Polyurethanes Made with Blends of Polycarbonates with Different Molecular Weights Showing Adequate Mechanical and Adhesion Properties and Fast Self-Healing at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PUs
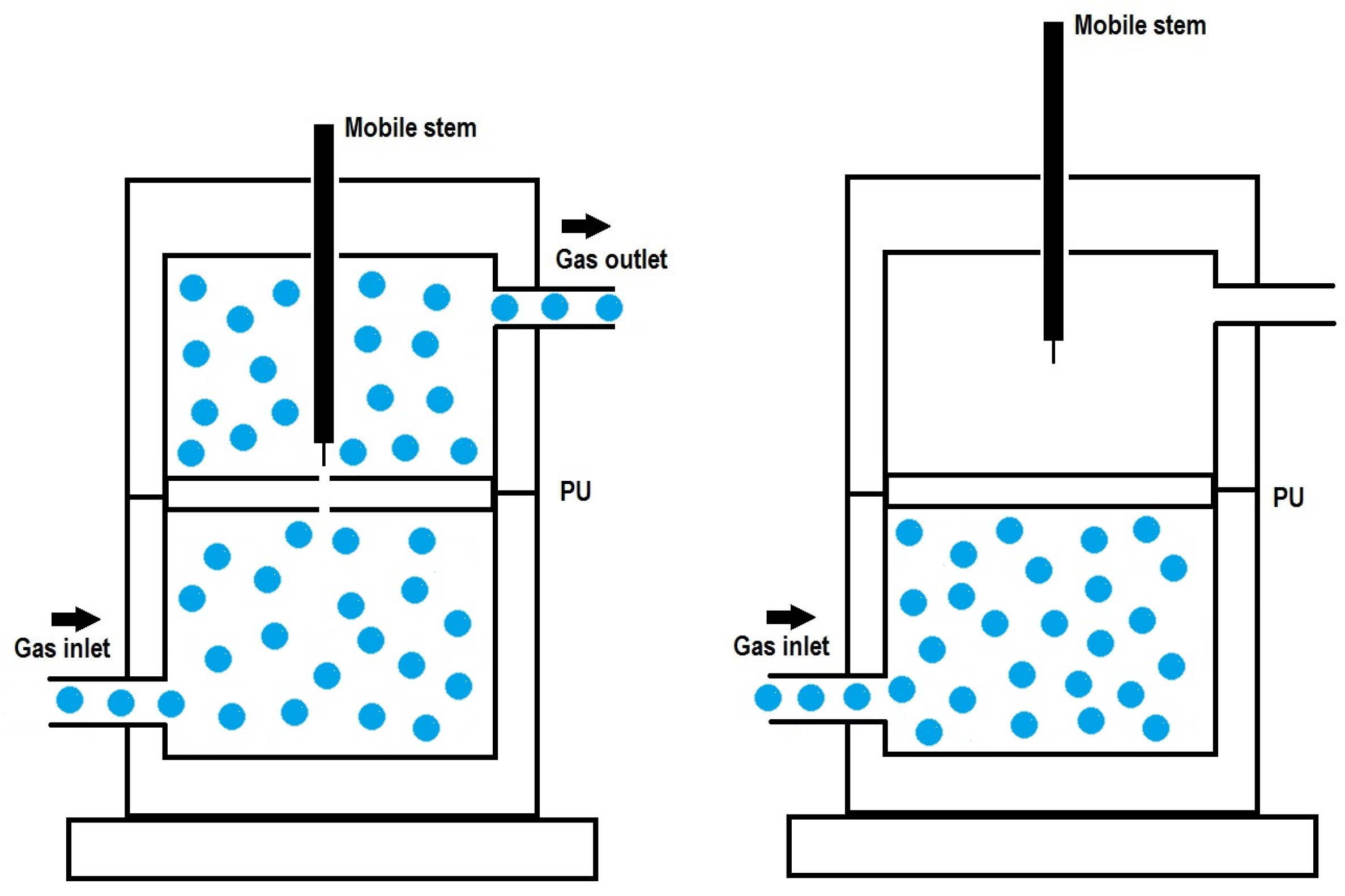
2.3. Experimental Techniques
3. Results and Discussion
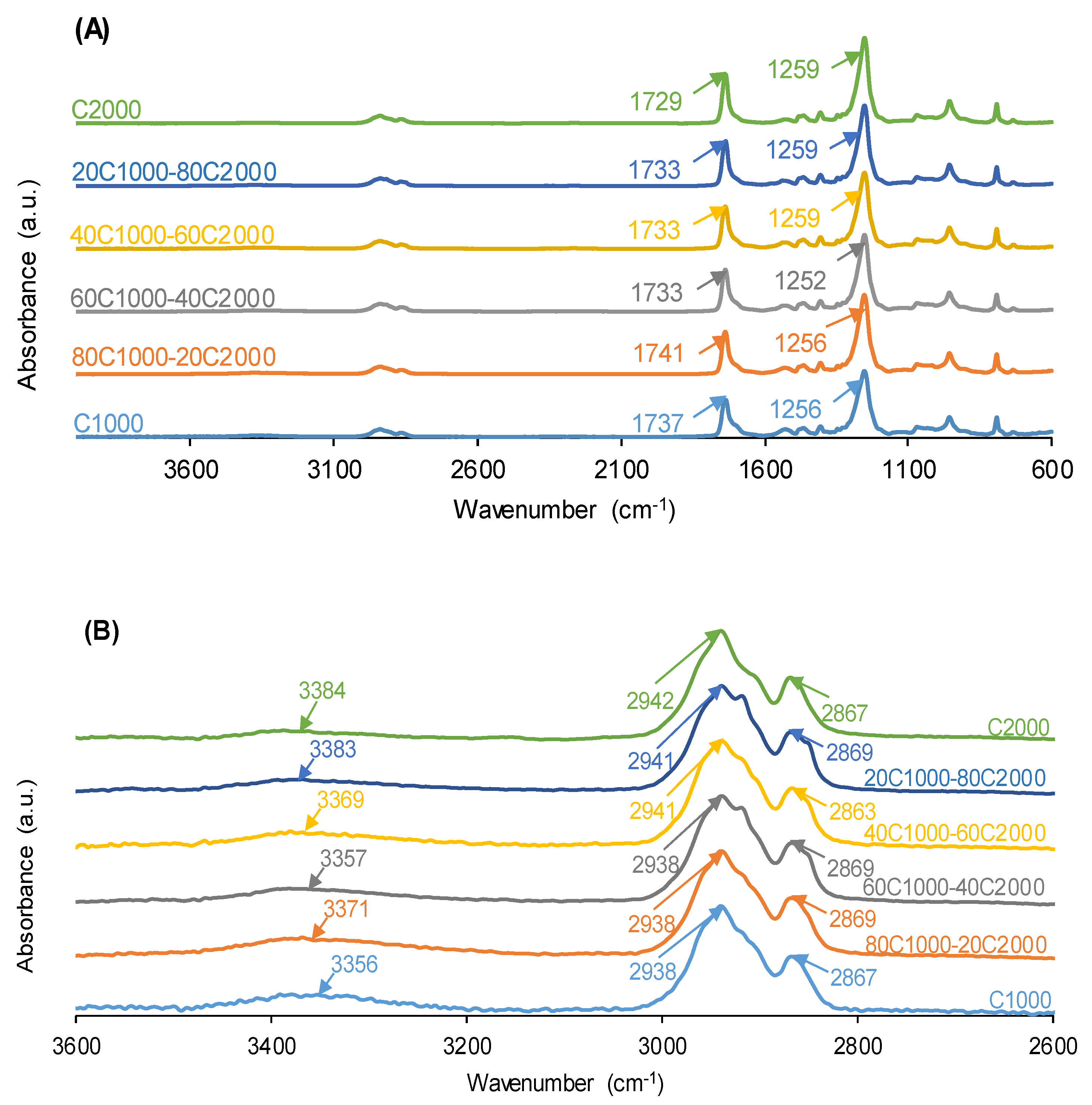
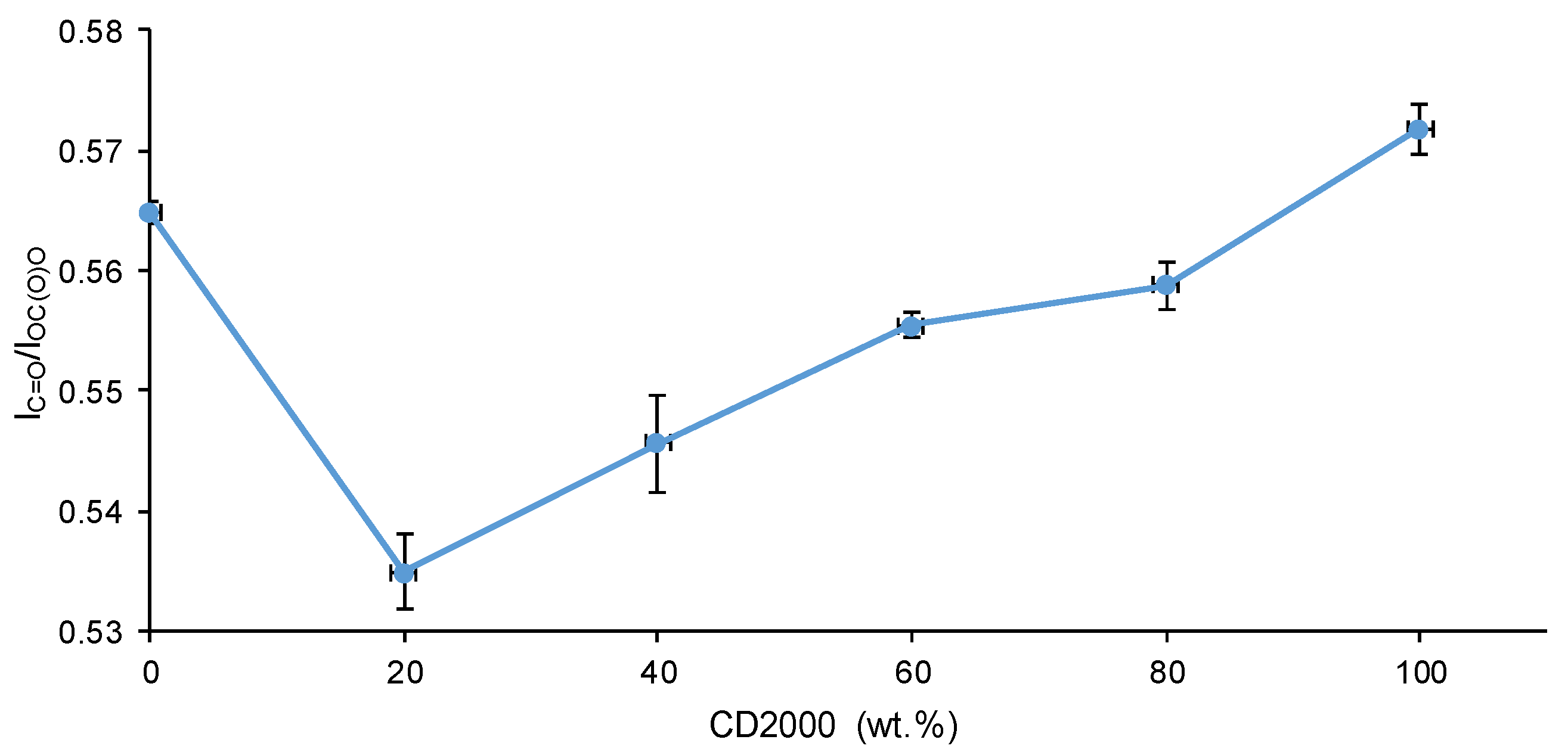
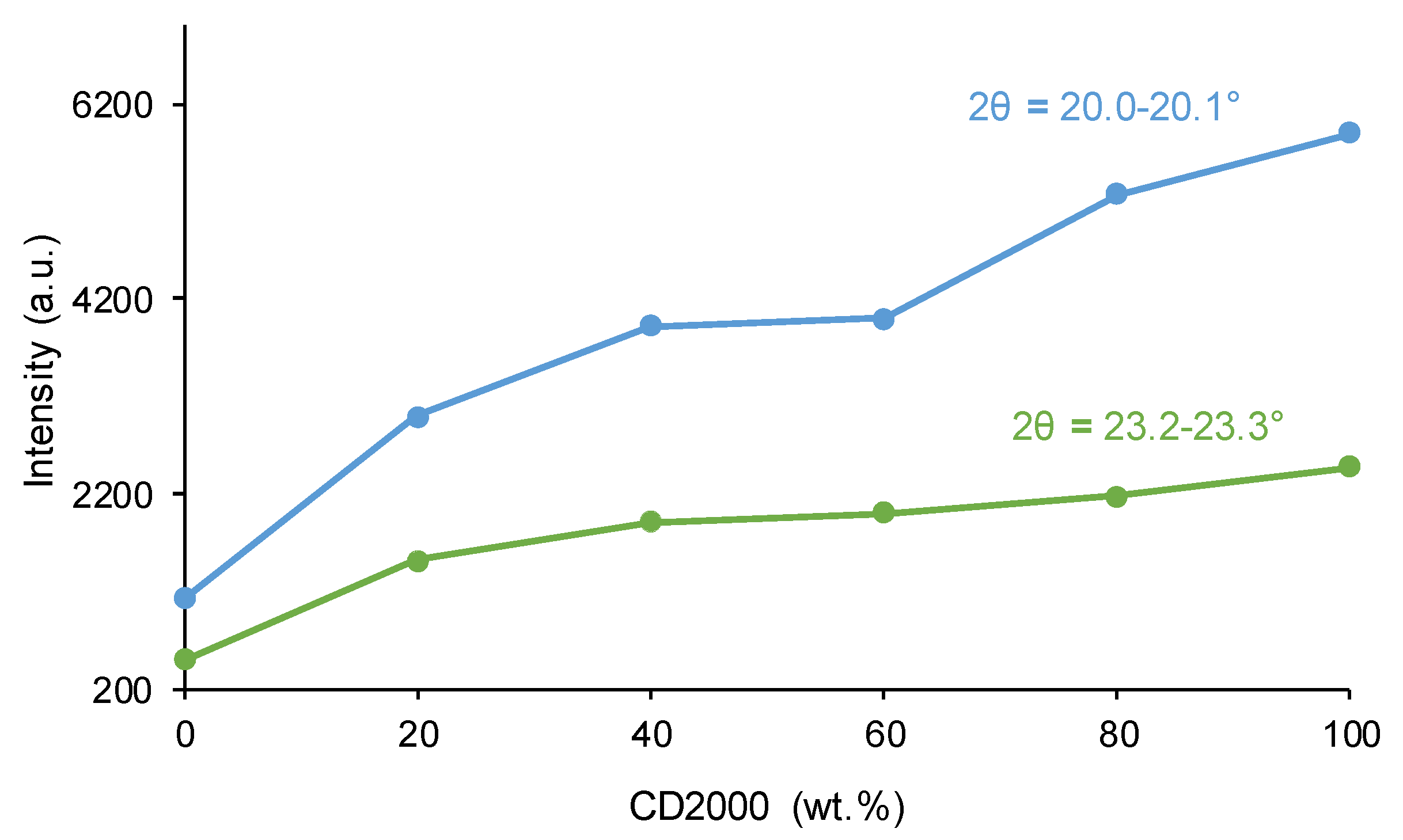
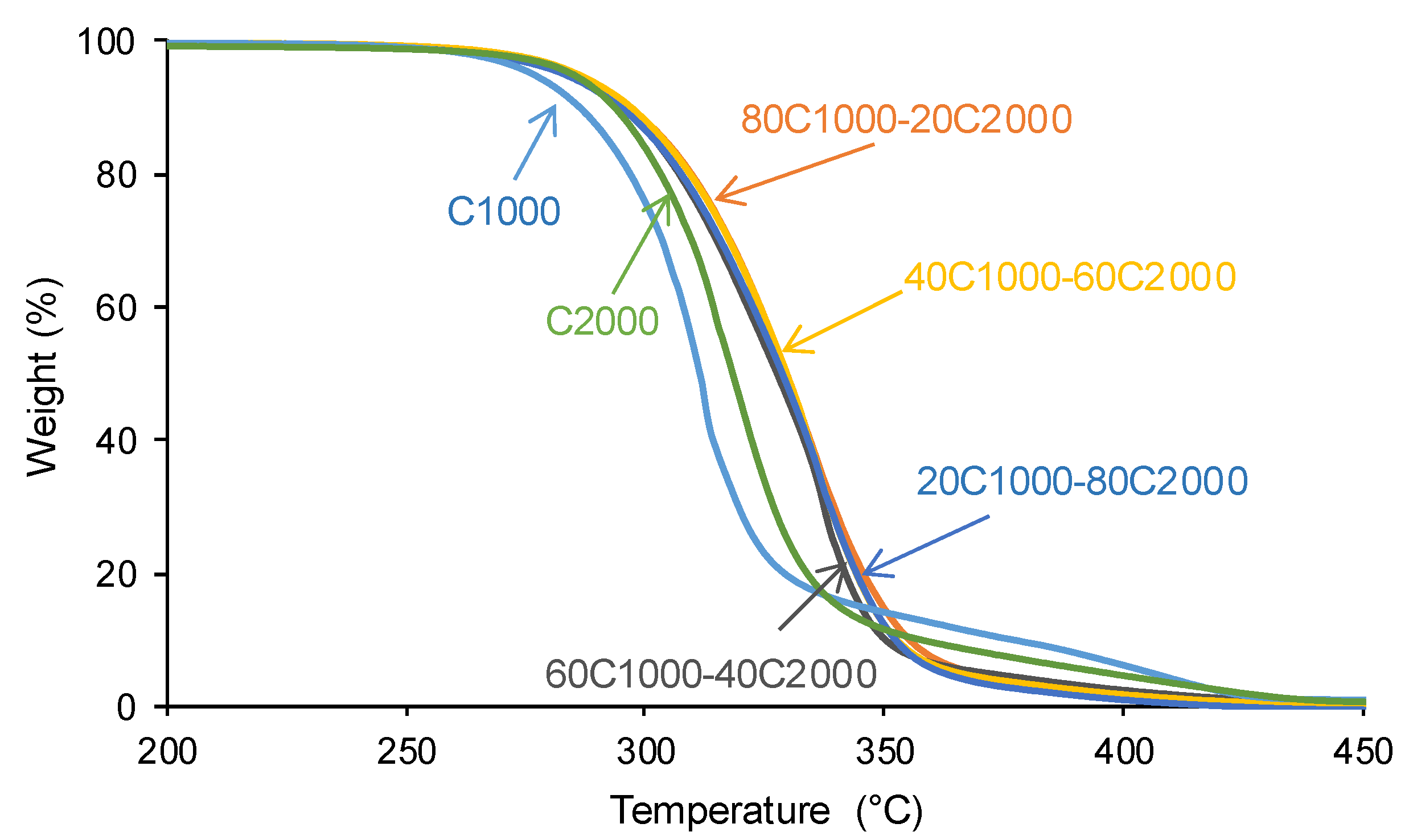
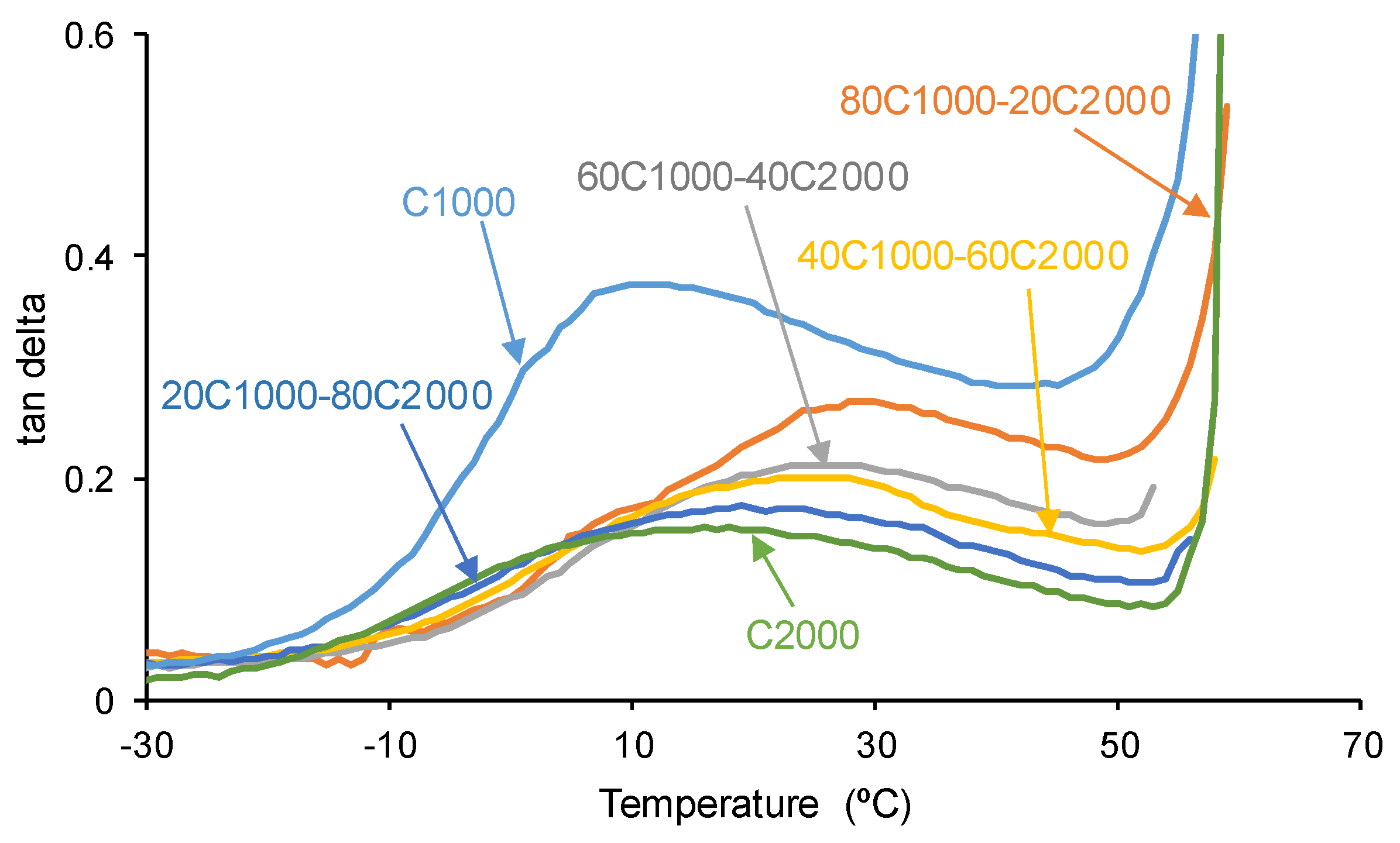
3.1. Structural Characterization and Degree of Micro-Phase Separation of PUs
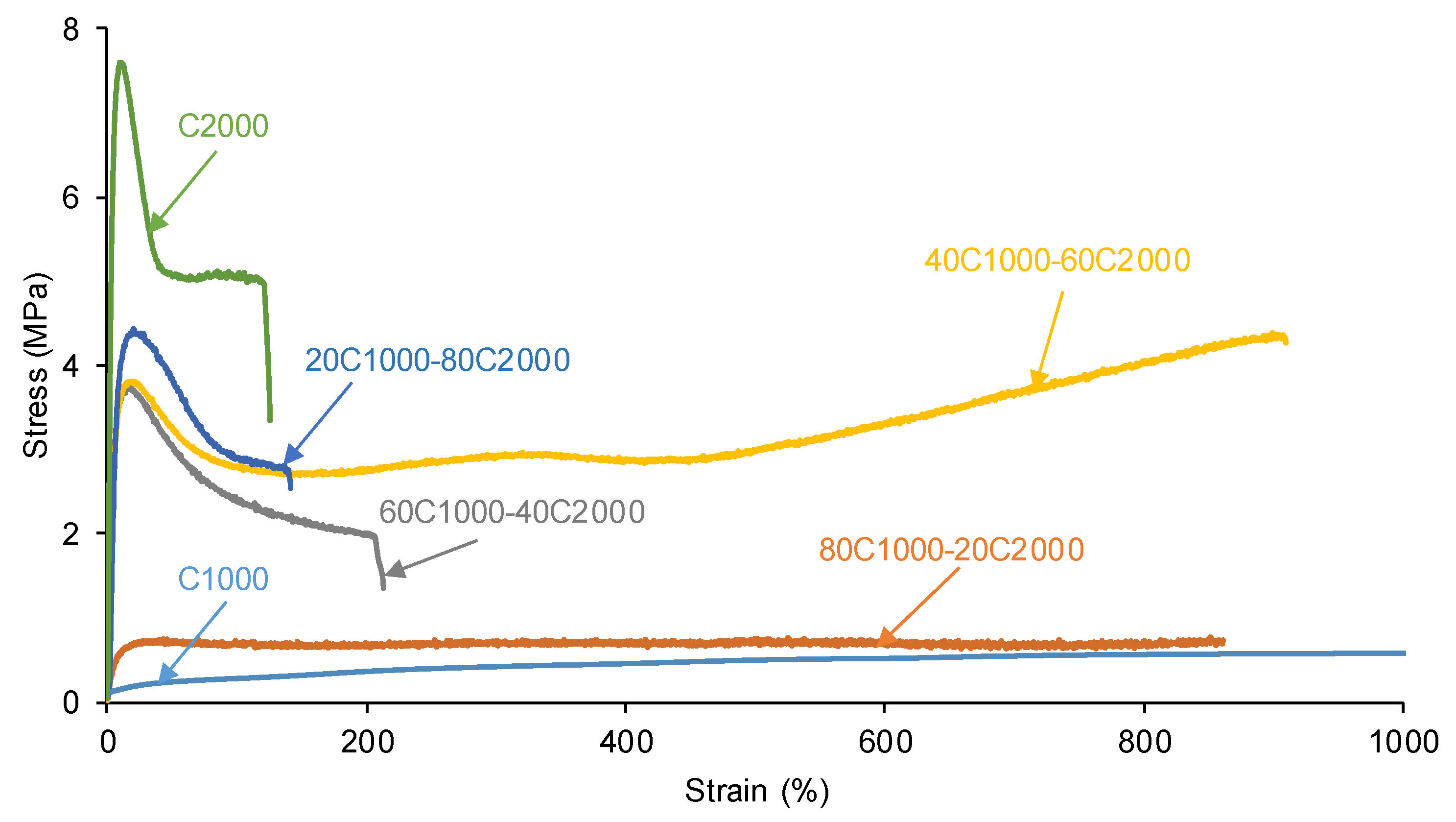
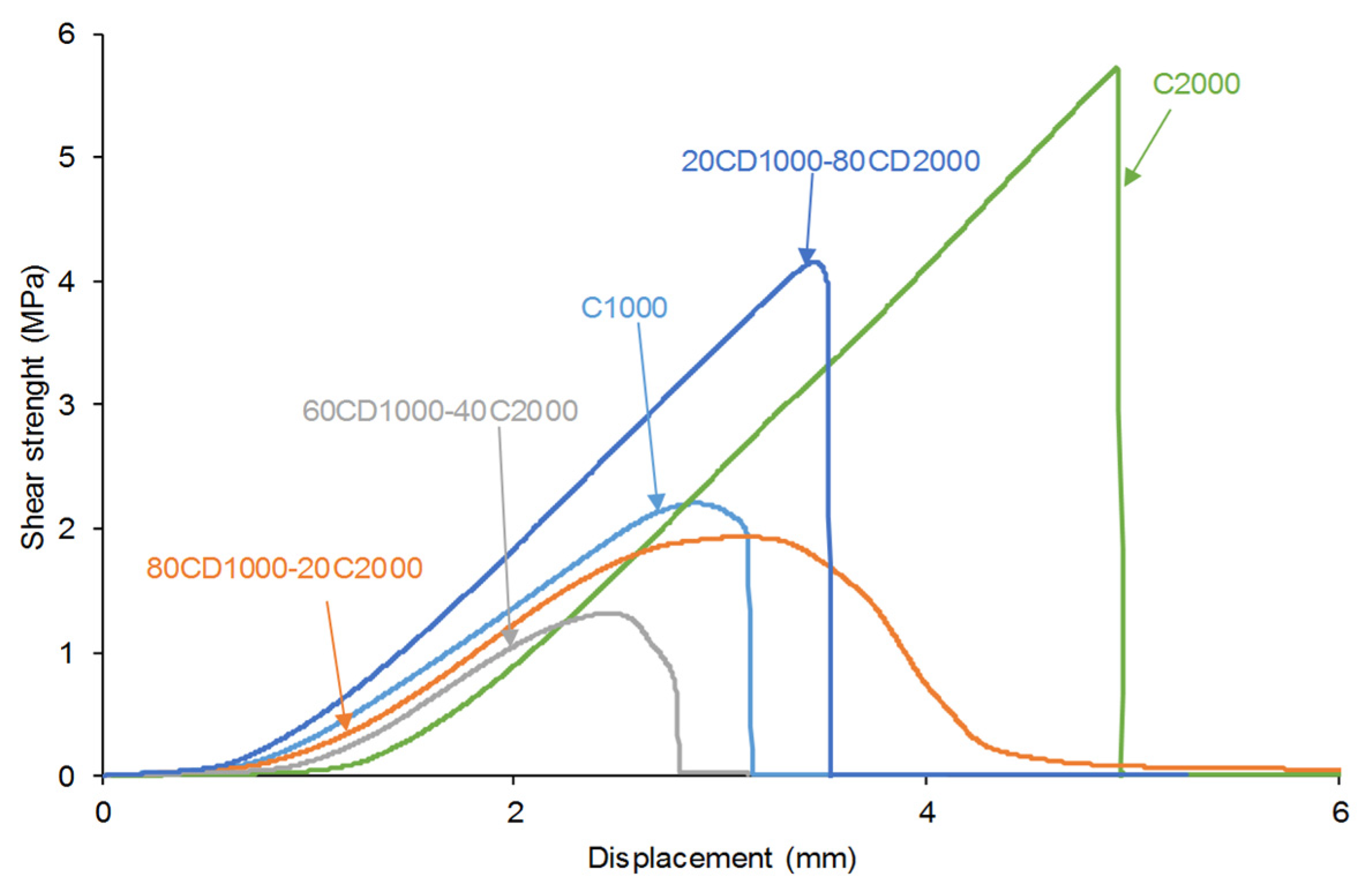
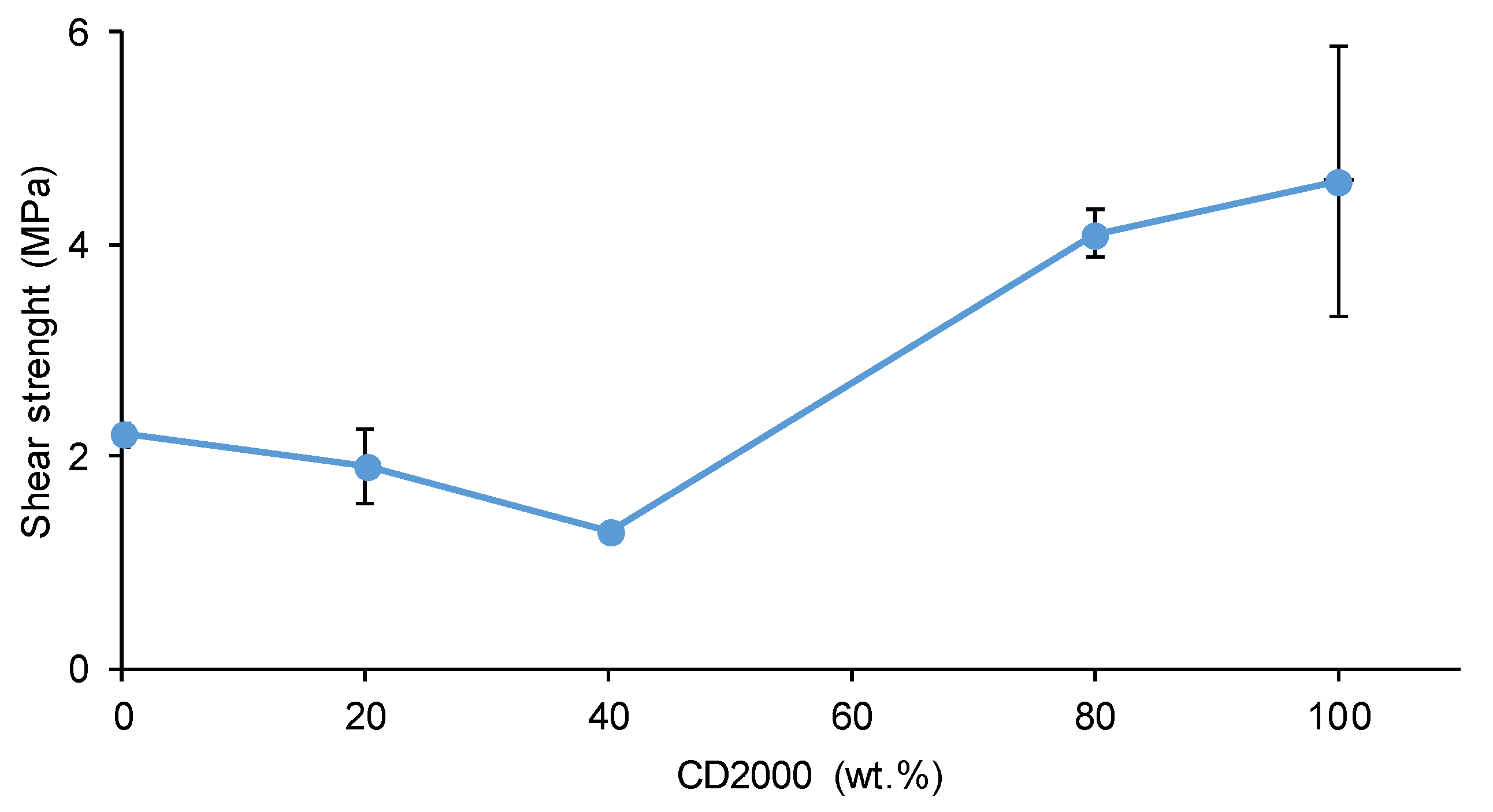
3.2. Mechanical Properties of the PUs
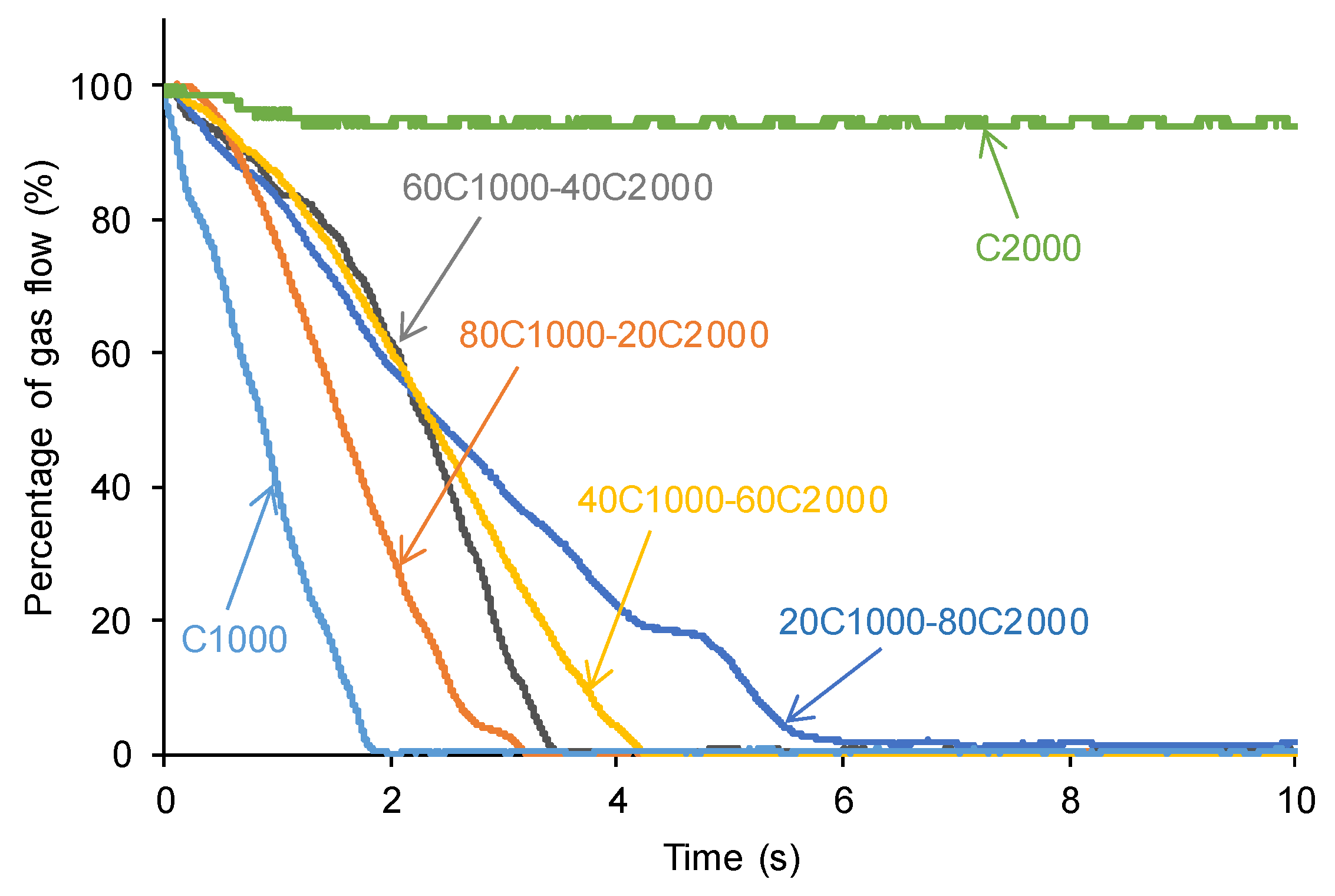
3.3. Self-Healing Assessment of the PUs at 20 °C
3.4. Proposed Mechanism of Self-Healing at 20 °C in PUs Obtained with CD1000 + CD2000 Blends
3.5. Adhesion of Stainless Steel/PU Adhesive/Stainless Steel Joints
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matos-Pérez, C.R.; White, J.D.; Wilker, J.J. Polymer composition and substrate influences on the adhesive bonding of a biomimetic, cross-linking polymer. J. Am. Chem. Soc. 2012, 134, 9498–9505. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, W.; Hu, J.; Xie, D.; Gerhard, E.; Nisic, M.; Shan, D.; Qian, G.; Zheng, S.; Yang, J. Synthesis and characterization of anti-bacterial and anti-fungal citrate-based mussel-inspired bioadhesives. Biomaterials 2016, 85, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Engels, H.W.; Pirkl, H.G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile materials and sustainable problem solvers for today’s challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Z.; Zhang, L.; Guo, Y.; Song, J.; Lou, J.; Guan, Q.; He, C.; You, Z. Strong, detachable, and self-healing dynamic crosslinked hot melt polyurethane adhesive. Mater. Chem. Front. 2019, 3, 1833–1839. [Google Scholar] [CrossRef]
- Han, F.; Shah, S.A.A.; Liu, X.; Zhao, F.; Xu, B.; Zhang, J.; Cheng, J. Preparation and properties of self-healing cross-linked polyurethanes based on blocking and deblocking reaction. React. Funct. Polym. 2019, 144, 104347. [Google Scholar] [CrossRef]
- Longfang, R.; Congcong, L.; Taotao, Q. Preparation of boroxine-based self-healing polyurethane with repeatable adhesive property under mild conditions. Mater. Today Chem. 2022, 26, 101126. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Zhang, X.; Du, Y.; Li, K.; Liu, Y.; Yu, G.; Jia, Y.; Song, S. Dual dynamic bonds self-healing polyurethane with superior mechanical properties over a wide temperature range. Eur. Polym. J. 2022, 163, 110934. [Google Scholar] [CrossRef]
- Urdl, K.; Kandelbauer, A.; Kern, W.; Müller, U.; Thebault, M.; Zikulnig-Rusch, E. Self-healing of densely crosslinked thermoset polymers—A critical review. Prog. Org. Coat. 2017, 104, 232–249. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Pan, C.; Li, D. Review of recent achievements in self-healing conductive materials and their applications. J. Mater. Sci. 2018, 53, 27–46. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, J.; Gan, L.; Long, M. Research progress in bio-based self-healing materials. Eur. Polym. J. 2020, 129, 109651. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Sun, A.; Jing, M.; Liu, X.; Wei, L.; Wu, K.; Fu, Q. A self-reinforcing and self-healing elastomer with high strength, unprecedented toughness and room-temperature reparability. Mater. Horiz. 2021, 8, 267–275. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Nevejans, S.; Reck, B.; Irusta, L.; Sardon, H.; Asua, J.M.; Ballard, N. Healable and self-healing polyurethanes using dynamic chemistry. Prog. Polym. Sci. 2021, 114, 101362. [Google Scholar] [CrossRef]
- Wong, C.S.; Hassan, N.I.; Su’ait, M.S.; Serra, M.A.P.; Gonzalez, J.A.M.; Granda, L.A.; Badri, K.H. Photo-activated self-healing bio-based polyurethanes. Ind. Crops Prod. 2019, 140, 111613. [Google Scholar] [CrossRef]
- Durand-Silva, A.; Cortés-Guzmán, K.P.; Johnson, R.M.; Perera, S.D.; Diwakara, S.D.; Smaldone, R.A. Balancing self-healing and shape stability in dynamic covalent photoresins for stereolithography 3D printing. ACS Macro Lett. 2021, 10, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, N.; Bode, S.; Bose, R.K.; Vitz, J.; Seifert, A.; Hoeppener, S.; Garcia, S.J.; Spange, S.; van der Zwaag, S.; Hager, M.D.; et al. Acylhydrazones as reversible covalent crosslinkers for self-healing polymers. Adv. Funct. Mater. 2015, 25, 3295–3301. [Google Scholar] [CrossRef]
- Chang, K.; Jia, H.; Gu, S.Y. A transparent, highly stretchable, self-healing polyurethane based on disulfide bonds. Eur. Polym. J. 2019, 112, 822–831. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Song, S. Synthesis and characterization of linear polyisoprene supramolecular elastomers based on quadruple hydrogen bonding. Polymers 2020, 12, 110. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, H.; Chen, S. New zwitterionic polyurethanes containing pendant carboxyl-pyridinium with shape memory, shape reconfiguration, and self-healing properties. Polymer 2019, 180, 121727. [Google Scholar] [CrossRef]
- Arévalo-Alquichire, S.; Morales-Gonzalez, M.; Navas-Gómez, K.; Diaz, L.E.; Gómez-Tejedor, J.A.; Serrano, M.A.; Valero, M.F. Influence of polyol/crosslinker blend composition on phase separation and thermo-mechanical properties of polyurethane thin films. Polymers 2020, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Mo, F.; Yang, Y.; Stadler, F.J.; Chen, S.; Yang, H.; Ge, Z. Development of zwitterionic polyurethanes with multi-shape memory effects and self-healing properties. J. Mater. Chem. A 2015, 3, 2924–2933. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.; Li, P.; Maitz, M.F.; Wang, K.; Shang, T.; Dai, S.; Fu, Y.; Zhao, Y.; Yang, Z.; et al. Adhesive and self-healing polyurethanes with tunable multifunctionality. Research 2022, 2022, 9795682. [Google Scholar] [CrossRef]
- Dehaghani, M.Z.; Kaffashi, B.; Haponiuk, J.T.; Piszczyk, L. Shape memory thin films of polyurethane: Does graphene content affect the recovery behavior of polyurethane nanocomposites? Polym. Compos. 2020, 41, 3376–3388. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Gao, G. Bioinspired adhesive hydrogels tackified by nucleobases. Adv. Funct. Mater. 2017, 27, 1703132. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Wen, X.; Wang, S.; Wang, H. Mechanically strong, wet adhesive, and self-healing polyurethane ionogel enhanced with a semi-interpenetrating network for underwater motion detection. ACS Appl. Mater. Interfaces 2022, 14, 54203–54214. [Google Scholar] [CrossRef]
- Li, T.; Xie, Z.; Xu, J.; Weng, Y.; Guo, B.H. Design of a self-healing cross-linked polyurea with dynamic cross-links based on disulfide bonds and hydrogen bonding. Eur. Polym. J. 2018, 107, 249–257. [Google Scholar] [CrossRef]
- Grande, A.M.; Bijleveld, J.C.; Garcia, S.J.; Van Der Zwaag, S. A combined fracture mechanical–rheological study to separate the contributions of hydrogen bonds and disulphide linkages to the healing of poly (urea-urethane) networks. Polymer 2016, 96, 26–34. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Zeng, W.; Jin, H.; Shang, X.; Zhou, R. Bioinspired fast room-temperature self-healing, robust, adhesive, and AIE fluorescent waterborne polyurethane via hierarchical hydrogen bonds and use as a strain sensor. ACS Appl. Mater. Interfaces 2023, 15, 35469–35482. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Mooney, D.J. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef] [PubMed]
- García-Pacios, V.; Costa, V.; Colera, M.; Martín-Martínez, J.M. Affect of polydispersity on the properties of waterborne polyurethane dispersions based on polycarbonate polyol. Int. J. Adhes. Adhes. 2010, 3, 456–465. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Chen, J.; Yao, M.; Liu, X.; Jiang, Z. Self-healing polycarbonate-based polyurethane with shape memory behavior. Macromol. Res. 2019, 27, 649–656. [Google Scholar] [CrossRef]
- Matějka, L.; Špírková, M.; Dybal, J.; Kredatusová, J.; Hodan, J.; Zhigunov, A.; Šlouf, M. Structure evolution during order–disorder transitions in aliphatic polycarbonate based polyurethanes. Self-healing polymer. Chem. Eng. J. 2019, 357, 611–624. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Chen, S.; Wang, G.; Han, S.; Guo, J.; Yang, L.; Hu, J. Physical cross-linked aliphatic polycarbonate with shape-memory and self-healing properties. J. Mol. Liq. 2023, 381, 121798. [Google Scholar] [CrossRef]
- Paez-Amieva, Y.; Martín-Martínez, J.M. Dynamic non-covalent exchange intrinsic self-healing at 20 °C mechanism of polyurethane induced by interactions among polycarbonate soft segments. Polymers 2024, 16, 924. [Google Scholar] [CrossRef]
- Paez-Amieva, Y.; Martín-Martínez, J.M. Influence of the molecular weight of the polycarbonate polyol on the intrinsic self-healing at 20 °C of polyurethanes. Polymers 2024, 16, 2724. [Google Scholar] [CrossRef] [PubMed]
- D638-14; Test Method for Tensile Properties of Plastics. ASMT: West Conshohocken, PA, USA, 2014.
- Paez-Amieva, Y.; Carpena-Montesinos, J.; Martín-Martínez, J.M. Innovative device and procedure for in situ quantification of the self-healing ability and kinetics of self-healing of polymeric materials. Polymers 2023, 15, 2152. [Google Scholar] [CrossRef]
- Paez-Amieva, Y.; Mateo-Oliveras, N.; Martín-Martínez, J.M. Polyurethanes made with blends of polyester and polycarbonate polyols—New evidences supporting the dynamic non-covalent exchange mechanism of intrinsic self-healing at 20 °C. Polymers 2024, 16, 2881. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, Y.; Kang, M.; Wang, J.; Wang, X.; Feng, Y.; Yin, N.; Li, Q. The effects of the molecular weight and structure of polycarbonatediols on the properties of waterborne polyurethanes. Prog. Org. Coat. 2015, 82, 46–56. [Google Scholar] [CrossRef]
- García-Pacios, V.; Jofre-Reche, J.A.; Costa, V.; Colera, M.; Martín-Martínez, J.M. Coatings prepared from waterborne poly-urethane dispersions obtained with polycarbonates of 1, 6-hexanediol of different molecular weights. Prog. Org. Coat. 2013, 76, 1484–1493. [Google Scholar] [CrossRef]
- Niemczyk, A.; Piegat, A.; Olalla, Á.S.; El Fray, M. New approach to evaluate microphase separation in segmented polyurethanes containing carbonate macrodiol. Eur. Polym. J. 2017, 93, 182–191. [Google Scholar] [CrossRef]
- Wang, Y.; Balbuena, P.B. Associations of alkyl carbonates: Intermolecular C−H···O interactions. J. Phys. Chem. A 2001, 43, 9972–9982. [Google Scholar] [CrossRef]
- Paez-Amieva, Y.; Martín-Martínez, J.M. Understanding the interactions between soft segments in polyurethanes: Structural synergies in blends of polyester and polycarbonate diol polyols. Polymers 2023, 15, 4494. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, Y.; Fan, W.; Zhou, R. A review on room-temperature self-healing polyurethane: Synthesis, self-healing mechanism and application. J. Leather Sci. Eng. 2022, 4, 24. [Google Scholar] [CrossRef]
- Dong, F.; Yang, X.; Guo, L.; Wang, Y.; Shaghaleh, H.; Huang, Z.; Xu, X.; Wang, S.; Liu, H. Self-healing polyurethane with high strength and toughness based on a dynamic chemical strategy. J. Mater. Chem. A 2022, 10, 10139–10149. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Du, X.; Du, Z.; Cheng, X.; Wang, H. A self-healing polyurethane-based composite coating with high strength and anti-corrosion properties for metal protection. Compos. Part B Eng. 2021, 225, 109273. [Google Scholar] [CrossRef]
- Samyn, F.; Ferreira, H.; Bui, K.; Muro-Puente, I.; Biget, C.; Lebeau, A.; Bellayer, S.; Casetta, M.; Jimenez, M. Eco-efficient, hydrophobic, self-healing and self-stratifying coating for polycarbonate. Prog. Org. Coat. 2024, 196, 108732. [Google Scholar] [CrossRef]
- Darla, V.R.; Ben, B.S.; Srinadh, K.V.S. Investigation on interfacial bonding strength between aluminum hub and CFRP bonded joints exposed to accelerated aging conditions. J. Adhes. Sci. Technol. 2024, 38, 31–43. [Google Scholar] [CrossRef]







| PU | Polyols Composition * | HS (wt.%) |
|---|---|---|
| C1000 | CD1000 | 22 |
| 80C1000-20C2000 | 80% CD1000 + 20% CD2000 | 21 |
| 60C1000-40C2000 | 60% CD1000 + 40% CD2000 | 19 |
| 40C1000-60C2000 | 40% CD1000 + 60% CD2000 | 17 |
| 20C1000-80C2000 | 20% CD1000 + 80% CD2000 | 15 |
| C2000 | CD2000 | 13 |
| Wavenumber (cm−1) | Percentage (%) | Assignment | |||||
|---|---|---|---|---|---|---|---|
| C1000 | 80C1000-20C2000 | 60C1000-40C2000 | 40C1000-60C2000 | 20C1000-80C2000 | C2000 | ||
| 1654–1664 | 9 | 4 | 4 | 3 | 3 | 3 | Bonded urea |
| 1695–1697 | 15 | 10 | 11 | 12 | 4 | 12 | Free urea |
| 1713–1725 | 14 | 18 | 18 | 15 | 16 | 11 | Bonded urethane |
| 1733–1735 | 38 | 43 | 34 | 40 | 48 | 40 | Carbonate-carbonate, free urethane |
| 1743–1745 | 24 | 24 | 33 | 29 | 29 | 34 | Free C=O (carbonate) |
| PU | Tg (°C) | °C) | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|---|
| C1000 | −21 | 0.29 | - | - | - | - |
| 80C1000-20C2000 | −26 | 0.40 | - | - | - | - |
| 60C1000-40C2000 | −27 | 0.35 | - | - | - | - |
| 40C1000-60C2000 | −29 | 0.35 | - | - | 44 | 1 |
| 20C1000-80C2000 | −29 | 0.22 | 21 | 2 | 44 | 14 |
| C2000 | −29 | 0.20 | 20 | 1 | 45 | 23 |
| PU | Tss (°C) | Ths (°C) |
|---|---|---|
| C1000 | −18 | 236 |
| 80C1000-20C2000 | −23 | 231 |
| 60C1000-40C2000 | −27 | 232 |
| 40C1000-60C2000 | −31 | 235 |
| 20C1000-80C2000 | −32 | 238 |
| C2000 | −36 | 240 |
| PU | tan delta | Ttan delta (°C) |
| C1000 | 0.38 | 10 |
| 80C1000-20C2000 | 0.27 | 30 |
| 60C1000-40C2000 | 0.21 | 26 |
| 40C1000-60C2000 | 0.20 | 26 |
| 20C1000-80C2000 | 0.17 | 24 |
| C2000 | 0.16 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paez-Amieva, Y.; Mateo-Oliveras, N.; Martín-Martínez, J.M. Polyurethanes Made with Blends of Polycarbonates with Different Molecular Weights Showing Adequate Mechanical and Adhesion Properties and Fast Self-Healing at Room Temperature. Materials 2024, 17, 5532. https://doi.org/10.3390/ma17225532
Paez-Amieva Y, Mateo-Oliveras N, Martín-Martínez JM. Polyurethanes Made with Blends of Polycarbonates with Different Molecular Weights Showing Adequate Mechanical and Adhesion Properties and Fast Self-Healing at Room Temperature. Materials. 2024; 17(22):5532. https://doi.org/10.3390/ma17225532
Chicago/Turabian StylePaez-Amieva, Yuliet, Noemí Mateo-Oliveras, and José Miguel Martín-Martínez. 2024. "Polyurethanes Made with Blends of Polycarbonates with Different Molecular Weights Showing Adequate Mechanical and Adhesion Properties and Fast Self-Healing at Room Temperature" Materials 17, no. 22: 5532. https://doi.org/10.3390/ma17225532
APA StylePaez-Amieva, Y., Mateo-Oliveras, N., & Martín-Martínez, J. M. (2024). Polyurethanes Made with Blends of Polycarbonates with Different Molecular Weights Showing Adequate Mechanical and Adhesion Properties and Fast Self-Healing at Room Temperature. Materials, 17(22), 5532. https://doi.org/10.3390/ma17225532









