Hot Uniaxial Pressing and Pressureless Sintering of AlCrCuFeMnNi Complex Concentrated Alloy—A Comparative Study
Abstract
1. Introduction
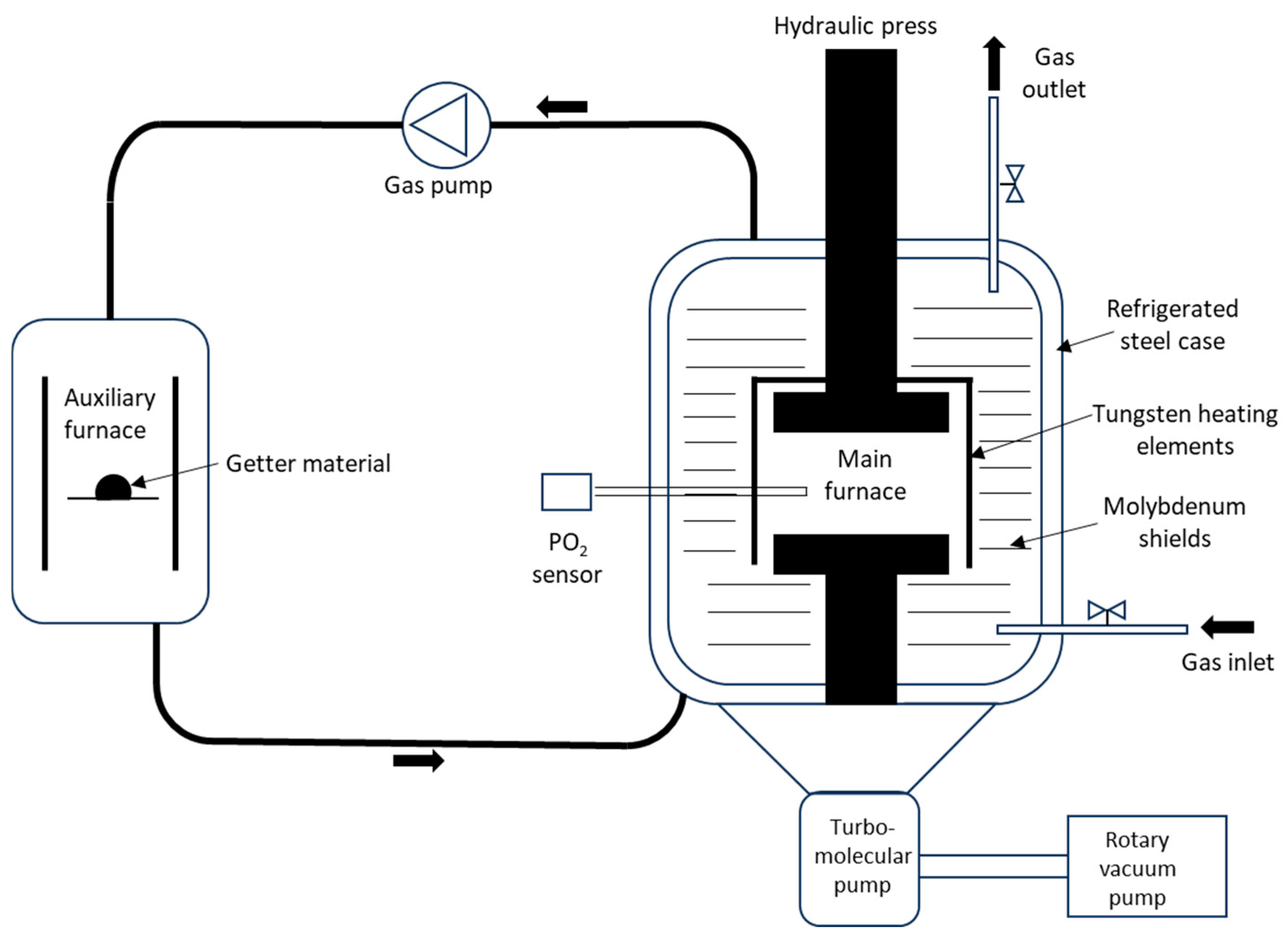
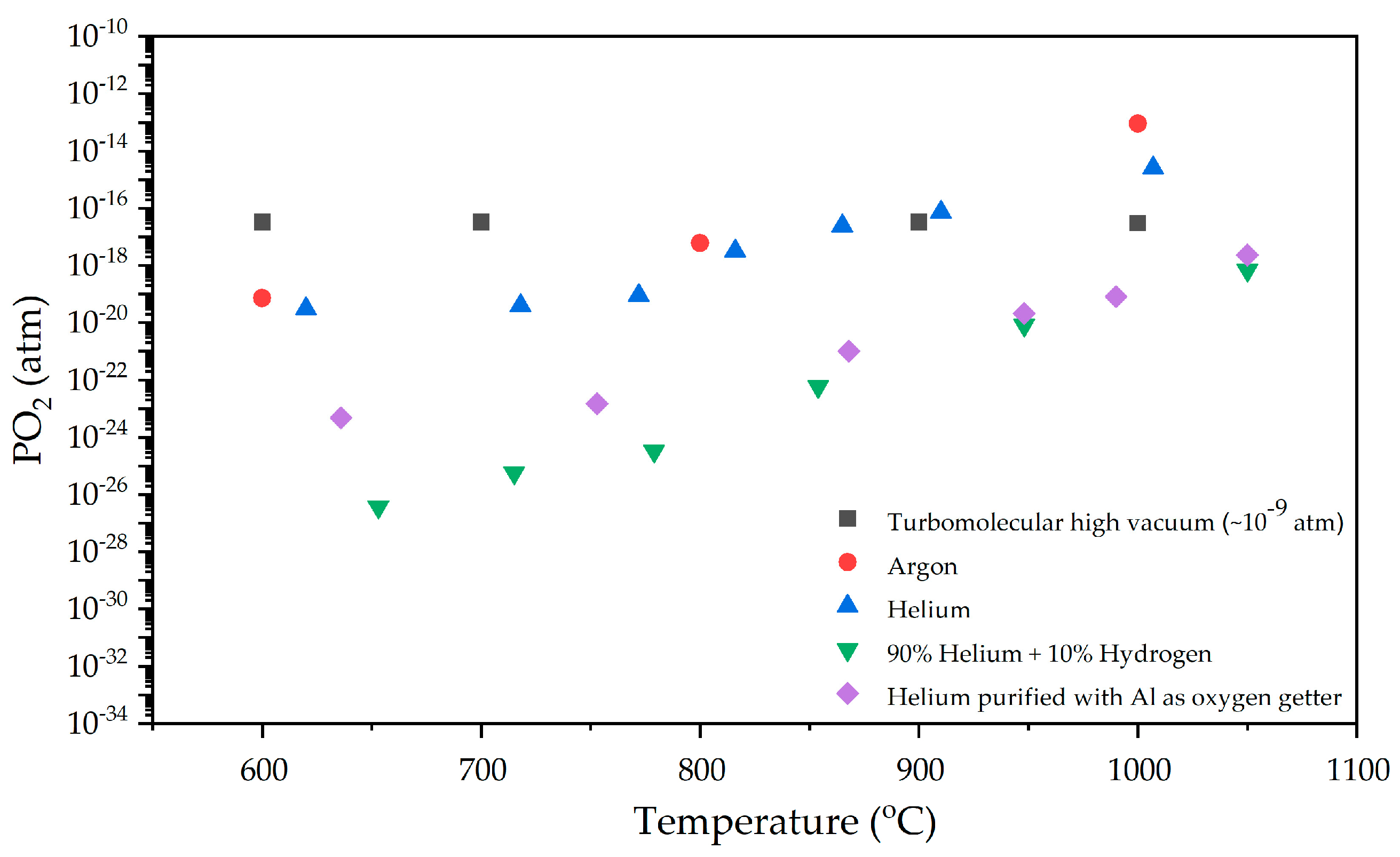
2. Materials and Methods
3. Results and Discussion
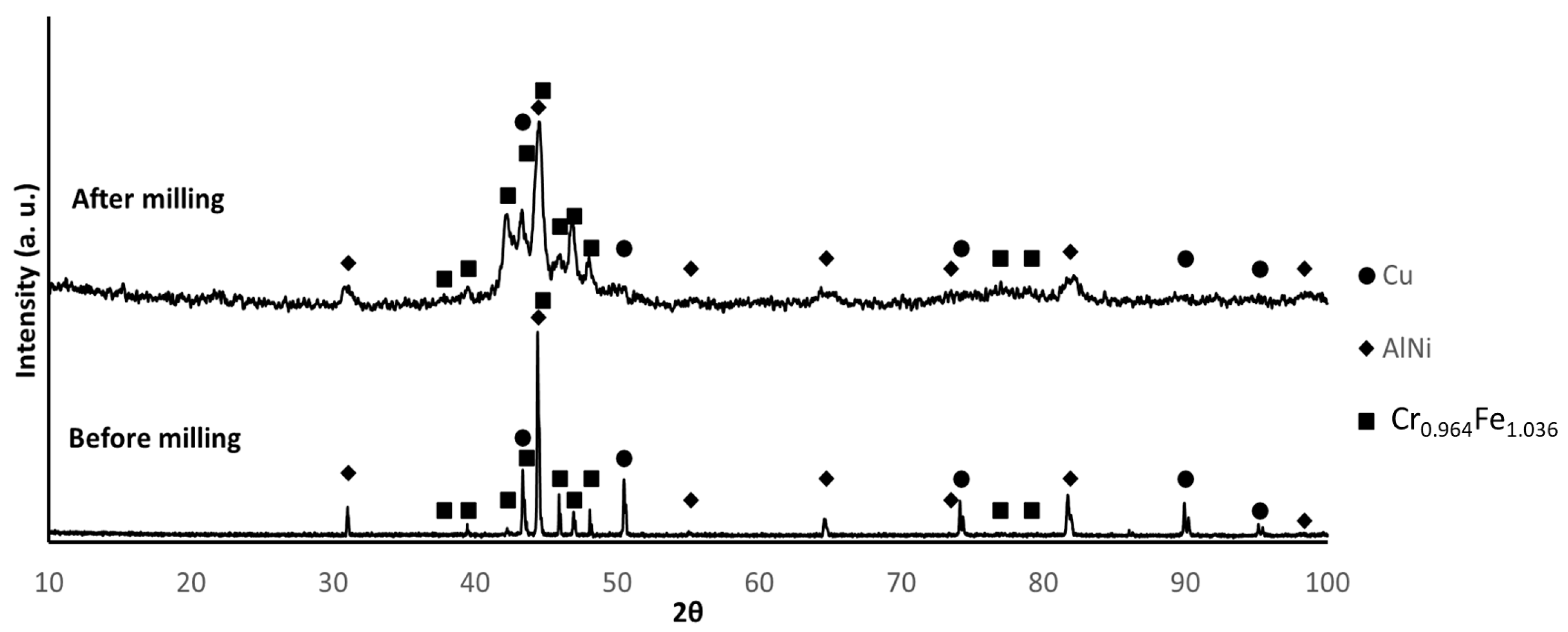
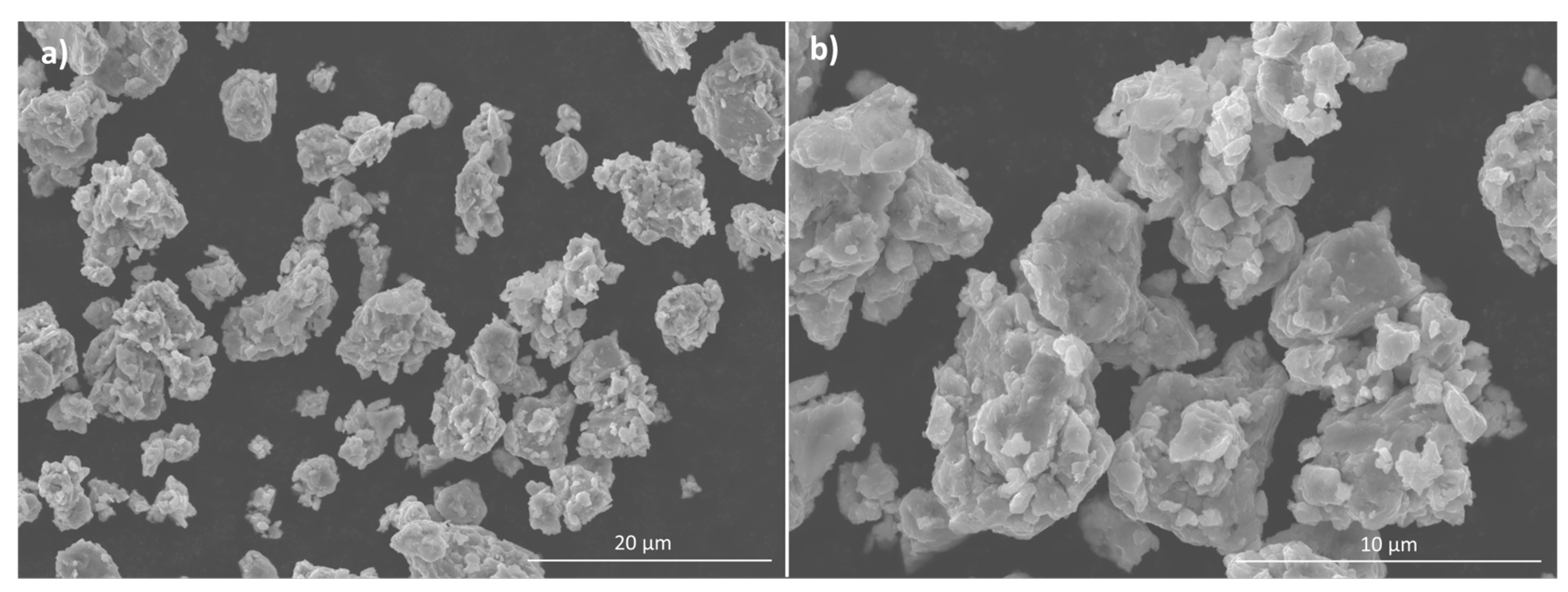
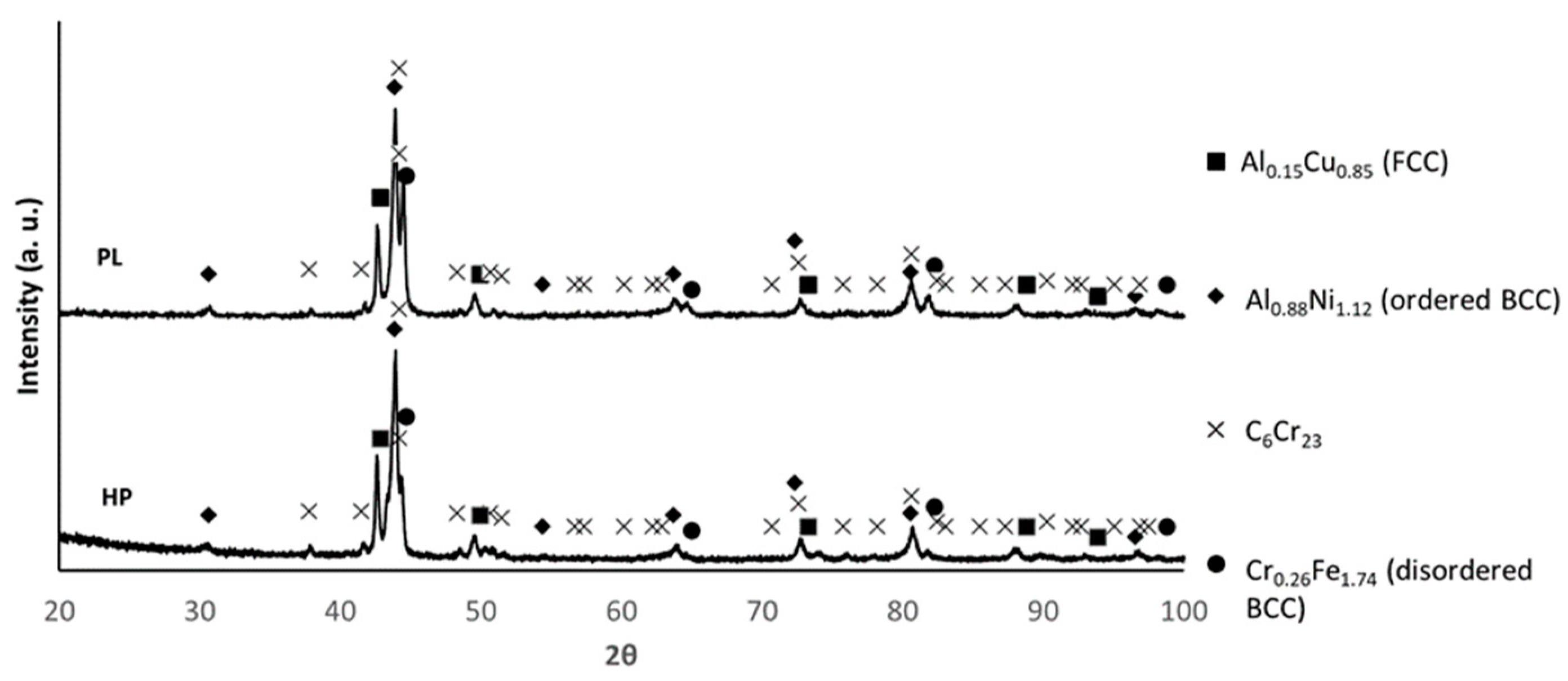
3.1. Powder Characterisation
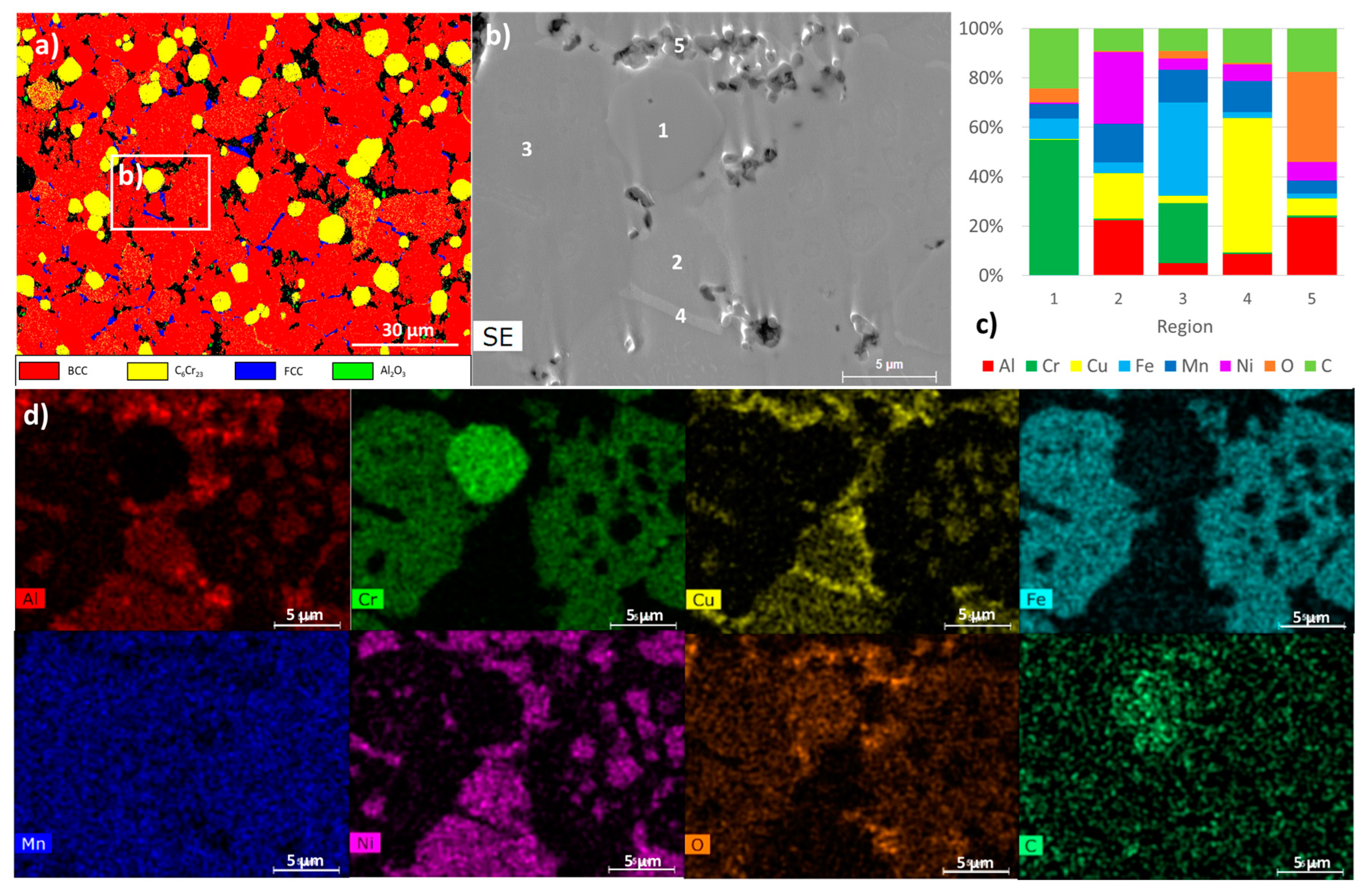
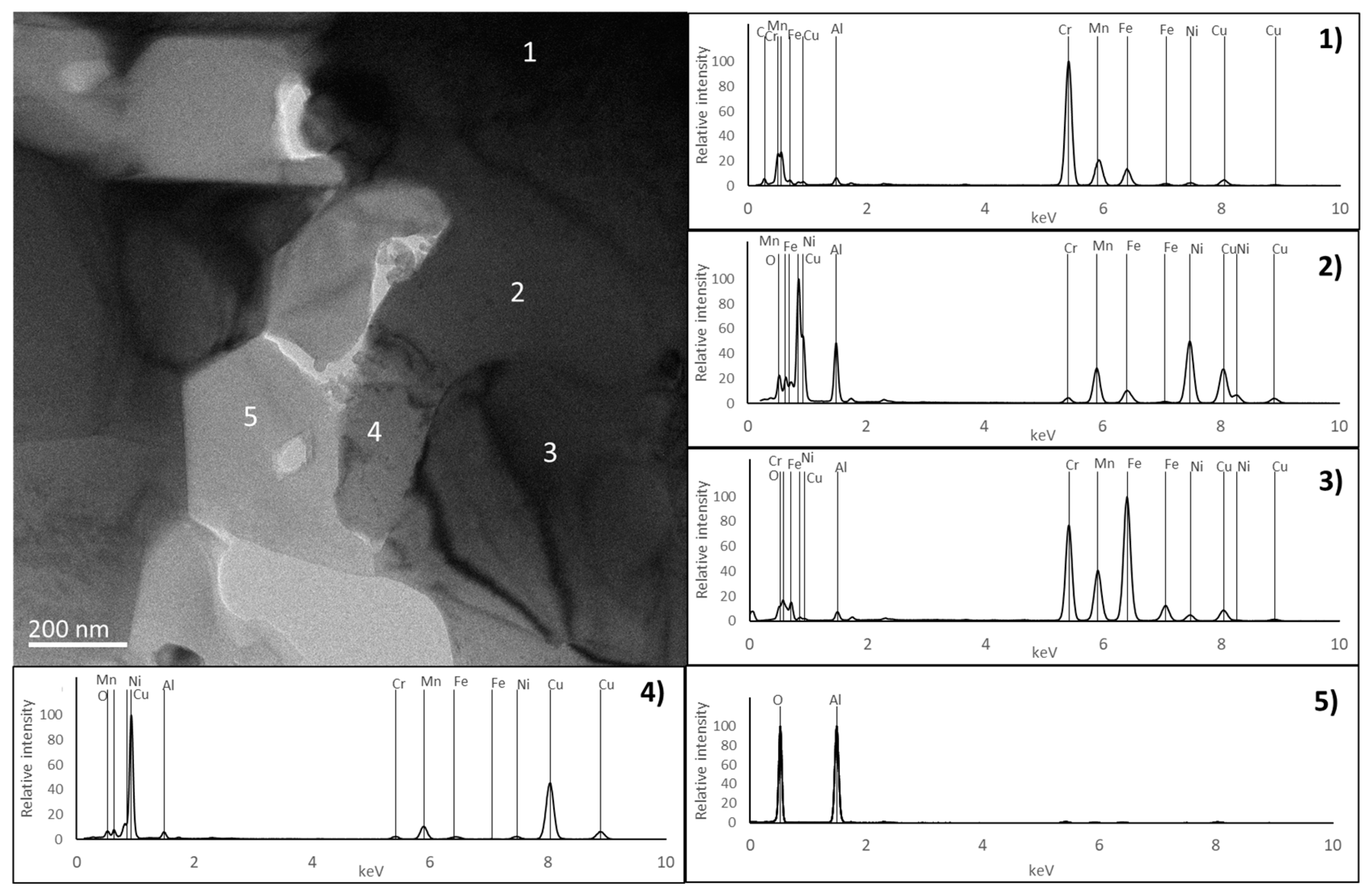
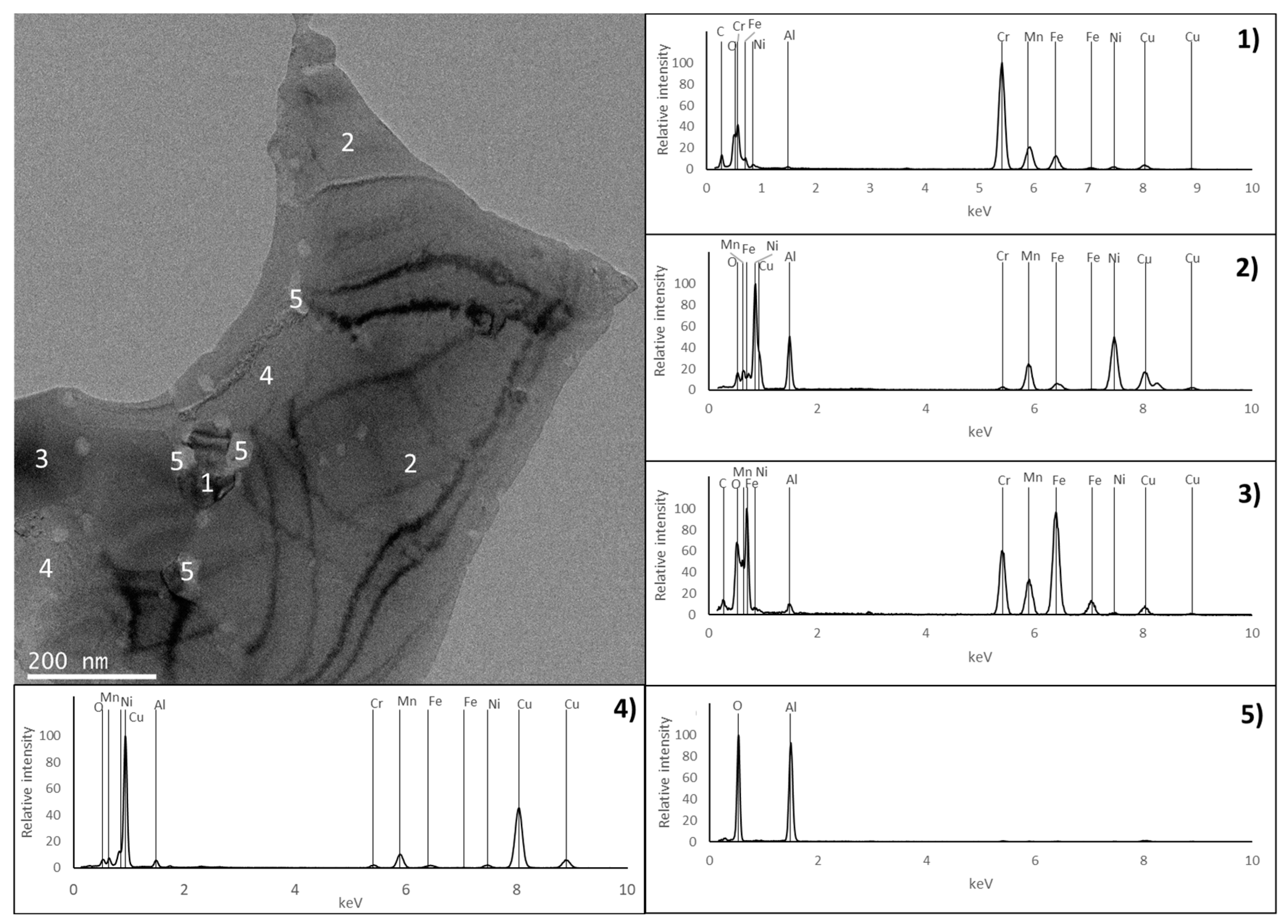
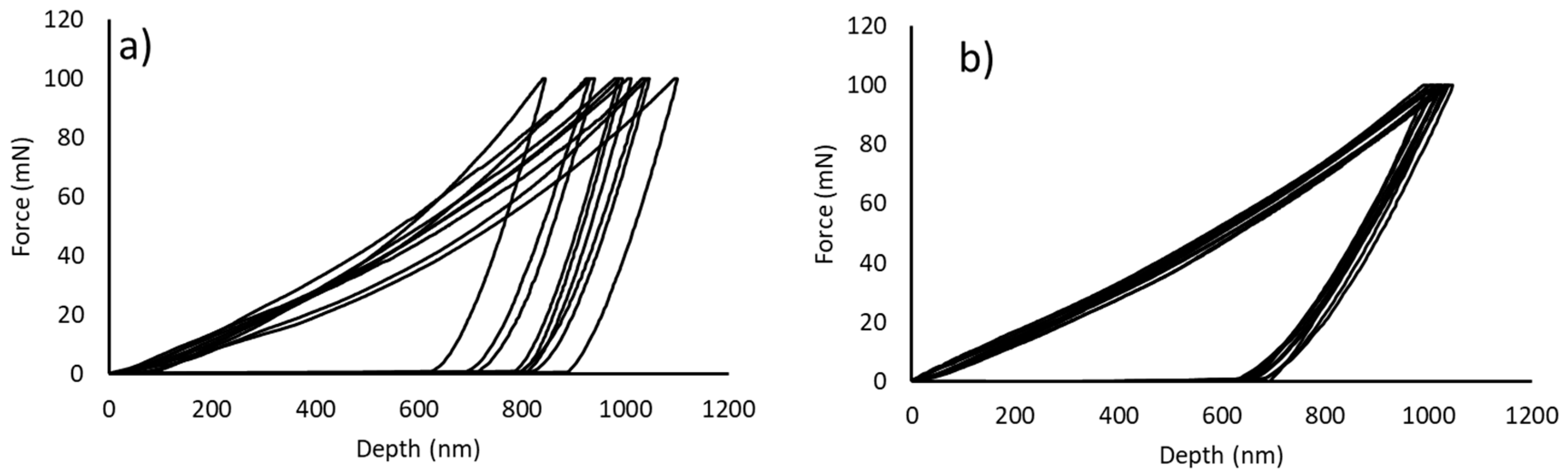
3.2. Characterisation of the Sintered Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys Fundamentals and Applications; Springer: Cham, Switzerland, 2016; pp. 1–115. [Google Scholar]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Gorsse, S.; Miracle, D.B.; Senkov, O.N. Mapping the world of complex concentrated alloys. Acta Mater. 2017, 135, 177–187. [Google Scholar] [CrossRef]
- Gorsse, S.; Couzinié, J.; Miracle, D.B. From high-entropy alloys to complex concentrated alloys. C. R. Phys. 2018, 19, 721–736. [Google Scholar] [CrossRef]
- Qiao, J.W.; Bao, M.L.; Zhao, Y.J.; Yang, H.J.; Wu, Y.C.; Zhang, Y.; Hawk, J.A.; Gao, M.C. Rare-earth high entropy alloys with hexagonal close-packed structure. J. Appl. Phys. 2018, 124, 195101. [Google Scholar] [CrossRef]
- Soare, V.; Mitrica, D.; Constantin, I.; Popescu, G.; Csaki, I.; Tarcolea, M.; Carcea, I. The mechanical and corrosion behaviors of as-cast and re-melted AlCrCuFeMnNi multi-component high-entropy alloy. Metall. Mater. Trans. 2015, 46, 1468–1473. [Google Scholar] [CrossRef]
- Nguyen, T.; Huang, M.; Li, H.; Hong, L.; Yang, S. Effect of Al content on microstructure and mechanical properties of As-cast AlxFeMnNiCrCu0.5 high-entropy alloys. Mater. Sci. Eng. A 2022, 832, 142495. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, X.; Wang, Q.; Liu, T.; Chen, X.; Wang, L.; Hui, X. Microstructure, mechanical properties, and oxidation behavior of AlxCr0.4CuFe0.4MnNi high entropy alloys. Adv. Eng. Mater. 2017, 19, 1600726. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhy, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- Chen, H.Y.; Tsai, C.W.; Tung, C.C.; Yeh, J.W.; Shun, T.T.; Yang, C.C.; Chen, S.K. Effect of the substitution of Co by Mn in Al-Cr-Cu-Fe-Co-Ni high-entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 685–698. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, S.; Mallick, A. Light weight MnTiAlNiFe high-entropy alloy (LWHEA) fabricated by powder metallurgy process: Mechanical, microstructure, and tribological properties. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Suhane, A. Mechanically alloyed high entropy alloys: Existing challenges and opportunities. J. Mater. Res. Technol. 2022, 17, 2431–2456. [Google Scholar] [CrossRef]
- Silva, T.; Lopes, A. Microstructural characterization of AlCrCuFeMnNi complex concentrated alloy prepared by pressureless sintering. Materials 2024, 17, 2378. [Google Scholar] [CrossRef] [PubMed]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S. High-Entropy Alloys; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Sharma, N.; Alam, S.N.; Ray, B.C. Fundamentals of Spark Plasma Sintering (SPS): An Ideal Processing Technique for Fabrication of Metal Matrix Nanocomposites; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Suárez, M.; Fernández, A.; Menéndez, J.L.; Torrecillas, R.; Kessel, H.U.; Hennicke, J.; Kirchner, R.; Kessel, T. Challenges and Opportunities for Spark Plasma Sintering: A Key Technology Form a New Generation of Materials; INTECH Open Access Publisher: London, UK, 2013; Chapter 13. [Google Scholar]
- Ren, B.; Zhao, R.; Jiang, A.; Yu, Y. Microstructure and oxidation behavior of CoCrxCuFeMnNi high-entropy alloys fabricated by vacuum hot-pressing sintering. Micron 2022, 158, 103291. [Google Scholar] [CrossRef]
- Stasiak, T.; Sow, M.A.; Addad, A.; Touzin, M.; Béclin, F.; Cordier, C. Processing and characterization of a mechanically alloyed and hot press sintered high entropy alloy from the Al-Cr-Fe-Mn-Mo family. Miner. Met. Mater. Soc. 2022, 74, 971–980. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, K.; Jiang, S. Microstructure and mechanical properties of Ti–22Al–25Nb alloy fabricated by vacuum hot pressing sintering. Mater. Sci. Eng. A 2014, 616, 93–98. [Google Scholar] [CrossRef]
- Chang, S.; Wang, C.; Huang, K. Influence of the vacuum hot pressing process on the microstructure, mechanical properties, and material characteristics of CrCoNiTa3 alloys. Vacuum 2023, 207, 111637. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, X.; Tang, Q.; Wang, W.; Yan, X.; Dai, P. Microstructure and mechanical properties of FeCoCrNiMnAlx high-entropy alloys prepared by mechanical alloying and hot-pressed sintering. J. Alloys Compd. 2019, 775, 742–751. [Google Scholar] [CrossRef]
- Hasegawa, M. Chapter 3.3—Ellingham Diagram. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 507–516. [Google Scholar]
- Safarian, J.; Engh, T.A. Vacuum Evaporation of Pure Metals. Metall. Mater. Trans. A 2013, 44, 747–753. [Google Scholar] [CrossRef]
- Yuhu, F.; Yunpeng, Z.; Hongyan, G.; Huimin, S.; Li, H. AlNiCrFexMo0.2CoCu high entropy alloys prepared by powder metallurgy. Rare Met. Mater. Eng. 2013, 42, 1127–1129. [Google Scholar] [CrossRef]
- Qiu, X. Microstructure and properties of AlCrFeNiCoCu high entropy alloy prepared by powder metallurgy. J. Alloys Compd. 2013, 555, 246–249. [Google Scholar] [CrossRef]
- Mane, R.; Panigrahi, B. Effect of alloying order on non-isothermal sintering kinetics of mechanically alloyed high entropy alloy powders. Mater. Lett. 2018, 217, 131–134. [Google Scholar] [CrossRef]
- Várez, A.; Levenfeld, B.; Torralba, J.M.; Matula, G.; Dobrzanski, L.A. Sintering in different atmospheres of T15 and M2 high speed steels produced by a modified metal injection moulding process. Mater. Sci. Eng. 2004, 366, 318–324. [Google Scholar] [CrossRef]
- Fang, Z.; Sun, P.; Wang, H. Hydrogen argon sintering of titanium to produce high density fine grain titanium alloys. Adv. Eng. Mater. 2012, 14, 383–387. [Google Scholar] [CrossRef]
- Schaffer, G.B.; Hall, B.J.; Bonner, S.J.; Huo, S.H.; Sercombe, T.B. The effect of the atmosphere and the role of pore filling on the sintering of aluminium. Acta Mater. 2006, 54, 131–138. [Google Scholar] [CrossRef]
- Pieczonka, T.; Schubert, T.; Baunack, S.; Kieback, B. Dimensional behaviour of aluminium sintered in different atmospheres. Mater. Sci. Eng. 2008, 478, 251–256. [Google Scholar] [CrossRef]
- Wolff, M.; Thomas, E.; Michael, D. Sintering of magnesium. Adv. Eng. Mater. 2010, 12, 829–836. [Google Scholar] [CrossRef]
- Yong, H.K.; Ryosuke, O.S.; Hiroshi, N.; Hirobumi, W.; Katsutoshi, O. Removal of oxygen and nitrogen from niobium by external gettering. J. Alloys Compd. 1997, 248, 251–258. [Google Scholar]
- Palka, K.; Pokrowiecki, R.; Krzywicka, M. Titanium for Consumer Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–75. [Google Scholar]
- Christian, G.; Raquel, C.; Herbert, D. The role of oxygen transfer in sintering of low alloy steel powder compacts: A review of the ‘‘Internal Getter’’ Effect. JOM 2016, 68, 920–927. [Google Scholar]
- Hans-Dieter, W.; Wolfgang, G. Fundamentals and principles of potentiometric gas sensors based upon solid electrolytes. Sens. Actuators B Chem. 1991, 4, 365–372. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. Mater. Res. Soc. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Okulov, I.V.; Wendrock, H.; Bönisch, M.; Volegov, A.S.; Calin, M.; Eckert, J.; Dargusch, M.S. Nanoindentation and wear properties of Ti and Ti-TiB composite materials produced by selective laser melting. Mater. Sci. Eng. A 2017, 688, 20–26. [Google Scholar] [CrossRef]
- Powder Diffraction File (PDF) Database 2024; International Centre for Diffraction Data: Newtown Square, PA, USA, 2024.
- Hongxia, W.; Yong, C.; Dongdong, S.; Changfeng, C. Effect of Cr/Mo carbides on corrosion behaviour of Fe_Mn_C twinning induced plasticity steel. Corros. Sci. 2020, 167, 108518. [Google Scholar]
- Bose, A.; German, R.M. Sintering atmosphere effects on tensile properties of heavy alloys. Metall. Trans. A 1988, 19, 2467–2476. [Google Scholar] [CrossRef]
- Broitman, E. Indentation hardness measurements at macro-, micro-, and nanoscale: A critical overview. Tribol. Lett. 2017, 65, 1–18. [Google Scholar] [CrossRef]
- German, R.M. Sintering: From Empirical Observations to Scientific Principles; Elsevier: New York, NY, USA, 2014; pp. 305–350. [Google Scholar]
- Kang, S.L. Densification, Grain Growth, and Microstructure; Elsevier: London, UK, 2005; pp. 67–74. [Google Scholar]
- Kim, W.; Griffith, W.M.; Froes, F.H. Surface oxides in P/M aluminum alloys. J. Met. 1985, 37, 27–33. [Google Scholar] [CrossRef]
- Preisler, D.; Stráský, J.; Janovská, M.; Becker, H.; Harcuba, P.; Janeček, M. Achieving high strength and low Young’s modulus by controlling the betastabilizers content in Ti-Nb-Ta-Zr-O alloys. MATEC Web Conf. 2020, 321, 05013. [Google Scholar] [CrossRef]


| HP | PL (Purified Helium) | PL (90%He + 10%H2) [14] | |
|---|---|---|---|
| Before sintering | |||
| Density (g/cm3) | - | 4.35 (60.1%) | 4.40 (60.8%) |
| After sintering | |||
| Density (g/cm3) | 6.87 (99.7%) | 6.48 (95.9%) | 6.50 (96.1%) |
| Phases | C6Cr23, Al0.88Ni1.12, Cr0.26Fe1.74, Al0.15Cu0.85 and Al2O3 | C6Cr23, Al0.88Ni1.12, Cr0.26Fe1.74, Al0.15Cu0.85 and Al2O3 | C6Cr23, Al0.88Ni1.12, Cr0.26Fe1.74, Al0.15Cu0.85 and Al2O3 |
| Average grain size (µm) | 1.2 | 10.5 | 10.7 |
| Vickers’ hardness HV2 (GPa) | 4.47 ± 0.03 | 3.64 ± 0.04 | 3.63 |
| Nanohardness (GPa) | 6.03 ± 0.16 | 5.17 ± 0.55 | - |
| Young’s modulus (GPa) | 78 ± 4 | 138 ± 11 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.; Simões, P.; Lopes, A. Hot Uniaxial Pressing and Pressureless Sintering of AlCrCuFeMnNi Complex Concentrated Alloy—A Comparative Study. Materials 2024, 17, 5457. https://doi.org/10.3390/ma17225457
Silva T, Simões P, Lopes A. Hot Uniaxial Pressing and Pressureless Sintering of AlCrCuFeMnNi Complex Concentrated Alloy—A Comparative Study. Materials. 2024; 17(22):5457. https://doi.org/10.3390/ma17225457
Chicago/Turabian StyleSilva, Tiago, Pedro Simões, and Augusto Lopes. 2024. "Hot Uniaxial Pressing and Pressureless Sintering of AlCrCuFeMnNi Complex Concentrated Alloy—A Comparative Study" Materials 17, no. 22: 5457. https://doi.org/10.3390/ma17225457
APA StyleSilva, T., Simões, P., & Lopes, A. (2024). Hot Uniaxial Pressing and Pressureless Sintering of AlCrCuFeMnNi Complex Concentrated Alloy—A Comparative Study. Materials, 17(22), 5457. https://doi.org/10.3390/ma17225457






