Effect of Deformed Prior Austenite Characteristics on Reverse Phase Transformation and Deformation Behavior of High-Strength Medium-Mn Steel
Abstract
1. Introduction
2. Experimental Procedures
2.1. Material and Thermomechanical Processing
2.2. Simulation Condition of Thermodynamic and Kinetics
2.3. Microstructure Characterization and Mechanical Properties Tests
3. Results and Discussion
3.1. Effect of Deformed Prior Austenite Characteristics on Microstructure Evolution During Prior Austenite Decomposition and Reverse Phase Transformation Processes
3.2. The Kinetics and Thermodynamics of Intercritical Austenite Formation in the Experimental Steel with Different Characteristics of Deformed Prior Austenite
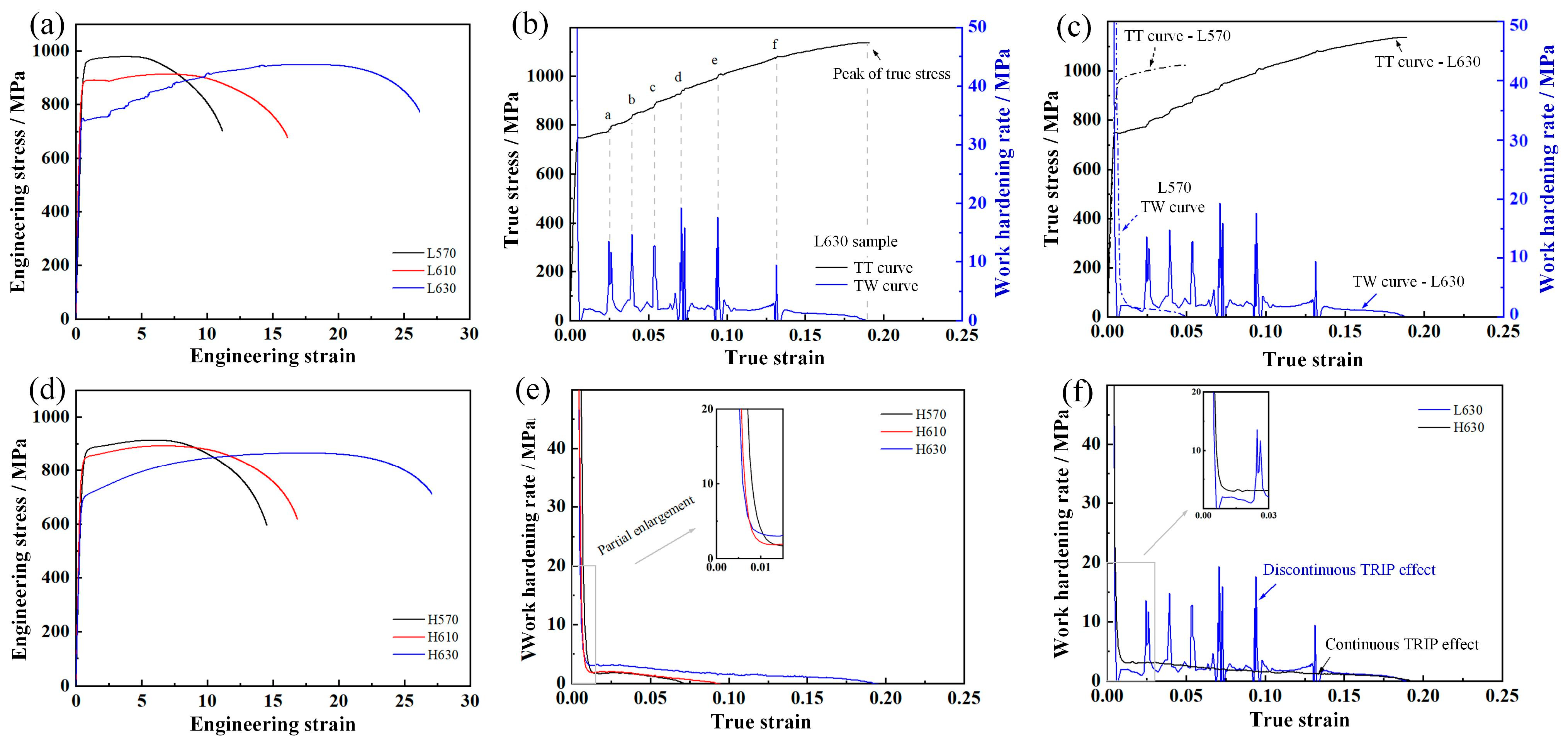
3.3. Effect of Deformed Prior Austenite Characteristics on Deformation Behavior
4. Conclusions
- (1)
- The recovery and recrystallization behavior of deformed prior austenite are significantly inhibited during rolling in the non-recrystallized zone. The morphology of PAGs changed from equiaxed type (rolling in the recrystallization region) to flat-elongated type (rolling in the non-recrystallization region). A refined martensite lath with high-density lattice defects can be obtained via the strain hardening of prior austenite.
- (2)
- The blocky intercritical austenite is mainly formed at PAGBs and packet boundaries with a high density of lattice defects, where the recrystallization of ferrite and the formation of intercritical austenite occur simultaneously. The lath-like RA is mainly formed along boundaries of lath-like ferrite with incomplete recovery. The strain hardening of deformed prior austenite by rolling in the non-recrystallization region can decrease lattice defects in the quenched martensite matrix.
- (3)
- The ΔGFCC-BCC is decreased with an increase in temperature and C/Mn content. The enrichment of C/Mn before intercritical austenite nucleation can reduce the critical temperature of ferrite to austenite transformation during the ART process. The dislocation and grain boundary can provide fast diffusion paths for C and Mn. The nucleation and growth of intercritical austenite are accelerated by high-density lattice defects and refined martensitic lath in the sample with flat-elongated PAGs.
- (4)
- The tensile deformation resistance is dependent on the work hardening effect (via TRIP effect and dislocation tangle and multiplication) and softening effect (via stress relaxation and dislocation glide). The optimum properties were obtained in the L630 sample, with a combination of yield strength of 748 MPa, tensile strength of 952 MPa, and total elongation of 26.2%, mainly attributed to the sustained TRIP effect and the laminated microstructure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zabihi-Gargari, M.; Emami, M.; Shahverdi, H.R.; Askari-Paykani, M. Influence of boron addition on microstructure and mechanical properties of medium-Mn advanced high-strength steel. J. Mater. Sci. Technol. 2024, 29, 5317–5329. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Biswal, S.; Barik, R.K.; Mahato, B.; Ghosh, M.; Mitra, R.; Chakrabarti, D. Comparative interplay of C and Mn on austenite stabilization and low temperature impact toughness of low C medium Mn steels. Mater. Charact. 2024, 208, 113658. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Gao, X.H.; Su, G.Q.; Du, L.X.; Liu, Z.G.; Hu, J. Corrosion behavior of low-C medium-Mn steel in simulated marine immersion and splash zone environment. J. Mater. Eng. Perform. 2017, 26, 2599–2607. [Google Scholar] [CrossRef]
- Dai, Z.B.; Chen, H.; Ding, R.; Lu, Q.; Zhang, C.; Yang, Z.G.; Zwaag, S.V.D. Fundamentals and application of solid-state phase transformations for advanced high strength steels containing metastable retained austenite. Mater. Sci. Eng. R 2021, 143, 100590. [Google Scholar] [CrossRef]
- Lee, H.; Jo, M.C.; Sohn, S.S.; Zargaran, A.; Ryu, J.H.; Kim, N.J.; Lee, S. Novel medium-Mn (austenite + martensite) duplex hot-rolled steel achieving 1.6 GPa strength with 20% ductility by Mn-segregation-induced TRIP mechanism. Acta Mater. 2018, 147, 247–260. [Google Scholar] [CrossRef]
- Liu, C.Q.; Xiong, F.; Liu, G.N.; Wang, Y.; Cao, Y.X.; Xue, Z.L.; Peng, Q.C. Austenite stability and deformation behavior in medium Mn steel processed by cyclic quenching ART heat treatment. Materials 2021, 23, 7132. [Google Scholar] [CrossRef]
- Mehrabi, A.; Zurob, H.; Benrabah, I.E.; Mcdermid, J.R. Austenite formation in a medium-Mn steel during intercritical annealing via in situ high-energy X-ray diffraction. J. Mater. Res. Technol. 2024, 30, 2158–2167. [Google Scholar] [CrossRef]
- Sedaghat-Nejad, R.; Shahverdi, H.R.; Askari-Paykani, M. Introduction and mechanical evaluation of a novel 3rd-generation medium manganese AHSS with 86 GPa% of PSE. Mater. Sci. Eng. A 2022, 843, 143104. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Q.H.; Yan, Y. Processing, microstructure, mechanical properties, and hydrogen embrittlement of medium-Mn steels: A review. J. Mater. Sci. Technol. 2024, 201, 444–457. [Google Scholar] [CrossRef]
- Shang, X.L.; Zhao, J.X.; Li, F.F.; Li, X.C.; Misra, R.D.K.; Xu, X.Y.; Wang, X.M. Heterogenous lamellar microstructure design to resist ductile-to-brittle transition of body-centered cubic structural metals. Mater. Res. Lett. 2024, 12, 493–499. [Google Scholar] [CrossRef]
- Chandan, A.K.; Mishra, G.; Kishore, K.; Bansal, G.K.; Sahoo, B.K.; Jena, P.S.M.; Kumar, S.; Rai, S.K.; Saha, R.; Kundu, S.; et al. Evading the strength-ductility compromise in medium manganese steel by a novel low temperature warm rolling treatment. Mater. Charact. 2023, 206, 113445. [Google Scholar] [CrossRef]
- Sahoo, B.K.; Srivastava, V.C.; Mahato, B.; Chowdhury, S.G. Microstructure-mechanical property evaluation and deformation mechanism in Al added medium Mn steel processed through intercritical rolling and annealing. Mater. Sci. Eng. A 2021, 799, 140100. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.Y.; Meng, Q.W.; Wang, L.Y.; Li, Y.Z.; Xu, W. Tailoring retained austenite and mechanical property improvement in Al-Si-V containing medium Mn steel via direct intercritical rolling. Mater. Sci. Eng. A 2022, 855, 143904. [Google Scholar] [CrossRef]
- Dong, Y.; Xiang, L.Y.; Zhu, C.J.; Du, Y.; Xiong, Y.; Zhang, X.Y.; Du, L.X. Analysis of phase transformation thermodynamics and kinetics and its relationship to structure-mechanical properties in a medium-Mn high strength steel. J. Mater. Res. Technol. 2023, 27, 5411–5423. [Google Scholar] [CrossRef]
- Strife, J.R.; Carr, M.J.; Ansell, G.S. The effect of austenite prestrain above the Md temperature on the martensitic transformation in Fe-Ni-Cr-C alloys. Metall. Trans. A 1977, 8, 1471–1484. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.X.; Liu, H.; Sun, G.S.; Xie, H.; Yi, H.L.; Misra, R.D.K. Structure–mechanical property relationship in a low-C medium-Mn ultrahigh strength heavy plate steel with austenite-martensite submicro-laminate structure. Mat. Sci. Eng. A 2015, 647, 144–151. [Google Scholar] [CrossRef]
- Dong, Y.; Tao, Z.; Sun, C.; Wu, H.Y.; Gao, X.H.; Du, L.X. Effect of Ti variation on microstructure evolution and mechanical properties of low carbon medium Mn heavy plate steel. J. Mater. Sci. 2019, 152, 21–35. [Google Scholar]
- Capurro, C.; Cicutti, C. Analysis of titanium nitrides precipitated during medium carbon steels solidification. J. Mater. Res. Technol. 2018, 7, 342–349. [Google Scholar] [CrossRef]
- Yan, W.; Shan, Y.Y.; Yang, K. Effect of TiN inclusions on the impact toughness of low-carbon microalloyed steels. Metall. Mater. Trans. A 2006, 37A, 2147–2158. [Google Scholar] [CrossRef]
- Cai, Z.H.; Jing, S.Y.; Li, H.Y.; Zhang, K.M.; Misra, R.D.K.; Ding, H.; Tang, Z.Y. The influence of microstructural characteristics on yield point elongation phenomenon in Fe-0.2C-11Mn-2Al steel. Mater. Sci. Eng. A 2019, 739, 17–25. [Google Scholar] [CrossRef]
- Varanasi, R.S.; Lipińska-Chwałek, M.; Mayer, J.; Gault, B.; Ponge, D. Mechanisms of austenite growth during intercritical annealing in medium manganese steels. Scripta Mater. 2022, 206, 114228. [Google Scholar] [CrossRef]
- Yu, W.L.; Qian, L.H.; Peng, X.; Wang, T.L.; Li, K.F.; Wei, C.Z.; Chen, Z.X.; Zhang, F.C.; Meng, J.Y. Roles of Al in enhancing the thermal stability of reverted austenite and mechanical properties of a medium-Mn TRIP steel containing 2.7 Mn. J. Mater. Res. Technol. 2023, 167, 119–136. [Google Scholar] [CrossRef]
- Jeong, M.S.; Park, T.M.; Kim, D.I.; Fujii, H.; Im, H.J.; Choi, P.P.; Lee, S.J.; Han, J. Improving toughness of medium-Mn steels after friction stir welding through grain morphology tuning. J. Mater. Res. Technol. 2022, 118, 243–254. [Google Scholar] [CrossRef]
- Liu, X.Q.; Sun, M.; Liang, J.H.; Hao, T.; Jiang, W.B.; Wang, Y.L.; Wang, X.P.; Fang, Q.F. Phase transition and defect relaxation behavior in a medium manganese steel. J. Alloys Compd. 2022, 904, 164003. [Google Scholar] [CrossRef]
- Shin, S.H.; Yoon, Y.C.; Lee, S.I.; Hwang, B.C. Improvement in low-temperature toughness of Fe-6.5Mn-0.08C Medium-Mn steel by multi-step heat treatment. J. Mater. Res. Technol. 2023, 26, 3558–3570. [Google Scholar] [CrossRef]
- Zou, Y.M.; Gao, Q.H.; Ding, H.; Tang, Z.Y. Effect of heterogeneous microstructure on martensitic transformation behavior and mechanical properties of a cold rolling medium Mn steel. Mater. Sci. Eng. A 2023, 885, 145630. [Google Scholar] [CrossRef]
- Kuzmina, M.; Ponge, D.; Raabe, D. Grain boundary segregation engineering and austenite reversion turn embrittlement into toughness: Example of a 9 wt.% medium Mn steel. Acta Mater. 2015, 86, 182–192. [Google Scholar] [CrossRef]
- Benzing, J.T.; Kwiatkowski da Silva, A.; Morsdorf, L.; Bentley, J.; Ponge, D.; Dutta, A.; Han, J.; McBride, J.R.; Van Leer, B.; Gault, B.; et al. Multi-scale characterization of austenite reversion and martensite recovery in a cold-rolled medium-Mn steel. Acta Mater. 2019, 166, 512–530. [Google Scholar] [CrossRef]
- Liu, G.; Li, B.; Xu, S.; Tong, S.; Sun, X. Effect of intercritical annealing temperature on multiphase microstructure evolution in ultra-low carbon medium manganese steel. Mater. Charact. 2021, 173, 110920. [Google Scholar] [CrossRef]
- Xu, Z.G.; Shen, X.; Allam, T.; Song, W.W.; Bleck, W. Austenite transformation and deformation behavior of a cold-rolled medium-Mn steel under different annealing temperatures. Mat. Sci. Eng. A 2022, 829, 142115. [Google Scholar] [CrossRef]
- Koohdar, H.; Nili-Ahmadabadi, M.; Habibi-Parsa, M.; Jafarian, H.R.; Bhattacharjee, T.; Tsuji, N. On the stability of reversely formed austenite and related mechanism of transformation in an Fe-Ni-Mn martensitic steel aided by electron backscattering diffraction and atom probe tomography. Metall. Mater. Trans. A 2017, 48, 5244–5257. [Google Scholar] [CrossRef]
- Envelope, H.K.P.; Roshanzadeh, F.; Jafarian, H.R. Correlation between microstructure and shape memory properties in an Fe-9.5Ni-6.5Mn dual phase steel developed by intercritical annealing and subsequent ageing. J. Mater. Res. Technol. 2022, 21, 4537–4547. [Google Scholar]
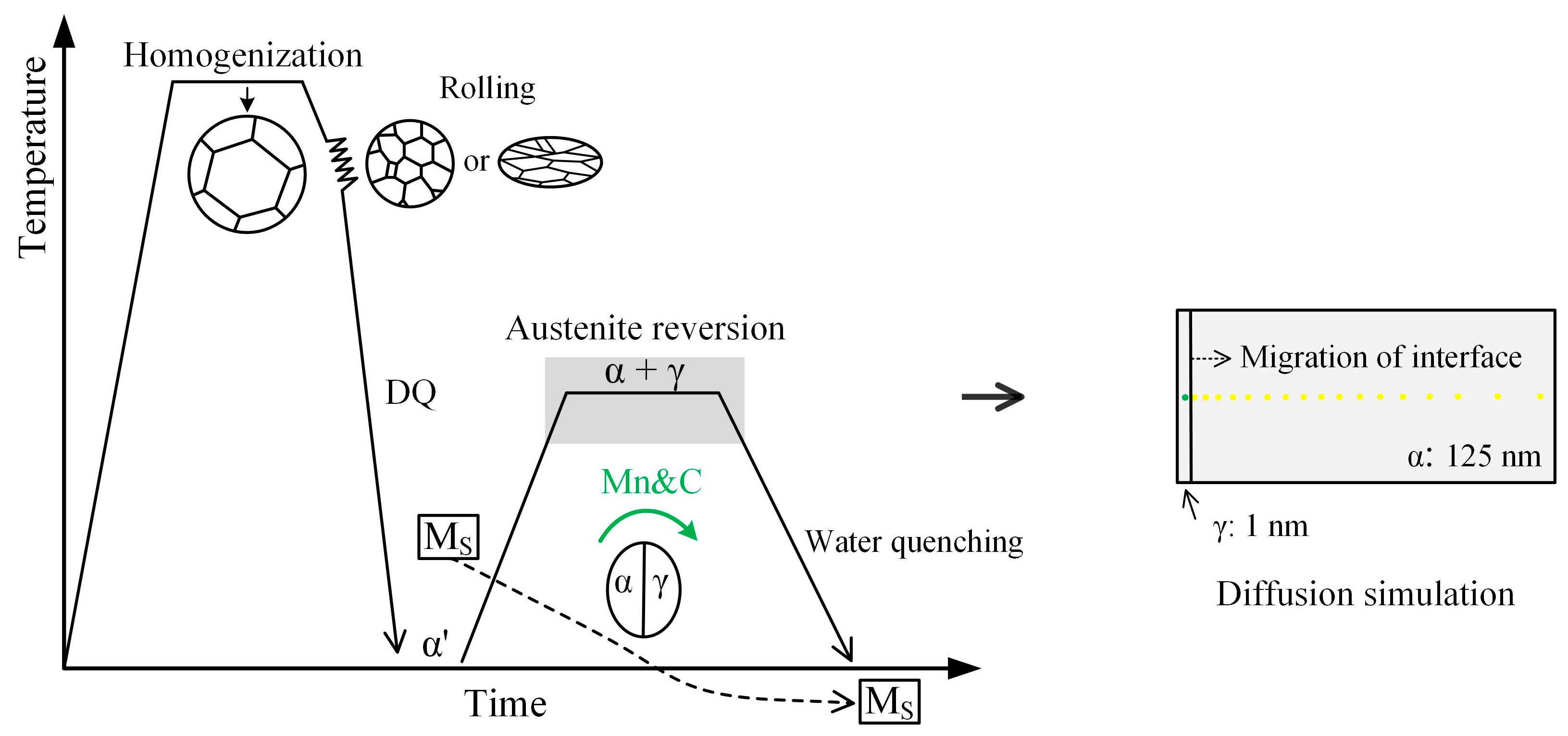







| C | Mn | Si | Al | Ti | N | Cr + Ni + Mo + Cu | Fe | Ae1 | Ae3 |
|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 5.8 | 0.2 | 0.02 | 0.02 | 0.004 | Trace | Bal. | 585 | 715 |
| Steel No. | Finish Rolling Temperature/°C | Annealing Temperature/°C |
|---|---|---|
| L570 | 820 | 570 |
| L610 | 820 | 610 |
| L650 | 820 | 650 |
| H570 | 1000 | 570 |
| H610 | 1000 | 610 |
| H650 | 1000 | 650 |
| Temperature (a) | 0.06C-5.8Mn Steel | 0.6C-5.8Mn Steel | 0.06C-10Mn Steel | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GBCC | GFCC | ΔG | GBCC | GFCC | ΔG | GBCC | GFCC | ΔG | |
| 570 °C | −34,678.3 | −34,510.3 | 168.0 | −33,786.5 | −33,978.6 | −192.1 | −35,326.0 | −35,608.2 | −282.2 |
| 610 °C | −37,246.6 | −37,243.2 | 3.4 | −36,285.9 | −36,710.9 | −425.0 | −37,992.4 | −38,375.1 | −382.7 |
| 630 °C | −38,565.6 | −38,631.2 | −65.6 | −37,569.2 | −38,099.4 | −530.2 | −39,362.6 | −39,780.3 | −417.7 |
| Steel No. | YS/MPa | UTS/MPa | Yield Ratio | TEL/% | VRA/% |
|---|---|---|---|---|---|
| L630 | 748 ± 3 | 952 ± 7 | 0.79 | 26.2 | 32.5 |
| L610 | 875 ± 5 | 898 ± 3 | 0.97 | 16.0 | 7.8 |
| L570 | 948 ± 8 | 970 ± 12 | 0.98 | 11.2 | 1.1 |
| H630 | 697 ± 7 | 868 ± 10 | 0.80 | 27.0 | 20.3 |
| H610 | 833 ± 3 | 880 ± 13 | 0.95 | 16.8 | 3.2 |
| H570 | 837 ± 4 | 908± 8 | 0.92 | 14.5 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Zhang, J.; Liu, T.; Ma, M.; Zhu, L.; Zhu, C.; Du, L. Effect of Deformed Prior Austenite Characteristics on Reverse Phase Transformation and Deformation Behavior of High-Strength Medium-Mn Steel. Materials 2024, 17, 5618. https://doi.org/10.3390/ma17225618
Dong Y, Zhang J, Liu T, Ma M, Zhu L, Zhu C, Du L. Effect of Deformed Prior Austenite Characteristics on Reverse Phase Transformation and Deformation Behavior of High-Strength Medium-Mn Steel. Materials. 2024; 17(22):5618. https://doi.org/10.3390/ma17225618
Chicago/Turabian StyleDong, Ying, Jingwen Zhang, Tao Liu, Mingxing Ma, Lei Zhu, Chengjun Zhu, and Linxiu Du. 2024. "Effect of Deformed Prior Austenite Characteristics on Reverse Phase Transformation and Deformation Behavior of High-Strength Medium-Mn Steel" Materials 17, no. 22: 5618. https://doi.org/10.3390/ma17225618
APA StyleDong, Y., Zhang, J., Liu, T., Ma, M., Zhu, L., Zhu, C., & Du, L. (2024). Effect of Deformed Prior Austenite Characteristics on Reverse Phase Transformation and Deformation Behavior of High-Strength Medium-Mn Steel. Materials, 17(22), 5618. https://doi.org/10.3390/ma17225618







