Preparation and Characterization of Novel Sulfoaluminate-Cement-Based Nonautoclaved Aerated Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sulfoaluminate-Cement-Based Nonautoclaved Aerated Concrete
2.3. Characterizations
3. Results
3.1. Effect of Water–Cement Ratio on the Properties of Sulfoaluminate-Cement-Based Nonautoclaved Aerated Concrete
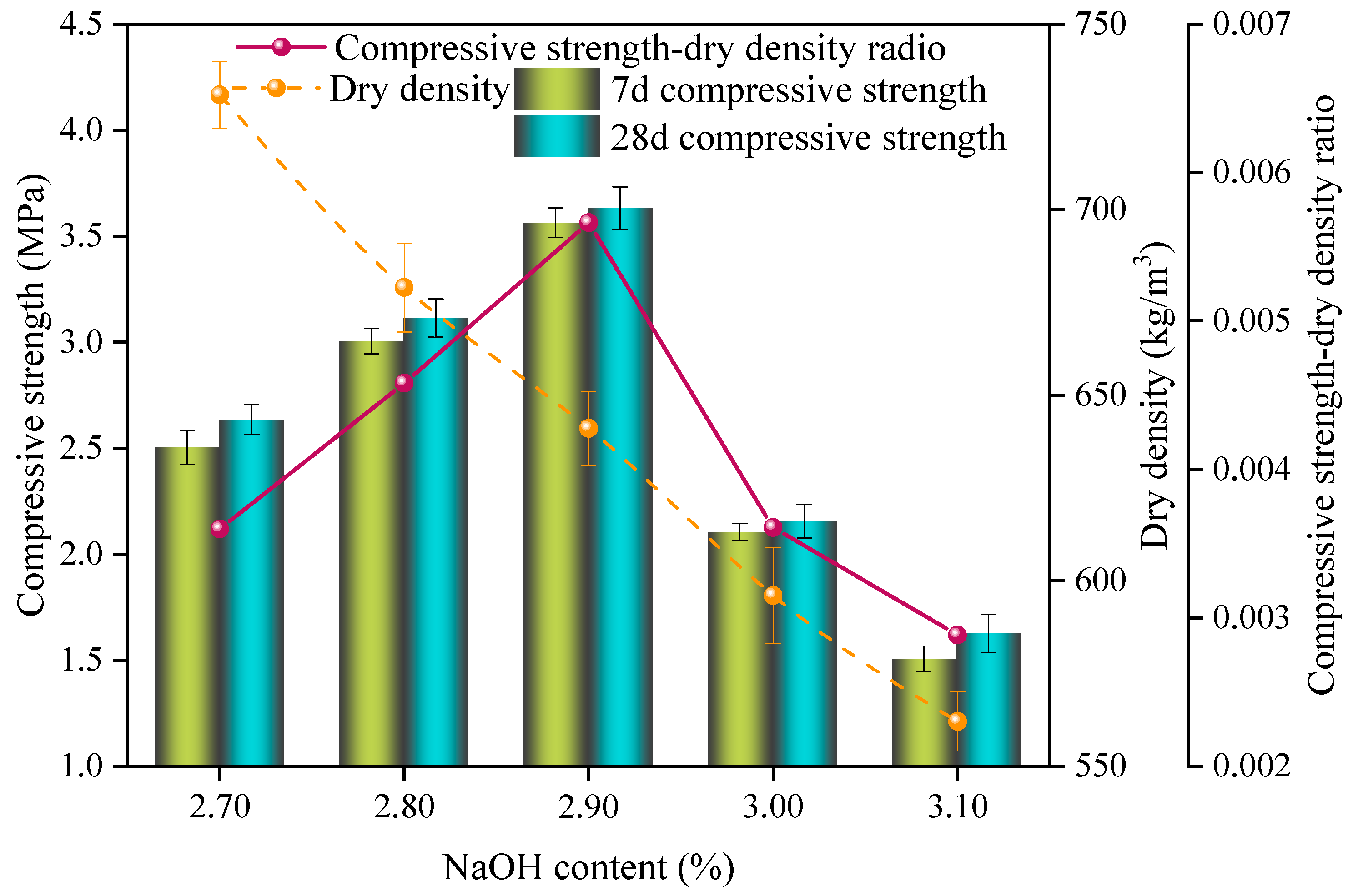
3.2. Effect of NaOH Content on the Properties of Sulfoaluminate-Cement-Based Nonautoclaved Aerated Concrete
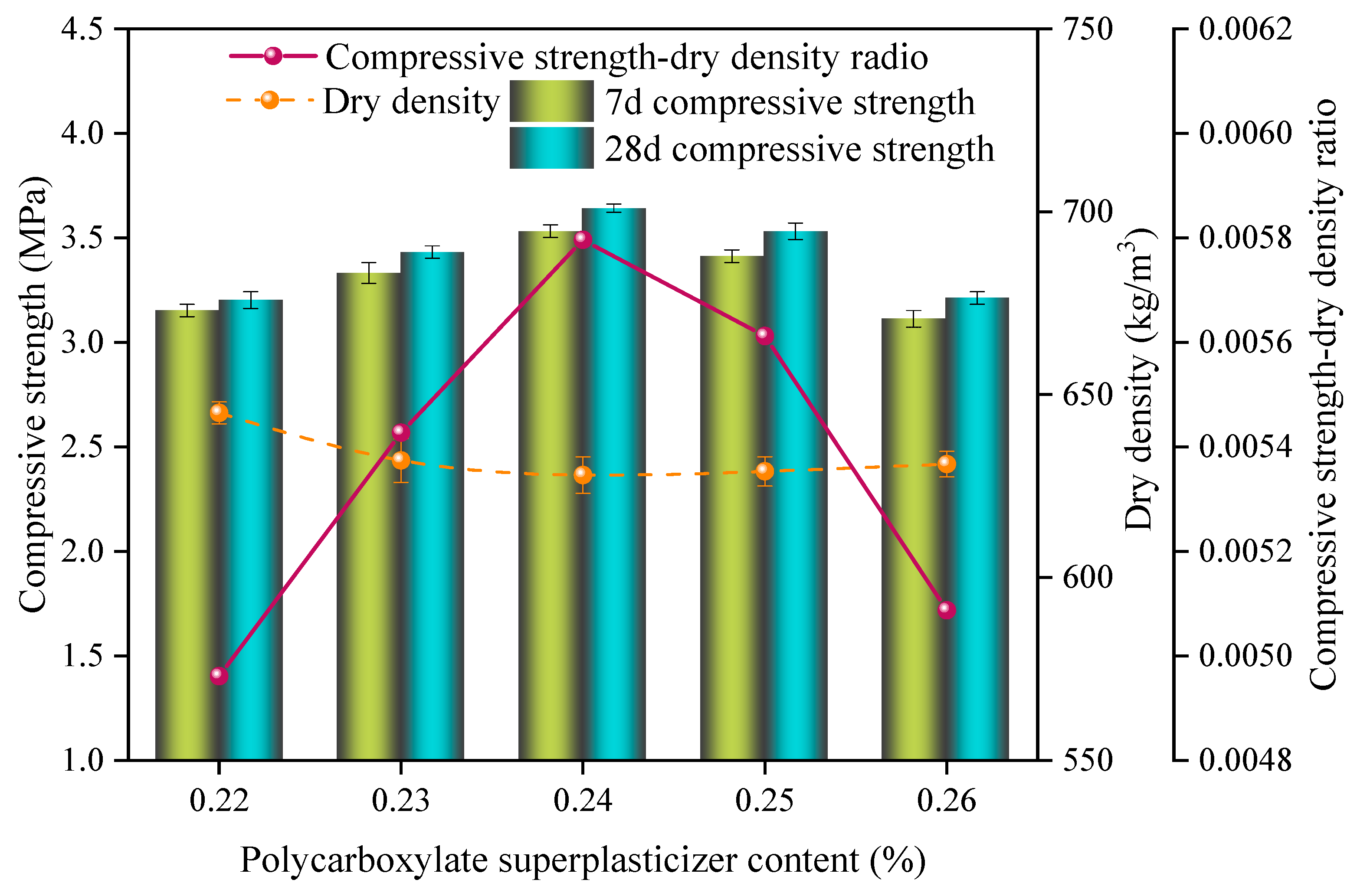
3.3. Effect of Admixture on the Properties of Sulfoaluminate-Cement-Based Nonautoclaved Aerated Concrete
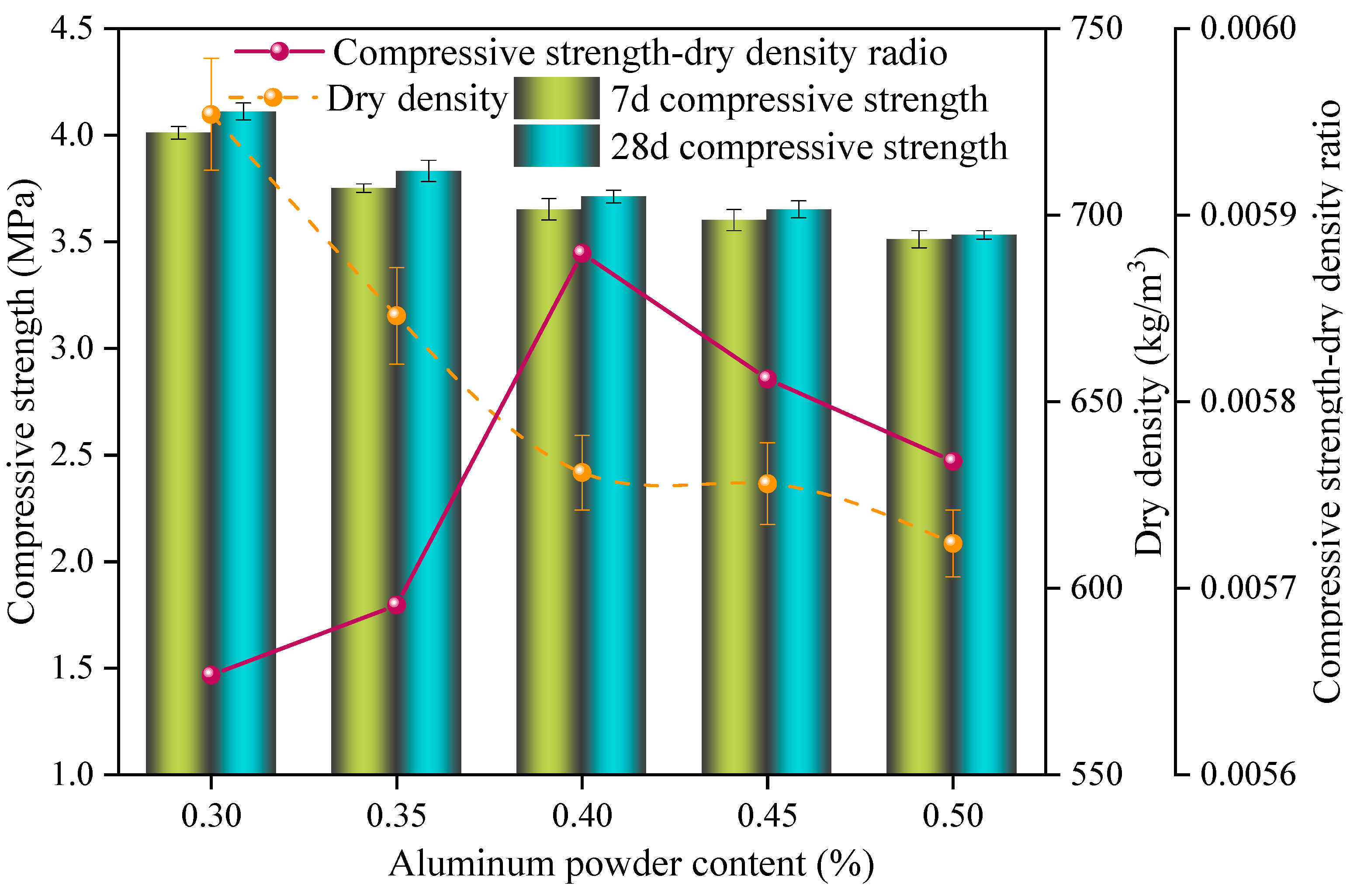
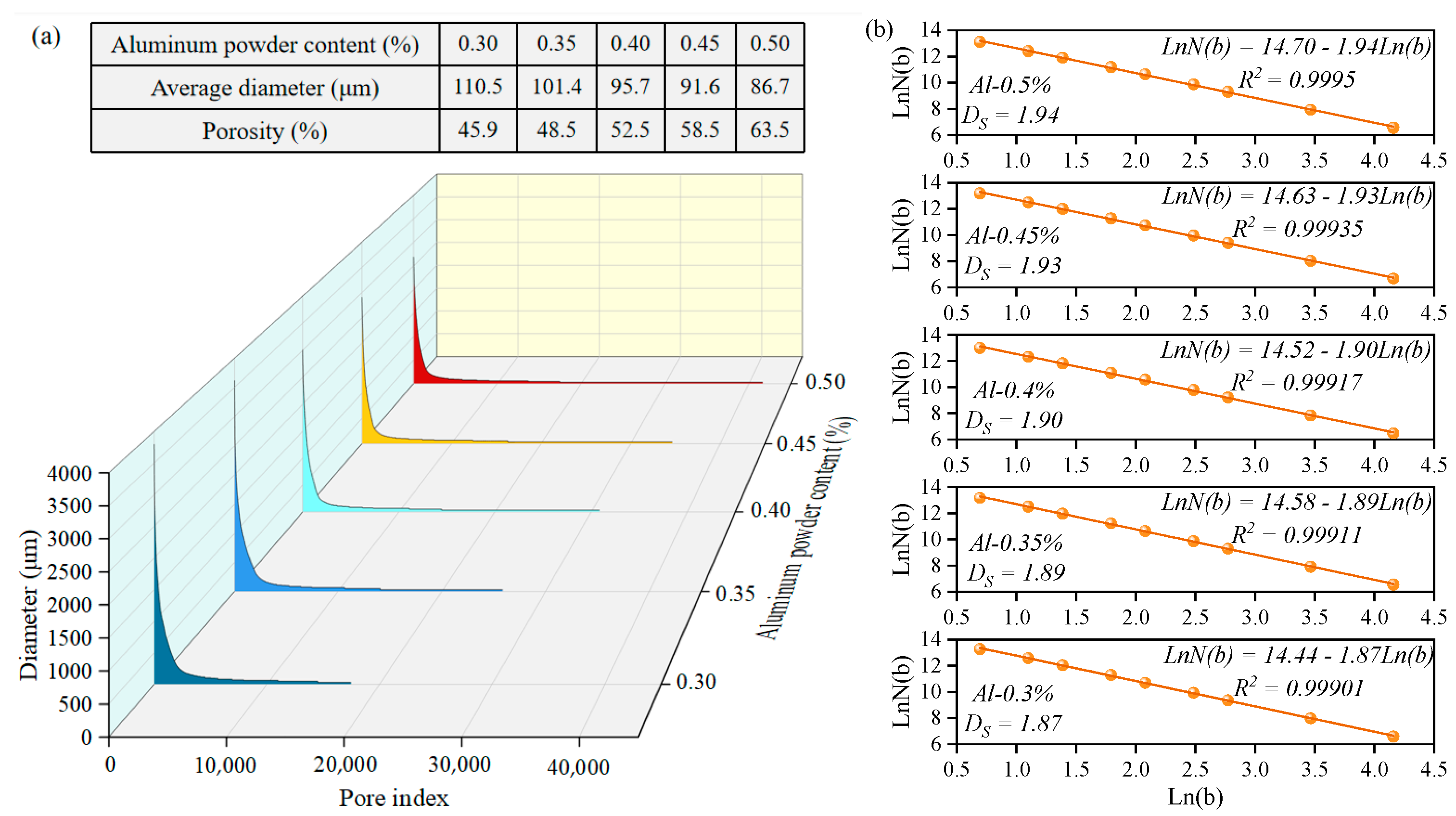
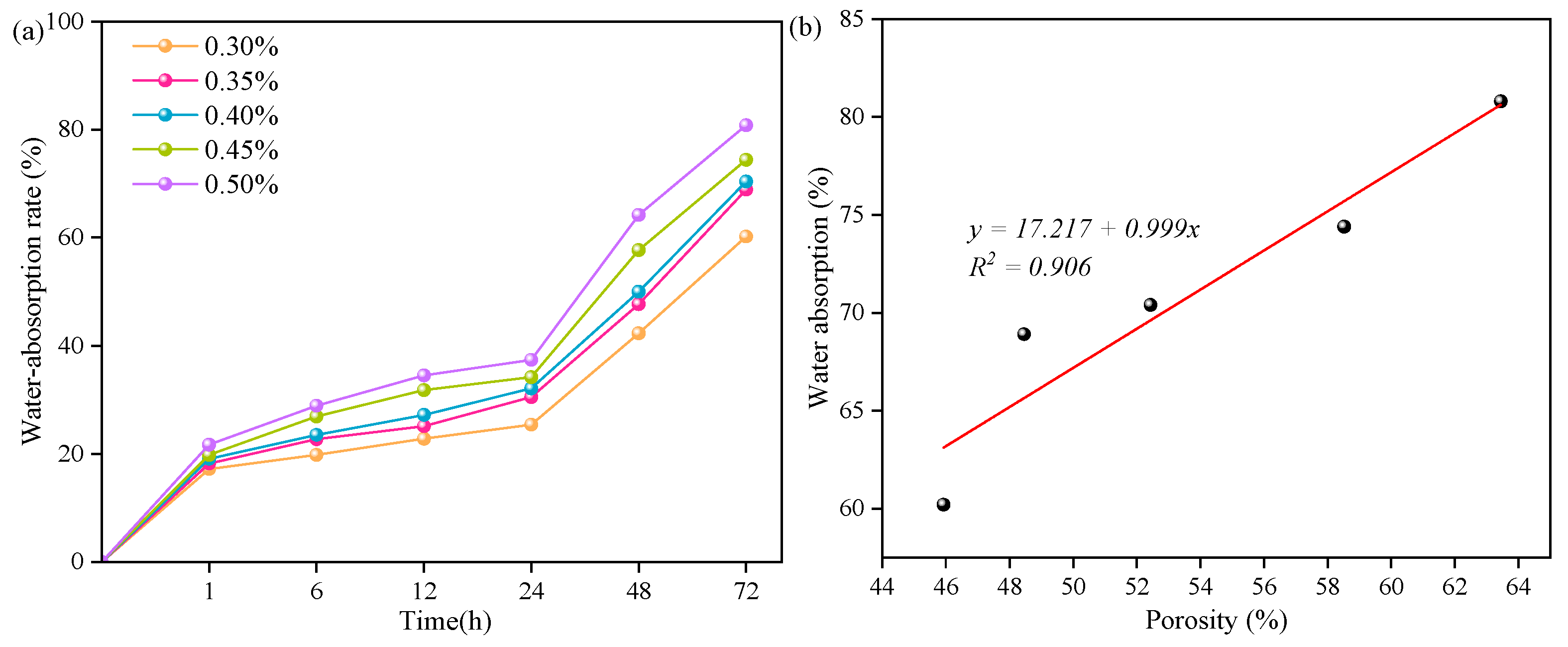
3.4. Effect of Different Aluminum Powder Contents on the Performance of Sulfoaluminate-Cement-Based Nonautoclaved Aerated Concrete
4. Conclusions
- (1)
- Compared to conventional autoclaved aerated concrete (AAC) and the existing nonautoclaved aerated concrete (NAAC), a sulfoaluminate-cement-based nonautoclaved aerated concrete (SAC-NAAC) was prepared by reducing the high-temperature autoclave process. It not only reduced the consumption of energy and filled the vacancy of preparing NAAC at 20 ± 2 °C, but the shorter setting time of the paste also greatly shortened the production cycle of the SAC-NAAC, laying the foundation for its mass production.
- (2)
- The dry density and compressive strength of the SAC-NAAC gradually decreased with the increase in the W/C and aluminum powder content. Conversely, as the NaOH content increased, the compressive strength first increased and then decreased, whereas the dry density decreased linearly. This phenomenon was attributed to the heightened alkalinity accelerating the aeration speed and the resulting mismatch between the slurry setting and aeration periods. Optimal physical properties of the slurry were achieved by adding additives. At a W/C ratio of 0.55 and a specific content ratio of polycarboxylate superplasticizer–borax–calcium stearate–sodium hydroxide of 0.24%:0.32%:0.36%:2.90%, the setting time of the slurry aligned with the aeration time of the aluminum powder, resulting in the optimal foaming state.
- (3)
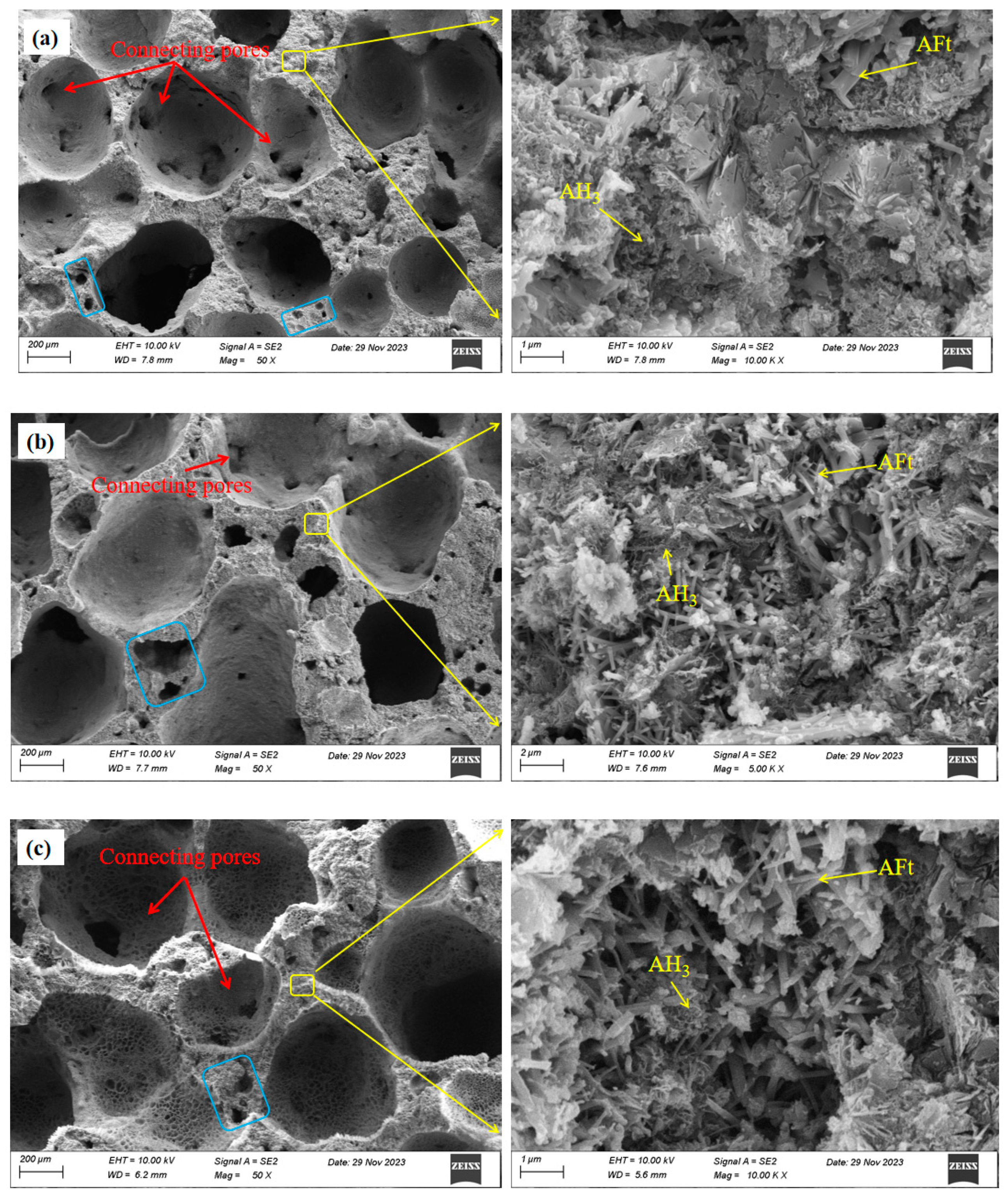
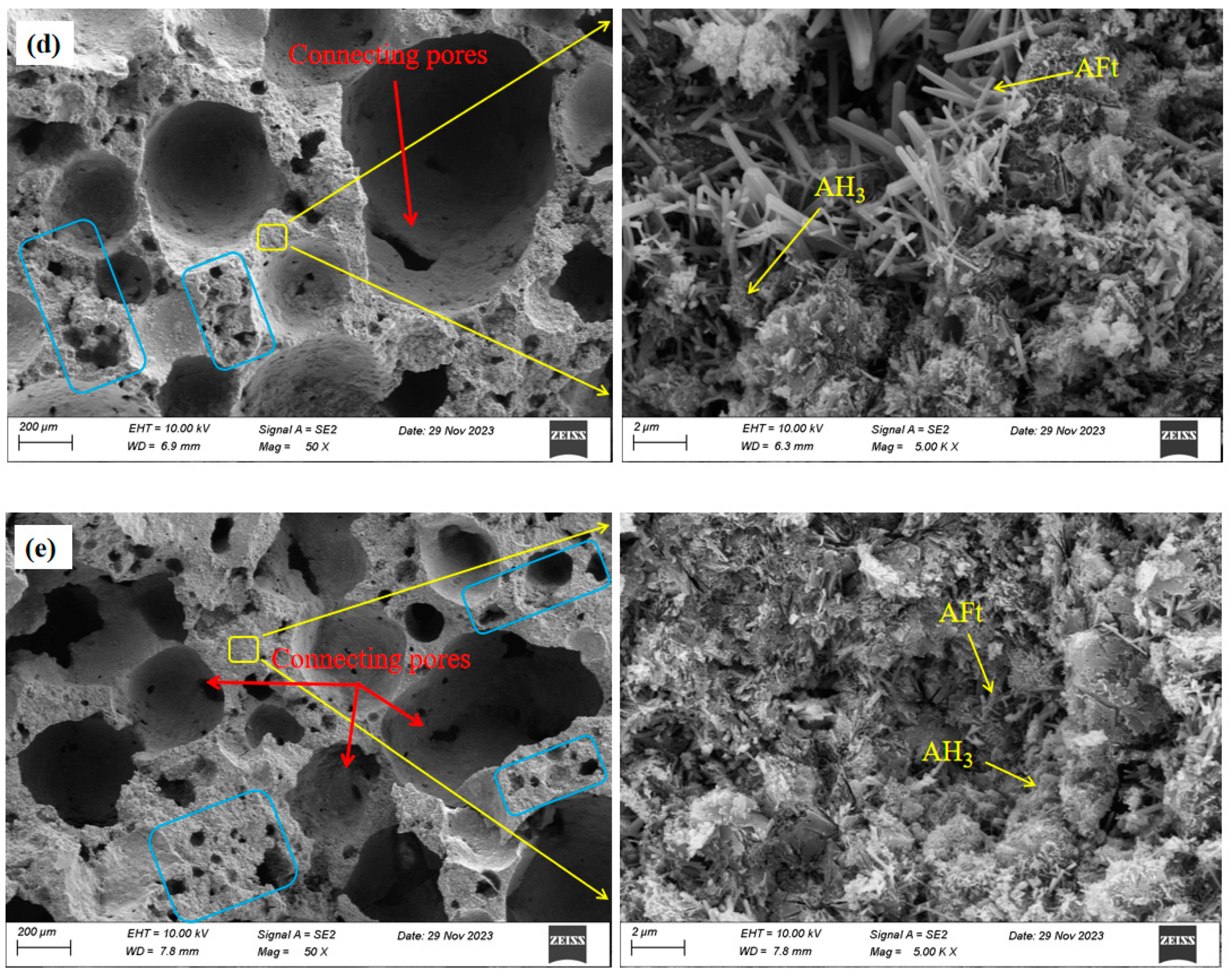
- An increase in the aluminum powder content led to an increase in the number of pores in the SAC-NAAC, a reduction in the average pore diameter, and the formation of thinner pore walls with small pores. This process resulted in an increased number of connected pores, elevating the porosity and bulk density of the SAC-NAAC. Additionally, the water absorption intensified with the increase in the aluminum powder content due to a greater number of connected pores in the low-density SAC-NAAC, which increased the porosity. Consequently, the correlation between the water absorption and porosity of the SAC-NAAC could be expressed as a linear function.
- (4)
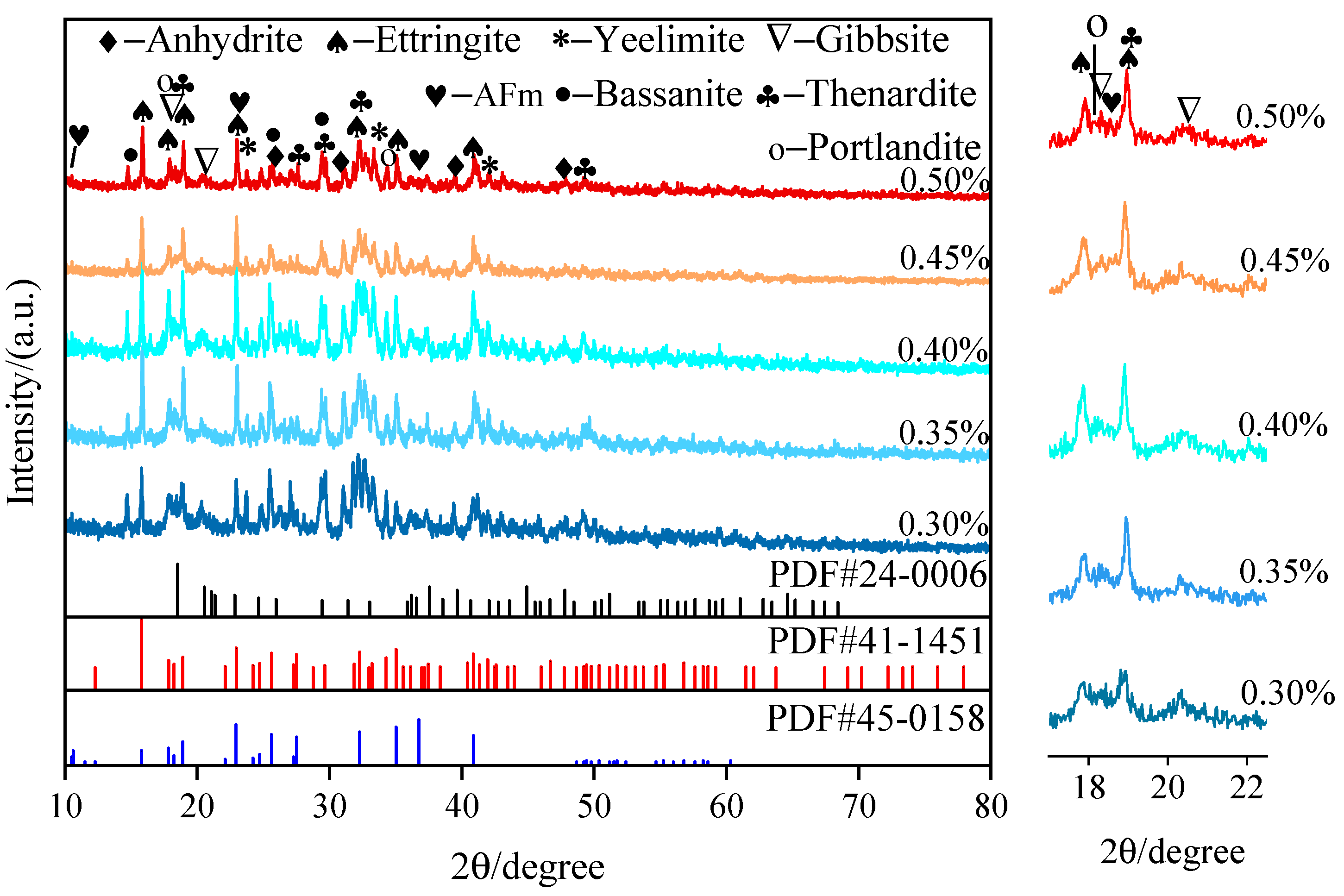
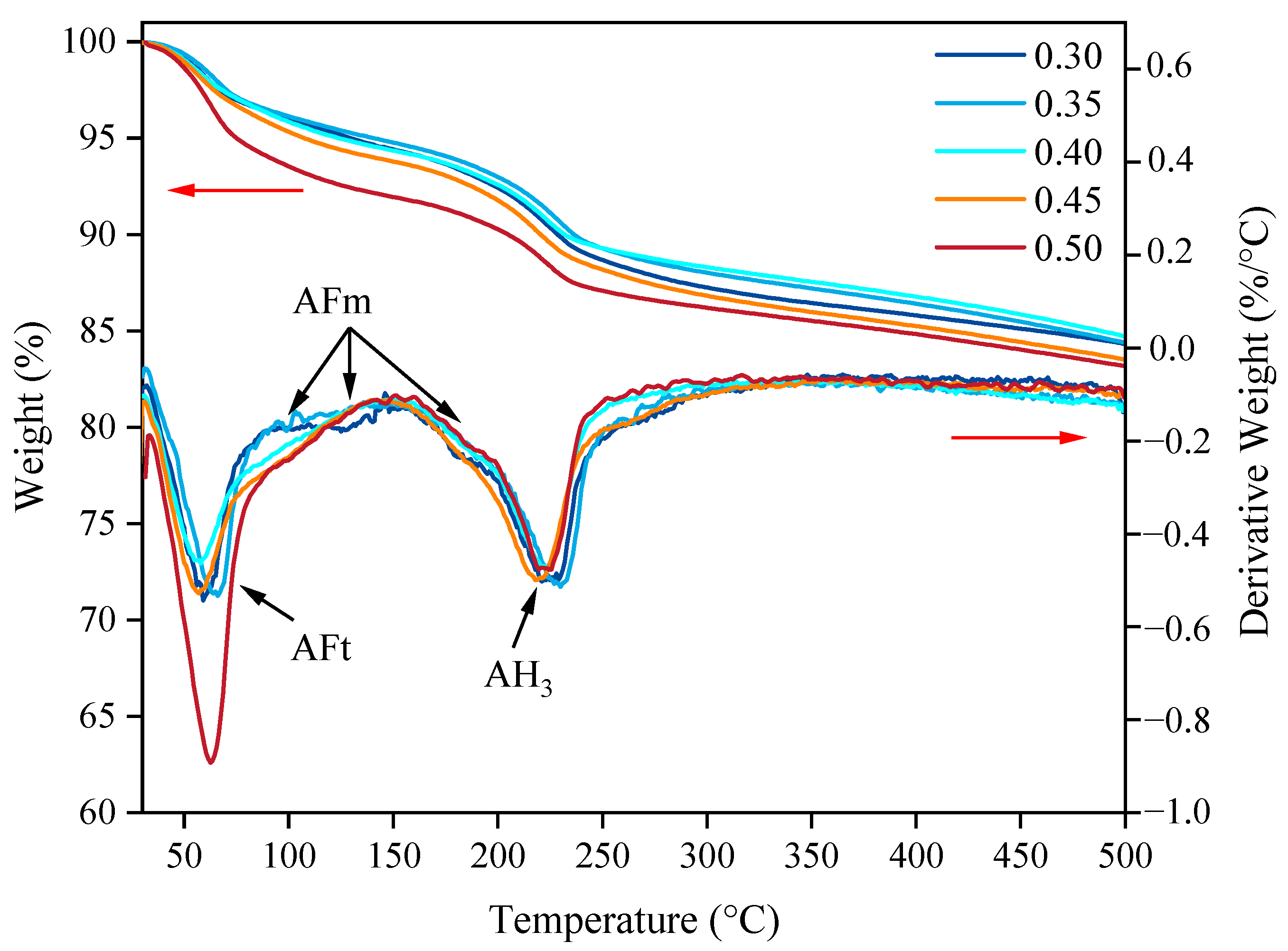
- AFt generated via SAC hydration provided early strength to the SAC-NAAC, and only a limited amount of AH3 was formed via SAC hydration. The introduction of NaOH altered the hydration process of yeelimite, and the high-alkalinity environment facilitated the formation of AH3 and the conversion of a portion of AFt into AFm. AH3 adhered to the surface of prismatic AFt in a microcrystalline gel state, filling the AFt skeleton and creating a dense structure, which further enhanced the mechanical properties of the SAC-NAAC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muthu Kumar, E.; Ramamurthy, K. Effect of fineness and dosage of aluminium powder on the properties of moist-cured aerated concrete. Constr. Build. Mater. 2015, 95, 486–496. [Google Scholar] [CrossRef]
- Michelini, E.; Ferretti, D.; Miccoli, L.; Parisi, F. Autoclaved aerated concrete masonry for energy efficient buildings: State of the art and future developments. Constr. Build. Mater. 2023, 402, 132996. [Google Scholar] [CrossRef]
- Liu, Y.; Leong, B.S.; Hu, Z.-T.; Yang, E.-H. Autoclaved aerated concrete incorporating waste aluminum dust as foaming agent. Constr. Build. Mater. 2017, 148, 140–147. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chang, J.-E.; Lai, Y.-C.; Chou, M.-I.M. A comprehensive study on the production of autoclaved aerated concrete: Effects of silica-lime-cement composition and autoclaving conditions. Constr. Build. Mater. 2017, 153, 622–629. [Google Scholar] [CrossRef]
- Bukhari, S.A.; Patil, D.; Gogate, N.G.; Minde, P.R. Utilization of waste materials in non-autoclaved aerated concrete blocks: State of art review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Z.; Niu, Y.; Li, J.; Zhang, Y. Study on the preparation and properties of high-porosity foamed concretes based on ordinary Portland cement. Mater. Des. 2016, 92, 949–959. [Google Scholar] [CrossRef]
- Fabien, A.; Sebaibi, N.; Boutouil, M. Effect of several parameters on non-autoclaved aerated concrete: Use of recycling waste perlite. Eur. J. Environ. Civ. Eng. 2019, 26, 1–18. [Google Scholar] [CrossRef]
- Xia, Y.; Yan, Y.; Hu, Z. Utilization of circulating fluidized bed fly ash in preparing non-autoclaved aerated concrete production. Constr. Build. Mater. 2013, 47, 1461–1467. [Google Scholar] [CrossRef]
- Esmaily, H.; Nuranian, H. Non-autoclaved high strength cellular concrete from alkali activated slag. Constr. Build. Mater. 2012, 26, 200–206. [Google Scholar] [CrossRef]
- Yang, L.; Yan, Y.; Hu, Z. Utilization of phosphogypsum for the preparation of non-autoclaved aerated concrete. Constr. Build. Mater. 2013, 44, 600–606. [Google Scholar] [CrossRef]
- Choudhury, S.; Jena, T. Influence of surfactant on foam generation and stabilization in cement slurry. Mater. Today Proc. 2023, 93, 340–345. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Pastore, T. CSA-based Portland-free binders to manufacture sustainable concretes for jointless slabs on ground. Constr. Build. Mater. 2018, 187, 691–698. [Google Scholar] [CrossRef]
- Li, T.; Huang, F.; Li, L.; Zhu, J.; Jiang, X.; Huang, Y. Preparation and properties of sulphoaluminate cement-based foamed concrete with high performance. Constr. Build. Mater. 2020, 263, 120945. [Google Scholar] [CrossRef]
- Kumar, K.; Ingole, S.B.; Saeedjassim, A.; YatikaGori; Kaushik, A.; Kumar, D.; Jain, A. Aluminum-foam by powder metallurgy: A review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Song, Y.; Dong, M.; Wang, Z.; Qian, X.; Yan, D.; Shen, S.; Zhang, L.; Sun, G.; Lai, J.; Ruan, S. Effects of red mud on workability and mechanical properties of autoclaved aerated concrete (AAC). J. Build. Eng. 2022, 61, 105238. [Google Scholar] [CrossRef]
- Soni, A.; Patel, J.; Poojalakshmi, E.S.; Gupta, N.; Gupta, N.; Thomas, B.S.; Ramaswamy, K.P. Study for the development of flyash and GGBS-based alkali activated foam concrete. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tambara, L.U.D.; Cheriaf, M.; Rocha, J.C.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis content on calcium sulfoaluminate (CSA) cement hydration. Cem. Concr. Res. 2020, 128, 105953. [Google Scholar] [CrossRef]
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Enhancing the chemical foaming process using superplasticizer in aerated geopolymer concrete. Constr. Build. Mater. 2022, 324, 126535. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Bullerjahn, F.; Ben Haha, M. Effect of retarders on the early hydration of calcium-sulpho-aluminate (CSA) type cements. Cem. Concr. Res. 2016, 84, 62–75. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, D.; Zhao, J.; Li, D.; Ng, S.; Rui, Y. Effect of calcium stearate based foam stabilizer on pore characteristics and thermal conductivity of geopolymer foam material. J. Build. Eng. 2018, 20, 21–29. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jitsangiam, P.; Rattanasak, U. Hydrophobicity and efflorescence of lightweight fly ash geopolymer incorporated with calcium stearate. J. Clean. Prod. 2022, 364, 132449. [Google Scholar] [CrossRef]
- Yao, X.; Liao, H.; Dong, H.; Yang, F.; Yao, Y.; Wang, W. Influence of water repellent on the property of solid waste based sulfoaluminate cement paste and its application in lightweight porous concrete. Constr. Build. Mater. 2021, 282, 122731. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Gong, T.; Li, B.; Zhao, Q. Regulation of pore structure of Brick-concrete recycled sand powder autoclaved aerated concrete and its relationship with key properties. Constr. Build. Mater. 2023, 392, 131849. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Ogawa, K.; Roy, D.M. C4A3S hydration, ettringite formation, and its expansion mechanism: III. Effect of CaO, NaOH and NaCl; conclusions. Cem. Concr. Res. 1982, 12, 247–256. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Najafi Kani, E.; Palomo Sanchez, A.; Fernandez-Jimenez, A. Rheology of activated phosphorus slag with lime and alkaline salts. Cem. Concr. Res. 2018, 113, 121–129. [Google Scholar] [CrossRef]
- Khan, H.A.; Khan, M.S.H.; Castel, A.; Sunarho, J. Deterioration of alkali-activated mortars exposed to natural aggressive sewer environment. Constr. Build. Mater. 2018, 186, 577–597. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q.; Gao, Z.; Chang, J. Nanostructural evolution of Al(OH)3 gel formed by the cubic and orthorhombic ye’elimite clinkers of calcium sulfoaluminate cements in an ultra-wide hydration temperature range. Cem. Concr. Res. 2021, 150, 106607. [Google Scholar] [CrossRef]
- Li, L.; Jiang, T.; Chen, B.; Wen, J.; Yang, G. Preparation and properties of foamed cement for lightweight thermal insulation with Ti-extraction blast furnace slag and sulfoaluminate cement by chemical foaming. Constr. Build. Mater. 2022, 337, 127634. [Google Scholar] [CrossRef]
- Paikara, R.K.; Gyawali, T.R. Influence of aluminum powder content and powder-to-sand ratio on the physical and mechanical properties of aerated lightweight mortar. Clean. Mater. 2023, 10, 100213. [Google Scholar] [CrossRef]
- Ahmida, F.; Sayah, G.M.; Zineb, D.; Quéneudec-t’Kint, M. Experimental study on the effect of lime and aluminium content on porosity, introduced porosity, compressive strength and thermal conductivity of a lightweight cellular concrete based on limestone sand. Constr. Build. Mater. 2023, 392, 131552. [Google Scholar] [CrossRef]
- Mohsen, A.; Ramadan, M.; Habib, A.O.; Abdel-Gawwad, H.A. Evaluating the role of magnesium aluminate nano spinel in phase composition, meso-porosity, compressive strength, and drying shrinkage of alkali-activated slag. Constr. Build. Mater. 2023, 409, 133857. [Google Scholar] [CrossRef]
- Lian, C.; Zhuge, Y.; Beecham, S. The relationship between porosity and strength for porous concrete. Constr. Build. Mater. 2011, 25, 4294–4298. [Google Scholar] [CrossRef]
- Martínez-Martínez, S.; Pérez-Villarejo, L.; Garzón, E.; Sánchez-Soto, P.J. Influence of firing temperature on the ceramic properties of illite-chlorite-calcitic clays. Ceram. Int. 2023, 49, 24541–24557. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, J.; Ji, J. AH3 phase in the hydration product system of AFt-AFm-AH3 in calcium sulfoaluminate cements: A microstructural study. Constr. Build. Mater. 2018, 167, 587–596. [Google Scholar] [CrossRef]
- Padilla-Encinas, P.; Fernández-Carrasco, L.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalinity on early-age hydration in calcium sulfoaluminate clinker. Cem. Concr. Res. 2022, 155, 106781. [Google Scholar] [CrossRef]




| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | SO3 |
|---|---|---|---|---|---|---|
| <10.5 | >34 | 1.5–3.5 | 41.5–43.5 | <3.5 | 1.0–2.0 | 7.5–9.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, F.; Chen, C.; Jiu, S.; Song, Q.; Chen, Y. Preparation and Characterization of Novel Sulfoaluminate-Cement-Based Nonautoclaved Aerated Concrete. Materials 2024, 17, 836. https://doi.org/10.3390/ma17040836
Peng F, Chen C, Jiu S, Song Q, Chen Y. Preparation and Characterization of Novel Sulfoaluminate-Cement-Based Nonautoclaved Aerated Concrete. Materials. 2024; 17(4):836. https://doi.org/10.3390/ma17040836
Chicago/Turabian StylePeng, Feifei, Chang Chen, Shaowu Jiu, Qiang Song, and Yanxin Chen. 2024. "Preparation and Characterization of Novel Sulfoaluminate-Cement-Based Nonautoclaved Aerated Concrete" Materials 17, no. 4: 836. https://doi.org/10.3390/ma17040836
APA StylePeng, F., Chen, C., Jiu, S., Song, Q., & Chen, Y. (2024). Preparation and Characterization of Novel Sulfoaluminate-Cement-Based Nonautoclaved Aerated Concrete. Materials, 17(4), 836. https://doi.org/10.3390/ma17040836





