Non-Isothermal Crystallization Kinetics and Properties of CaO-Al2O3-SiO2 (CAS) Glass-Ceramics from Eggshell Waste, Zeolite, and Pumice
Abstract
1. Introduction
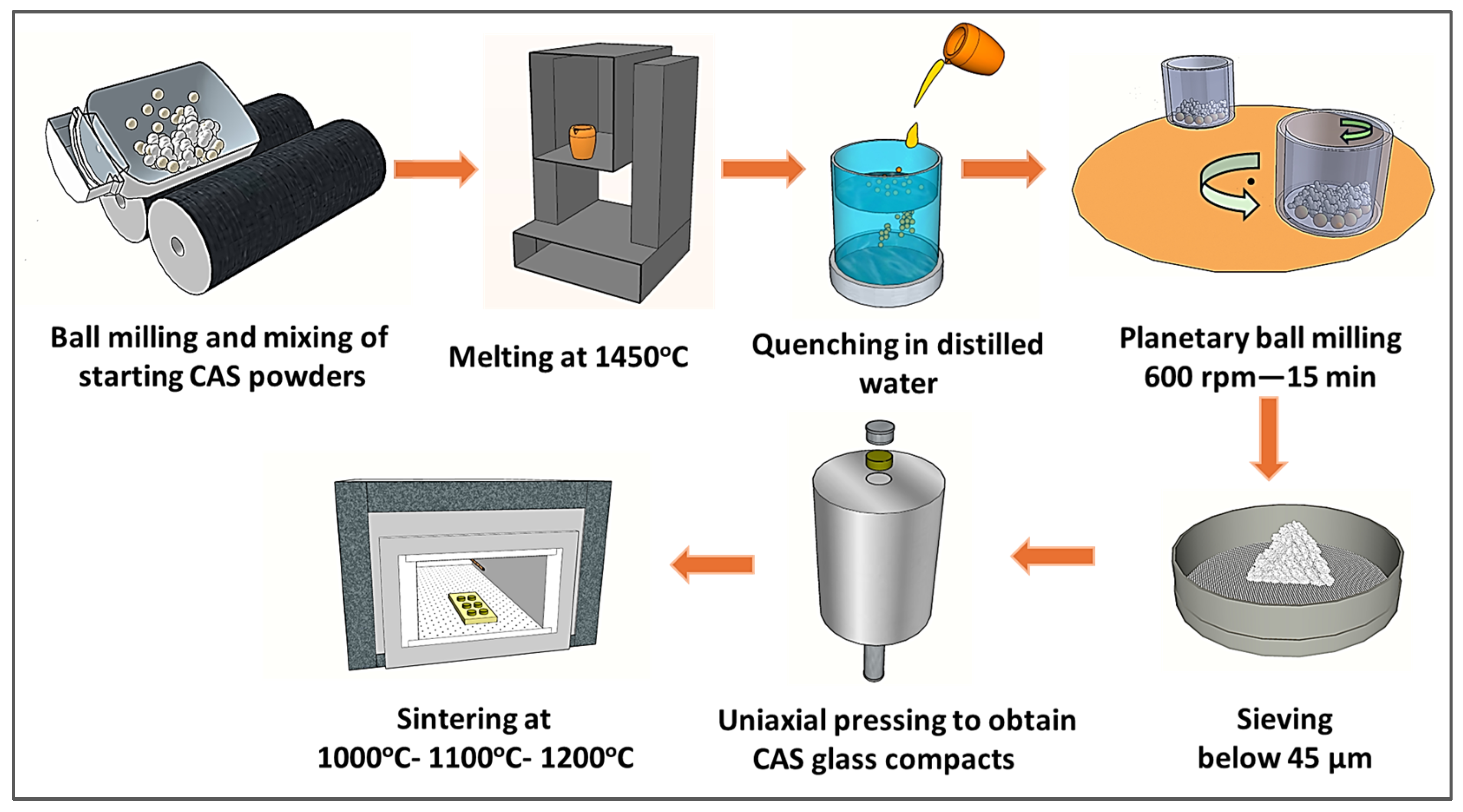
2. Materials and Methods
3. Results and Discussion
3.1. X-Ray Diffraction Analysis of CAS Glasses and Glass-Ceramics
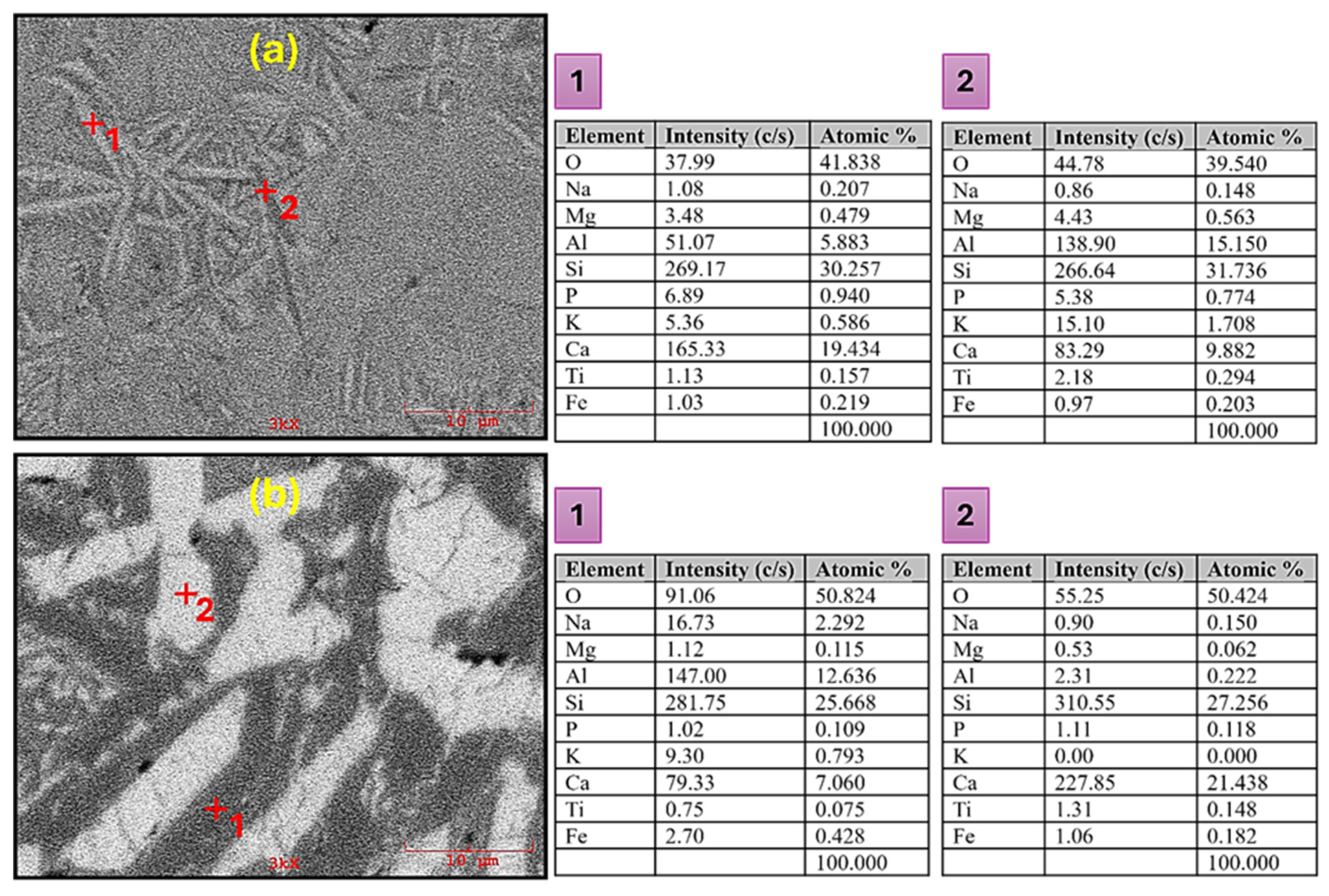
3.2. Microstructural Evaluation of CAS Glass-Ceramics
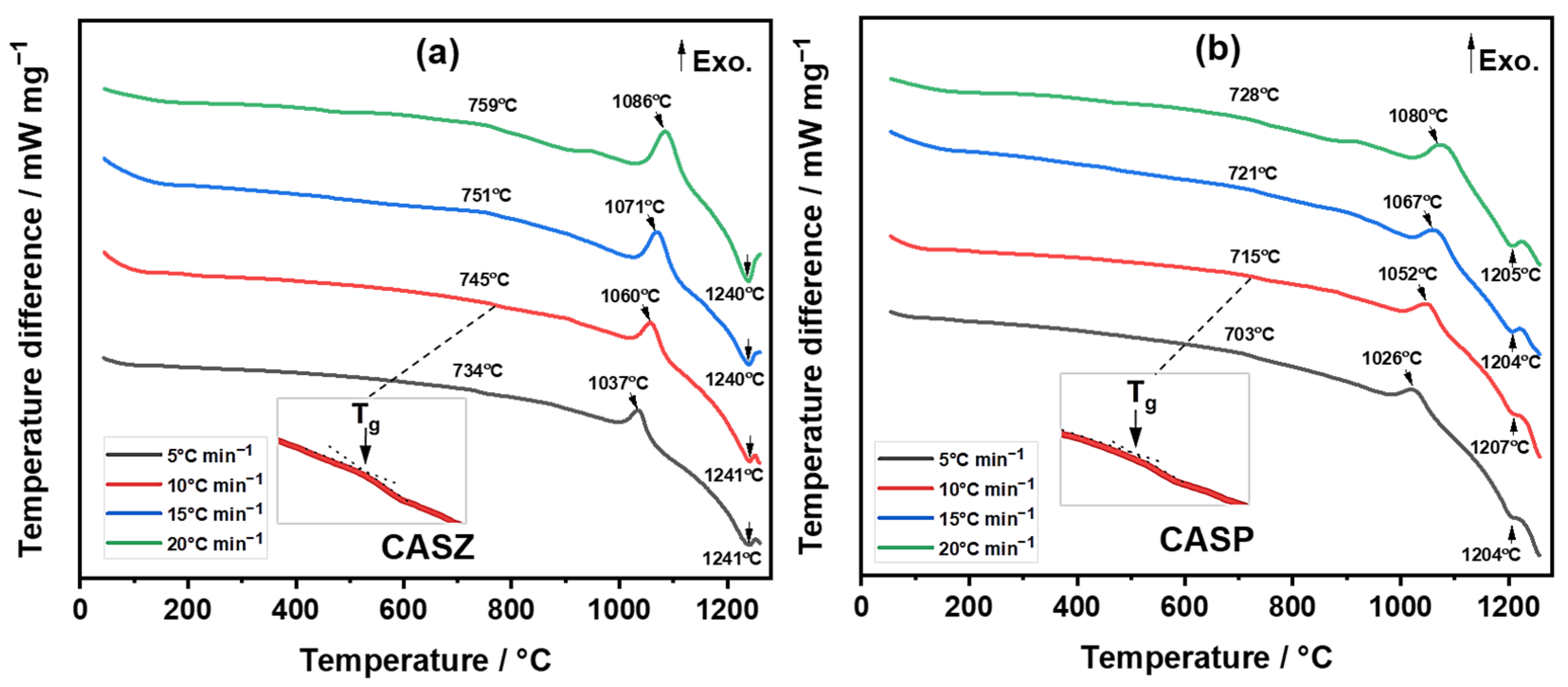
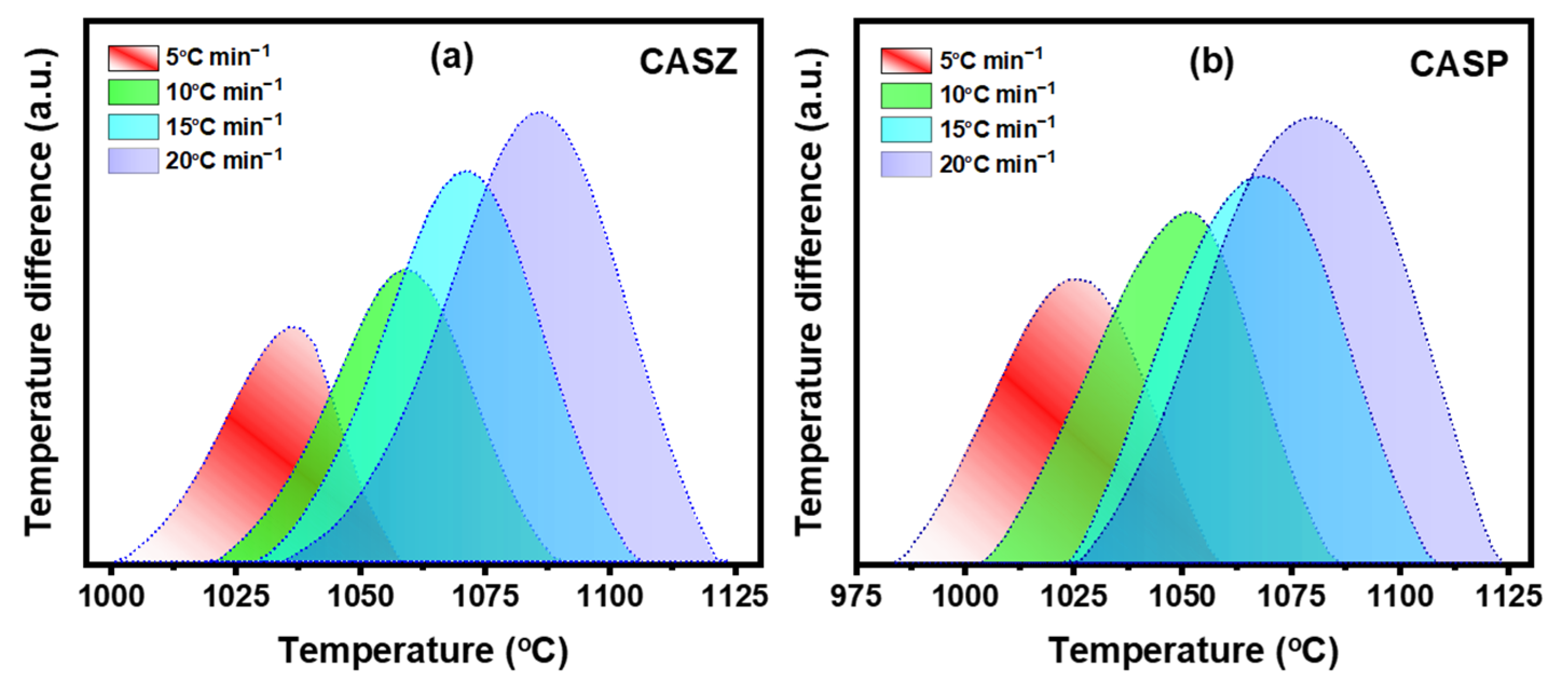
3.3. Differential Thermal Analysis (DTA)
3.4. Non-Isothermal Crystallization Kinetics
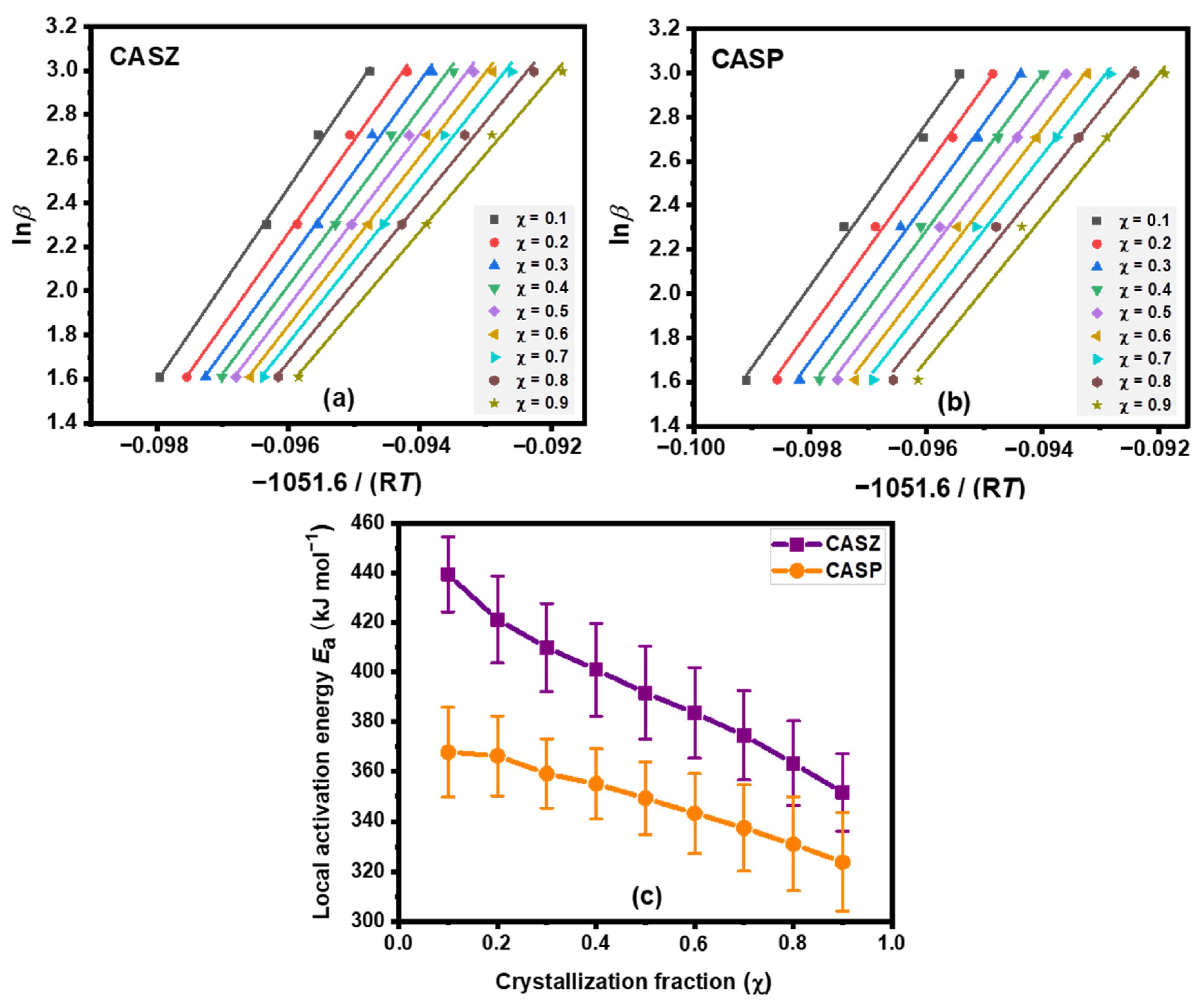
3.4.1. Kissinger Method
3.4.2. Ozawa Method
3.4.3. Matusita Method
3.4.4. Local Activation Energy
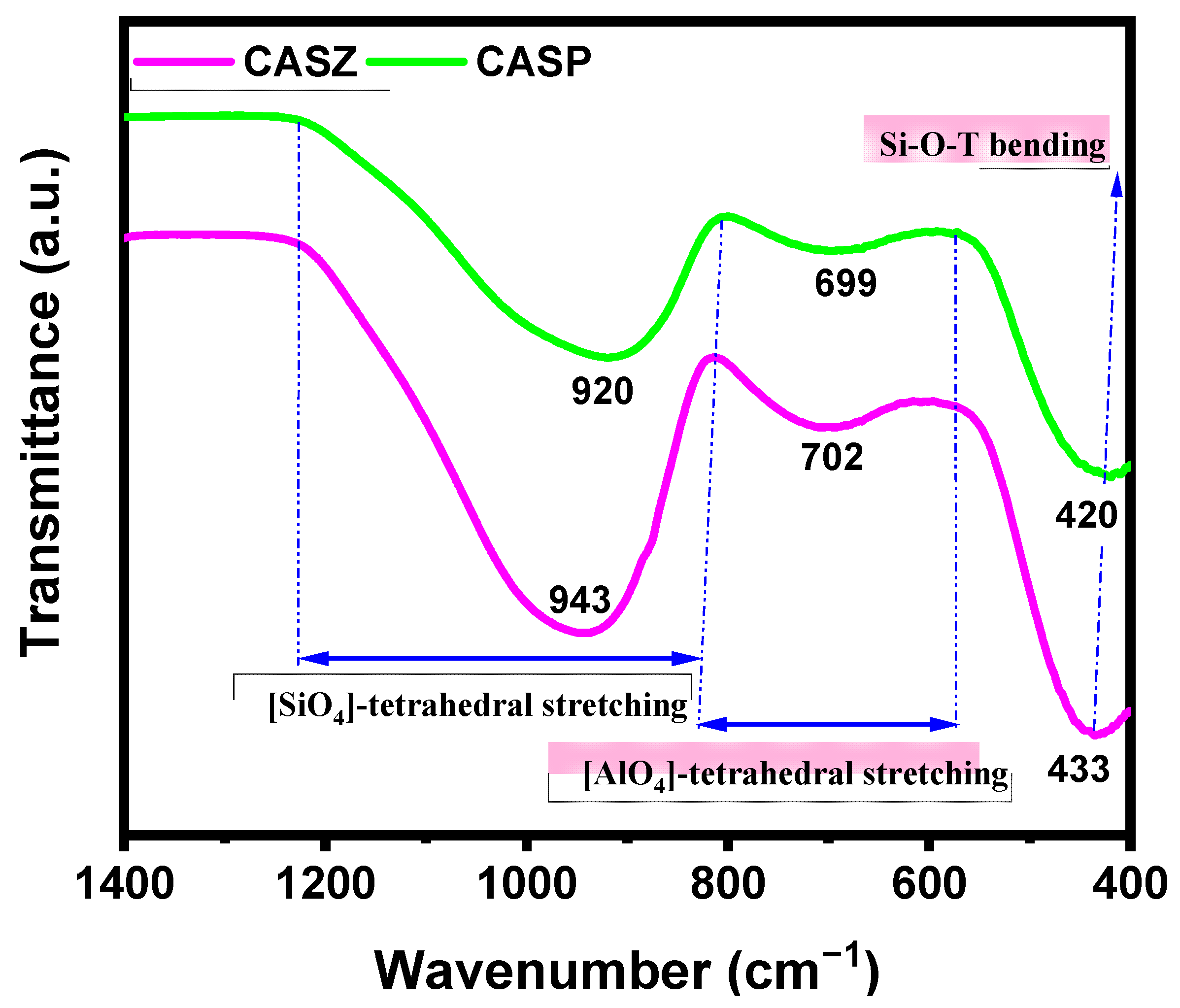
3.5. FTIR Analysis
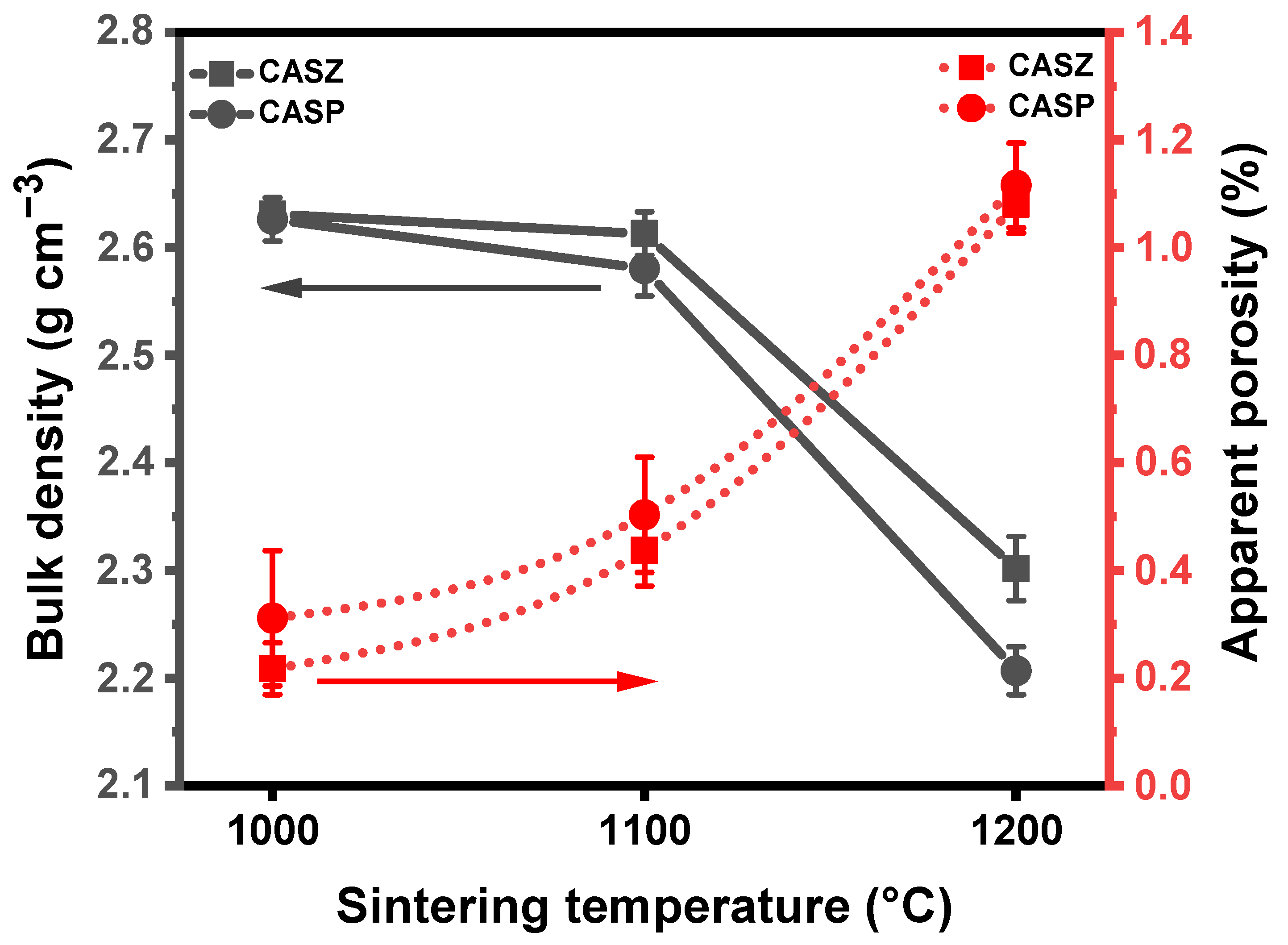
3.6. Physical Properties of Glass-Ceramics
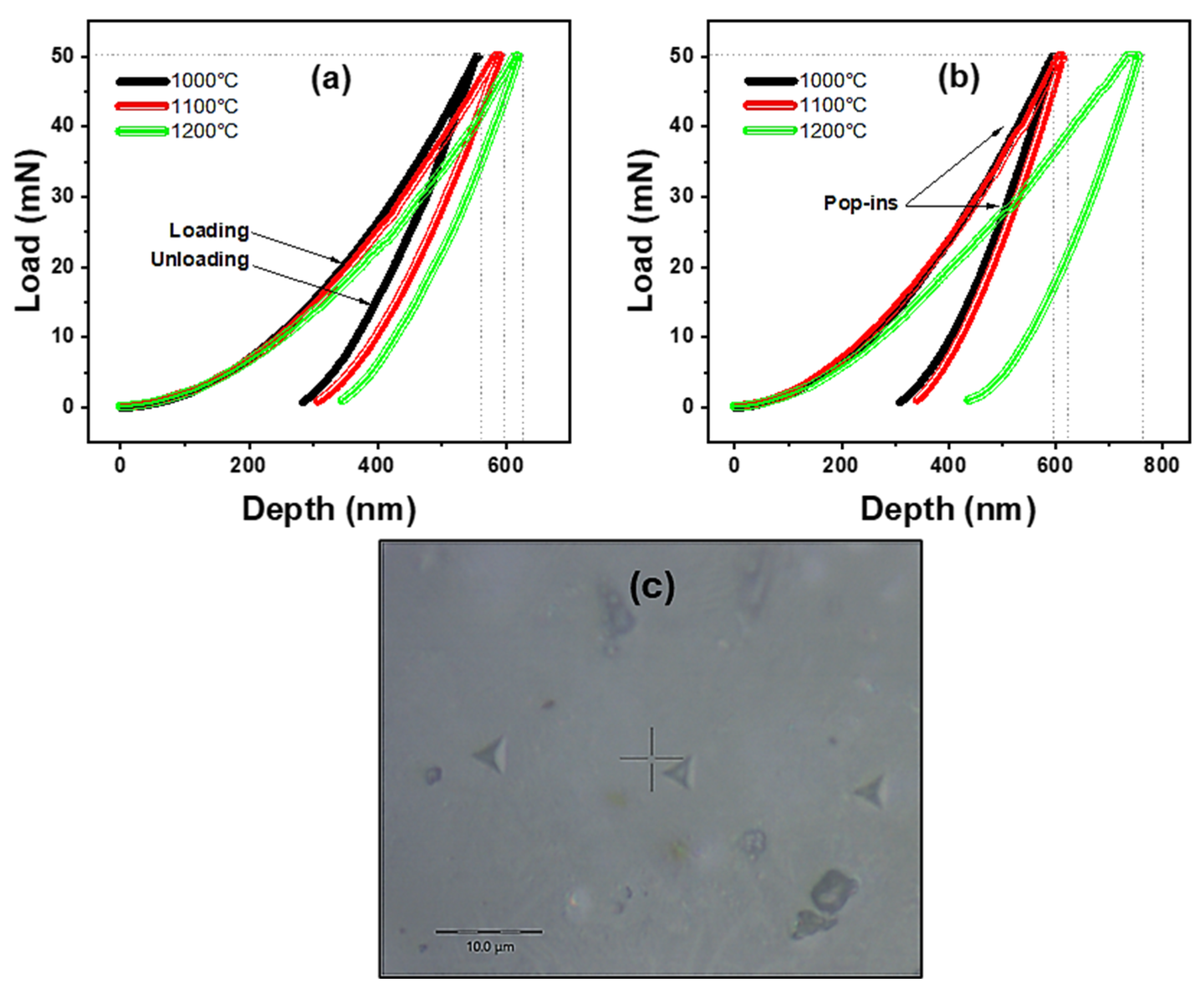
3.7. Mechanical Properties of CAS Glass-Ceramics
4. Conclusions
- The crystallization peak temperature and crystallization activation energy decreased with the increasing content of modifying metal oxides in the glass structure. While the activation energy required for the crystallization of CASZ, calculated using different approaches, ranged between 406 and 428 kJ mol−1, it ranged between 356 and 378 kJ mol−1 for CASP. The average local activation energy values calculated from different crystallization fractions of the CASZ and CASP glasses were 392.96 ± 17.39 kJ mol⁻1 and 348.15 ± 16.46 kJ mol⁻1, respectively.
- The Avrami parameter (n) for CASZ and CASP glasses was calculated as 3.33 and 2.89 on average from the determined temperatures, respectively. However, the value of n is not constant and tends to decrease with increasing temperature. This indicates that the crystal growth in CAS glasses varies from three-dimensional to one-dimensional during crystallization and the crystallization mechanism is volume crystallization.
- XRD analyses revealed that anorthite and wollastonite were the crystalline phases detected in both glass-ceramics after sintering at 1000 °C and 1100 °C, while increasing the temperature to 1200 °C resulted in the disappearance of wollastonite peaks and the formation of intense peaks corresponding to the pseudowollastonite crystalline phase.
- SEM examinations demonstrated the existence of porosity within the glass-ceramic samples. Initially, the crystals exhibited a needle-like structure; however, as the sintering temperature elevated, they underwent morphological changes and exhibited rod-like characteristics after the final sintering at 1200 °C.
- The highest bulk density of the glass-ceramic samples was achieved at the initial sintering temperature of 1000 °C, with calculated values of 2.63 g cm−3 for CASZ and 2.62 g cm−3 for CASP.
- Depending on the sintering temperature, the Vickers hardness of the CASZ-coded glass-ceramics ranged from 916 to 614 HV, while CASP ranged from 747 to 569 HV. Additionally, the elastic modulus was found to vary between 111 and 92 GPa and 99 and 87 GPa for these two compositions, respectively. Increasing sintering temperatures resulted in a decrease in mechanical properties for both glass-ceramics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holand, W.; Beall, G.H. Glass-Ceramic Technology; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Montoya-Quesada, E.; Manzano-Ramírez, A.; Rivera-Vargas, G.; Juárez-Hernández, M.E.; Neira-Velázquez, G. Effect of ZnO content on the physical, mechanical and chemical properties of glass-ceramics in the CaO-SiO₂-Al₂O₃ system. Ceram. Int. 2020, 46, 4322–4328. [Google Scholar] [CrossRef]
- Buchner, S.; Meboldt, M.; Ermanni, P.; Gasser, P. Mechanical and tribological properties of a sintered glass-ceramic compared to granite and porcelainized stoneware. Wear 2011, 271, 875–880. [Google Scholar] [CrossRef]
- Ozabaci, M.; Erol, M.; Yücel, B.; Ovecoglu, L. Preparation and characterization of CaO-Al₂O₃-SiO₂ (CAS) glass-ceramics. J. Non-Cryst. Solids 2016, 454, 8–12. [Google Scholar] [CrossRef]
- He, F.; Ping, C.M.; Cheng, J.S. Phase Evolutionary Process of CaO-Al₂O₃-SiO₂ System Glass and Glass-Ceramic. Adv. Mater. Res. 2011, 168, 1947–1952. [Google Scholar] [CrossRef]
- Cheng, J.S.; Ping, C.M.; Hu, X.J.; Liu, Y.B. Effects of Phosphorus and Fluorine on the Crystallization and Structure of CaO-Al₂O₃-SiO₂ System Glass-Ceramic. Key Eng. Mater. 2008, 368, 1412–1414. [Google Scholar] [CrossRef]
- Li, H.; Cheng, J.S.; Long, X.J. Study on Corrosion Mechanism of CAS Glass-Ceramic to Refractories by EPMA. Key Eng. Mater. 2004, 280, 1663–1666. [Google Scholar] [CrossRef]
- Mukherjee, D.P.; Das, S.K. SiO₂–Al₂O₃–CaO glass-ceramics: Effects of CaF₂ on crystallization, microstructure and properties. Ceram. Int. 2013, 39, 571–578. [Google Scholar] [CrossRef]
- Wu, J.; Wang, F.; Gong, Y.; Huang, W. Crystallization behavior and properties of K₂O–CaO–Al₂O₃–SiO₂ glass-ceramics. Ceram. Int. 2013, 39, 7743–7750. [Google Scholar] [CrossRef]
- Minouei, H.; Soltanpour, M.; Babaei, S.; Shokouhimehr, M. Non-isothermal nano-crystallization kinetics in amorphous Ni₅₅Nb₃₅Si₁₀ alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 358–364. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Yu, Z.; Zhou, H.; Wen, Z. Study on non-isothermal crystallization kinetics of the BaO-CaO-Al₂O₃-B₂O₃-SiO₂ glass for IT-SOFCs sealing. Ceram. Int. 2018, 44, 21277–21283. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.; Izadi, H.; Delshad, M.; Tabari, M.; Maziar, R.; Yaghoubnejad, A. Crystallization kinetics of glass-ceramics by differential thermal analysis. Ceram.-Silikaty 2011, 55, 188–194. [Google Scholar]
- Erol, M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. Production of glass-ceramics obtained from industrial wastes by means of controlled nucleation and crystallization. Chem. Eng. J. 2007, 132, 335–343. [Google Scholar] [CrossRef]
- Faridi, H.; Arabhosseini, A. Application of eggshell wastes as valuable and utilizable products: A review. Res. Agric. Eng. 2018, 64, 104–111. [Google Scholar] [CrossRef]
- Ding, Q.; Tian, X.; Cheng, W.; Liu, J.; Wang, F. Conversion of waste eggshell into difunctional Au/CaCO₃ nanocomposite for 4-Nitrophenol electrochemical detection and catalytic reduction. Appl. Surf. Sci. 2020, 510, 145526. [Google Scholar] [CrossRef]
- Hossain, S.S.; Sharma, A.; Alam, T.; Acharya, S.K. A comparative study of physico-mechanical, bioactivity and hemolysis properties of pseudo-wollastonite and wollastonite glass-ceramic synthesized from solid wastes. Ceram. Int. 2020, 46, 833–843. [Google Scholar] [CrossRef]
- Tisler, Z.; Lapčík, L.; Lapčíková, B.; Kozánková, J.; Veselý, P.; Študent, J.; Lapčík, V. Clinoptilolite foams prepared by alkali activation of natural zeolite and their post-synthesis modifications. Microporous Mesoporous Mater. 2019, 282, 169–178. [Google Scholar] [CrossRef]
- Poyraz, H.B.; Erginel, N.; Ay, N. The use of pumice (pumicite) in transparent roof tile glaze composition. J. Eur. Ceram. Soc. 2006, 26, 741–746. [Google Scholar] [CrossRef]
- Wongsa, A.; Kunther, W.; Nawa, T.; Sata, V.; Chindaprasirt, P. Use of crushed clay brick and pumice aggregates in lightweight geopolymer concrete. Constr. Build. Mater. 2018, 188, 1025–1034. [Google Scholar] [CrossRef]
- Jones, T.; Quinn, J.; Tollerfield, C.; LaRiviere, J. Pumice attrition in an air-jet. Powder Technol. 2017, 308, 298–305. [Google Scholar] [CrossRef]
- Ayawanna, J.; Kingnoi, N.; Laorodphan, N. A feasibility study of eggshell-derived porous glass–ceramic orbital implants. Mater. Lett. 2019, 241, 39–42. [Google Scholar] [CrossRef]
- Loh, Z.W.; Zaid, M.H.M.; Matori, K.A.; Kechik, M.M.A.; Fen, Y.W.; Mayzan, M.Z.H.; Cheong, W.M. Phase transformation and mechanical properties of new bioactive glass-ceramics derived from CaO–P2O5–Na2O–B2O3–SiO2 glass system. J. Mech. Behav. Biomed. Mater. 2023, 143, 105889. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.Q.A.; Sa’at, N.K.; Zaid, M.H.M.; Zainuddin, N.; Mayzan, M.Z.H. Effect of Na2CO3/Al2O3 ratio on the calcium fluoroaluminosilicate-based bioactive glass-ceramics derived from waste materials. Mater. Chem. Phys. 2024, 312, 128556. [Google Scholar] [CrossRef]
- Sharma, G.; Singh, K. Dielectric and optical properties of glasses and glass-ceramics synthesized from agro-food wastes. Mater. Chem. Phys. 2020, 246, 122754. [Google Scholar] [CrossRef]
- Hongxu, C.; Azis, R.A.S.; Zaid, M.H.M.; Matori, K.A.; Ismail, I. Influence of sintering temperature on structure, physical, and optical properties of wollastonite based glass-ceramic derived from waste eggshells and waste soda-lime-silica glasses. Sci. Sinter. 2024, 56, 89–103. [Google Scholar] [CrossRef]
- Estrada-Guel, I.; Montes, J.A.; Ramirez-Balderrama, K.A.; Soto-Figueroa, C.; Ruiz Santos, R. Synthesis and characterization of low-cost glass-ceramic foams for insulating applications using glass and pumice wastes. J. Appl. Res. Technol. 2020, 18, 44–50. [Google Scholar] [CrossRef]
- Tore, I.; Civan, L. Evaluation of pumice in glaze compositions for ceramics. Int. J. Sci. Technol. Res. 2015, 1, 22–29. [Google Scholar]
- Oztürk, Z.B.; Can, A. Sürdürülebilir endüstriyel özellikleri taşıyan seramik sağlık gereci sırları üretiminde mikronize pomzanın kullanımı. Gazi Univ. Eng. Arch. Fac. J. 2023, 38, 1967–1978. [Google Scholar] [CrossRef]
- Ibrahim, J.E.F.; Tihtih, M.; Kurovics, M.; Gömze, L.A.; Kocserha, I. Innovative glass-ceramic foams prepared by alkali activation and reactive sintering of clay containing zeolite (zeolite-poor rock) and sawdust for thermal insulation. J. Build. Eng. 2022, 59, 105160. [Google Scholar] [CrossRef]
- Ivanov, K. Extrusion method for producing microgranular foam-glass ceramic from zeolite rocks. Refract. Ind. Ceram. 2021, 62, 157–161. [Google Scholar] [CrossRef]
- Vereshagin, V.I.; Sokolova, S.N. Granulated foam glass–ceramic material from zeolitic rocks. Constr. Build. Mater. 2008, 22, 999–1003. [Google Scholar] [CrossRef]
- Wei, G.; Luo, F.; Miao, Y.; Han, W.; Xie, Y.; Huang, W.; Xu, Z.; Lu, X. Treatment of zeolite adsorbed material as a potential nuclear waste glass-ceramic matrix. J. Am. Ceram. Soc. 2022, 105, 257–267. [Google Scholar] [CrossRef]
- Yuan, B.; Luo, F.; Miao, Y.; Shi, M.; Zhao, Y.; Huang, W.; Xu, Z.; Lu, X. Immobilization of simulated An4+ radioactively contaminated zeolite: Solidify mechanism and theory investigation. J. Solid State Chem. 2022, 311, 123095. [Google Scholar] [CrossRef]
- Jinshu, C.; Huiming, C.; Peng, Z.; Li, J.; Huang, Y. Stress analysis of CaO−Al₂O₃−SiO₂ system glass-ceramic with different thickness. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2005, 20, 126–127. [Google Scholar] [CrossRef]
- Piva, J.H.; Nogueira, P.C.; Nogueira, R.A.; Andrade, J.M.; Reis, J.A.; Oliveira, E.S.; Souza, G.P.; Nagata, A.G. Sintering and crystallization of plates prepared from coarse glass ceramic frits. Ceram. Int. 2013, 39, 9137–9144. [Google Scholar] [CrossRef]
- He, F.; Cheng, J.S.; Liu, Y.B.; Zhang, T.H. Fabrication and characterization of glass–ceramics materials developed from steel slag waste. Mater. Des. 2012, 42, 198–203. [Google Scholar] [CrossRef]
- Zhao, T.; Han, Y.; Li, Y.; Li, H.; Wang, X. Influence of SiO₂ contents on the microstructure and mechanical properties of lithium disilicate glass-ceramics by reaction sintering. J. Non-Cryst. Solids 2019, 512, 148–154. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, Y. On formation of CaO–Al₂O₃–SiO₂ glass–ceramics by vitrification of incinerator fly ash. Chemosphere 2003, 51, 817–824. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, D.; Ma, L.; Deng, C.; Li, L.; Chen, K.; Yang, X. Effect of La2O3 on crystallization of glass-ceramics. J. Non-Cryst. Solids 2020, 536, 120007. [Google Scholar] [CrossRef]
- Bao, Z.; Li, S.; Zhang, X.; Shen, X.; Hu, X. Preparation, properties and formation mechanism of transparent anorthite-based glass-ceramic glaze with high hardness. Ceram. Int. 2024, 50, 20964–20974. [Google Scholar] [CrossRef]
- Banijamali, S.; Khakbiz, M.; Vakilian, F. Preparation of glass–ceramic glazes for fast firing applications by CaF₂ substitution with B₂O₃ in the CaO–CaF₂–Al₂O₃–SiO₂ system. Ceram. Int. 2013, 39, 8815–8822. [Google Scholar] [CrossRef]
- Mukherjee, D.P.; Das, S.K. Effects of nano silica on synthesis and properties of glass ceramics in SiO₂–Al₂O₃–CaO–CaF₂ glass system: A comparison. J. Non-Cryst. Solids 2013, 368, 98–104. [Google Scholar] [CrossRef]
- Fröberg, L.; Hupa, L.; Hupa, M. Corrosion of the crystalline phases of matte glazes in aqueous solutions. J. Eur. Ceram. Soc. 2009, 29, 7–14. [Google Scholar] [CrossRef]
- Shan, T.; Song, M.; Lei, X.; Chen, J.; Zhang, S. Microstructure and mechanical properties of SiC joint using anorthite based glass-ceramic as filler. Mater. Res. Express 2017, 4, 015504. [Google Scholar] [CrossRef]
- Tunali, A.; Ozel, E.; Turan, S. Production and characterisation of granulated frit to achieve anorthite based glass–ceramic glaze. J. Eur. Ceram. Soc. 2015, 35, 1089–1095. [Google Scholar] [CrossRef]
- Vernaz, E.; Gin, S.; Veyer, C. Waste Glass. Compr. Nucl. Mater. 2012, 5, 451–483. [Google Scholar] [CrossRef]
- Panyata, S.; Meethong, N.; Intawin, P.; Tippayasam, C.; Tippawan, P. Non-isothermal crystallization kinetics of bismuth germanate glass-ceramics. Ceram. Int. 2017, 43, S407–S411. [Google Scholar] [CrossRef]
- Li, H.; Zhao, T.; Yuan, X.; Liu, Z.; Wang, X.; He, L.; Tang, J.; Liu, S.; Qi, X. In situ observation of Li₂O-Al₂O₃-SiO₂ glass crystallization process and kinetics analysis. J. Cryst. Growth 2020, 547, 125816. [Google Scholar] [CrossRef]
- Danewalia, S.; Singh, K.; Arya, S. Influence of vanadium oxide on non-isothermal crystallization kinetics of zinc lithium borate glasses. J. Non-Cryst. Solids 2021, 553, 120471. [Google Scholar] [CrossRef]
- Lopes, A.; Rodrigues, S.; Gomes, A.C.; Nunes, P.; Nunes, E.; Carvalho, M.L. Crystallization kinetics of a barium–zinc borosilicate glass by a non-isothermal method. J. Alloys Compd. 2014, 591, 268–274. [Google Scholar] [CrossRef]
- Deng, L.; He, X.; Wu, J.; Liu, Z.; Wang, Y.; Yao, C.; Zhang, Q.; Fan, S.; Li, L. Crystallization behavior and structure of CaO–MgO–Al₂O₃–SiO₂ glass ceramics prepared from Cr-bearing slag. Mater. Chem. Phys. 2021, 261, 124249. [Google Scholar] [CrossRef]
- Gaddam, A.; Weigel, C.; Friederich, M.; Bornhöft, H.; Fey, T.; Sieber, H. The roles of P₂O₅ and SiO₂/Li₂O ratio on the network structure and crystallization kinetics of non-stoichiometric lithium disilicate based glasses. J. Non-Cryst. Solids 2018, 481, 512–521. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Savabieh, H.; Ajalloueian, F.; Abdizadeh, H.; Saidi, A. Kinetics of crystallization in 13.2 Li₂O-67.6 SiO₂-14.49 Al₂O₃-3.3 TiO₂-0.4 BaO-0.97 ZnO glass ceramic powder: Part I: A model-free vs. model-fitting approach. Ceram. Int. 2019, 45, 8856–8865. [Google Scholar] [CrossRef]
- Matusita, K.; Sakka, S. Kinetic study of crystallization of glass by differential thermal analysis—Criterion on application of Kissinger plot. J. Non-Cryst. Solids 1980, 38, 741–746. [Google Scholar] [CrossRef]
- Matusita, K.; Komatsu, T.; Yokota, R. Kinetics of non-isothermal crystallization process and activation energy for crystal growth in amorphous materials. J. Mater. Sci. 1984, 19, 291–296. [Google Scholar] [CrossRef]
- Intawin, P.; Tippayasam, C.; Phuruangrat, A.; Sukon, P.; Panyata, S.; Meethong, N. Effects of TiO₂ content and thermal parameters on crystallization kinetics and mechanical properties of phosphate based glass system. Thermochim. Acta 2020, 690, 178699. [Google Scholar] [CrossRef]
- Guo, J.; Han, H.; Cui, X.; Li, C.; Liu, C.; Yang, Z. Glass and glass-ceramics from red mud tailings: Understanding the evolution mechanism. Ceram. Int. 2023, 49, 27430–27438. [Google Scholar] [CrossRef]
- Karamanov, A.; Avramov, I.; Arrizza, L.; Pascova, R.; Gutzow, I. Variation of Avrami parameter during non-isothermal surface crystallization of glass powders with different sizes. J. Non-Cryst. Solids 2012, 358, 1486–1490. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Z.; Peng, C.; Li, X.; Jiang, H. Influence of particle size on sinterability, crystallisation kinetics and flexural strength of wollastonite glass-ceramics from waste glass and fly ash. Mater. Chem. Phys. 2014, 148, 449–456. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, P.; Sharma, V.; Gupta, D.C.; Thakur, A.; Singh, R. Glass-forming ability and thermal stability of Se₁₀₀−ₓ (Ge₂Sb₂Te₅)ₓ glassy alloys. J. Therm. Anal. Calorim. 2018, 134, 923–931. [Google Scholar] [CrossRef]
- El-Sonbaty, S.S.; Shoker, H.; Abdel-Bary, M. Thermal stability, glass transition and crystallization kinetics of Se₉₅−ₓ Sb₅ Inₓ chalcogenide. Appl. Phys. A 2018, 124, 186. [Google Scholar] [CrossRef]
- El Boukili, A.; Erramli, H.; Bazzi, L.; Rachid, R. Non-isothermal crystallization and spectroscopic study of Na₂Pb₁₋ₓNiₓP₂O₇ (0≤ₓ≤1) glasses. J. Non-Cryst. Solids 2020, 544, 120208. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, H.; Sun, S.; Jia, S.; Liu, Y. Effect of TiO2 content on the crystallization behavior and properties of CaO-Al2O3-SiO2 glass ceramic fillers for high temperature joining application. J. Alloys Compd. 2018, 732, 141–148. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; Yang, J.; Chen, C.; Wang, X.; Wu, J. Preparation and characterization of CaO–Al₂O₃–SiO₂ glass-ceramics from molybdenum tailings. Mater. Chem. Phys. 2017, 197, 57–64. [Google Scholar] [CrossRef]
- Fang, J.; Sun, L.; Guo, S.; Liu, C.; Zhang, J. Study of Li2O addition on crystallization behavior and thermal expansion properties of CaO-Al2O3–SiO2 (CAS) glass-ceramic and its application for joining SiC ceramic. J. Eur. Ceram. Soc. 2021, 41, 1817–1827. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, Y.; Su, Y.; Liu, S.; Xu, F.; Yin, W.; Guo, Z.; Zhang, P.; Zhang, M.; Yin, D. Non-isothermal crystallization kinetics of a Fe–Cr–Mo–B–C amorphous powder. J. Alloys Compd. 2020, 823, 153783. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, Y.; Qiao, J.; Zhao, Z.; Wang, G.; Xu, X.; Shen, L.; Lu, Z.; Wang, H.; Li, S.; et al. Comparative study of thermal stability and crystallization kinetics between melt-spun and bulk Pd₇₇.₅Cu₆Si₁₆.₅ metallic glasses. J. Mater. Res. Technol. 2022, 17, 2203–2219. [Google Scholar] [CrossRef]
- Li, Z.; Chen, D.; Wang, Y.; Zhu, H.; Zhou, M.; Fan, X. Effect of Fe₂O₃ on the crystallization behavior, microstructure and properties of glass-ceramics prepared from the modified tailings after reduction of copper slag. Mater. Today Commun. 2022, 31, 103516. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, M.; Zhang, X.; Yuan, X.; Wang, X.; Dong, J. Effect of Al₂O₃ on viscosity and structure of SiO₂-FeO-Al₂O₃-Fe₂O₃-CaO-MgO slag system. JOM 2023, 75, 1221–1229. [Google Scholar] [CrossRef]
- Xing, X.; Li, X.; Zheng, Y.; Yang, Y.; Zhao, X.; Yan, Y.; Huang, X. Effect of MgO and K₂O on high-Al silicon–manganese alloy slag viscosity and structure. Minerals 2020, 10, 810. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Z.; Jiang, X.; Zhang, L.; Zhang, S. Effect of glass-ceramics network intermediate Al₂O₃ content on up-conversion luminescence in Er³⁺/Yb³⁺ co-doped NaYF₄ oxy-fluoride glass-ceramics. J. Eur. Ceram. Soc. 2023, 43, 3591–3599. [Google Scholar] [CrossRef]
- Cormier, L. Glasses: Aluminosilicates. Encycl. Mater. Tech. Ceram. Glasses 2021, 2, 496–518. [Google Scholar] [CrossRef]
- Bouhadja, M.; Jakse, N.; Pasturel, A. Striking role of non-bridging oxygen on glass transition temperature of calcium aluminosilicate glass-formers. J. Chem. Phys. 2014, 140, 234507. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, F.; Wu, J.; Liu, Z.; Gong, Y.; Yuan, X.; Wang, H.; Wang, X. Insight into the dual effect of Fe₂O₃ addition on the crystallization of CaO-MgO-Al₂O₃-SiO₂ glass-ceramics. J. Non-Cryst. Solids 2019, 513, 144–151. [Google Scholar] [CrossRef]
- Kucharczyk, S.; Wolek, M.; Bucko, M.; Mazur, J.; Oleksy, M. The effect of CaO/SiO₂ molar ratio of CaO-Al₂O₃-SiO₂ glasses on their structure and reactivity in alkali activated system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 194, 163–171. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Li, X.-M.; Xing, X.-D. Effect of B₂O₃ content on viscosity and structure of SiO₂−MgO−FeO-based slag. Trans. Nonferrous Met. Soc. China 2022, 32, 2403–2413. [Google Scholar] [CrossRef]
- Lutpi, H.A.; Mohd Ghazali, M.J.; Ismail, K.N.; Azhari, M.F.; Kuntjoro, W.; Razak, M.A.A.; Raza, M. Effect of sintering treatment time on the sintering behaviour and thermal shock resistance of Li₂O-Al₂O₃-SiO₂ glass-ceramics. J. Asian Ceram. Soc. 2021, 9, 507–518. [Google Scholar] [CrossRef]
- Zheng, F.; Jiang, C.; Cui, X.; Yang, Y.; Liu, C.; Li, C.; Yang, Z. Effective utilization of extracted titanium tailing to prepare high performance glass-ceramic and their formation mechanism. Ceram. Int. 2021, 47, 17391–17399. [Google Scholar] [CrossRef]
- Kraipok, A.; Tippawan, P.; Intawin, P.; Panyata, S.; Meethong, N.; Tippayasam, C. Investigation of phase formation and mechanical properties of lithium disilicate glass-ceramic doped CeO2. J. Non-Cryst. Solids 2021, 561, 120772. [Google Scholar] [CrossRef]
- Gualberto, H.R.; Lopes, R.S.; Silva, F.A.D.; Schnurr, E.; Poiate Junior, E.; Andrade, M.C.D. Influence of Sintering Temperature on Mechanical Properties of Glass-Ceramics Produced with Windshield Waste. Int. J. Chem. Eng. 2019, 2019, 2531027. [Google Scholar] [CrossRef]
- Teixeira, J.D.; de Oliveira, A.P.N.; Boehs, L.; Cesconeto, F.R.; Siligardi, C.; Pereira, M.A. Sintering behavior of LZS glass-ceramics. Mater. Sci. Forum 2012, 727, 1028–1033. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, Y.; Vlassak, J.J. Novel technique for measuring the mechanical properties of porous materials by nanoindentation. J. Mater. Res. 2006, 21, 715–724. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Gross, K.A. Micromechanical properties of single crystal hydroxyapatite by nanoindentation. Acta Biomater. 2009, 5, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, J.; Li, W.; Zhang, Y.; Zhu, Q.; Zhang, J.; Li, X.; Tan, Z.; Zhang, X. Nanoindentation pop-in in oxides at room temperature: Dislocation activation or crack formation? J. Am. Ceram. Soc. 2021, 104, 4728–4741. [Google Scholar] [CrossRef]
- Raoelison, R.; Amram, D.; Latychevskaia, T.; Weber, S.; Klemenz, M.; Nguyen, N.T.; Michler, J. Effect of the nanopores in the Al-Cu intermetallic phase on nanoindentation instabilities at the Al/Cu interface of a magnetic pulse impact weld. Materialia 2023, 32, 101955. [Google Scholar] [CrossRef]







| Compound (wt.%) | Eggshell | Zeolite | Pumice |
|---|---|---|---|
| CaO | 51.76 | 2.0 | 3.25 |
| Al2O3 | 0.04 | 13.2 | 13.21 |
| SiO2 | 0.10 | 71.9 | 72.45 |
| K2O | 0.08 | 3.5 | 5.07 |
| Na2O | 0.11 | 0.3 | 3.17 |
| Fe2O3 | 0.12 | 1.4 | 1.94 |
| MgO | 0.36 | 1.1 | 0.65 |
| TiO2 | - | 0.1 | 0.14 |
| P2O5 | 0.17 | - | - |
| MnO | - | 0.1 | 0.08 |
| SO3 | 0.597 | - | - |
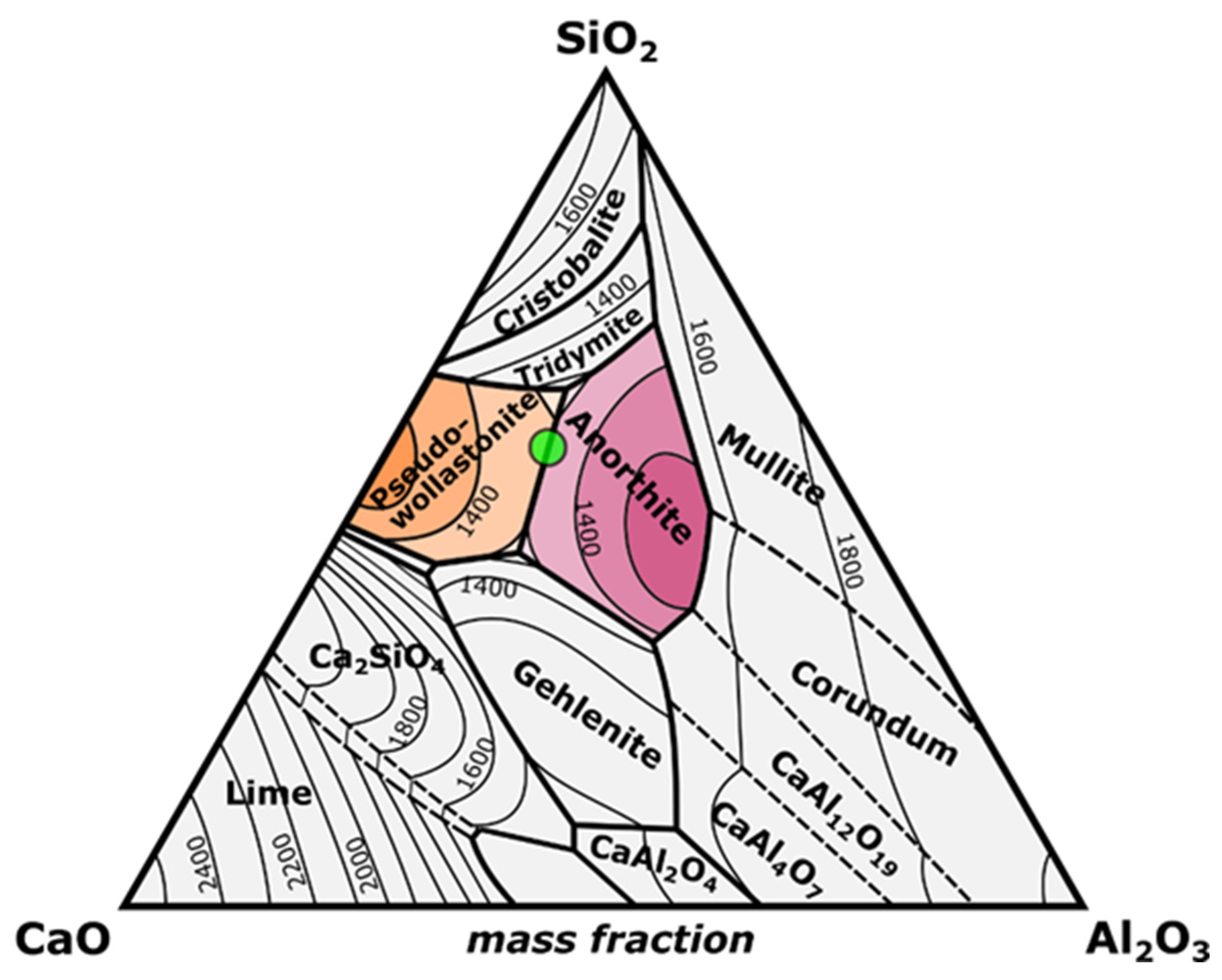
| Sample Name (wt.%) | CaO | Al2O3 | SiO2 |
|---|---|---|---|
| CASZ | 27.5 | 15 | 57.5 |
| CASP | 27.5 | 15 | 57.5 |
| Glass | β (°C min−1) | Tg (°C) | Tp (°C) | Tm (°C) |
|---|---|---|---|---|
| CASZ | 5 | 734 | 1037 | 1241 |
| 10 | 745 | 1060 | 1241 | |
| 15 | 751 | 1071 | 1240 | |
| 20 | 759 | 1086 | 1240 | |
| CASP | 5 | 703 | 1026 | 1204 |
| 10 | 715 | 1052 | 1207 | |
| 15 | 721 | 1067 | 1204 | |
| 20 | 728 | 1080 | 1205 |
| Crystallization Mechanism | n | |
|---|---|---|
| Volume crystallization | Three-dimensional crystal growth | 4 |
| Two-dimensional crystal growth | 3 | |
| One-dimensional crystal growth | 2 | |
| Surface crystallization | 1 | |
| Sample | Kissinger (kJ mol−1) | Ozawa (kJ mol−1) | Matusita (kJ mol−1) | Avrami Constant (n) |
|---|---|---|---|---|
| CASZ | 406.63 | 428.81 | 409.20 | 3.33 |
| CASP | 356.22 | 378.25 | 369.44 | 2.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydın, B.; Toplan, H.Ö.; Toplan, N. Non-Isothermal Crystallization Kinetics and Properties of CaO-Al2O3-SiO2 (CAS) Glass-Ceramics from Eggshell Waste, Zeolite, and Pumice. Materials 2024, 17, 5630. https://doi.org/10.3390/ma17225630
Aydın B, Toplan HÖ, Toplan N. Non-Isothermal Crystallization Kinetics and Properties of CaO-Al2O3-SiO2 (CAS) Glass-Ceramics from Eggshell Waste, Zeolite, and Pumice. Materials. 2024; 17(22):5630. https://doi.org/10.3390/ma17225630
Chicago/Turabian StyleAydın, Bahadır, Hüseyin Özkan Toplan, and Nil Toplan. 2024. "Non-Isothermal Crystallization Kinetics and Properties of CaO-Al2O3-SiO2 (CAS) Glass-Ceramics from Eggshell Waste, Zeolite, and Pumice" Materials 17, no. 22: 5630. https://doi.org/10.3390/ma17225630
APA StyleAydın, B., Toplan, H. Ö., & Toplan, N. (2024). Non-Isothermal Crystallization Kinetics and Properties of CaO-Al2O3-SiO2 (CAS) Glass-Ceramics from Eggshell Waste, Zeolite, and Pumice. Materials, 17(22), 5630. https://doi.org/10.3390/ma17225630






