Photocatalytic Hydrogen Evolution of TiZrNbHfTaOx High-Entropy Oxide Synthesized by Mechano-Thermal Method
Abstract
1. Introduction
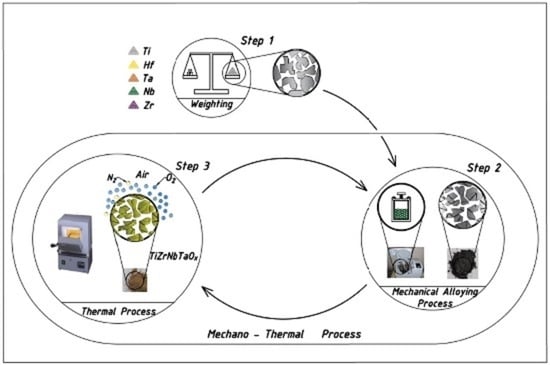
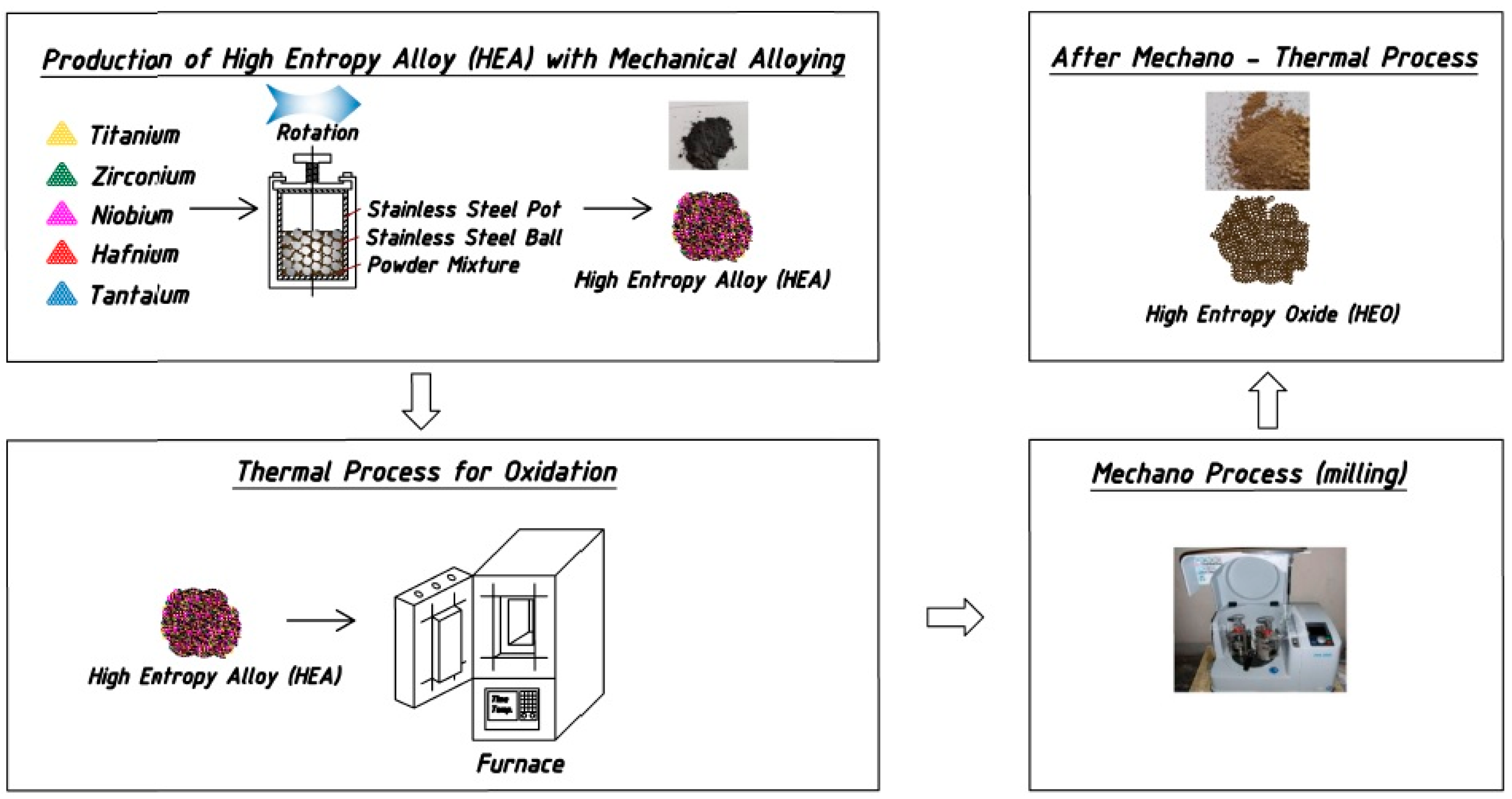
2. Materials and Methods
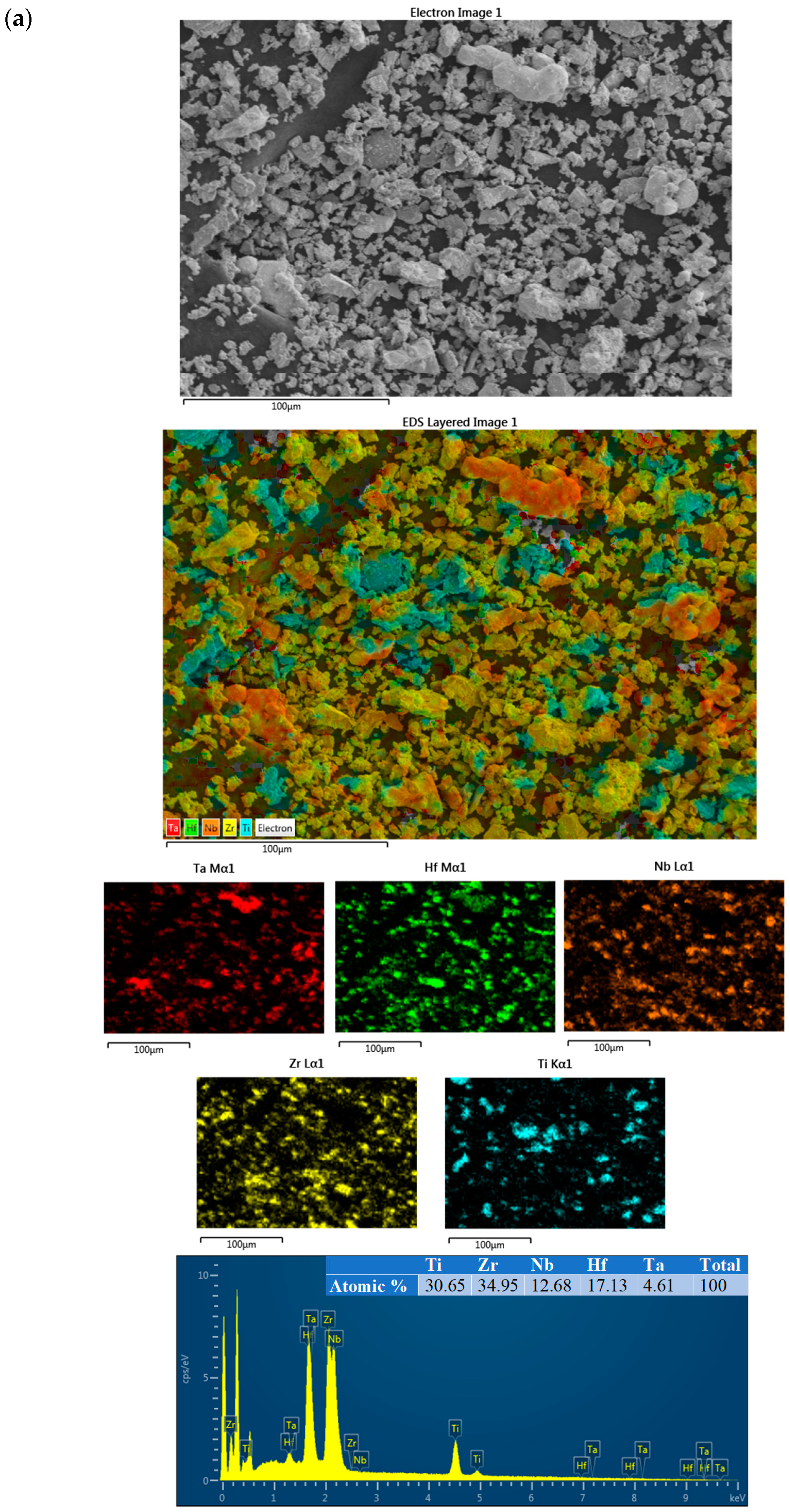
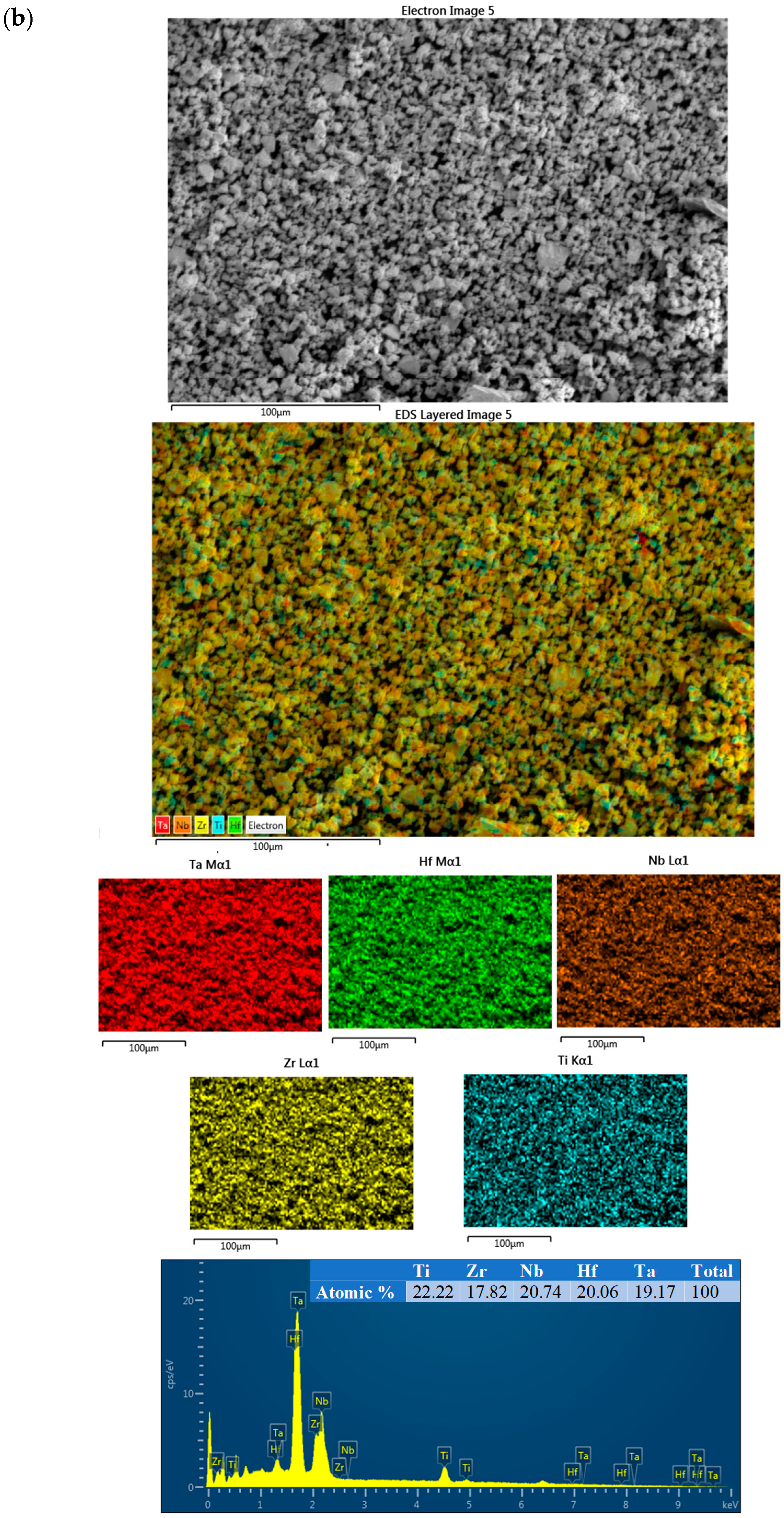
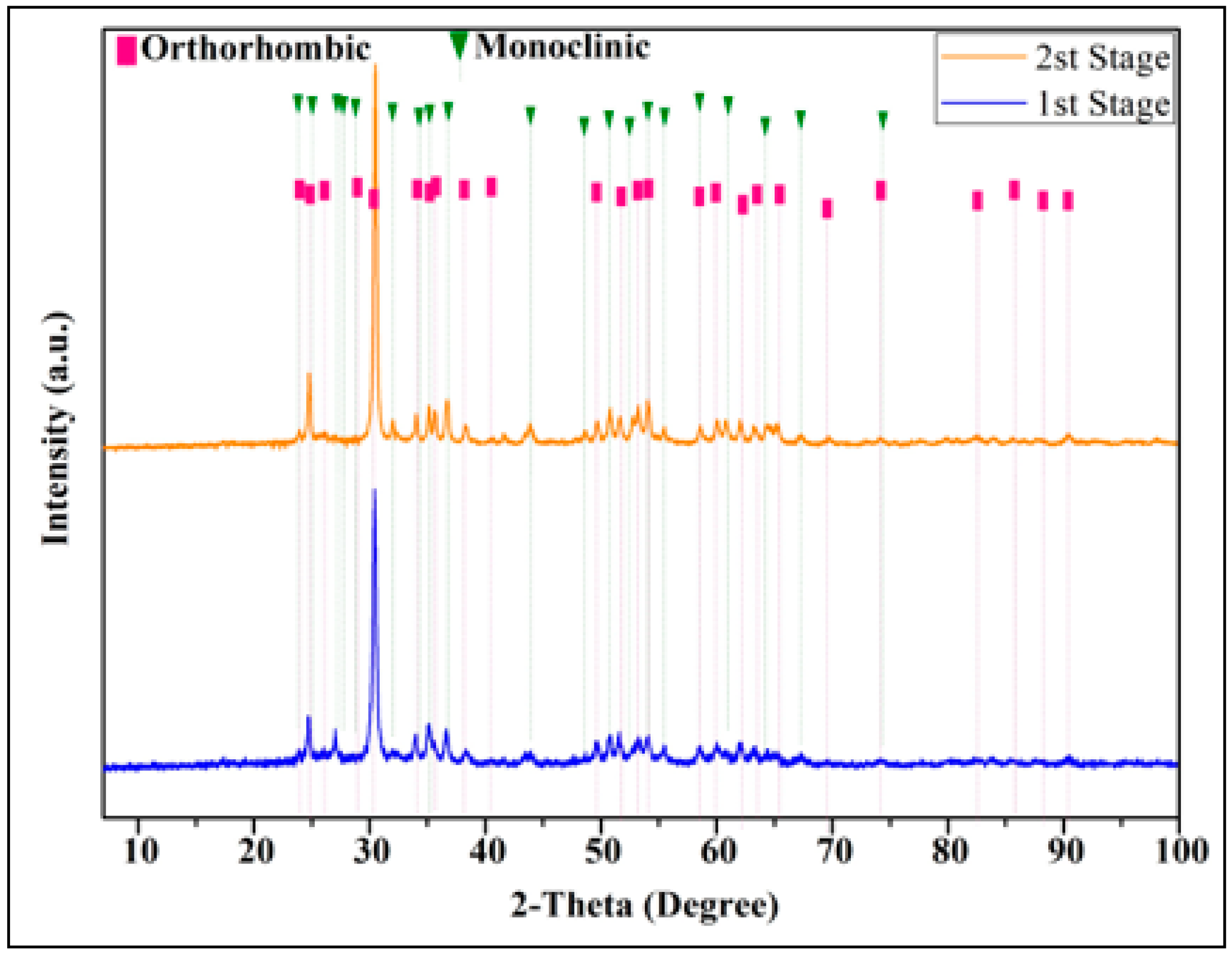
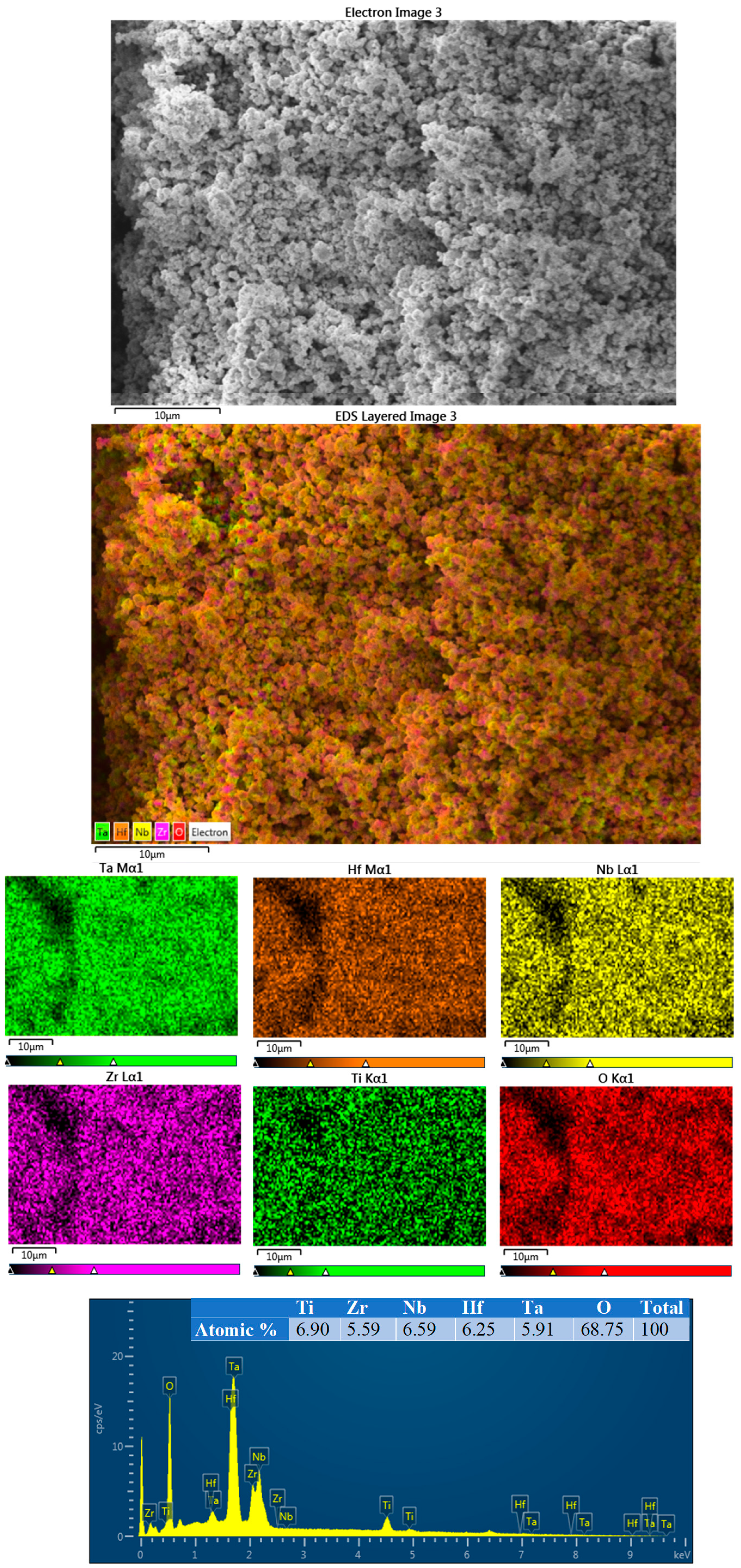
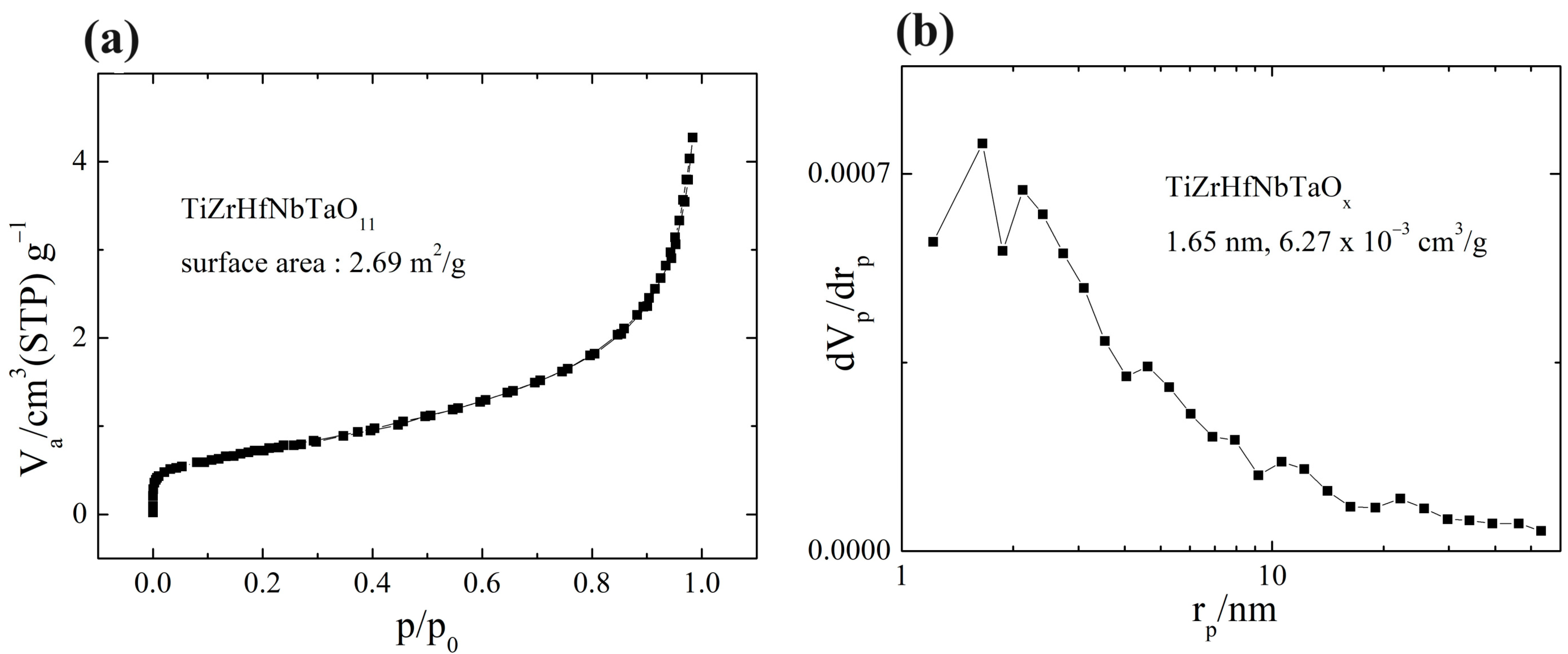
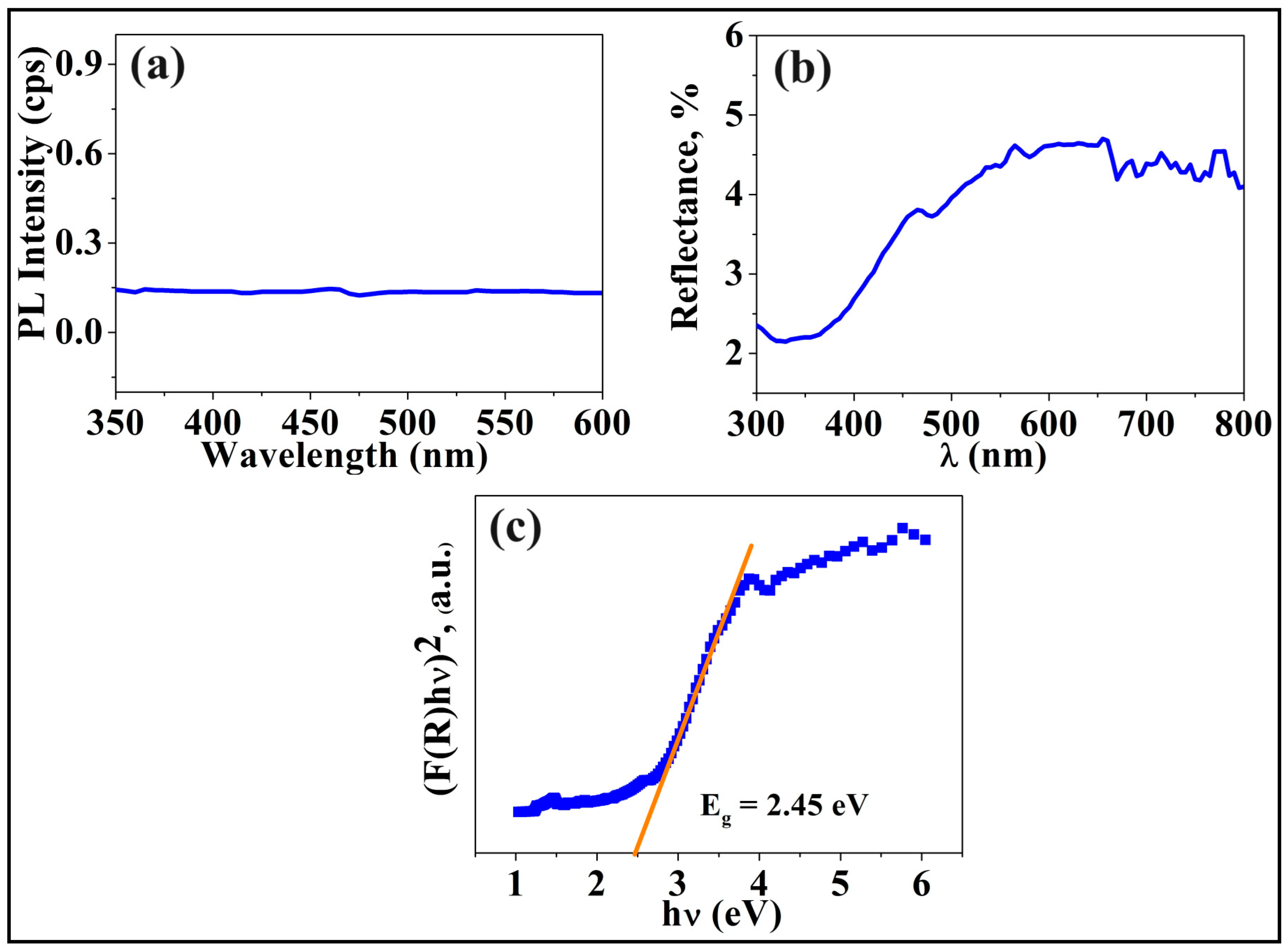
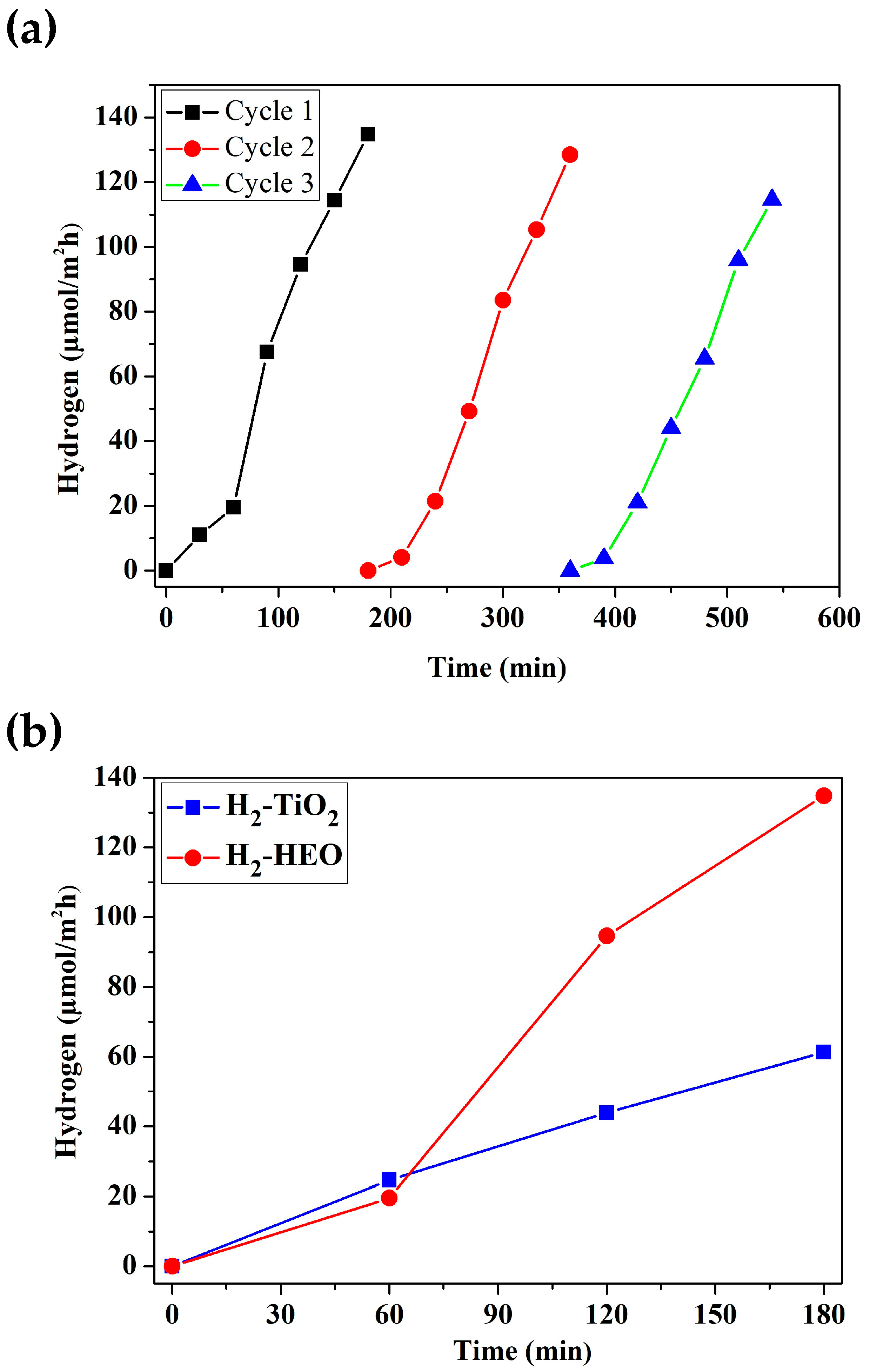
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravanan, A.; Vo, D.V.B.; Jeevanantham, S.; Bhuvaneswari, V.; Narayanan, V.A.; Yaashikaa, P.; Swetha, S.; Reshma, B. A comprehensive review on different approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Hong, Q.; Cui, L.; Hong, P. The impact of carbon emissions trading on energy efficiency: Evidence from quasi-experiment in China’s carbon emissions trading pilot. Energy Econ. 2022, 110, 106025. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, A.F.; Cao, C.S.; Zhao, B. Applications of MOFs: Recent advances in photocatalytic hydrogen production from water. Coord. Chem. Rev. 2019, 390, 50–75. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R.; López, N.; Palomares, E. Photocatalytic water splitting: Advantages and challenges. Sustain. Energy Fuels 2021, 5, 4560–4569. [Google Scholar] [CrossRef]
- Kim, Y.; Jun, S.E.; Lee, G.; Nam, S.; Jang, H.W.; Park, S.H.; Kwon, K.C. Recent Advances in Water-Splitting Electrocatalysts Based on Electrodeposition. Materials 2023, 16, 3044. [Google Scholar] [CrossRef]
- Piqué, O.; Illas, F.; Calle-Vallejo, F. Designing water splitting catalysts using rules of thumb: Advantages, dangers and alternatives. Phys. Chem. Chem. Phys. 2020, 22, 6797–6803. [Google Scholar] [CrossRef]
- Lee, G.; Jun, S.E.; Kim, Y.; Park, I.H.; Jang, H.W.; Park, S.H.; Kwon, K.C. Multicomponent Metal Oxide- and Metal Hydroxide-Based Electrocatalysts for Alkaline Water Splitting. Materials 2023, 16, 3280. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Moniz, S.J.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Du, H.; Liu, Y.N.; Shen, C.C.; Xu, A.W. Nanoheterostructured photocatalysts for improving photocatalytic hydrogen production. Chin. J. Catal. 2017, 38, 1295–1306. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Moridon, S.T.N.; Arifin, K.; Mohamed, M.A.; Minggu, L.J.; Yunus, R.M.; Kassim, M.B. TiO2 Nanotubes Decorated with Mo2C for Enhanced Photoelectrochemical Water-Splitting Properties. Materials 2023, 16, 6261. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Amato, L.S.; Maddalena, P. Charge Carrier Processes and Optical Properties in TiO2 and TiO2-Based Heterojunction Photocatalysts: A Review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Gawlak, K.; Popiolek, D.; Pisarek, M.; Sulka, G.D.; Zaraska, L. CdS-Decorated Porous Anodic SnOx Photoanodes with Enhanced Performance under Visible Light. Materials 2022, 15, 3848. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Pooja Devi, P.; Basu, S. Revolutionizing the Role of Solar Light Responsive BiVO4/BiOBr Heterojunction Photocatalyst for the Photocatalytic Deterioration of Tetracycline and Photoelectrocatalytic Water Splitting. Materials 2023, 16, 5661. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Fan, Z.; Nakabayashi, M.; Ju, H.; Pastukhova, N.; Xiao, Y.; Feng, C.; Shibata, N.; Domen, K.; Li, Y. Interface engineering of Ta3N5 thin film photoanode for highly efficient photoelectrochemical water splitting. Nat. Commun. 2022, 13, 729. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wang, R.; Wu, M.Z.; Yuan, Y.P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic carbon nitride (g–C3N4)–based metal-free photocatalysts for water splitting: A review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Edalati, P.; Shen, X.F.; Watanabe, M.; Ishihara, T.; Arita, M.; Fuji, M.; Edalati, K. High-entropy oxynitride as a low-bandgap and stable photocatalyst for hydrogen production. J. Mater. Chem. A 2021, 9, 15076–15086. [Google Scholar] [CrossRef]
- Feng, X.; Feng, C.; Lu, Y. A Lightweight AlTiVNb High-Entropy Alloy Film with High Strength-Ductility Synergy and Corrosion Resistance. Materials 2022, 15, 8568. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y. History of high-entropy materials. In High-Entropy Materials: A Brief Introduction; Springer: Singapore, 2019; pp. 1–33. [Google Scholar]
- Edalati, P.; Itagoe, Y.; Ishihara, H.; Ishihara, T.; Emami, H.; Arita, M.; Fuji, M.; Edalati, K. Visible-light photocatalytic oxygen production on a high-entropy oxide by multiple-heterojunction introduction. J. Photochem. Photobiol. A Chem. 2022, 433, 114167. [Google Scholar] [CrossRef]
- Akrami, S.; Murakami, Y.; Watanabe, M.; Ishihara, T.; Arita, M.; Fuji, M.; Edalati, K. Defective high-entropy oxide photocatalyst with high activity for CO2 conversion. Appl. Catal. B Environ. 2022, 303, 120896. [Google Scholar] [CrossRef]
- Edalati, P.; Wang, Q.; Razavi-Khosroshahi, H.; Fuji, M.; Ishihara, T.; Edalati, K. Photocatalytic hydrogen evolution on a high-entropy oxide. J. Mater. Chem. A 2020, 8, 3814–3821. [Google Scholar] [CrossRef]
- Seyyedin, S.; Zangi, H.; Bozorgmehr, M.; Ghasemi, B.; Tavallaei, M.M.; Adib, S. The effect of mechanical alloying time on the microstructural and mechanical properties of spark plasma sintered Ta–10W. Mater. Sci. Eng. A 2020, 798, 140024. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying: A critical review. Mater. Res. Lett. 2022, 10, 619–647. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Shundo, Y.; Watanabe, M.; Ishihara, T.; Fuji, M.; Edalati, K. Significant CO2 photoreduction on a high-entropy oxynitride. Chem. Eng. J. 2022, 449, 137800. [Google Scholar] [CrossRef]
- Rishel, D.; Smee, J.; Kammenzind, B. The corrosion behavior of hafnium in high-temperature water environments. J. Nucl. Mater. 2002, 303, 210–225. [Google Scholar] [CrossRef]
- Cox, B. Some thoughts on the mechanisms of in-reactor corrosion of zirconium alloys. J. Nucl. Mater. 2005, 336, 331–368. [Google Scholar] [CrossRef]
- Bermúdez, M.D.; Carrión, F.J.; Martínez-Nicolás, G.; López, R. Erosion–corrosion of stainless steels, titanium, tantalum and zirconium. Wear 2005, 258, 693–700. [Google Scholar] [CrossRef]
- Pan, T.; Chen, Y.; Zhang, B.; Hu, J.; Li, C. Corrosion behavior of niobium coated 304 stainless steel in acid solution. Appl. Surf. Sci. 2016, 369, 320–325. [Google Scholar] [CrossRef]
- Souza, J.C.; Apaza-Bedoya, K.; Benfatti, C.A.; Silva, F.S.; Henriques, B. A comprehensive review on the corrosion pathways of titanium dental implants and their biological adverse effects. Metals 2020, 10, 1272. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, F.; Zhao, M.; Xu, J.; Zhou, J.; Wang, Y. Ultra-small yellow defective TiO2 nanoparticles for co-catalyst free photocatalytic hydrogen production. Nano Energy 2016, 24, 63–71. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Qin, J.; Shen, X.; Yu, R.; Ma, M.; Liu, R. Porous carbon-doped TiO2 on TiC nanostructures for enhanced photocatalytic hydrogen production under visible light. J. Catal. 2017, 347, 36–44. [Google Scholar] [CrossRef]
- Esmat, M.; El-Hosainy, H.; Tahawy, R.; Jevasuwan, W.; Tsunoji, N.; Fukata, N.; Ide, Y. Nitrogen doping-mediated oxygen vacancies enhancing co-catalyst-free solar photocatalytic H2 production activity in anatase TiO2 nanosheet assembly. Appl. Catal. B Environ. 2021, 285, 119755. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, İ.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Zhou, W.; Li, W.; Wang, J.Q.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered mesoporous black TiO2 as highly efficient hydrogen evolution photocatalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, J.C.; Ho, C.; Hou, Y.; Fu, X. Photocatalytic activity of a hierarchically macro/mesoporous titania. Langmuir 2005, 21, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Zhu, J.; Wang, S.; Cao, Y.; Qian, X.; Li, H. Self-assembly of active Bi2O3/TiO2 visible photocatalyst with ordered mesoporous structure and highly crystallized anatase. J. Phys. Chem. C 2008, 112, 6258–6262. [Google Scholar] [CrossRef]
- Lo, A.Y.; Taghipour, F. Ordered mesoporous photocatalysts for CO2 photoreduction. J. Mater. Chem. A 2021, 9, 26430–26453. [Google Scholar] [CrossRef]



| Catalyst Name | Eg (eV) | BET Surface Area (m2/g) | Result (µmol/m2 h) |
|---|---|---|---|
| Ag2O/TiO2 | 2.86 | 24.65 | 135.90 |
| Au–TiO2 | 2.76 | 77.14 | 93.34 |
| GO–TiO2 | 2.58 | 42.72 | 88.95 |
| TiO2–ZnO | 3.06 | 85.91 | 15.02 |
| CdS | 2.40 | 24.57 | 48.84 |
| Au–CdS | 2.40 | 8.47 | 472.26 |
| CdS/Ti | 2.63 | 138.41 | 0.34 |
| Bi2S3/Pt/ZnO | 2.71 | 27.45 | 0.84 |
| ZnO/ZnS | 3.40 | 34.67 | 11.20 |
| In2O3/Ta2O5 | 2.80 | 43.75 | 12.18 |
| Rh/Cr2O3/GaZn | 2.60 | 48.30 | 30.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güler, Ö.; Boyrazlı, M.; Albayrak, M.G.; Güler, S.H.; Ishihara, T.; Edalati, K. Photocatalytic Hydrogen Evolution of TiZrNbHfTaOx High-Entropy Oxide Synthesized by Mechano-Thermal Method. Materials 2024, 17, 853. https://doi.org/10.3390/ma17040853
Güler Ö, Boyrazlı M, Albayrak MG, Güler SH, Ishihara T, Edalati K. Photocatalytic Hydrogen Evolution of TiZrNbHfTaOx High-Entropy Oxide Synthesized by Mechano-Thermal Method. Materials. 2024; 17(4):853. https://doi.org/10.3390/ma17040853
Chicago/Turabian StyleGüler, Ömer, Mustafa Boyrazlı, Muhammet Gökhan Albayrak, Seval Hale Güler, Tatsumi Ishihara, and Kaveh Edalati. 2024. "Photocatalytic Hydrogen Evolution of TiZrNbHfTaOx High-Entropy Oxide Synthesized by Mechano-Thermal Method" Materials 17, no. 4: 853. https://doi.org/10.3390/ma17040853
APA StyleGüler, Ö., Boyrazlı, M., Albayrak, M. G., Güler, S. H., Ishihara, T., & Edalati, K. (2024). Photocatalytic Hydrogen Evolution of TiZrNbHfTaOx High-Entropy Oxide Synthesized by Mechano-Thermal Method. Materials, 17(4), 853. https://doi.org/10.3390/ma17040853