The Doping of a Carbon Coating with Phosphorus as a Potential Way of Improving the Biological Properties of Diamond-like Carbon
Abstract
1. Introduction
2. Materials and Methods
- Sample preparation and coating synthesis
- Sample sterilization
- Chemical composition
- XPS
- FTIR
- Raman spectroscopy
- Surface free energy (SFE)
- Surface morphology and topography
- Mechanical properties
- Biological response
- Microbial colonization
- Mammalian cell viability
- Response to contact with blood (thrombogenicity assessment)
3. Results and Discussion
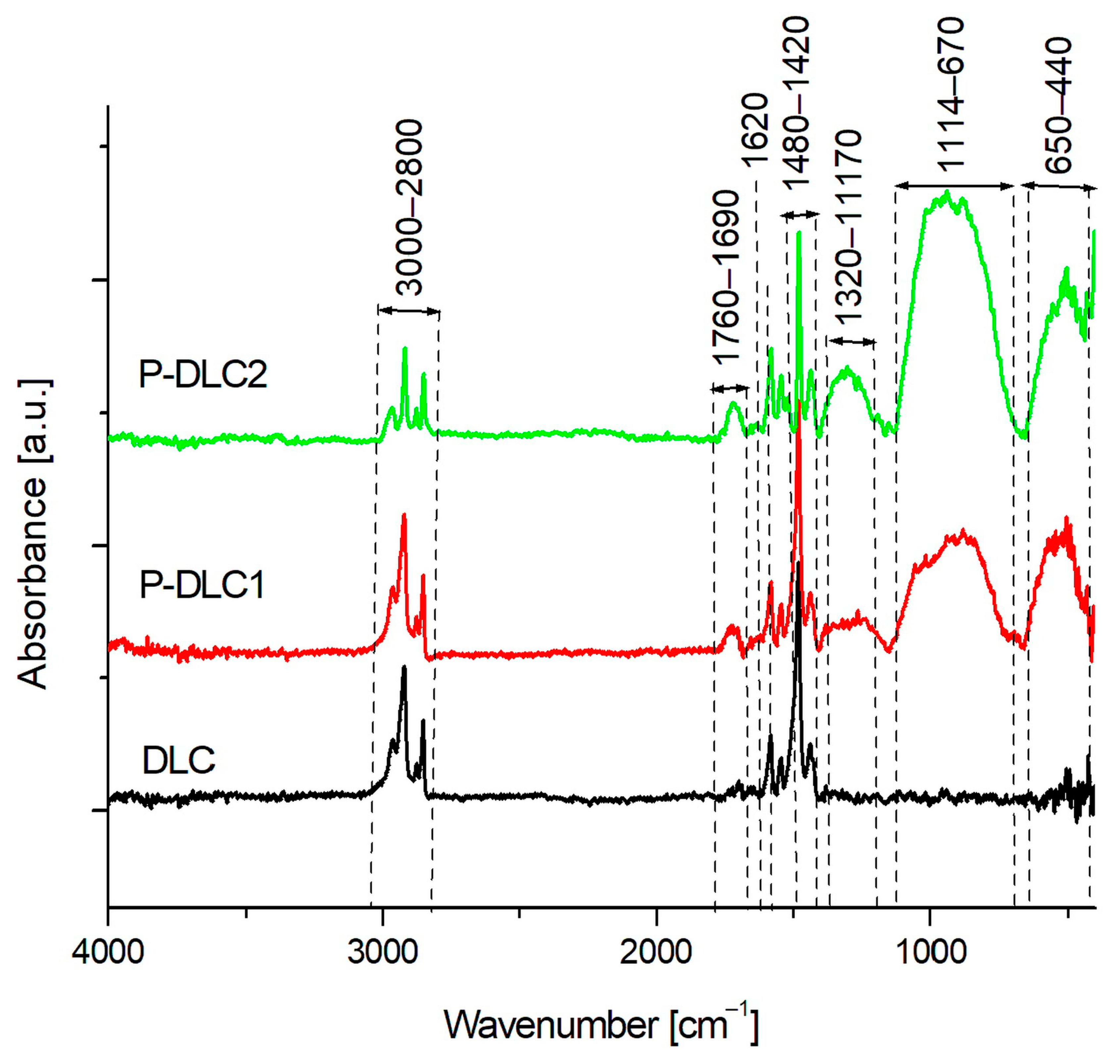
3.1. Chemical Composition
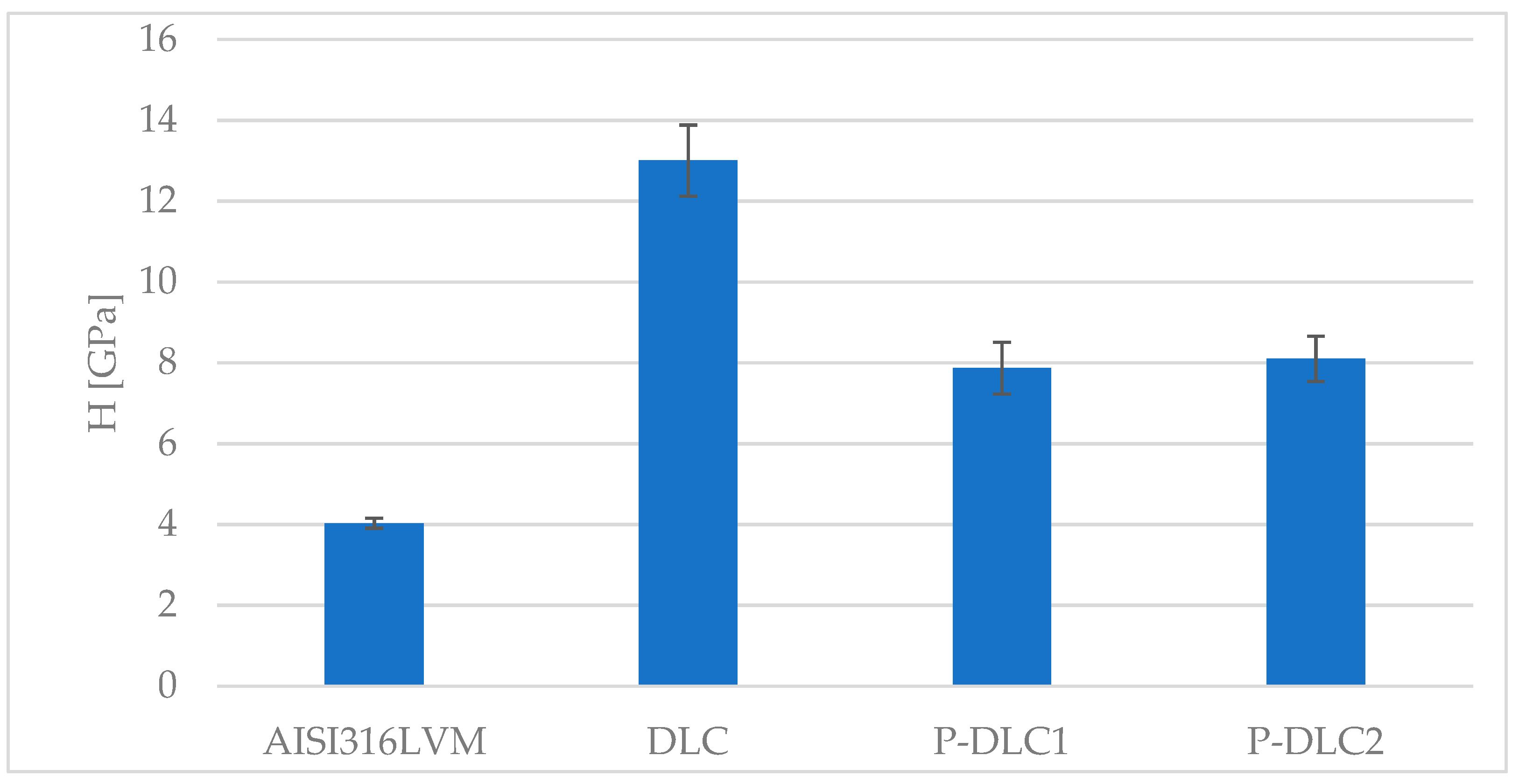
3.2. Mechanical and Surface Properties
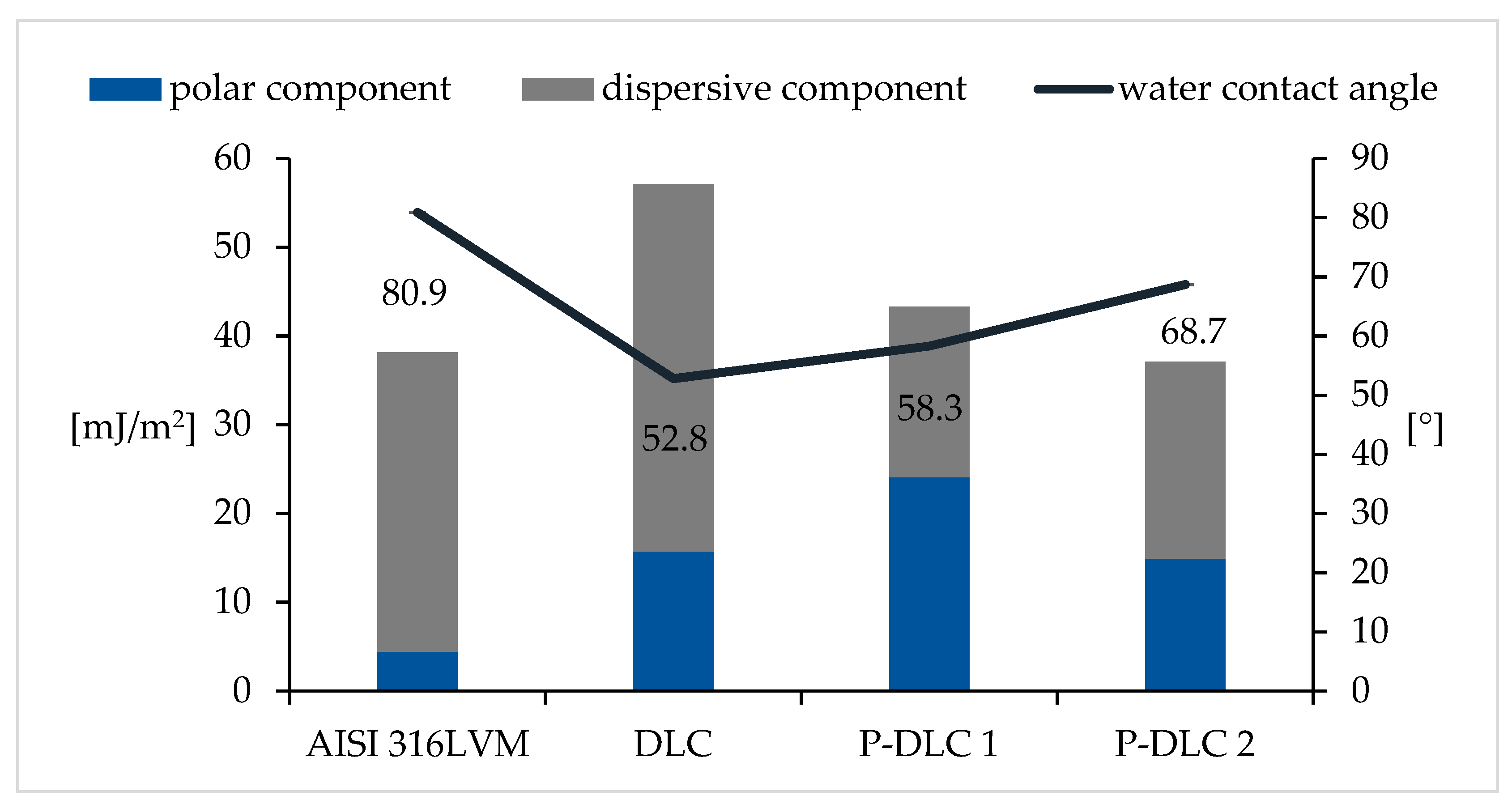
3.3. Surface Energy and Wettability
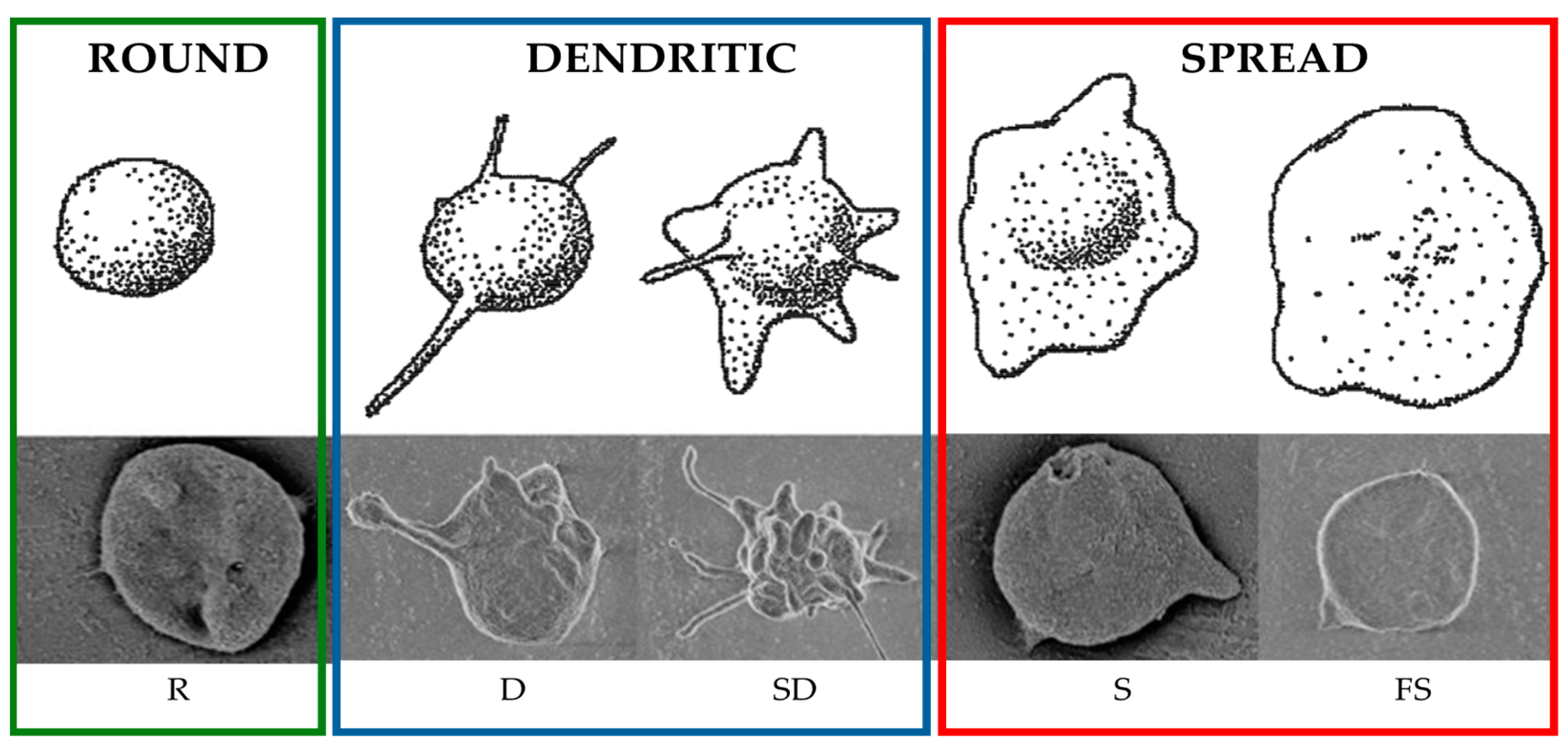
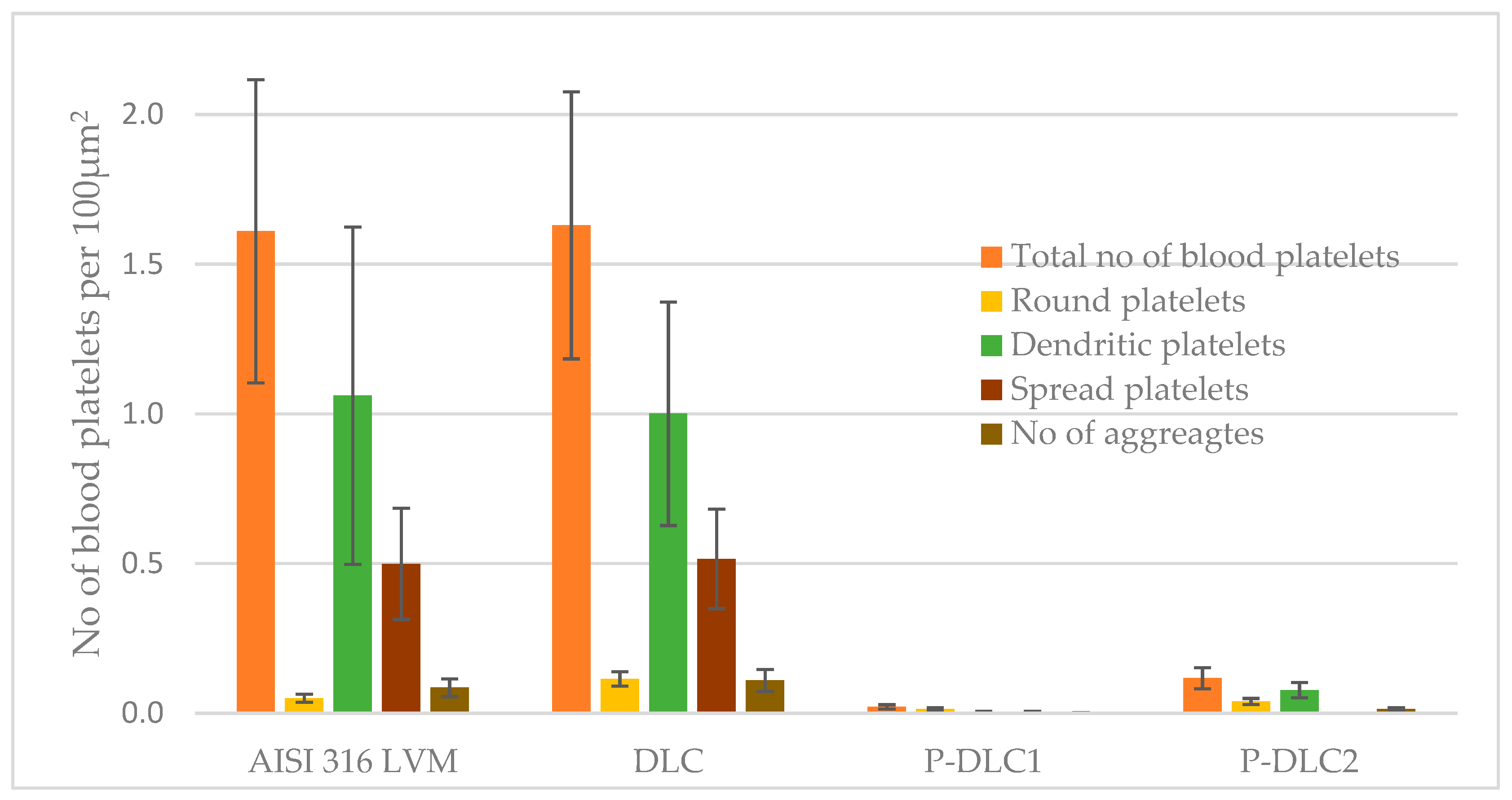
3.4. Biological Effect
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, Y.; Peng, J.; Wang, Z.; Xiao, Y.; Qiu, X. Diamond-like Carbon Coatings in the Biomedical Field: Properties, Applications and Future Development. Coatings 2022, 12, 1088. [Google Scholar] [CrossRef]
- Stoica, A.; Manakhov, A.; Polčák, J.; Ondračka, P.; Buršíková, V.; Zajíčková, R.; Medalová, J.; Zajíčková, L. Cell proliferation on modified DLC thin films prepared by plasma enhanced chemical vapor deposition. Biointerphases 2015, 10, 029520. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD); European Commission. Hip and Knee Replacement. Health at a Glance: Europe 2019: State of Health in the EU Cycle; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Jastrzebski, K.; Białecki, J.; Jastrzebska, A.; Kaczmarek, A.; Para, M.; Niedzielski, P.; Bociaga, D. Induced Biological Response in Contact with Ag-and Cu-Doped Carbon Coatings for Potential Orthopedic Applications. Materials 2021, 14, 1861. [Google Scholar] [CrossRef]
- Manninen, N.K.; Calderon, S.; Carvalho, V.I.; Henriques, M.; Cavaleiro, A.; Carvalho, S. Antibacterial Ag/a-C nanocomposite coatings: The influence of nano-galvanic a-C and Ag couples on Ag ionization rates. Appl. Surf. Sci. 2016, 377, 283–291. [Google Scholar] [CrossRef]
- Birkett, M.; Zia, A.W.; Devarajan, D.K.; Panayiotidis, S.M.I.; Joyce, T.J.; Tambuwala, M.; Serrano-Aroca, A. Multi-functional bioactive silver- and copper-doped diamond-like carbon coatings for medical implants. Acta Biomater. 2023, 167, 54–68. [Google Scholar] [CrossRef]
- Heaney, R.P. Phosphorus. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Washington, DC, USA, 2012; pp. 447–500. [Google Scholar]
- Rui, S.; Kubota, T.; Ohata, Y.; Yamamoto, K.; Fujiwara, M.; Takeyari, S.; Ozono, K. Phosphate promotes osteogenic differentiation through non-canonical Wnt signaling pathway in human mesenchymal stem cells. Bone 2022, 164, 116525. [Google Scholar] [CrossRef]
- Anselme, K.; Ponche, A.; Bigerell, M. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 2: Biological aspects. Proc. I. Mech. E. Part H J. Eng. Med. 2010, 224, 1487–1507. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Kochanowska, I.; Chaberek, S.; Wojtowicz, A.; Marczyński, B.; Włodarski, K.; Dytko, M.; Ostrowski, K. Expression of genes for bone morphogenetic proteins BMP-2, BMP-4 and BMP-6 in various parts of the human skeleton. BMC Musculoskelet. Disord. 2007, 8, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Regan, E.M.; Uney, J.B.; Dick, A.D.; Zhang, Y.; Nunez-Yanez, J.; McGeehan, J.P.; Claeyssens, F.; Kelly, S. Differential patterning of neuronal, glial and neural progenitor cells on phosphorus-doped and UV irradiated diamond-like carbon. Biomaterials 2010, 31, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Dolai, S.; Hussain, S.; Bhar, R.; Pal, A.K. Phosphorus doping of diamond-like carbon films by radio frequency CVD-cum-evaporation technique. Diam. Relat. Mater. 2018, 82, 70–78. [Google Scholar] [CrossRef]
- Bociaga, D.; Kaminska, M.; Sobczyk-Guzenda, A.; Jastrzebski, K.; Swiatek, L.; Olejnik, A. Surface properties and biological behaviour of Si-DLC coatings fabricated by a multi-target DC–RF magnetron sputtering method for medical application. Diam. Relat. Mater. 2016, 67, 41–50. [Google Scholar] [CrossRef]
- Jelinek, M.; Kocourek, T.; Zemek, J.; Mikšovský, J.; Kubinová, S.; Remsa, J.; Kopeček, J.; Jurek, K. Chromium-doped DLC for implants prepared by laser-magnetron deposition. Mater. Sci. Eng. C 2015, 46, 381–386. [Google Scholar] [CrossRef]
- Yetim, A.F.; Kovacı, H.; Kasapoğlu, A.E.; Bozkurt, Y.B.; Çelik, A. Influences of Ti, Al and V metal doping on the structural, mechanical and tribological properties of DLC films. Diam. Relat. Mater. 2021, 120, 108639. [Google Scholar] [CrossRef]
- Gao, K.; Wei, X.; Liu, G.; Zhang, B.; Zhang, J. Electrodeposition and biocompatibility of palladium and phosphorus doped amorphous hydrogenated carbon films. Chem. Phys. 2020, 537, 110857. [Google Scholar] [CrossRef]
- Batory, D.; Gorzedowski, J.; Rajchel, B.; Szymanski, W.; Kolodziejczyk, L. Silver implanted diamond-like carbon coatings. Vacuum 2014, 110, 78–86. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hammad, A.H.; Abdelghany, A.M. Optical and structural investigations of zinc phosphate glasses containing vanadium ions. J. Non-Cryst. Solids 2016, 433, 14–19. [Google Scholar] [CrossRef]
- Nayab, R.S.; Sasikala, T.; Mohan, B.A.; Rama, M.L.; Jayasankar, C.K. Optical spectroscopy, 1.06μm emission properties of Nd3+-doped phosphate based glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 180, 13–197. [Google Scholar] [CrossRef]
- Corbrjdge DE, C. Infra-red analysis of phosphorus compounds. J. Appl. Chem. 1956, 6, 456–465. [Google Scholar] [CrossRef]
- Wan, S.; Hu, H.; Chen, G.; Zhang, J. Synthesis and characterization of high voltage electrodeposited phosphorus doped DLC films. Electrochem. Commun. 2008, 10, 461–465. [Google Scholar] [CrossRef]
- Yang, C.J.; Nguyen, D.D.; Lai, J.Y. Poly(l-Histidine)-Mediated On-Demand Therapeutic Delivery of Roughened Ceria Nanocages for Treatment of Chemical Eye Injury. Adv. Sci. 2023, 10, e2302174. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, G.; Wang, S.; Xi, L.; Qin, G.; Wei, J.; Chen, X. Pulsed laser deposition of the protective and Anti-reflective DLC film. Infrared Phys. Technol. 2021, 119, 103949. [Google Scholar] [CrossRef]
- Akaike, S.; Kobayashi, D.; Aono, Y.; Hiratsuka, M.; Hirata, A.; Hayakawa, T.; Nakamura, Y. Relationship between static friction and surface wettability of orthodontic brackets coated with diamond-like carbon (DLC), fluorine- or silicone-doped DLC coatings. Diam. Relat. Mater. 2016, 61, 109–114. [Google Scholar] [CrossRef]
- Domínguez-Meister, S.; Rojas, T.C.; Frías, J.E.; Sánchez-López, J.C. Silver effect on the tribological and antibacterial properties of a-C:Ag coatings. Tribol. Int. 2019, 140, 140105837. [Google Scholar] [CrossRef]
- Kwok SC, H.; Wang, J.; Chu, P.K. Surface energy, wettability, and blood compatibility phosphorus doped diamond-like carbon films. Diam. Relat. Mater. 2005, 14, 78–85. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, X.; Zhang, S.; Lei, L.; Ma, W.; Li, D.; Wang, W.; Zhao, Q.; Xing, B. Bacterial toxicity of exfoliated black phosphorus nanosheets. Ecotoxicol. Environ. Saf. 2018, 161, 507–514. [Google Scholar] [CrossRef]
- Chai, S.; Zhou, L.; Pei, S.; Zhu, Z.; Chen, B. P-Doped Carbon Quantum Dots with Antibacterial Activity. Micromachines 2021, 12, 1116. [Google Scholar] [CrossRef]
- Miao, Y.; Shi, X.; Li, Q.; Hao, L.; Liu, L.; Liu, X.; Chen, Y.; Wang, Y. Engineering natural matrices with black phosphorus nanosheets to generate multi-func- tional therapeutic nanocomposite hydrogels. Biomater. Sci. 2019, 7, 4046–4059. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Fasolino, I.; Caporali, M.; Serrano-Ruiz, M.; Soriente, A.; Peruzzini, M.; Ambrosio, L. Exfoliated Black Phosphorus Promotes in Vitro Bone Regeneration and Suppresses Osteosarcoma Progression through Cancer-Related Inflammation Inhibition. ACS Appl. Mater. Interfaces 2019, 11, 9333–9342. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, X.; Park, S.; Terzic, A.; Lu, L. Size-dependent osteogenesis of black phosphorus in nanocomposite hydrogel scaffolds. J. Biomed. Mater. Res. A 2022, 110, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Magne, D.; Bluteau, G.; Faucheux, C.; Palmer, G.; Vignes-Colombeix, C.; Pilet, P.; Rouillon, T.; Caverzasio, J.; Weiss, P.; Daculsi, G.; et al. Phosphate is a specific signal for ATDC5 chondrocyte maturation and apoptosis-associated mineralization: Possible implication of apoptosis in the regulation of endochondral ossification. J. Bone Miner. Res. 2003, 18, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.; Zhao, L. Antibacterial, angiogenic, and osteogenic activities of Ca, P, Co, F, and Sr compound doped titania coatings with different Sr content. Sci. Rep. 2019, 9, 14203. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.K.; Denby, L.; Patel, R.K.; Mark, P.B.; Kettlewell, S.; Smith, G.L.; Clancy, M.J.; Delles, C.; Jardine, A.G. Deleterious effects of phosphate on vascular and endothelial function via disruption to the nitric oxide pathway. Nephrol. Dial. Transplant. 2017, 32, 1617–1627. [Google Scholar] [CrossRef]
- Szuto, E.; Taketani, Y.; Tanaka, R.; Harada, N.; Isshiki, M.; Sato, M.; Nashiki, K.; Amo, K.; Yamamoto, H.; Higashi, Y.; et al. Dietary phosphorus acutely impairs endothelial function. J. Am. Soc. Nephrol. 2009, 20, 1504–1512. [Google Scholar]
- Goodman, S.L. Sheep, pig, and human platelet-material interactions with model cardiovascular biomaterials. J. Biomed. Mater. Res. 1999, 45, 240–250. [Google Scholar] [CrossRef]
- Kwok, S.C.H.; Wan, G.J.; Ho JP, Y.; Chu, P.K.; Bilek, M.M.M.; McKenzie, D.R. Characteristics of phosphorus-doped diamond-like carbon films synthesized by plasma immersion ion implantation and deposition (PIII and D). Surf. Coat. Technol. 2007, 201, 6643–6646. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, J.; Liu, M.; Dai, Z.; Han, X.; Han, J. Platelet adhesion on phosphorus-incorporated tetrahedral amorphous carbon films. Appl. Surf. Sci. 2008, 255, 279. [Google Scholar] [CrossRef]




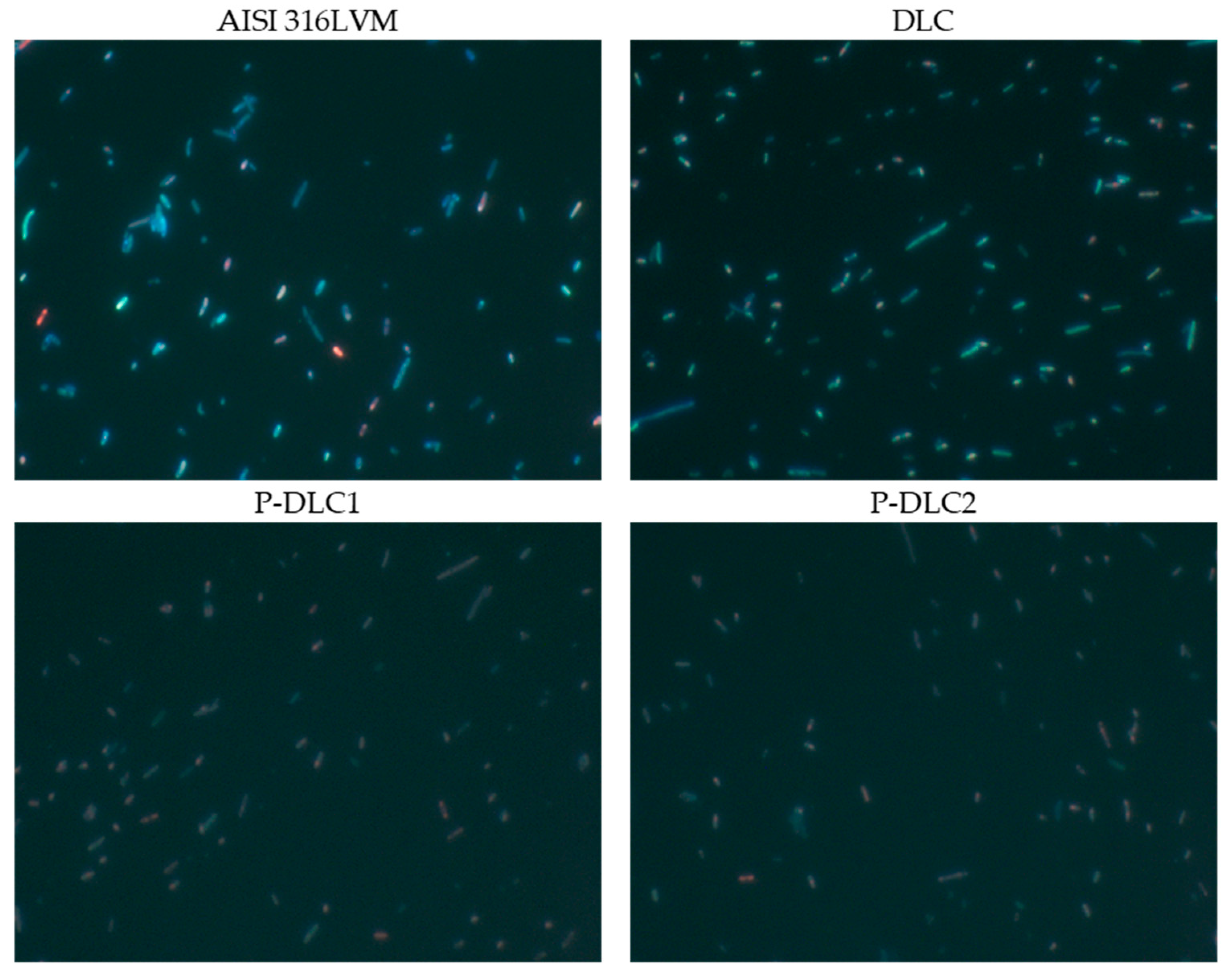


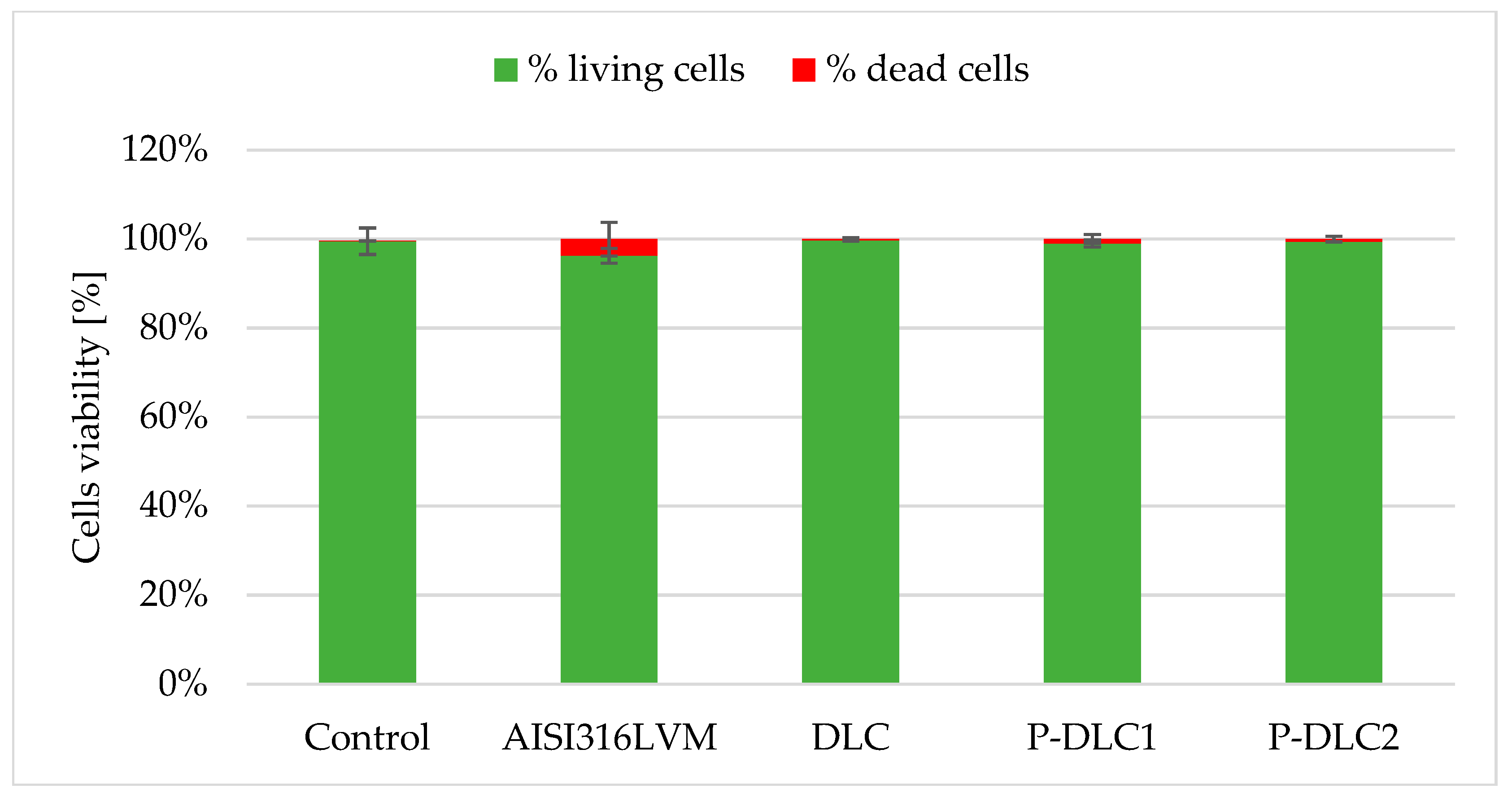
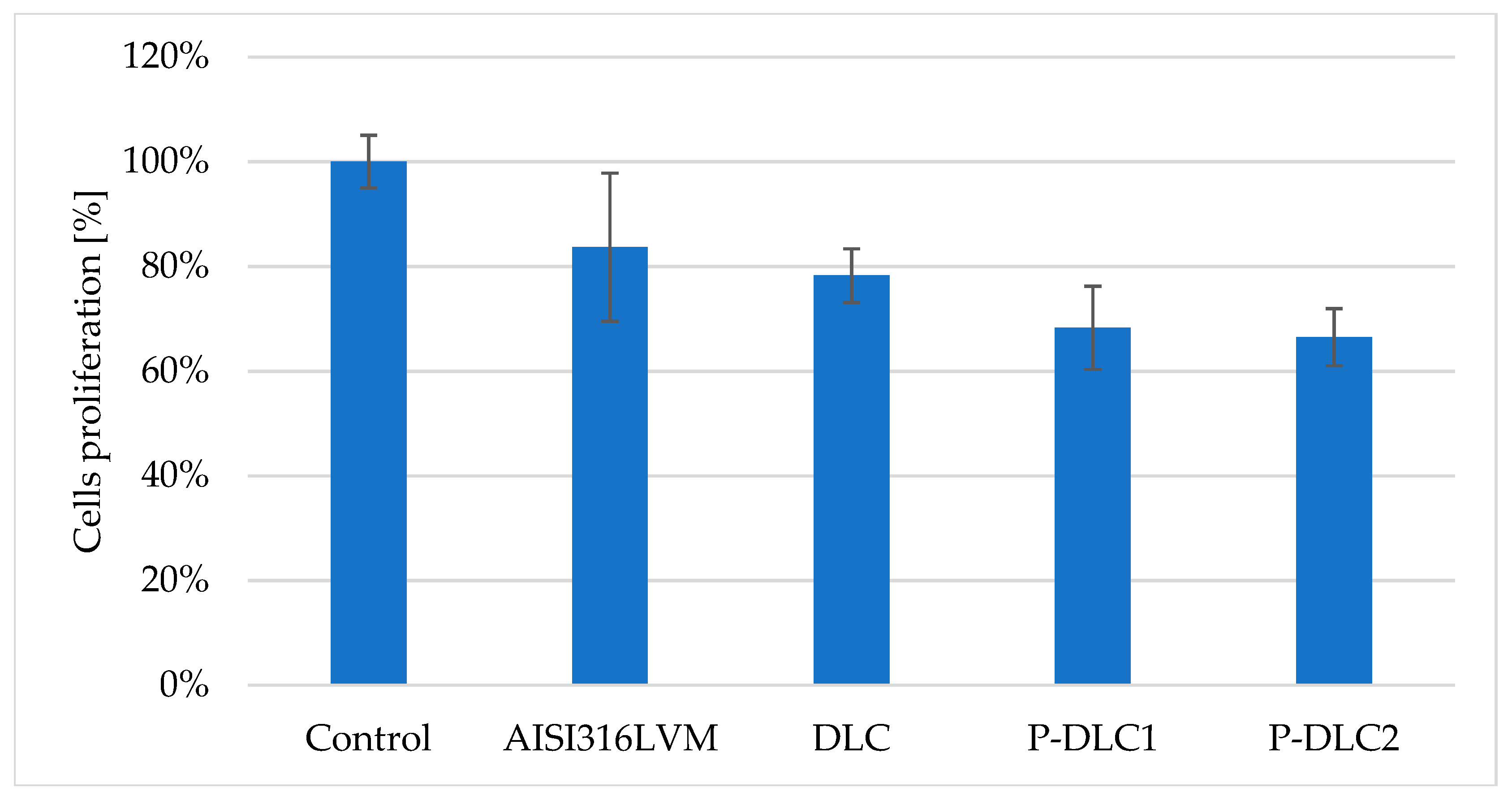
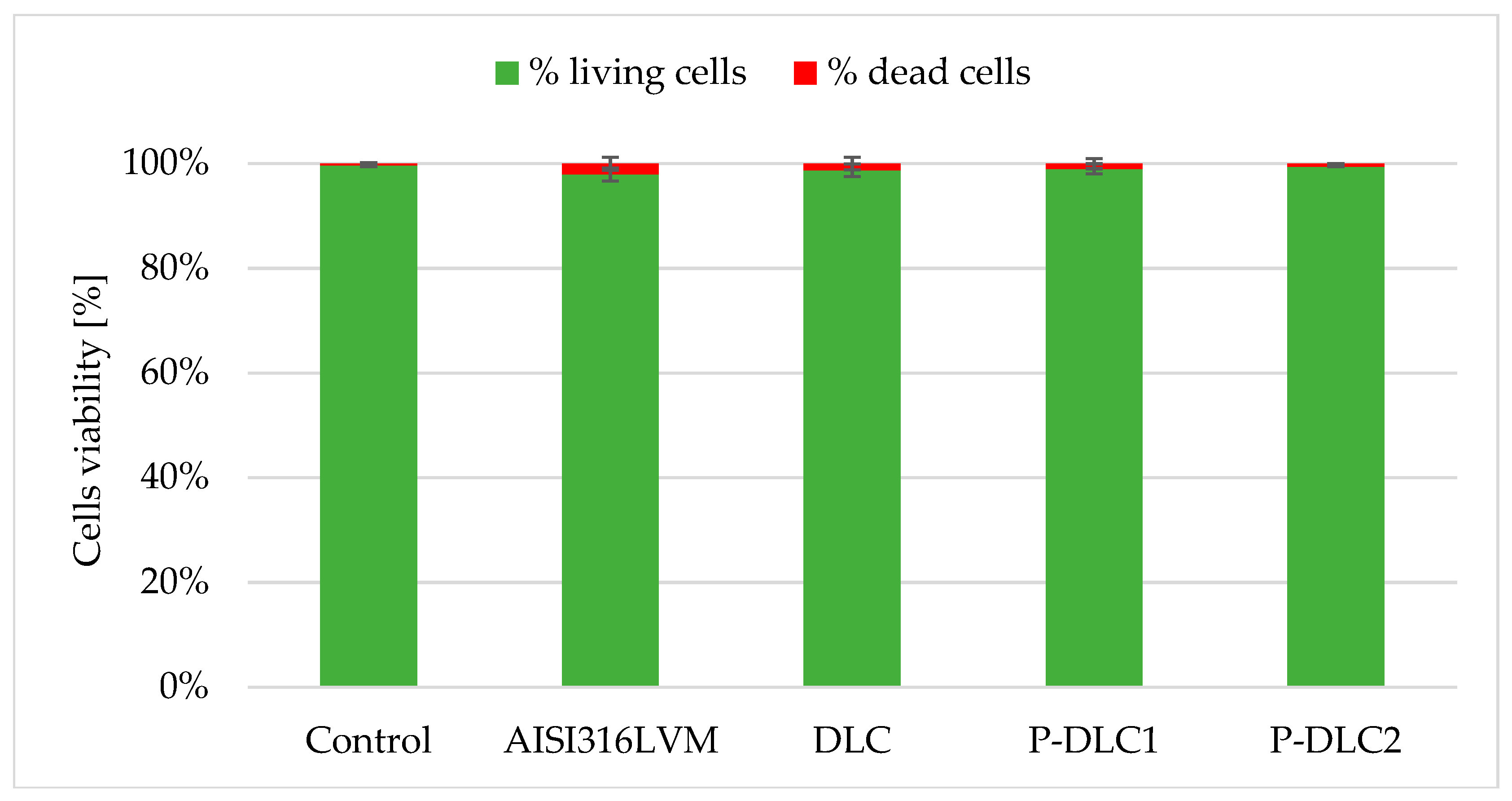

| C [at%] | O [at%] | P [at%] | |
|---|---|---|---|
| DLC | 73.50 | 26.50 | 0.00 |
| P-DLC 1 | 66.50 | 31.86 | 1.64 |
| P-DLC 2 | 63.71 | 32.03 | 4.26 |
| sp2 (C=C) | sp3 (C−C) | C−O | C=O | COO-R | sp3/sp2 | |
|---|---|---|---|---|---|---|
| DLC | 78.10 | 10.37 | 5.90 | 1.80 | 3.87 | 0.13 |
| P-DLC 1 | 70.80 | 11.50 | 10.23 | 2.47 | 5.00 | 0.16 |
| P-DLC 2 | 72.05 | 10.25 | 10.00 | 4.55 | 3.10 | 0.14 |
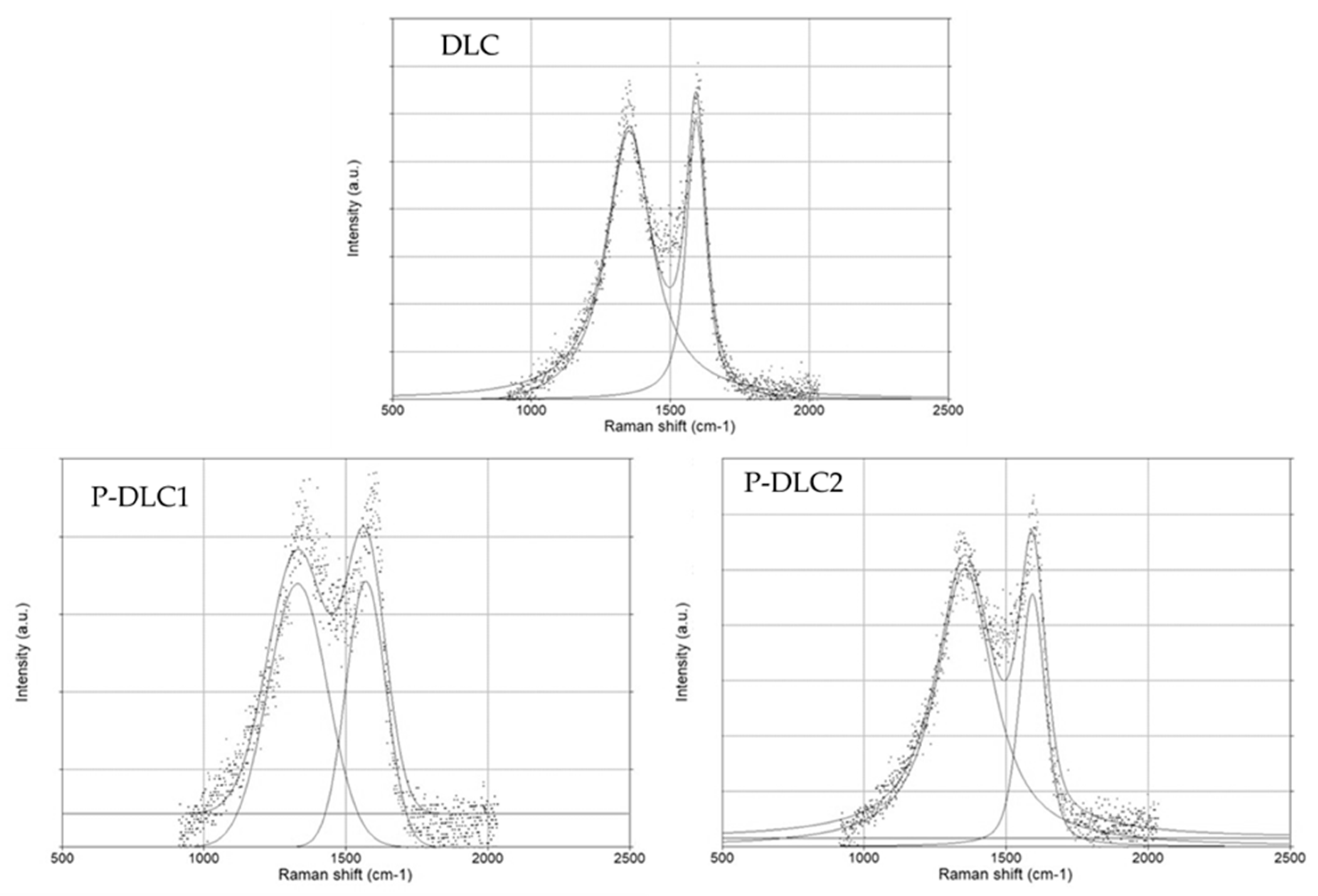
| D pos | G pos | G FWHM | ID | IG | ID/IG | |
|---|---|---|---|---|---|---|
| DLC | 1352.43 | 1594.35 | 68.41 | 236.97 | 246.71 | 0.96 |
| P-DLC1 | 1360.93 | 1591.28 | 120.13 | 201.89 | 207.31 | 0.97 |
| P-DLC2 | 1366.76 | 1589.83 | 105.20 | 185.31 | 167.93 | 1.10 |
| Scanning Area of 10 × 10 µm | Scanning Area of 1 × 1 µm | |
|---|---|---|
| DLC | 11.94 ± 1.94 nm | 4.301 ± 0.56 nm |
| P-DLC1 | 21.57 ± 1.86 nm | 16.99 ± 3.89 nm |
| P-DLC2 | 31.56 ± 4.16 nm | 17.19 ± 2.81 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jastrzębski, K.; Grabarczyk, J.; Niedzielski, P.; Jędrzejczak, A.; Sobczyk-Guzenda, A.; Szymański, W.; Kamińska, M.; Skibska, B. The Doping of a Carbon Coating with Phosphorus as a Potential Way of Improving the Biological Properties of Diamond-like Carbon. Materials 2024, 17, 5859. https://doi.org/10.3390/ma17235859
Jastrzębski K, Grabarczyk J, Niedzielski P, Jędrzejczak A, Sobczyk-Guzenda A, Szymański W, Kamińska M, Skibska B. The Doping of a Carbon Coating with Phosphorus as a Potential Way of Improving the Biological Properties of Diamond-like Carbon. Materials. 2024; 17(23):5859. https://doi.org/10.3390/ma17235859
Chicago/Turabian StyleJastrzębski, Krzysztof, Jacek Grabarczyk, Piotr Niedzielski, Anna Jędrzejczak, Anna Sobczyk-Guzenda, Witold Szymański, Marta Kamińska, and Beata Skibska. 2024. "The Doping of a Carbon Coating with Phosphorus as a Potential Way of Improving the Biological Properties of Diamond-like Carbon" Materials 17, no. 23: 5859. https://doi.org/10.3390/ma17235859
APA StyleJastrzębski, K., Grabarczyk, J., Niedzielski, P., Jędrzejczak, A., Sobczyk-Guzenda, A., Szymański, W., Kamińska, M., & Skibska, B. (2024). The Doping of a Carbon Coating with Phosphorus as a Potential Way of Improving the Biological Properties of Diamond-like Carbon. Materials, 17(23), 5859. https://doi.org/10.3390/ma17235859









