Formation Mechanism of Polypyrrole-Coated Hollow Glass Microspheres (PPy@HGMs) Composite Powder
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
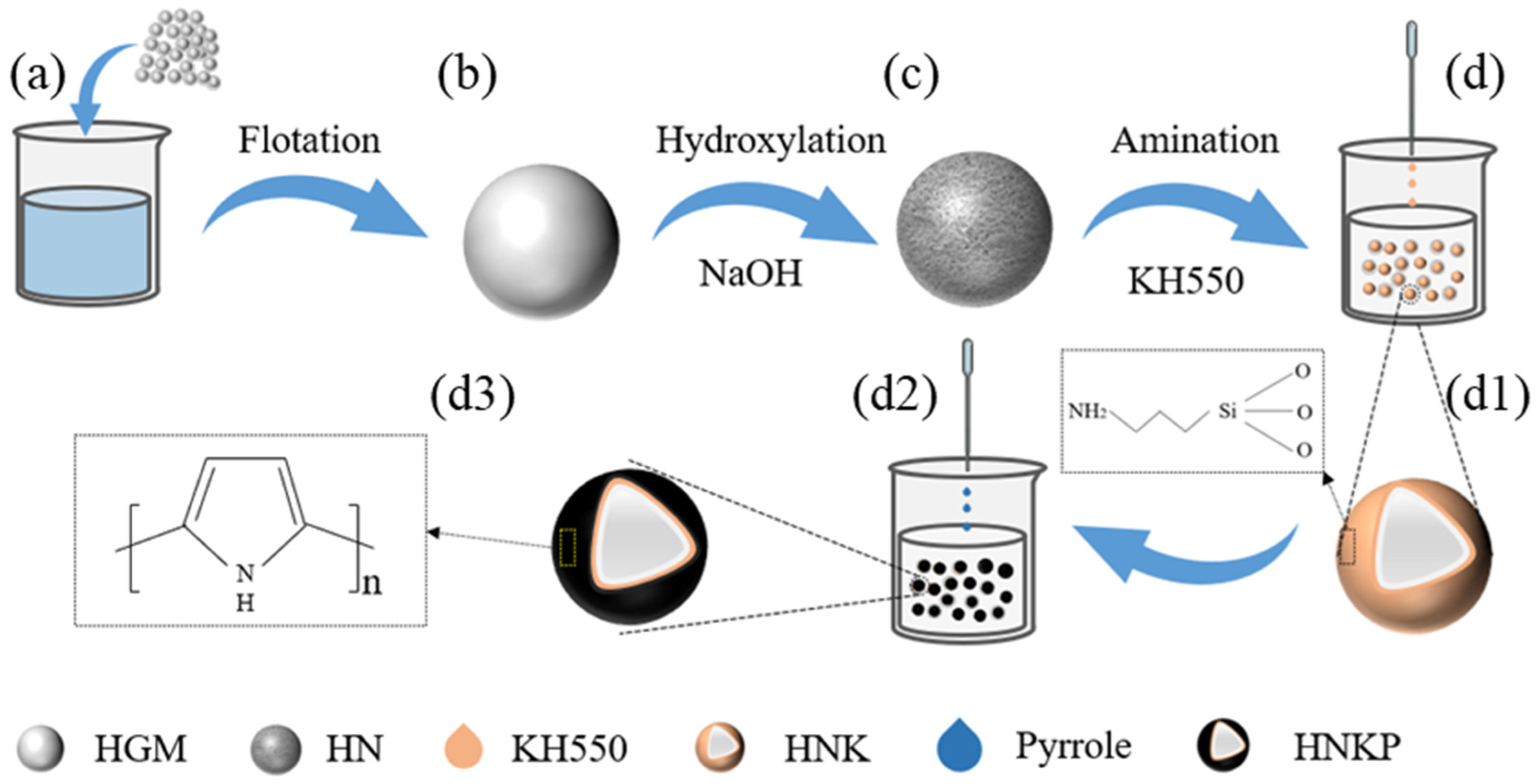
2.2. Treatment Process
- (1)
- Flotation treatment.
- (2)
- Hydroxylation treatment.
- (3)
- Amination treatment.
- (4)
- Coated polypyrrole
2.3. Characterization
3. Results and Discussion
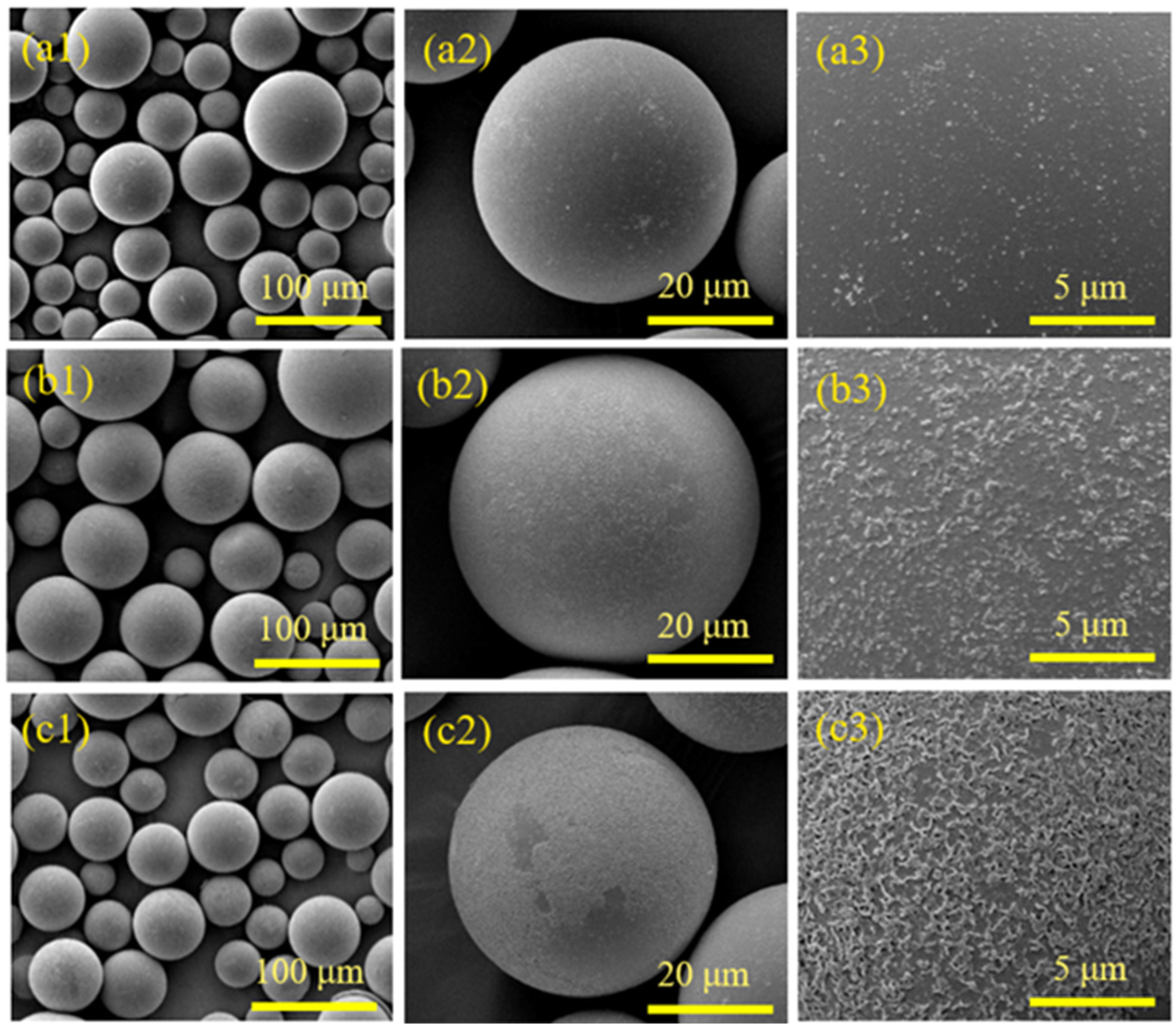
3.1. HGMs Surface Pretreatment
3.1.1. Flotation
3.1.2. Hydroxylation
3.1.3. Amination
3.2. Influence of Process Parameters on the Surface Effect of HGMs
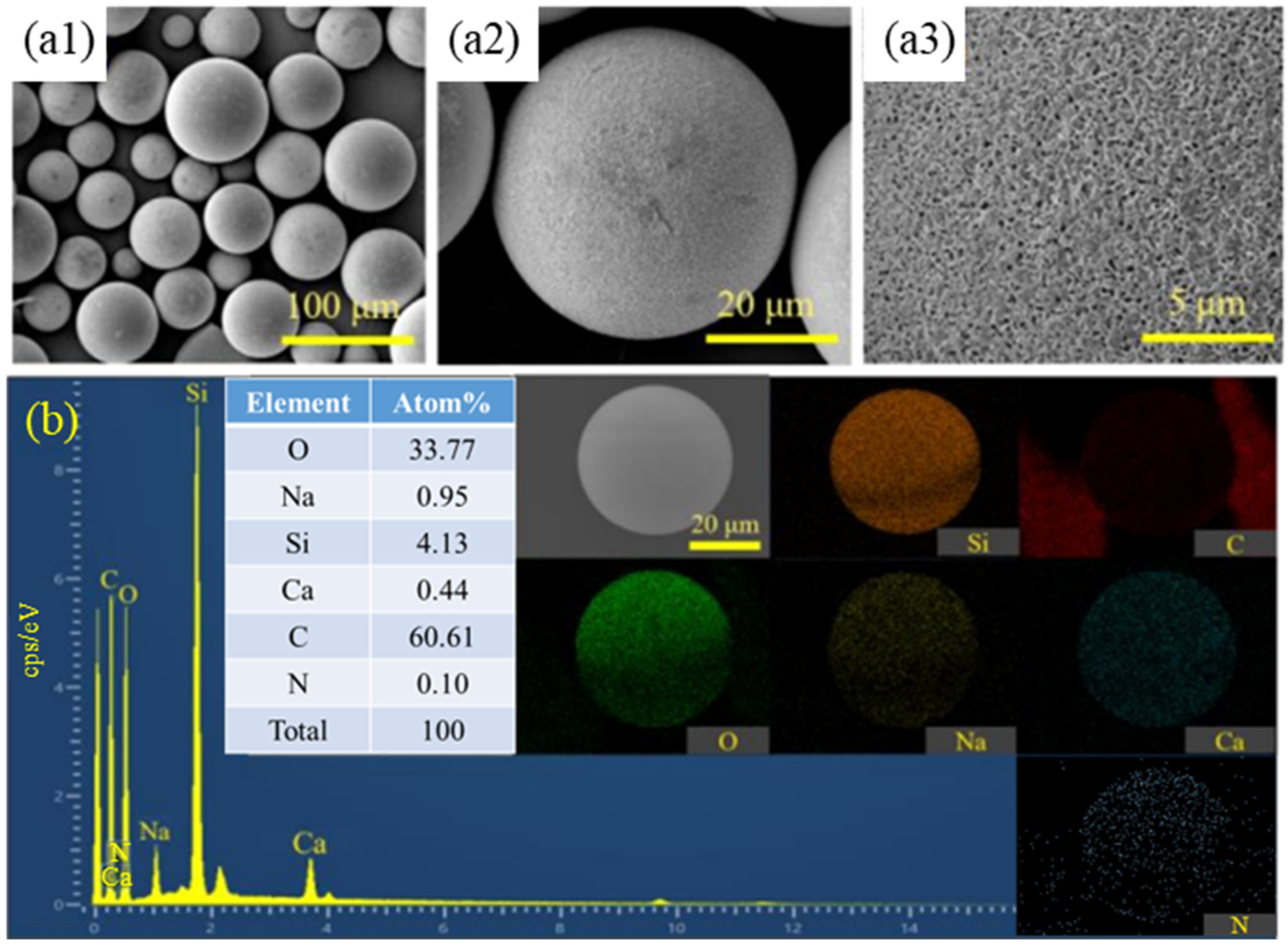
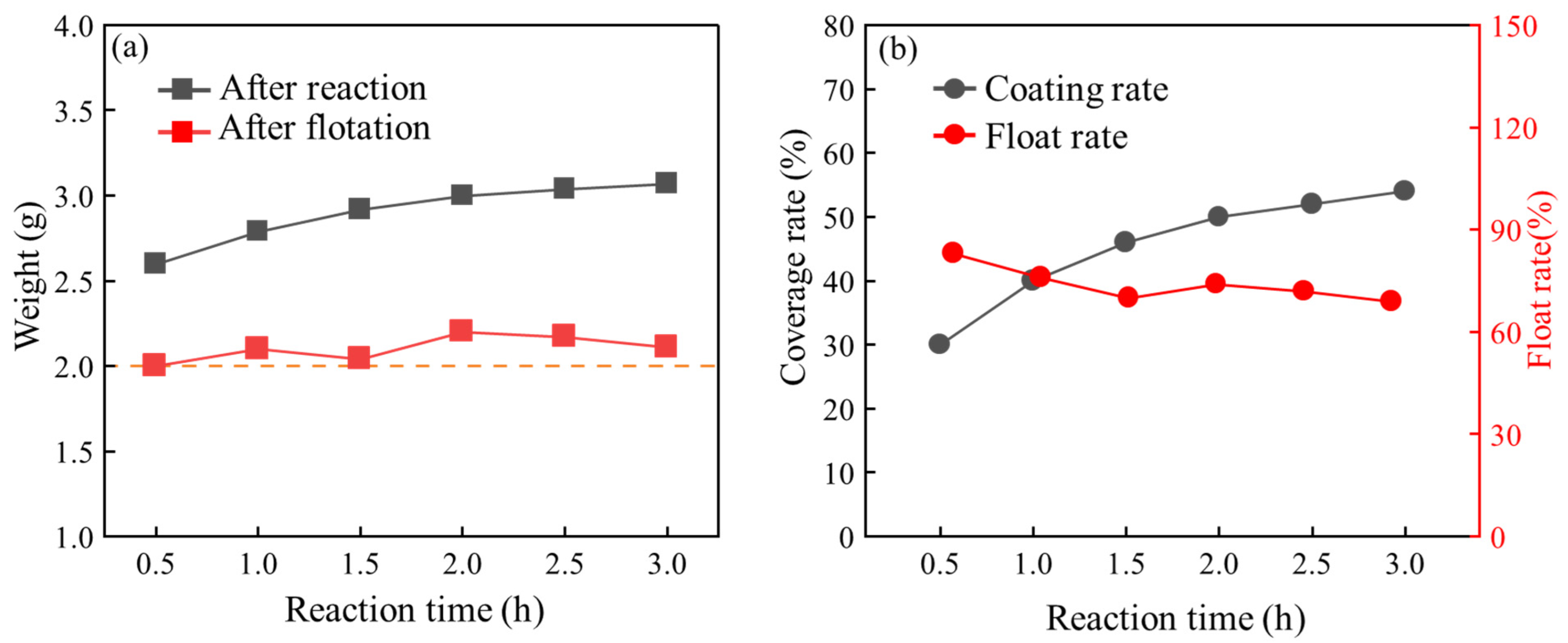
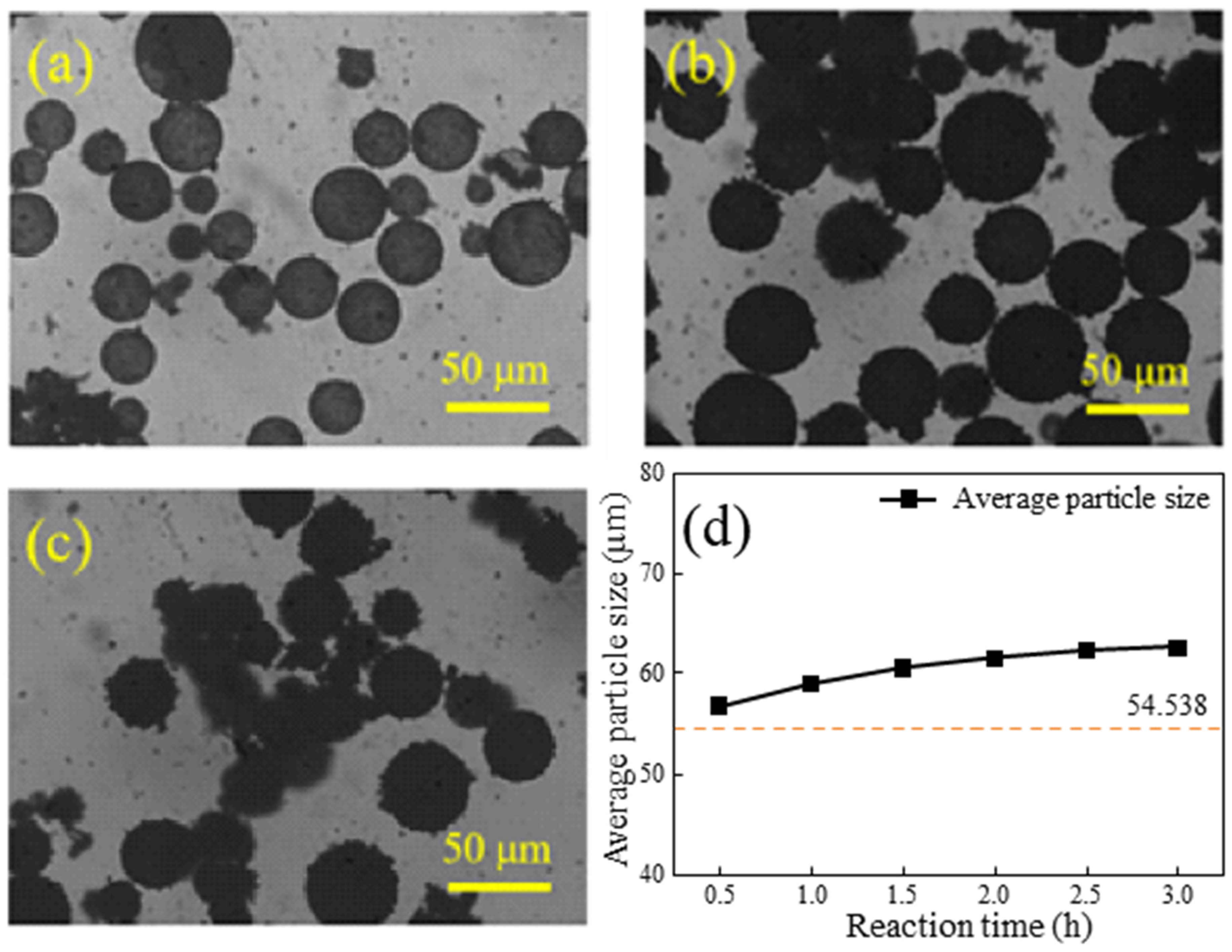
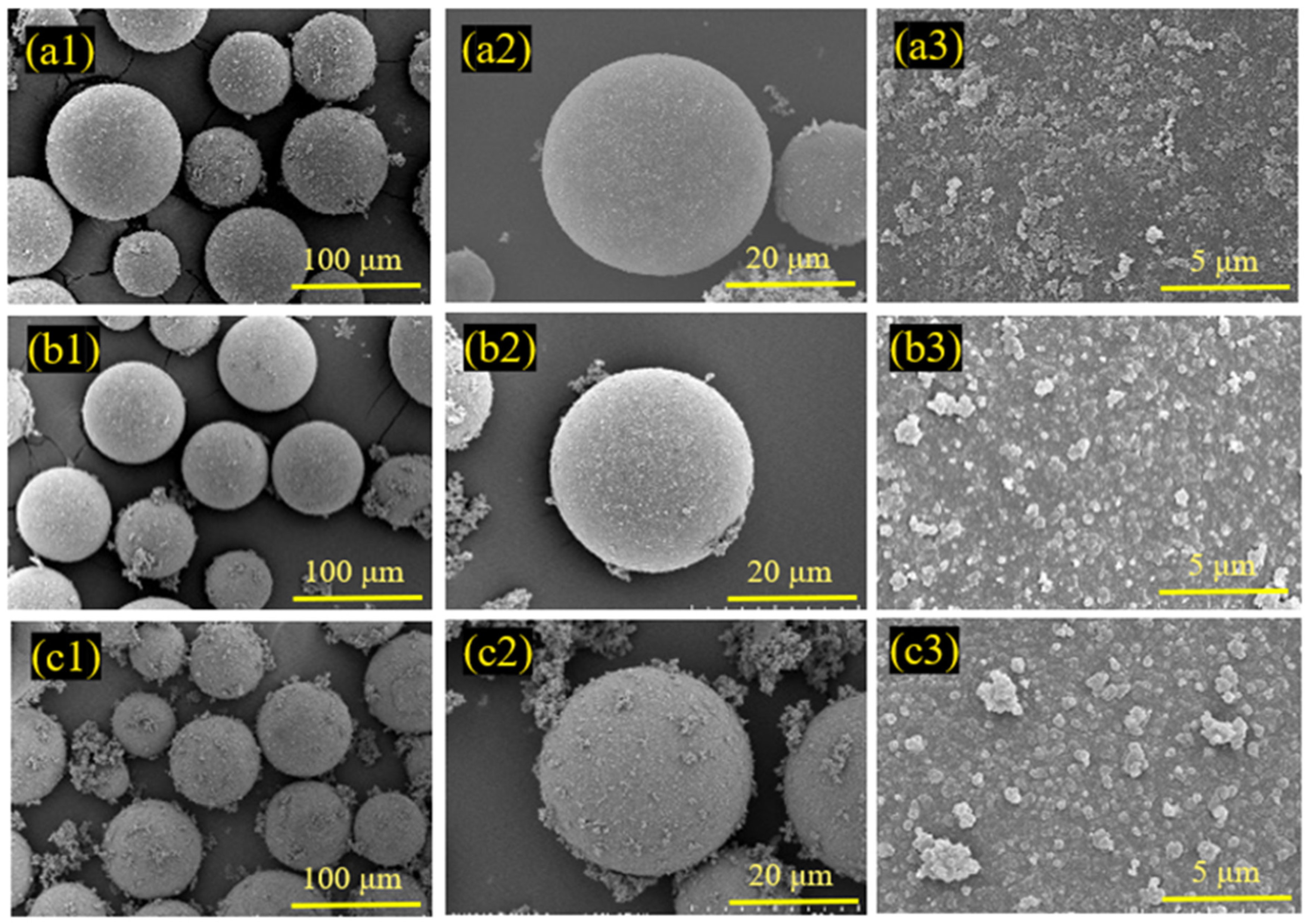
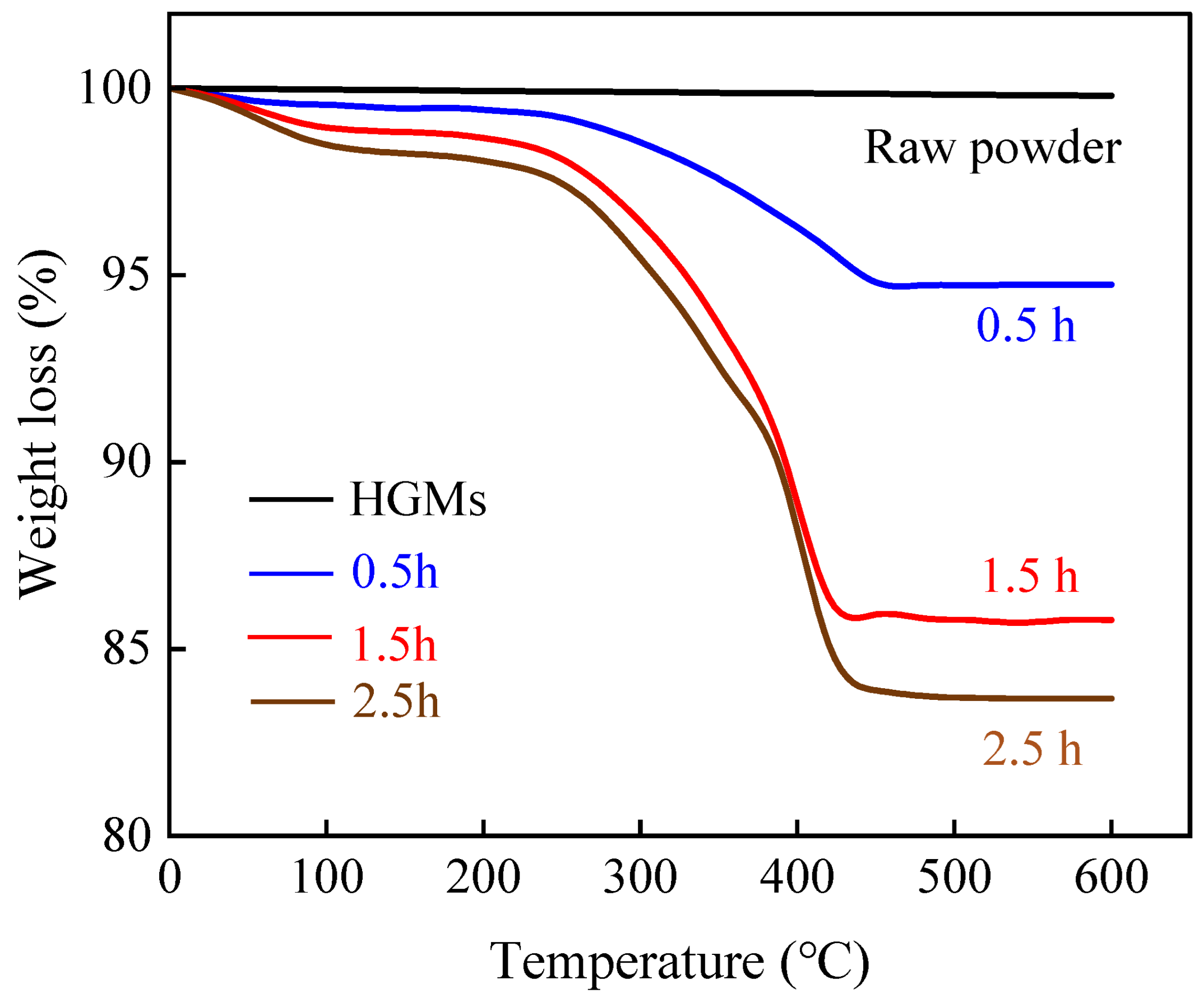
3.3. Influence of Reaction Time on Coating Effect
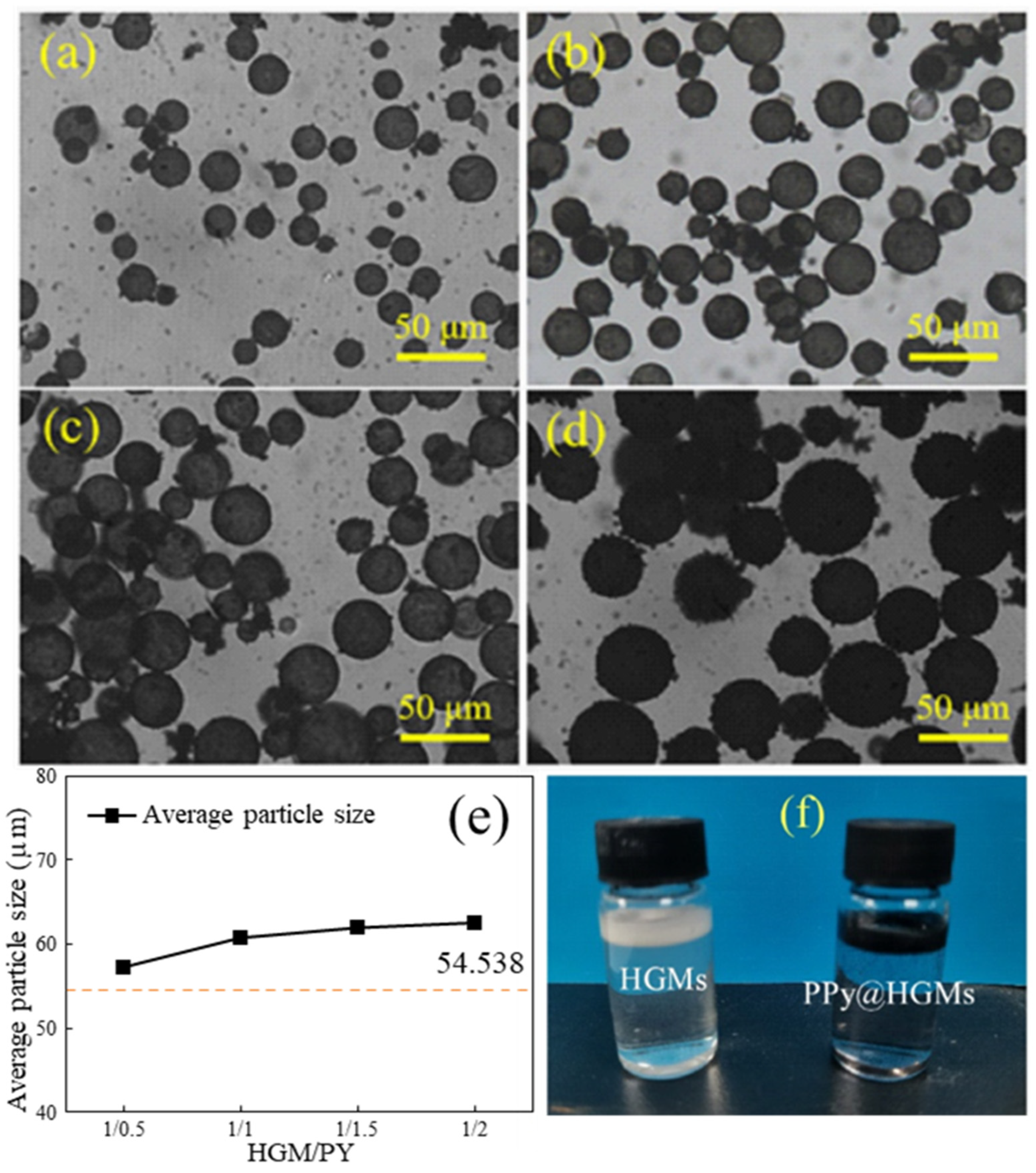
3.4. Influence of Microsphere/Pyrrole Mass Ratio on Coating Effect
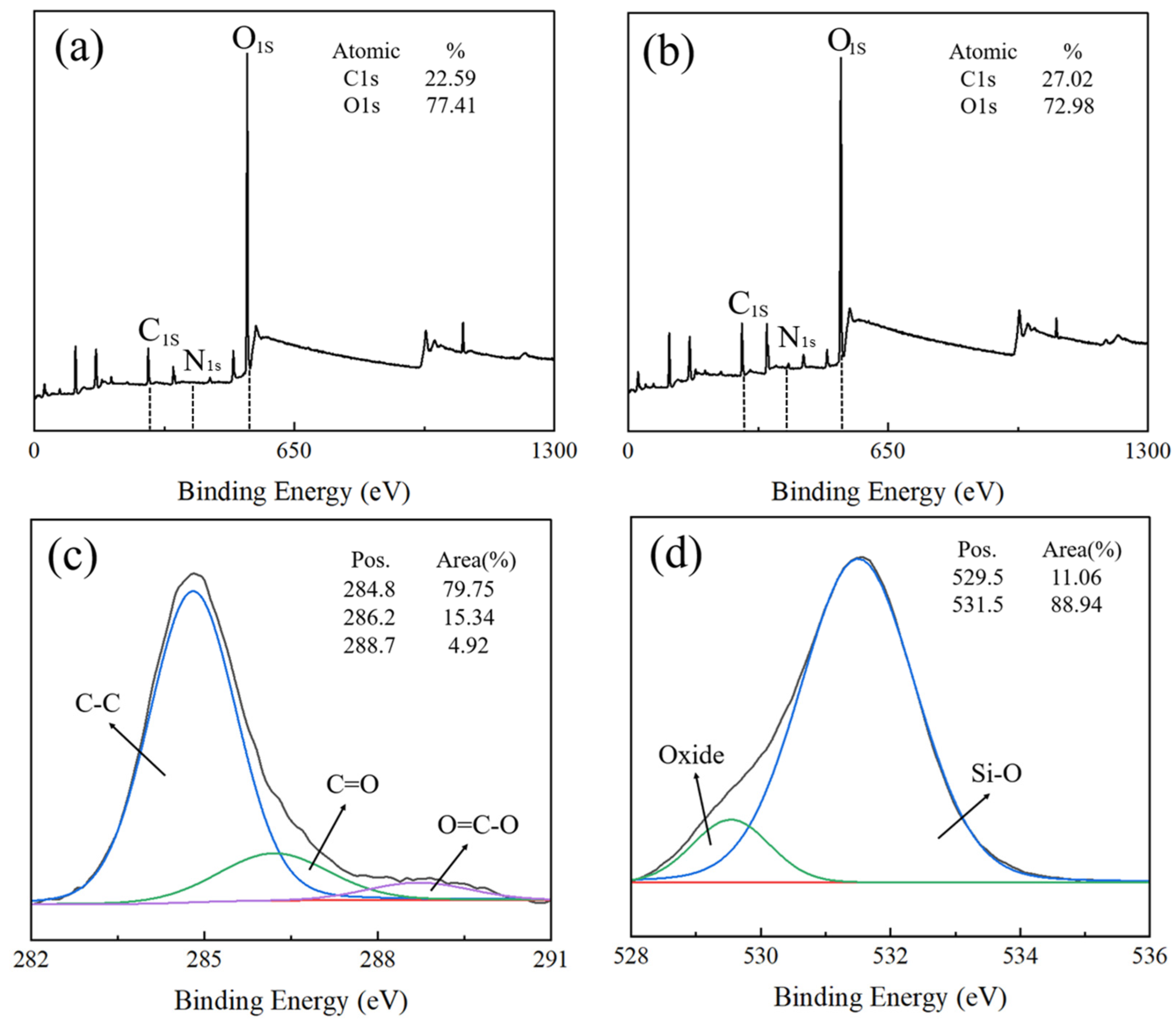
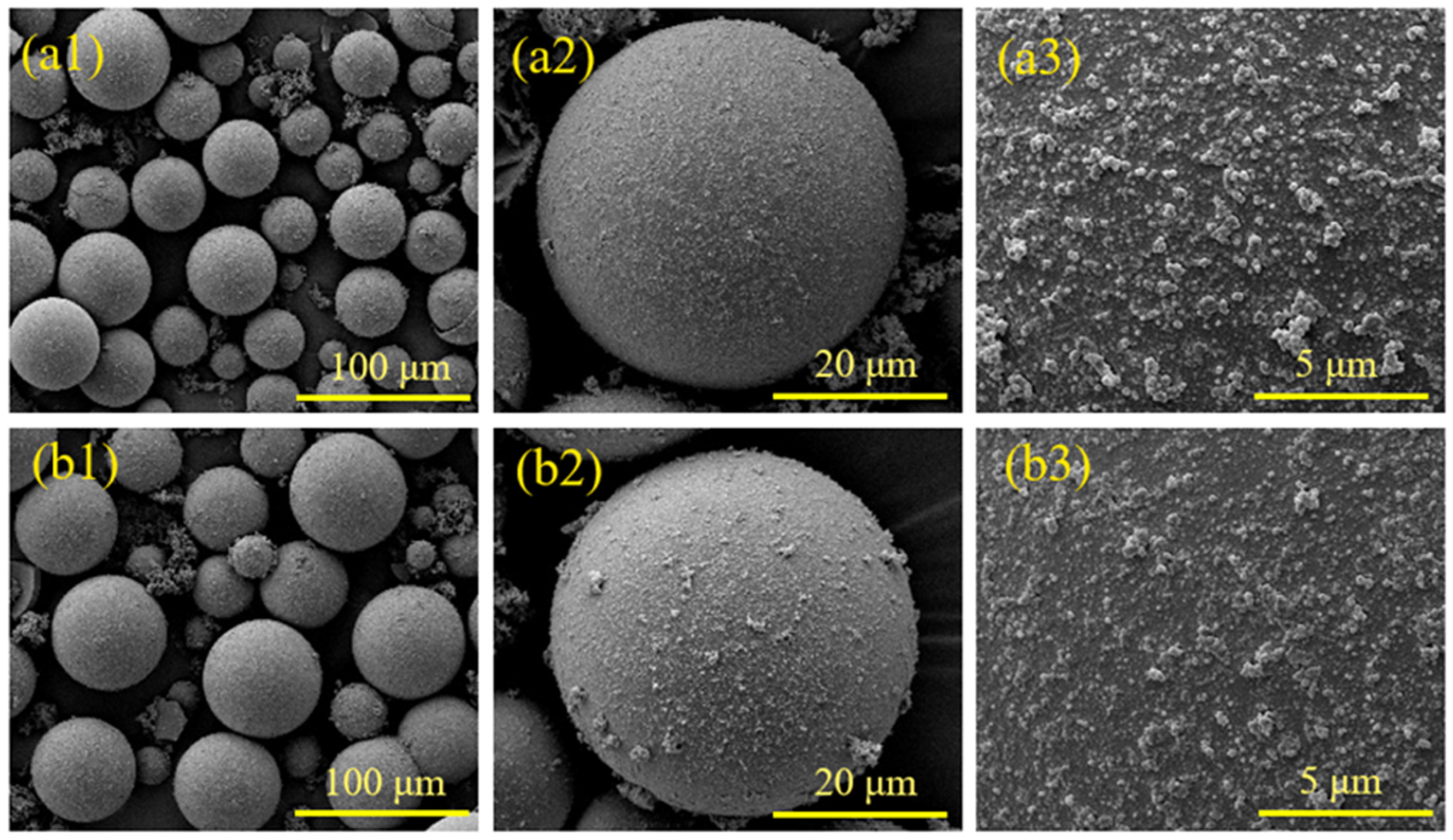
4. PPy@HGMs Surface Coating Mechanisms
5. Conclusions
- (1)
- The development of a new process that integrates flotation, hydroxylation, amination pretreatment, and in situ surface polymerization is a significant advancement. This integrated approach addresses the challenges associated with the coating of HGMs, which is a complex task due to their low density, high specific surface area, thin cavity shells, and poor surface adhesion.
- (2)
- Achieving a uniform coating of PPy on HGMs is a notable accomplishment. This uniformity is crucial for the performance of HGMs in various applications, as it ensures consistent properties across the material.
- (3)
- Flotation treatment can effectively remove defective particles in HGMs and reduce their density. The density of HGMs decreased from 0.3841 g/cm3 to 0.3111 g/cm3 after flotation.
- (4)
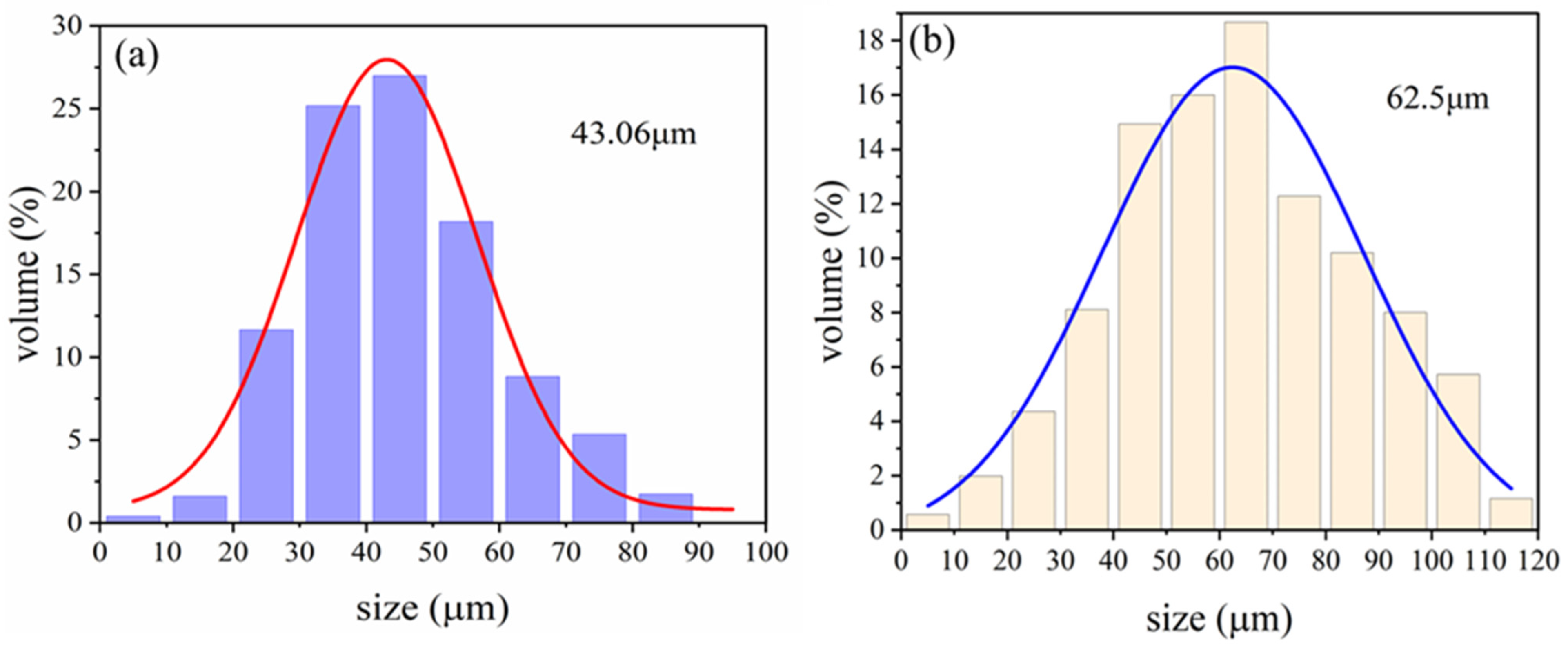
- Different reaction durations and microsphere/PPy mass ratios in the in situ polymerization reaction influence the extent of PPy encapsulation and the size of the encapsulated particles. The coated HGMs retained their lightweight properties. The in situ polymerization resulting in a uniform encapsulation of spherical PPy, with an average particle size increase of 14.60%, is a significant result. This indicates that the coating process not only improves the functionality of HGMs but also enhances their physical properties.
- (5)
- The mechanism of PPy coating on HGMs: The amino groups on the surface of HGMs attract pyrrole, leading to the enrichment of pyrrole and promoting the growth of the PPy chain. This process contributes to the homogeneous dispersion of PPy on the surface of HGMs. The Lewis acid properties of PPy react strongly with the Lewis-type electron-donating amino groups in the silane coupling agent, enhancing the bonding strength of the product and facilitating the formation of a robust interface.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Zheng, K.; Zhang, X.; Wang, Y.; Xiao, C.; Chen, L.; Tian, X. Hollow microsphere-infused porous poly (vinylidene fluoride)/multiwall carbon nanotube composites with excellent electromagnetic shielding and low thermal transport. J. Mater. Sci. 2018, 53, 6042–6052. [Google Scholar] [CrossRef]
- Chen, W.; Qin, Y.; He, X.; Su, Y.; Wang, J. Light-weight carbon fiber/silver-coated hollow glass spheres/epoxy composites as highly effective electromagnetic interference shielding material. J. Reinf. Plast. Compos. 2022, 41, 497–508. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Liu, F.; Dang, Z. Experimental and Computational Study of the Thermal Insulation Properties of Hollow Glass Microspheres. Energy Technol. 2024, 12, 2301308. [Google Scholar] [CrossRef]
- Song, L.; Zong, L.-S.; Wang, J.-Y.; Jian, X.-G. Preparation and Performance of HGM/PPENK-based High Temperature-resistant Thermal Insulating Coatings. Chin. J. Polym. Sci. 2021, 39, 770–778. [Google Scholar] [CrossRef]
- Wang, L.; Cui, H.; Han, X.; Wang, X. Floating microparticles of ZnIn2S4 @hollow glass microsphere for enhanced photocatalytic activity. Int. J. Hydrogen Energy 2021, 46, 9678–9689. [Google Scholar] [CrossRef]
- Huang, Z.; Chi, B.; Guan, J.; Liu, Y. Facile method to synthesize silver nanoparticles on the surface of hollow glass microspheres and their microwave shielding properties. RSC Adv. 2014, 4, 18645–18651. [Google Scholar] [CrossRef]
- Wang, P.; He, B.; An, Z.; Xiao, W.; Song, X.; Yan, K.; Zhang, J. Hollow glass microspheres embedded in porous network of chitosan aerogel used for thermal insulation and flame retardant materials. Int. J. Biol. Macromol. 2024, 256, 128329. [Google Scholar] [CrossRef]
- Qiu, R.; Wang, B.; Shang, J.; Hu, G.; Yu, L.; Gao, X. Modifying hollow glass microspheres to obtain self-floating separation adsorbents for adsorbing pollutants in wastewater: A review. J. Mol. Liq. 2024, 404, 124965. [Google Scholar] [CrossRef]
- Yang, J.; Jeon, D.; Kang, H.; Shang, X.; Moon, J. Hydrophobic treatment on hollow glass microspheres for enhancing the flowability of lightweight high-performance cementitious composites. Constr. Build. Mater. 2023, 409, 133856. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, H.; Wang, T.; Zeng, H. Cu-plated hollow glass microspheres for hydrogen production and degradation. J. Mater. Sci. Mater. Electron. 2016, 27, 5183–5189. [Google Scholar] [CrossRef]
- Niazi, P.; Karevan, M.; Javanbakht, M. Mechanical and thermal insulation performance of hollow glass microsphere (HGMs)/fumed silica/polyester microcomposite coating. Prog. Org. Coat. 2023, 176, 107387. [Google Scholar] [CrossRef]
- Yu, Z.; Du, X.; Zhu, P.; Zhao, T.; Sun, R.; Chen, J.; Wang, N.; Li, W. Surface modified hollow glass microspheres-epoxy composites with enhanced thermal insulation and reduced dielectric constant. Mater. Today Commun. 2022, 32, 104046. [Google Scholar] [CrossRef]
- Mahmoud, M.; Kraxner, J.; Elsayed, H.; Bernardo, E.; Galusek, D. Fabrication and environmental applications of glass microspheres: A review. Ceram. Int. 2023, 49, 39745–39759. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, L.; Liu, W.; Qiao, Y.; Zhang, L.; Bai, C.; Su, S.; Shen, J.; Zheng, T. Constructing ‘bayberry shape’ structure on HGMs surface using CNTs to enhance the mechanical properties of HGMs/epoxy composites. Compos. Commun. 2024, 47, 101885. [Google Scholar] [CrossRef]
- Afolabi, O.A.; Kanny, K.; Mohan, T.P. Processing of hollow glass microspheres (HGMs) filled epoxy syntactic foam composites with improved structural characteristics. Sci. Eng. Compos. Mater. 2021, 28, 116–127. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Peng, H.; Wang, L.; Lu, X.; Huang, Y.; Chen, J.; Shao, T. Buoyant ALG/HA/HGMs composite adsorbents for highly efficient removal of copper from aqueous solution and contaminated kaolin soil. Chem. Eng. J. 2017, 327, 244–256. [Google Scholar] [CrossRef]
- Zeng, L.W.; Bian, J.J. Ultralow-k hollow glass microsphere filled perfluoroalkoxy composites. Int. J. Appl. Ceram. Technol. 2023, 20, 1692–1700. [Google Scholar] [CrossRef]
- An, Y.; Zheng, P.; Ma, X. Preparation and visible-light photocatalytic properties of the floating hollow glass microspheres-TiO2/Ag3PO4 composites. RSC Adv. 2019, 9, 721–729. [Google Scholar] [CrossRef]
- Zhang, H. Silver plating on hollow glass microsphere and coating finishing of PET/cotton fabric. J. Ind. Text. 2013, 42, 283–296. [Google Scholar] [CrossRef]
- Kanwal, R.; Maqsood, M.F.; Raza, M.A.; Inam, A.; Waris, M.; Rehman, Z.U.; Mehdi, S.M.Z.; Abbas, N.; Lee, N. Polypyrrole coated carbon fiber/magnetite/graphene oxide reinforced hybrid epoxy composites for high strength and electromagnetic interference shielding. Mater. Today Commun. 2024, 38, 107684. [Google Scholar] [CrossRef]
- Chen, X.; Qi, S. Synthesis and microwave absorption enhancement of polyaniline/SrFe12O19/hollow glass microsphere composite with core-shell structure. J. Mater. Sci. Mater. Electron. 2016, 27, 13099–13104. [Google Scholar] [CrossRef]
- Xu, R.; Wang, W.; Yu, D. Preparation of silver-plated Hollow Glass Microspheres and its application in infrared stealth coating fabrics. Prog. Org. Coatings 2019, 131, 1–10. [Google Scholar] [CrossRef]
- Chen, Q.; Han, B. Microporous Polycarbazole Materials: From Preparation and Properties to Applications. Macromol. Rapid Commun. 2018, 39, e1800040. [Google Scholar] [CrossRef]
- Li, X.; Xue, Y.; Zhang, D.; Chen, Y. Robust silicone rubber with high transparency by loading with porous hollow glass microsphere. J. Polym. Res. 2023, 30, 426. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhang, F.; Li, N.; Shi, J.-F.; Jia, L.-C.; Yan, D.-X.; Li, Z.-M. Carbon-based aerogels and foams for electromagnetic interference shielding: A review. Carbon 2023, 205, 10–26. [Google Scholar] [CrossRef]
- Huo, S.; Wang, J.; Wu, X. Morphology, thermal and mechanical performances of SR composites containing sepiolite and HGMs as binary fillers. J. Polym. Eng. 2017, 37, 197–204. [Google Scholar] [CrossRef]
- Yang, J.; Liao, X.; Wang, G.; Chen, J.; Guo, F.; Tang, W.; Wang, W.; Yan, Z.; Li, G. Gradient structure design of lightweight and flexible silicone rubber nanocomposite foam for efficient electromagnetic interference shielding. Chem. Eng. J. 2020, 390, 124589. [Google Scholar] [CrossRef]
- Bu, F.; Song, P.; Liu, Y.; Wang, J.; Wu, X.; Liu, L.; Xu, C.; Zhang, J. The Effective Surface Metallization of Hollow Glass Microspheres for Flexible Electromagnetic Shielding Film. J. Wuhan Univ. Technol. Sci. Ed. 2022, 37, 779–786. [Google Scholar] [CrossRef]
- Krakowiak, K.J.; Nannapaneni, R.G.; Moshiri, A.; Phatak, T.; Stefaniuk, D.; Sadowski, L.; Qomi, M.J.A. Engineering of high specific strength and low thermal conductivity cementitious composites with hollow glass microspheres for high-temperature high-pressure applications. Cem. Concr. Compos. 2020, 108, 103514. [Google Scholar] [CrossRef]
- Imran, M.; Pal, S.; Kahaly, M.U.; Rahaman, A. Radially grown carbon nanomaterials on hollow glass microspheres and their application in composite foams with excellent electromagnetic interference shielding. Polym. Compos. 2021, 42, 3786–3798. [Google Scholar] [CrossRef]
- Bu, F.; Zhang, J.; Yu, S.; Li, Q.; Li, G.; Wang, J.; Wu, X.; Goto, T. Effective surface pretreatment of hollow glass microspheres via a combined KF roughening and alkali washing strategy for the following metallization. Adv. Powder Technol. 2020, 31, 2305–2314. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S.I.; Choe, C.R.; Park, M.; Rim, S.; Kim, J. Preparation and characterization of epoxy composites filled with functionalized nanosilica particles obtained via sol–gel process. Polymer 2001, 42, 879–887. [Google Scholar] [CrossRef]
- Dai, P.; Tao, M.; Li, X.; Xue, Z.; Zeng, J.; Chen, L. Effect of lime (CaOH+) on chalcopyrite-arsenopyrite separation in high gradient magnetic separation: Experiment and molecular simulation. Sep. Purif. Technol. 2024, 333, 125831. [Google Scholar] [CrossRef]
- Yan, K.; Xie, X.; Li, B.; Yuan, J.; Zhang, J. Influence of the real density and structure imperfection of hollow glass microspheres on the compression strength. Mater. Sci. Eng. A 2011, 528, 8671–8675. [Google Scholar] [CrossRef]
- Shao, X.; Han, S.; Kang, Y.; Yang, X.; Yang, L.; Zhang, Q.; Wang, X. The plasma-coupling agent synergistically modified the acoustic matching layer for the preparation of a gas ultrasonic flowmeter. Mater. Sci. Semicond. Process. 2024, 179, 108484. [Google Scholar] [CrossRef]
- Sai, B.K.; Tambe, P. Surface modified hollow glass microsphere reinforced 70/30 (wt/wt) PC/ABS blends: Influence on rheological, mechanical, and thermo-mechanical properties. Compos. Interfaces 2022, 29, 617–641. [Google Scholar] [CrossRef]
- Wang, P.; Zhong, S.; Yan, K.; Liao, B.; Guo, Y.; Zhang, J. Effect of hollow glass microspheres surface modification on the compressive strength of syntactic foams. J. Mater. Res. Technol. 2024, 30, 2264–2271. [Google Scholar] [CrossRef]
- Ma, Y.; Du, Y.; Zhao, J.; Yuan, X.; Hou, X. Preparation and Characterization of Furan–Matrix Composites Blended with Modified Hollow Glass Microsphere. Polymers 2020, 12, 1480. [Google Scholar] [CrossRef]
- Weng, Y.; Jin, Z.; Xie, S.; Zhang, M. Integration of hierarchical magnesium silicate with polypyrrole-coated hollow Fe3O4 hybrids as a synergistic adsorbent toward efficient water treatment. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 681, 132745. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, L.; Wang, C.; Feng, S.; Zhang, N.; Xiang, S.; Feng, T.; Yang, M.; Zhang, K.; Yang, B. Utilizing in-situ polymerization of pyrrole to fabricate composited hollow nanospindles for boosting oxygen evolution reaction. Appl. Catal. B Environ. 2020, 274, 119112. [Google Scholar] [CrossRef]
- Ren, J.; Wang, C.; Zhang, H.; Liu, X.; Yang, T.; Zheng, W.; Li, T.; Ma, Y. Magnetic Core@Shell Fe3O4@Polypyrrole@Sodium Dodecyl Sulfate Composite for Enhanced Selective Removal of Dyestuffs and Heavy Metal Ions from Complex Wastewater. Langmuir 2023, 39, 10098–10111. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Ahmed, J.; Algethami, J.S.; Jalalah, M.; Alsareii, S.A.; Alsaiari, M.; Harraz, F.A. Visible-light responsive Au nanoparticle-decorated polypyrrole-carbon black/SnO2 ternary nanocomposite for ultrafast removal of insecticide imidacloprid and methylene blue. J. Ind. Eng. Chem. 2023, 121, 287–298. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, X. Well encapsulated hollow borosilicate glass sphere@polypyrrole composite with low density, designable thickness and conductivity. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 386, 158–165. [Google Scholar] [CrossRef]
- Perruchot, C.; Chehimi, M.M.; Delamar, M.; Cabet-Deliry, E.; Miksa, B.; Slomkowski, S.; Khan, M.A.; Armes, S.P. Chemical deposition and characterization of thin polypyrrole films on glass plates: Role of organosilane treatment. Colloid Polym. Sci. 2000, 278, 1139–1154. [Google Scholar] [CrossRef]







| Number | Reagent Name | Chemical Formula | Concentration |
|---|---|---|---|
| 1 | KH550 | C9H23NO3Si | 1% |
| 2 | Ethyl alcohol | C2H5OH | 24% |
| 3 | Deionized water | H2O | 75% |
| 4 | 5-Sulfosalicylic acid | C7H6O5S | 1.0 mol/L |
| 5 | Ethyl alcohol | C2H5OH | 7.0% |
| 6 | Deionized water | H2O | 93.0% |
| 7 | Pyrrole | C4H5N | 1.0–4.0 g |
| 8 | Ferric chloride | FeCl3 | 1.0 mol/L |
| Number | Technological Parameter | Numerical Value |
|---|---|---|
| 1 | HGMs/pyrrole | 1:2 |
| 2 | Water:ethyl alcohol | 10:1 |
| 3 | 5-Sulfosalicylic acid concentration | 1 mol/L |
| 4 | Fecl3 concentration | 1 mol/L |
| 5 | Temperature | 0 °C |
| 6 | Reaction time | 0.5–3.0 h |
| Number | Technological Parameter | Numerical Value |
|---|---|---|
| 1 | HGMs/pyrrole | 0.5–2.0 |
| 2 | Water:ethyl alcohol | 10:1 |
| 3 | 5-Sulfosalicylic acid concentration | 1 mol/L |
| 4 | Fecl3 concentration | 1 mol/L |
| 5 | Temperature | 0 °C |
| 6 | Reaction time | 3.0 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Zhang, J.; Wang, N.; Liu, L.; Wang, J.; Liu, Y.; Li, G.; Xu, C. Formation Mechanism of Polypyrrole-Coated Hollow Glass Microspheres (PPy@HGMs) Composite Powder. Materials 2024, 17, 5595. https://doi.org/10.3390/ma17225595
Du Y, Zhang J, Wang N, Liu L, Wang J, Liu Y, Li G, Xu C. Formation Mechanism of Polypyrrole-Coated Hollow Glass Microspheres (PPy@HGMs) Composite Powder. Materials. 2024; 17(22):5595. https://doi.org/10.3390/ma17225595
Chicago/Turabian StyleDu, Yao, Jianfeng Zhang, Ning Wang, Lei Liu, Jun Wang, Yahui Liu, Gaiye Li, and Chuanhua Xu. 2024. "Formation Mechanism of Polypyrrole-Coated Hollow Glass Microspheres (PPy@HGMs) Composite Powder" Materials 17, no. 22: 5595. https://doi.org/10.3390/ma17225595
APA StyleDu, Y., Zhang, J., Wang, N., Liu, L., Wang, J., Liu, Y., Li, G., & Xu, C. (2024). Formation Mechanism of Polypyrrole-Coated Hollow Glass Microspheres (PPy@HGMs) Composite Powder. Materials, 17(22), 5595. https://doi.org/10.3390/ma17225595





