processing parameter effects for carbon fibre reinforced thermoplastic composites manufactured by laser-assisted automated fibre placement (AFP). Compos. Part A Appl. 2021, 140, 106160. [Google Scholar] [CrossRef]
- Boon, Y.D.; Joshi, S.C.; Bhudolia, S.K. Filament Winding and Automated Fiber Placement with In Situ Consolidation for Fiber Reinforced Thermoplastic Polymer Composites. Polymers 2021, 13, 1951. [Google Scholar] [CrossRef]
- Donough, M.J.; Shafaq; St John, N.A.; Philips, A.W.; Prusty, B.G. Process modelling of In-situ consolidated thermoplastic composite by automated fibre placement—A review. Compos. Part A Appl. 2022, 163, 107179. [Google Scholar] [CrossRef]
- O’Shaughnessey, P.G.; Dube, M.; Villegas, I.F. Modeling and experimental investigation of induction welding of thermoplastic composites and comparison with other welding processes. J. Compos. Mater. 2016, 50, 2895–2910. [Google Scholar] [CrossRef]
- Pappada, S.; Salomi, A.; Montanaro, J.; Passaro, A.; Caruso, A.; Maffezzoli, A. Fabrication of a thermoplastic matrix composite stiffened panel by induction welding. Aerosp. Sci. Technol. 2015, 43, 314–320. [Google Scholar] [CrossRef]
- Farahani, R.D.; Dube, M. Novel Heating Elements for Induction Welding of Carbon Fiber/Polyphenylene Sulfide Thermoplastic Composites. Adv. Eng. Mater. 2017, 19, 1700294. [Google Scholar] [CrossRef]
- Mai, K.C.; Zhang, M.Q.; Zeng, H.M.; Qi, S.C. Double melting phenomena of polyphenylene sulfide and its blends. J. Appl. Polym. Sci. 1994, 51, 57–62. [Google Scholar] [CrossRef]
- Ghanbari, L.N.; Crater, E.R.; Enos, N.R.; McNair, O.D.; Moore, R.B.; Wiggins, J.S. Polyphenylene sulfide for high-rate composite manufacturing: Impacts of processing parameters on chain architecture, rheology, and crystallinity. Polym. Degrad. Stab. 2023, 218, 110580. [Google Scholar] [CrossRef]
- Um, C.; Jung, D.; Yu, S.Y.; Lee, S.S.; Lee, H.W.; Lee, P.C.; Ko, Y.K.; Jeoung, S.K.; Ha, J.U. Study on Physical Properties of Polyphenylene Sulfide Composites with Variothermal Mold Temperatures. Polym. Korea 2020, 44, 798–803. [Google Scholar] [CrossRef]
- Zuo, P.Y.; Tcharkhtchi, A.; Shirinbayan, M.; Fitoussi, J.; Bakir, F. Effect of thermal aging on crystallization behaviors and dynamic mechanical properties of glass fiber reinforced polyphenylene sulfide (PPS/GF) composites. J. Polym. Res. 2020, 27, 77. [Google Scholar] [CrossRef]
- Hu, S.H.; Yang, F.; Xiang, M.; Cao, Y.; Wu, T.; Fu, Q. Competition of memory effect and thermal degradation on the crystallization behavior of polyphenylene sulfide. Polymer 2022, 262, 125427. [Google Scholar] [CrossRef]
- Ferrara, J.A.; Seferis, J.C.; Sheppard, C.H. Dual-mechanism kinetics of polyphenylene sulfide (PPS) melt-crystallization. J. Therm. Anal. Calorim. 1994, 42, 467–484. [Google Scholar] [CrossRef]
- Jog, J.P.; Bulakh, N.; Nadkarni, V.M. Crystallization of polyphenylene sulfide. B Mater. Sci. 1994, 17, 1079–1089. [Google Scholar] [CrossRef]
- Furushima, Y.; Nakada, M.; Yoshida, Y.; Okada, K. Crystallization/Melting Kinetics and Morphological Analysis of Polyphenylene Sulfide. Macromol. Chem. Phys. 2018, 219, 1700481. [Google Scholar] [CrossRef]
- Oshima, S.; Higuchi, R.; Kato, M.; Minakuchi, S.; Yokozeki, T.; Aoki, T. Cooling rate-dependent mechanical properties of polyphenylene sulfide (PPS) and carbon fiber reinforced PPS (CF/PPS). Compos. Part A Appl. 2023, 164, 107250. [Google Scholar] [CrossRef]
- Sangroniz, L.; Cavallo, D.; Müller, A.J. Self-Nucleation Effects on Polymer Crystallization. Macromolecules 2020, 53, 4581–4604. [Google Scholar] [CrossRef]
- Candau, N.; Fernandes, J.P.C.; Vasmer, E.; Maspoch, M.L. Cellulose nanocrystals as nucleating agents for the strain induced crystallization in natural rubber. Soft Matter 2022, 18, 8663–8674. [Google Scholar] [CrossRef]
- Yang, J.H.; Xu, T.; Lu, A.; Zhang, Q.; Tan, H.; Fu, Q. Preparation and properties of poly (p-phenylene sulfide)/multiwall carbon nanotube composites obtained by melt compounding. Compos. Sci. Technol. 2009, 69, 147–153. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Naffakh, M. Tuning the properties of carbon fiber-reinforced poly(phenylene sulphide) laminates via incorporation of inorganic nanoparticles. Polymer 2012, 53, 2369–2378. [Google Scholar] [CrossRef]
- Yan, P.; Yang, F.; Xiang, M.; Wu, T.; Fu, Q. New insights into the memory effect on the crystallization behavior of poly(phenylene sulfide). Polymer 2020, 195, 122439. [Google Scholar] [CrossRef]
- Ren, Y.; Lu, Y.G.; Zhang, B.; Su, J.Y.; Liu, W.P.; Zhou, J.F.; Yu, M.H.; Zhu, S. Control of the crystalline morphology of polyphenylene sulfide in secondary thermoforming by the strong memory effect of crystallization. Polymer 2023, 281, 126067. [Google Scholar] [CrossRef]
- Chen, X.; Mamun, A.; Alamo, R.G. Effect of Level of Crystallinity on Melt Memory Above the Equilibrium Melting Temperature in a Random Ethylene 1-Butene Copolymer. Macromol. Chem. Phys. 2015, 216, 1220–1226. [Google Scholar] [CrossRef]
- Gao, H.; Vadlamudi, M.; Alamo, R.G.; Hu, W. Monte Carlo Simulations of Strong Memory Effect of Crystallization in Random Copolymers. Macromolecules 2013, 46, 6498–6506. [Google Scholar] [CrossRef]
- Reid, B.O.; Vadlamudi, M.; Mamun, A.; Janani, H.; Gao, H.; Hu, W.; Alamo, R.G. Strong Memory Effect of Crystallization above the Equilibrium Melting Point of Random Copolymers. Macromolecules 2013, 46, 6485–6497. [Google Scholar] [CrossRef]
- De Stefano, F.; Cicolella, A.; Barreca, A.; Scoti, M.; De Rosa, C. Melt memory in propene-pentene isotactic copolymers: The case of defects hosted in the crystals. Polym. Chem. 2023, 14, 5260–5273. [Google Scholar] [CrossRef]
- Liao, Y.L.; Pan, L.; Ma, Z.; Cavallo, D.; Liu, G.M.; Wang, D.J.; Müller, A.J. How 4-(7-Octen-1-yl)-N,N-diphenylaniline co-units induce strong melt memory effects in isotactic polypropylene random copolymers. Polymer 2023, 282, 126184. [Google Scholar] [CrossRef]
- Sangroniz, L.; Safari, M.; de Ilarduya, A.M.; Sardon, H.; Cavallo, D.; Müller, A.J. Disappearance of Melt Memory Effect with Comonomer Incorporation in Isodimorphic Random Copolyesters. Macromolecules 2023, 56, 7879–7888. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Janke, A.; Androsch, R. Crystal self-nucleation in polyamide 11 of different semicrystalline morphology. Polymer 2019, 184, 121864. [Google Scholar] [CrossRef]
- Joijode, A.S.; Hawkins, K.; Tonelli, A.E. Improving Poly(ethylene terephthalate) Through Self-nucleation. Macromol. Mater. Eng. 2013, 298, 1190–1200. [Google Scholar] [CrossRef]
- Shen, J.F.; Zhou, Y.F.; Lu, Y.G.; Wang, B.H.; Shen, C.Y.; Chen, J.B.; Zhang, B. Later Stage Melting of Isotactic Polypropylene. Macromolecules 2020, 53, 2136–2144. [Google Scholar] [CrossRef]
- Balzano, L.; Rastogi, S.; Peters, G. Self-Nucleation of Polymers with Flow: The Case of Bimodal Polyethylene. Macromolecules 2011, 44, 2926–2933. [Google Scholar] [CrossRef]
- Vassiliadou, O.; Chrysostomou, V.; Pispas, S.; Klonos, P.A.; Kyritsis, A. Molecular dynamics and crystallization in polymers based on ethylene glycol methacrylates (EGMAs) with melt memory characteristics: From linear oligomers to comb-like polymers. Soft Matter 2021, 17, 1284–1298. [Google Scholar] [CrossRef]
- Sangroniz, L.; Ocando, C.; Cavallo, D.; Müller, A.J. Melt Memory Effects in Poly(butylene succinate) Studied by Differential Fast Scanning Calorimetry. Polymers 2020, 12, 2796. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, W. Role of Chain Dynamics in the Melt Memory Effect of Crystallization. Macromolecules 2020, 53, 7887–7898. [Google Scholar] [CrossRef]
- Arandia, I.; Mugica, A.; Zubitur, M.; Arbe, A.; Liu, G.; Wang, D.; Mincheva, R.; Dubois, P.; Muller, A.J. How Composition Determines the Properties of Isodimorphic Poly(butylene succinate-ran-butylene azelate) Random Biobased Copolymers: From Single to Double Crystalline Random Copolymers. Macromolecules 2015, 48, 43–57. [Google Scholar] [CrossRef]
- Liu, X.R.; Wang, Y.; Wang, Z.F.; Cavallo, D.; Muller, A.J.; Zhu, P.; Zhao, Y.; Dong, X.; Wang, D.J. The origin of memory effects in the crystallization of polyamides: Role of hydrogen bonding. Polymer 2020, 188, 122117. [Google Scholar] [CrossRef]
- Peters, O.A.; Still, R.H. The thermal degradation of poly (phenylene sulphide)—Part 1. Polym. Degrad. Stab. 1993, 42, 41–48. [Google Scholar] [CrossRef]
- Reents, W.D., Jr.; Kaplan, M.L. Mass spectral analysis of poly (p-phenylene sulphide). Polymer 1982, 23, 310–313. [Google Scholar] [CrossRef]
- Yan, P.; Peng, W.X.; Yang, F.; Cao, Y.; Xiang, M.; Wu, T.; Fu, Q. Investigation on thermal degradation mechanism of poly(phenylene sulfide). Polym. Degrad. Stab. 2022, 197, 109863. [Google Scholar] [CrossRef]
- Lian, D.; Zhang, R.; Lu, J.; Dai, J. Performances and structure changes of neat PPS fiber and nano Ti-SiO2-modified PPS fiber after over-temperature oxidation. High Perform. Polym. 2018, 30, 328–338. [Google Scholar] [CrossRef]
- Hawkins, R.T. Chemistry of the cure of poly (p-phenylene sulfide). Macromolecules 1976, 9, 189–194. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Liao, Y.; Zheng, Y.N.; Ikeda, K.; Okabe, R.; Wu, R.F.; Ozaki, R.; Xu, J.; Xu, Q.Y. Influence of Cooling Rate on Crystallization Behavior of Semi-Crystalline Polypropylene: Experiments and Mathematical Modeling. Polymers 2022, 14, 3646. [Google Scholar] [CrossRef] [PubMed]
- Boelle, S.; Alms, J.; Weihermueller, M.; Robisch, M.; Wipperfuerth, J.; Hopmann, C.; Dahlmann, R. Modelling of the melting point shift in semi-crystalline thermoplastics dependent on prior cooling rate and heating rate. Polymer 2022, 254, 125100. [Google Scholar] [CrossRef]
- Heidrich, D.; Gehde, M. Calorimetric analysis of the crystallization of PBT considering the dynamic cooling condition of real processing. Polym. Test. 2020, 85, 106459. [Google Scholar] [CrossRef]
- Moghadam, A.S.N.; Rafizadeh, M.; Taromi, F.A. Non-isothermal crystallization kinetics of polyethylene terephthalate: A study based on Tobin, Hay and Nakamura models. Iran. Polym. J. 2023, 32, 125–137. [Google Scholar] [CrossRef]
- Speranza, V.; Salomone, R.; Pantani, R. Effects of Pressure and Cooling Rates on Crystallization Behavior and Morphology of Isotactic Polypropylene. Crystals 2023, 13, 922. [Google Scholar] [CrossRef]
- Volpe, V.; Speranza, V.; Schrank, T.; Berer, M.; Pantani, R. An investigation of crystallization kinetics of polyoxymethylene in processing conditions. Polym. Adv. Technol. 2022, 34, 634–645. [Google Scholar] [CrossRef]
- Ren, J.Y.; Ouyang, Q.F.; Ma, G.Q.; Li, Y.; Lei, J.; Huang, H.D.; Jia, L.C.; Lin, H.; Zhong, G.J.; Li, Z.M. Enhanced dielectric and ferroelectric properties of poly (vinylidene fluoride) through annealing oriented crystallites under high pressure. Macromolecules 2022, 55, 2014–2027. [Google Scholar] [CrossRef]
- Bair, H.E.; Salovey, R.; Huseby, T.W. Melting and annealing of polyethylene single crystals. Polymer 1967, 8, 9–20. [Google Scholar] [CrossRef]
- Schick, C.; Androsch, R. The Origin of Annealing Peaks in Semicrystalline Polymers: Enthalpy Recovery or Melting? Macromolecules 2020, 53, 8751–8756. [Google Scholar] [CrossRef]
- Hu, W.B. Principles of Polymer Crystallization; Chemical Industry Press: Beijing, China, 2013. [Google Scholar]
- Hoffman, J.D.; Davis, G.T.; Lauritzen, J.I., Jr. The rate of crystallization of linear polymers with chain folding. In Treatise on Solid State Chemistry: Volume 3 Crystalline and Noncrystalline Solids; Springer: Berlin/Heidelberg, Germany, 1976; pp. 497–614. [Google Scholar]
- Yin, Y.G.; Song, Y.; Xiong, Z.J.; Zhang, X.Q.; de Vos, S.; Wang, R.Y.; Joziasse, C.A.P.; Liu, G.M.; Wang, D.J. Effect of the melting temperature on the crystallization behavior of a poly(L-lactide)/poly(D-lactide) equimolar mixture. J. Appl. Polym. Sci. 2016, 133, 43015. [Google Scholar] [CrossRef]
- Supaphol, P.; Lin, J.S. Crystalline memory effect in isothermal crystallization of syndiotactic polypropylenes: Effect of fusion temperature on crystallization and melting behavior. Polymer 2001, 42, 9617–9626. [Google Scholar] [CrossRef]
- Kong, W.L.; Zhu, B.; Su, F.M.; Wang, Z.; Shao, C.G.; Wang, Y.M.; Liu, C.T.; Shen, C.Y. Melting temperature, concentration and cooling rate-dependent nucleating ability of a self-assembly aryl amide nucleator on poly(lactic acid) crystallization. Polymer 2019, 168, 77–85. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Li, Z.; Li, X.; Su, J.; Li, Y.; Gao, Y.; Zhou, J.; Ji, C.; Zhu, S.; Yu, M. The Influence of Thermal Parameters on the Self-Nucleation Behavior of Polyphenylene Sulfide (PPS) during Secondary Thermoforming. Materials 2024, 17, 890. https://doi.org/10.3390/ma17040890
Ren Y, Li Z, Li X, Su J, Li Y, Gao Y, Zhou J, Ji C, Zhu S, Yu M. The Influence of Thermal Parameters on the Self-Nucleation Behavior of Polyphenylene Sulfide (PPS) during Secondary Thermoforming. Materials. 2024; 17(4):890. https://doi.org/10.3390/ma17040890
Chicago/Turabian StyleRen, Yi, Zhouyang Li, Xinguo Li, Jiayu Su, Yue Li, Yu Gao, Jianfeng Zhou, Chengchang Ji, Shu Zhu, and Muhuo Yu. 2024. "The Influence of Thermal Parameters on the Self-Nucleation Behavior of Polyphenylene Sulfide (PPS) during Secondary Thermoforming" Materials 17, no. 4: 890. https://doi.org/10.3390/ma17040890
APA StyleRen, Y., Li, Z., Li, X., Su, J., Li, Y., Gao, Y., Zhou, J., Ji, C., Zhu, S., & Yu, M. (2024). The Influence of Thermal Parameters on the Self-Nucleation Behavior of Polyphenylene Sulfide (PPS) during Secondary Thermoforming. Materials, 17(4), 890. https://doi.org/10.3390/ma17040890