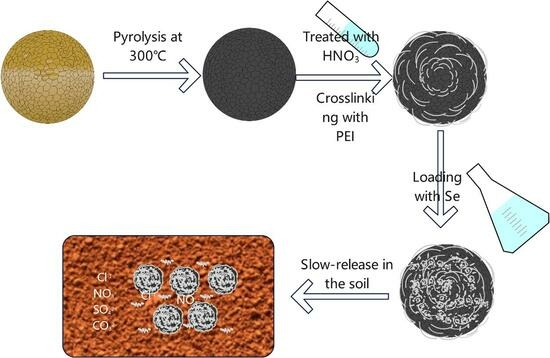
Effects of Pyrolysis Temperature and Acid-Base Pre-Treatment on the Synthesis of Biochar-Based Slow-Release Selenium Fertilizer and Its Release in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biochar and PEI-Modified Biochar
2.2. PEI-Modified Biochar-Based Se Fertilizer Loading Experiment
2.2.1. Batch Adsorption
2.2.2. Adsorption Kinetics
2.2.3. The Loading Capacity of Selenite
2.3. PEI-Modified Biochar Se Fertilizer Releasing Experiment
2.3.1. The Maximum Exchange-Releasing Potential of PEI-Modified Biochar Se Fertilizer
2.3.2. Release Character of Se in Red and Brown Soil
2.4. Characterization of Biochar and Determination of Selenium
2.5. Analysis Statics
3. Results and Discussion
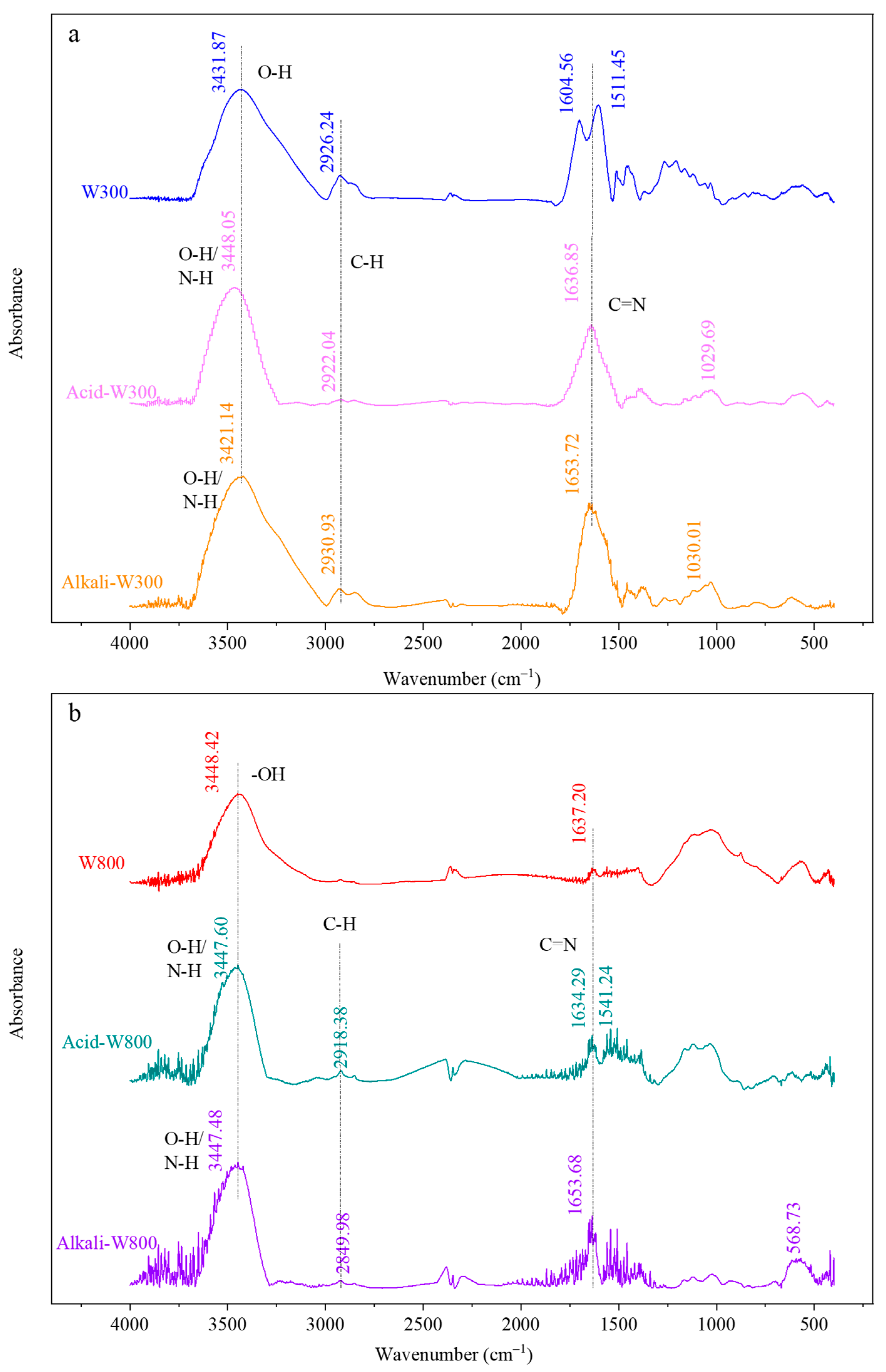
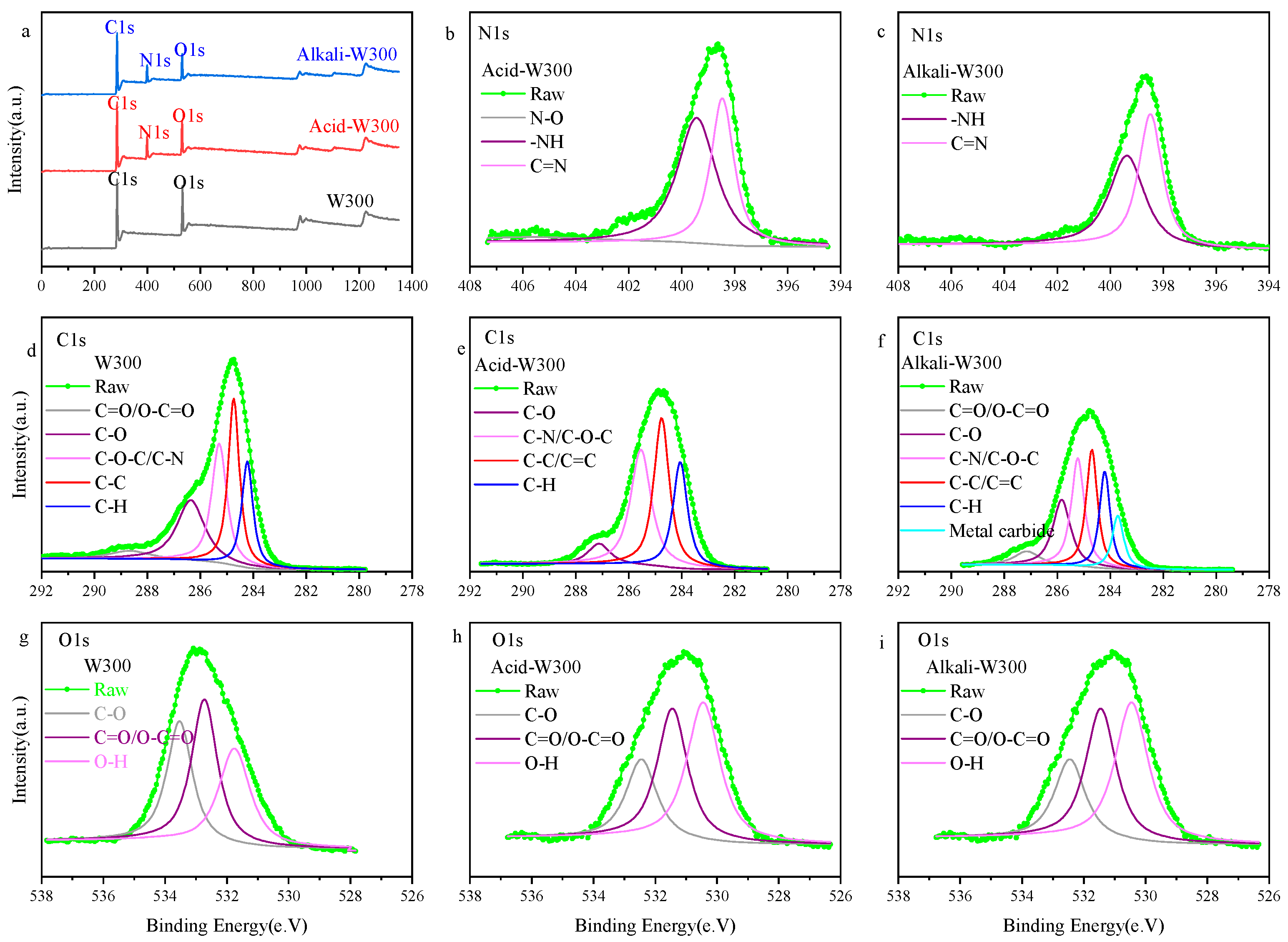
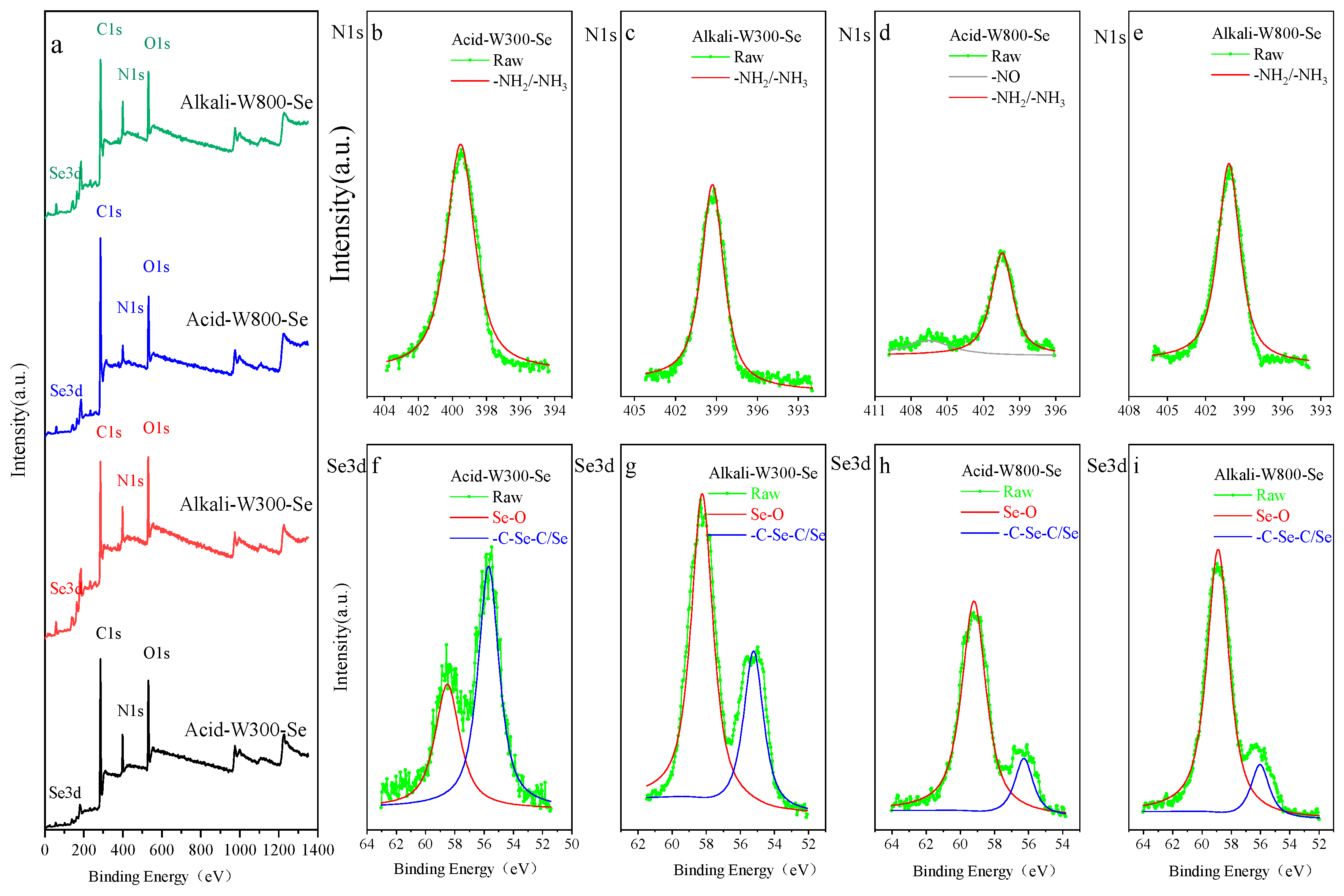
3.1. Characterization of Biochar before and after Modification
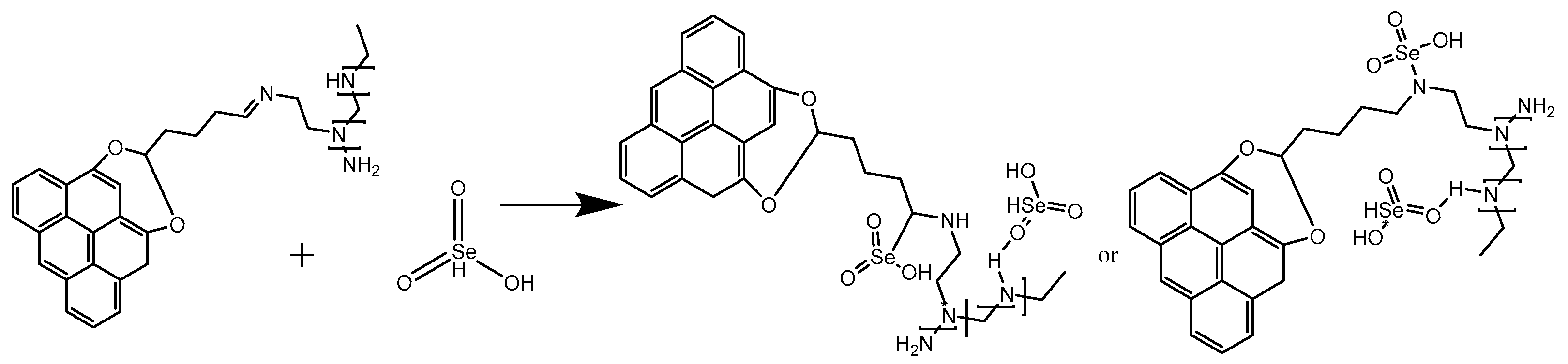
3.2. Loading Characteristics of Modified Biochar on Sodium Selenite
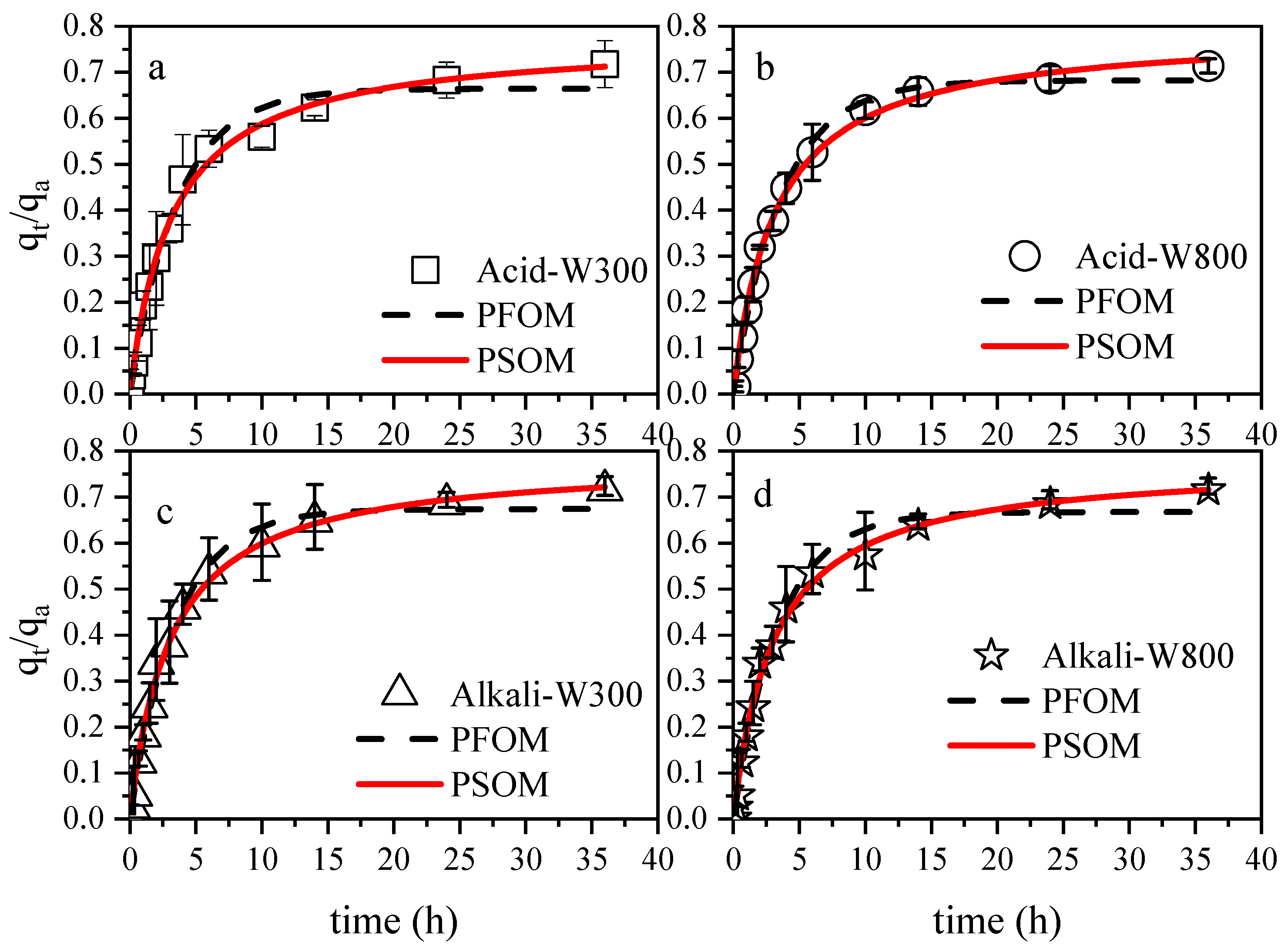
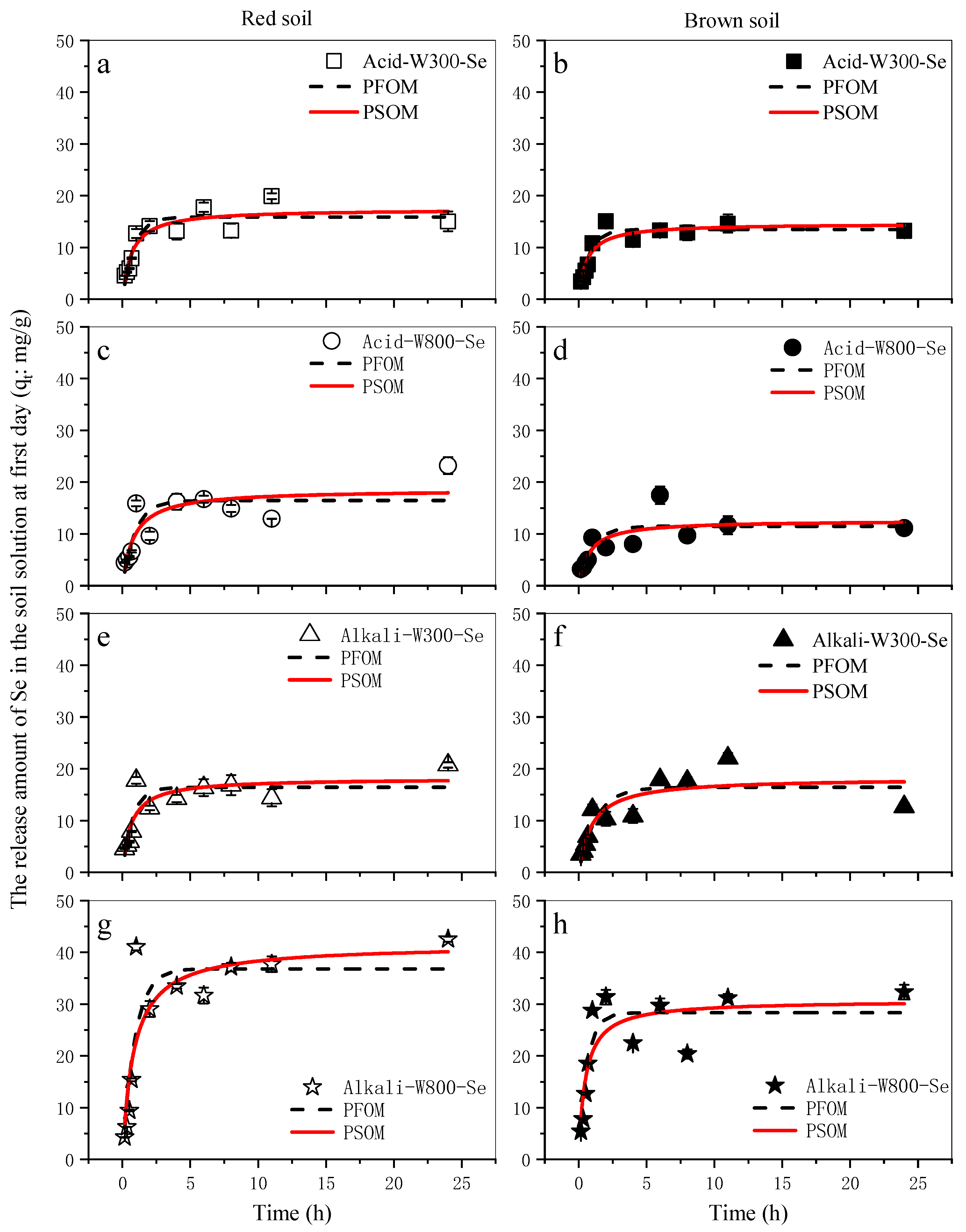
3.3. Release Characteristics of Modified Biochar-Based Selenium in Red and Brown Soils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. J. Anim. Sci. Biotechnol. 2022, 13, 58. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef] [PubMed]
- Banuelos, G.S.; Lin, Z.-Q.; Caton, J. Selenium in soil-plant-animal systems and its essential role for human health. Front. Plant Sci. 2023, 14, 1237646. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Cominetti, C.; Seale, L.A. Editorial: Selenium, Human Health and Chronic Disease. Front. Nutr. 2022, 8, 827759. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Muehling, K.H. Selenium Exerts an Intriguing Alteration of Primary and Secondary Plant Metabolites: Advances, Challenges, and Prospects. Crit. Rev. Plant Sci. 2023, 42, 34–52. [Google Scholar] [CrossRef]
- Bjorklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef] [PubMed]
- Tangjaidee, P.; Swedlund, P.; Xiang, J.; Yin, H.; Quek, S.Y. Selenium-enriched plant foods: Selenium accumulation, speciation, and health functionality. Front. Nutr. 2023, 9, 962312. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Hatfield, D.L. Editorial to Special Issue Molecular Biology of Selenium in Health and Disease. Int. J. Mol. Sci. 2022, 23, 808. [Google Scholar] [CrossRef]
- Kieliszek, M.; Bano, I. Selenium as an important factor in various disease states—A review. EXCLI J. 2022, 21, 948–966. [Google Scholar] [CrossRef]
- Acosta-Luque, M.P.; López, J.E.; Henao, N.; Zapata, D.; Giraldo, J.C.; Saldarriaga, J.F. Remediation of Pb-contaminated soil using biochar-based slow-release P fertilizer and biomonitoring employing bioindicators. Sci. Rep. 2023, 13, 1657. [Google Scholar] [CrossRef]
- Mutonhodza, B.; Joy, E.J.M.; Bailey, E.H.; Lark, M.R.; Kangara, M.G.M.; Broadley, M.R.; Matsungo, T.M.; Chopera, P. Linkages between soil, crop, livestock, and human selenium status in Sub-Saharan Africa: A scoping review. Int. J. Food Sci. Technol. 2022, 57, 6336–6349. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Thivet, J.; Laisne, E.; Srivastava, V.; Grimm, A.; Lima, E.C.; Bergna, D.; Hu, T.; Naushad, M.; Lassi, U. Synthesis of novel mesoporous selenium-doped biochar with high-performance sodium diclofenac and reactive orange 16 dye removals. Chem. Eng. Sci. 2023, 281, 119129. [Google Scholar] [CrossRef]
- Ok, Y.S.; Bhatnagar, A.; Hou, D.Y.; Bhaskar, T.; Masek, O. Advances in algal biochar: Production, characterization and applications Preface. Bioresour. Technol. 2020, 317, 123982. [Google Scholar] [CrossRef]
- Pandey, D.; Daverey, A.; Dutta, K.; Arunachalam, K. Enhanced adsorption of Congo red dye onto polyethyleneimine-impregnated biochar derived from pine needles. Environ. Monit. Assess. 2022, 194, 880. [Google Scholar] [CrossRef]
- Truong, Q.-M.; Nguyen, T.-B.; Chen, C.-W.; Chen, W.-H.; Bui, X.-T.; Dong, C.-D. KHCO3-activated high surface area biochar derived from brown algae: A case study for efficient adsorption of Cr(VI) in aqueous solution. Environ. Res. 2024, 247, 118227. [Google Scholar] [CrossRef] [PubMed]
- Mustaffa, M.R.A.; Pandian, K.; Chitraputhirapillai, S.; Kuppusamy, S.; Dhanushkodi, K. Synthesis of biochar-embedded slow-release nitrogen fertilizers: Mesocosm and field scale evaluation for nitrogen use efficiency, growth and rice yield. Soil Use Manag. 2023, 40, e12959. [Google Scholar] [CrossRef]
- Ngo, T.; Shahsavari, E.; Shah, K.L.; Surapaneni, A.; Ball, A.S. Improving bioenergy production in anaerobic digestion systems utilising chicken manure via pyrolysed biochar additives: A review. Fuel 2022, 316, 123374. [Google Scholar] [CrossRef]
- Witczak, A.; Pokorska-Niewiada, K.; Tomza-Marciniak, A.; Witczak, G.; Cybulski, J.; Aftyka, A. The Problem of Selenium for Human Health-Removal of Selenium from Water and Wastewater. Water 2023, 15, 2230. [Google Scholar] [CrossRef]
- El-Sayed, M.; Nada, A.A. Polyethylenimine functionalized amorphous carbon fabricated from oil palm leaves as a novel adsorbent for Cr(VI) and Pb(II) from aqueous solution. J. Water Process Eng. 2017, 16, 296–308. [Google Scholar] [CrossRef]
- Chun, Y.; Lee, S.K.; Yoo, H.Y.; Kim, S.W. Recent Advancements in Biochar Production According to Feedstock Classification, Pyrolysis Conditions, and Applications: A Review. Bioresources 2021, 16, 6512–6547. [Google Scholar] [CrossRef]
- Hall, J.A.; Bobe, G.; Filley, S.J.; Pirelli, G.J.; Bohle, M.G.; Wang, G.J.; Davis, T.Z.; Banuelos, G.S. Effects of Amount and Chemical Form of Selenium Amendments on Forage Selenium Concentrations and Species Profiles. Biol. Trace Elem. Res. 2023, 201, 4951–4960. [Google Scholar] [CrossRef]
- Sami, H.; Ashraf, K.; Sultan, K.; Alamri, S.; Abbas, M.; Javied, S.; Zaman, Q.U. Remediation potential of biochar and selenium for mitigating chromium-induced stress in spinach to minimize human health risk. S. Afr. J. Bot. 2023, 163, 237–249. [Google Scholar] [CrossRef]
- Vojodi Mehrabani, L.; Hassanpouraghdam, M.B.; Rasouli, F.; Okcu, Z.; Alina Marc, R. Foliar application of graphene oxide, nano-Fe, and selenium mitigates salinity depression on Ocimum basilicum. Turk. J. Agric. For. 2023, 47, 510–528. [Google Scholar] [CrossRef]
- He, Y.; Chen, J.; Lv, J.; Huang, Y.; Zhou, S.; Li, W.; Li, Y.; Chang, F.; Zhang, H.; Wagberg, T.; et al. Separable amino-functionalized biochar/alginate beads for efficient removal of Cr(VI) from original electroplating wastewater at room temperature. J. Clean. Prod. 2022, 373, 133790. [Google Scholar] [CrossRef]
- Truong, H.B.; Ike, I.A.; Ok, Y.S.; Hur, J. Polyethyleneimine modification of activated fly ash and biochar for enhanced removal of natural organic matter from water via adsorption. Chemosphere 2020, 243, 125454. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, C.; Zeng, W. Ultra-high selective removal of CR and Cr(VI) from aqueous solutions using polyethyleneimine functionalized magnetic hydrochar: Application strategy and mechanisms insight. Chem. Eng. J. 2022, 448, 137464. [Google Scholar] [CrossRef]
- Ezzati, R.; Ezzati, S.; Azizi, M. Exact solution of the Langmuir rate equation: New Insights into pseudo-first-order and pseudo-second-order kinetics models for adsorption. Vacuum 2024, 220, 112790. [Google Scholar] [CrossRef]
- Goldan, E.; Nedeff, V.; Barsan, N.; Culea, M.; Panainte-Lehadus, M.; Mosnegutu, E.; Tomozei, C.; Chitimus, D.; Irimia, O. Assessment of Manure Compost Used as Soil Amendment—A Review. Processes 2023, 11, 1167. [Google Scholar] [CrossRef]
- Xiong, Y.; Tian, X.; Qiu, T.; Cong, X.; Zheng, X.; Chen, S.; You, A.; Cheng, S.; Wu, M.; Xu, D. Effects of SeNPs Fertilizer on Se and Microelement Contents, Eating and Cooking Qualities, and Volatile Organic Compounds in Rice Grains. Sustainability 2023, 15, 10553. [Google Scholar] [CrossRef]
- Gao, Z.; Shan, D.; He, J.; Huang, T.; Mao, Y.; Tan, H.; Shi, H.; Li, T.; Xie, T. Effects and mechanism on cadmium adsorption removal by CaCl2-modified biochar from selenium-rich straw. Bioresour. Technol. 2023, 370, 128563. [Google Scholar] [CrossRef]
- Lei, C.; Yi, B.; Deng, W.; Chen, M.; Wang, Y. Effect of metal cationic on the adsorption of selenium from sewage by biochar loaded with zero-valent iron. Desalination Water Treat. 2022, 245, 202–216. [Google Scholar] [CrossRef]
- Ma, Q.; Haider, F.U.; Farooq, M.; Adeel, M.; Shakoor, N.; Wu, J.; Xu, J.; Wang, X.W.; Luo, P.; Cai, L. Selenium treated foliage and biochar treated soil for improved lettuce (Lactuca sativa L.) growth in Cd-polluted soil. J. Clean. Prod. 2022, 335, 130267. [Google Scholar] [CrossRef]
- Khalid, U.; Inam, M.A. The Influence of Pyrolysis Temperature on the Performance of Cotton Stalk Biochar for Hexavalent Chromium Removal from Wastewater. Water Air Soil Pollut. 2024, 235, 114. [Google Scholar] [CrossRef]
- Mo, Y.Y.; Vincent, T.; Faur, C.; Guibal, E. Se(VI) sorption from aqueous solution using alginate/polyethylenimine membranes: Sorption performance and mechanism. Int. J. Biol. Macromol. 2020, 147, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Sadr, A.M.; Shahidi, A.; Siuki, A.K.; Bagheri, H. Electrokinetic remediation of chromium-contaminated saline soil coupled with permeable reactive barrier of biochar. Environ. Earth Sci. 2024, 83, 29. [Google Scholar] [CrossRef]
- Serrano, M.F.; Lopez, J.E.; Henao, N.; Saldarriaga, J.F. Phosphorus-Loaded Biochar-Assisted Phytoremediation to Immobilize Cadmium, Chromium, and Lead in Soils. ACS Omega 2024, 9, 3574–3587. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Gasparatos, D.; Ioannou, D.; Kalderis, D.; Massas, I. Selenium Biofortification of Lettuce Plants (Lactuca sativa L.) as Affected by Se Species, Se Rate, and a Biochar Co-Application in a Calcareous Soil. Agronomy 2022, 12, 131. [Google Scholar] [CrossRef]
- Abedian-Dehaghani, N.; Sadjadi, S.; Heravi, M.M. Selenium and nitrogen co-doped biochar as an efficient metal-free catalyst for oxidation of aldehydes. J. Mol. Struct. 2022, 1264, 133237. [Google Scholar] [CrossRef]
- Fu, C.; He, Y.; Yang, C.; He, J.; Sun, L.; Pan, Y.; Deng, L.; Huang, R.; Li, M.; Chang, K. Utilizing biochar to decorate nanoscale FeS for the highly effective decontamination of Se(IV) from simulated wastewater. Ecotoxicol. Environ. Saf. 2023, 263, 115285. [Google Scholar] [CrossRef]
- Alam, M.Z.; Hoque, M.A.; Carpenter-Boggs, L. Mycorrhizal fungi, biochar, and selenium increase biomass of Vigna radiata and reduce arsenic uptake. Toxicol. Environ. Chem. 2022, 104, 84–102. [Google Scholar] [CrossRef]
- Huang, P.; Yang, W.; Johnson, V.E.; Si, M.; Zhao, F.; Liao, Q.; Su, C.; Yang, Z. Selenium-sulfur functionalized biochar as amendment for mercury-contaminated soil: High effective immobilization and inhibition of mercury re-activation. Chemosphere 2022, 306, 135552. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, G.; Parhat, R.; Jin, Y.; Wang, X.; Wu, W.; Xu, W.; Wang, Y.; Chen, W. Exogenous Selenium and Biochar Application Modulate the Growth and Selenium Uptake of Medicinal Legume Astragalus Species. Plants 2023, 12, 1957. [Google Scholar] [CrossRef]
- Padilla, J.T.; Watts, D.W.; Szogi, A.A.; Johnson, M.G. Evaluation of a pH- and time-dependent model for the sorption of heavy metal cations by poultry litter-derived biochar. Chemosphere 2024, 347, 140688. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Pagano, L.; Bartoli, M.; Fedeli, R.; Malcevschi, A.; Sidoli, M.; Magnani, G.; Pontiroli, D.; Ricco, M.; Marmiroli, M.; et al. Accumulation and Release of Cadmium Ions in the Lichen Evernia prunastri (L.) Ach. and Wood-Derived Biochar: Implication for the Use of Biochar for Environmental Biomonitoring. Toxics 2024, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.H.; Alnusairi, G.S.H.; Khan, A.A.; Alnusaire, T.S.; Fakhr, M.A.; Abdulmajeed, A.M.; Aldesuquy, H.S.; Yahya, M.; Najeeb, U. Biochar and Selenium Nanoparticles Induce Water Transporter Genes for Sustaining Carbon Assimilation and Grain Production in Salt-Stressed Wheat. J. Plant Growth Regul. 2023, 42, 1522–1543. [Google Scholar] [CrossRef]



| Samples * | C | H | O | N | S | H/C | O/C | (O + N)/C |
|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | ||||
| W0 | 47.00 | 7.20 | 40.50 | 0.20 | 0.02 | 0.15 | 0.86 | 0.87 |
| W300 | 67.90 | 5.40 | 25.80 | 0.30 | 0.15 | 0.08 | 0.38 | 0.38 |
| W800 | 91.73 | 1.90 | 5.90 | 0.40 | 0.07 | 0.02 | 0.06 | 0.07 |
| Acid-W300 | 49.16 | 8.40 | 30.70 | 11.7 | 0.04 | 0.17 | 0.62 | 0.86 |
| Acid-W800 | 80.40 | 1.70 | 14.30 | 1.50 | 0.08 | 0.02 | 0.18 | 0.20 |
| Alkali-W300 | 56.20 | 7.60 | 29.70 | 6.30 | 0.03 | 0.14 | 0.53 | 0.64 |
| Alkali-W800 | 76.90 | 4.28 | 13.00 | 5.78 | 0.03 | 0.06 | 0.17 | 0.24 |
| Biochar Selenium | Loading Capacity of Se | The Maximum Exchangeable Release of Se | Se-Exchangeable Release Rate |
|---|---|---|---|
| mg/g | mg/g | % | |
| Acid-W300-Se | 287.1 ± 9.3 ab | 178.6 ± 33.5 ab | 62.2 |
| Alkali-W300-Se | 278.8 ± 17.6 ab | 198.9 ± 29.4 ab | 71.3 |
| Acid-W800-Se | 247.8 ± 4.9 b | 176.7 ± 2.7 b | 71.1 |
| Alkali-W800-Se | 314.1 ± 1.6 a | 275.0 ± 29.5 a | 87.5 |
| Leaching Solution | Desorbent | PFOM | PSOM | ||||
|---|---|---|---|---|---|---|---|
| k1d | qa | R2 | k2d | qa | R2 | ||
| Red soil extract | Acid-W300-Se | 1.193 | 15.867 | 0.826 | 1.527 | 17.388 | 0.826 |
| Alkali-W300-Se | 1.349 | 16.407 | 0.73 | 1.611 | 18.147 | 0.744 | |
| Acid-W800-Se | 1.057 | 16.469 | 0.646 | 1.203 | 18.559 | 0.692 | |
| Alkali-W800-Se | 1.104 | 36.838 | 0.771 | 1.214 | 41.487 | 0.76 | |
| Brown soil extract | Acid-W300-Se | 1.309 | 13.455 | 0.903 | 1.716 | 14.61 | 0.855 |
| Alkali-W300-Se | 0.787 | 16.423 | 0.722 | 1.032 | 18.202 | 0.741 | |
| Acid-W800-Se | 0.983 | 11.501 | 0.616 | 1.306 | 12.615 | 0.633 | |
| Alkali-W800-Se | 1.596 | 28.32 | 0.765 | 2.055 | 30.692 | 0.706 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, J.; Wang, S.; Yu, J.; Gao, Y.; Tang, Z.; Yang, Q. Effects of Pyrolysis Temperature and Acid-Base Pre-Treatment on the Synthesis of Biochar-Based Slow-Release Selenium Fertilizer and Its Release in Soil. Materials 2024, 17, 879. https://doi.org/10.3390/ma17040879
Chu J, Wang S, Yu J, Gao Y, Tang Z, Yang Q. Effects of Pyrolysis Temperature and Acid-Base Pre-Treatment on the Synthesis of Biochar-Based Slow-Release Selenium Fertilizer and Its Release in Soil. Materials. 2024; 17(4):879. https://doi.org/10.3390/ma17040879
Chicago/Turabian StyleChu, Jun, Suikai Wang, Jie Yu, Yuting Gao, Zhenya Tang, and Qiliang Yang. 2024. "Effects of Pyrolysis Temperature and Acid-Base Pre-Treatment on the Synthesis of Biochar-Based Slow-Release Selenium Fertilizer and Its Release in Soil" Materials 17, no. 4: 879. https://doi.org/10.3390/ma17040879
APA StyleChu, J., Wang, S., Yu, J., Gao, Y., Tang, Z., & Yang, Q. (2024). Effects of Pyrolysis Temperature and Acid-Base Pre-Treatment on the Synthesis of Biochar-Based Slow-Release Selenium Fertilizer and Its Release in Soil. Materials, 17(4), 879. https://doi.org/10.3390/ma17040879