Interaction and Diffusion Mechanism of Moisture in Power Capacitor Insulating Oil Based on Molecular Simulation
Abstract
1. Introduction
2. Model Construction and Simulation Details
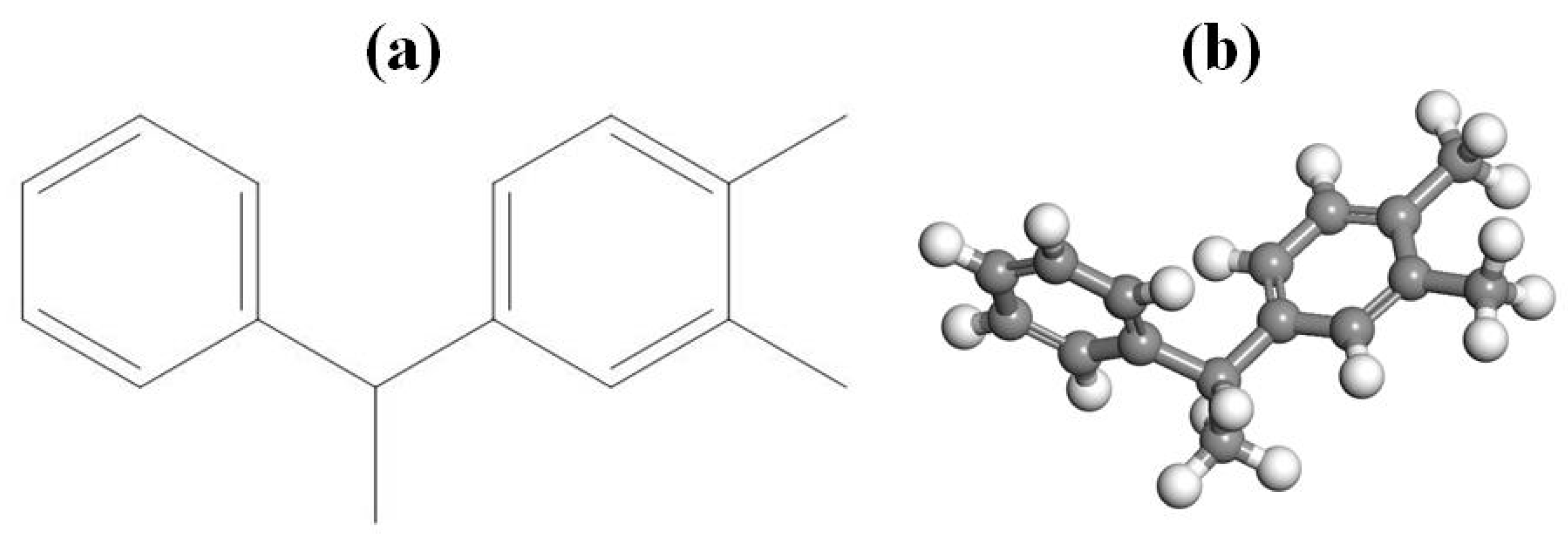
2.1. Model Construction
2.2. Simulation Details
3. Results and Discussion
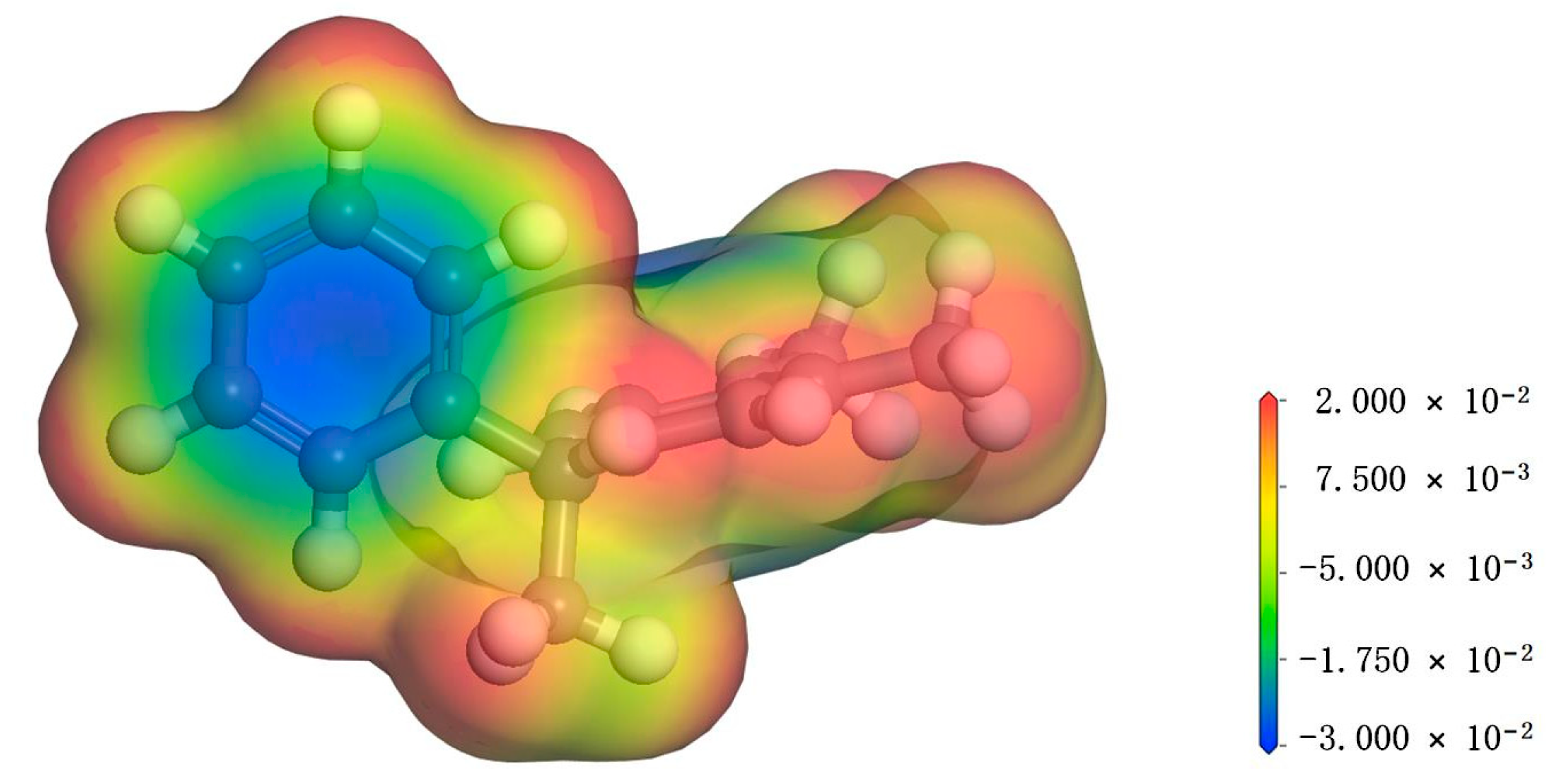
3.1. Electrostatic Potential Analysis of PXE Insulating Oil
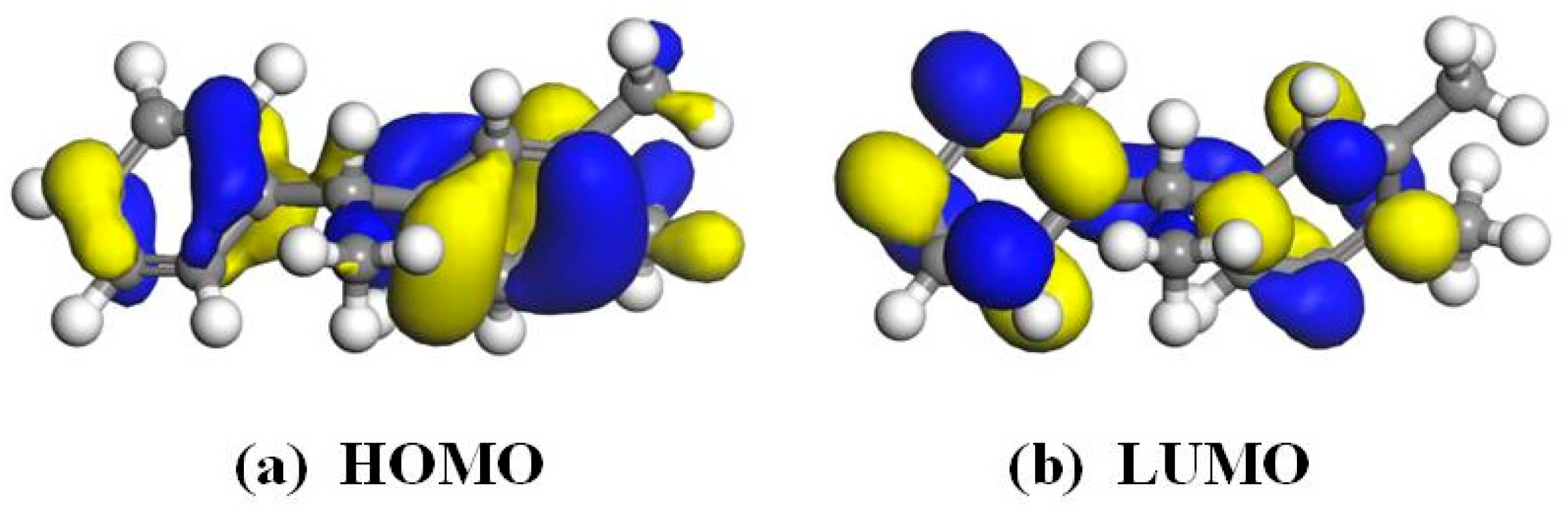
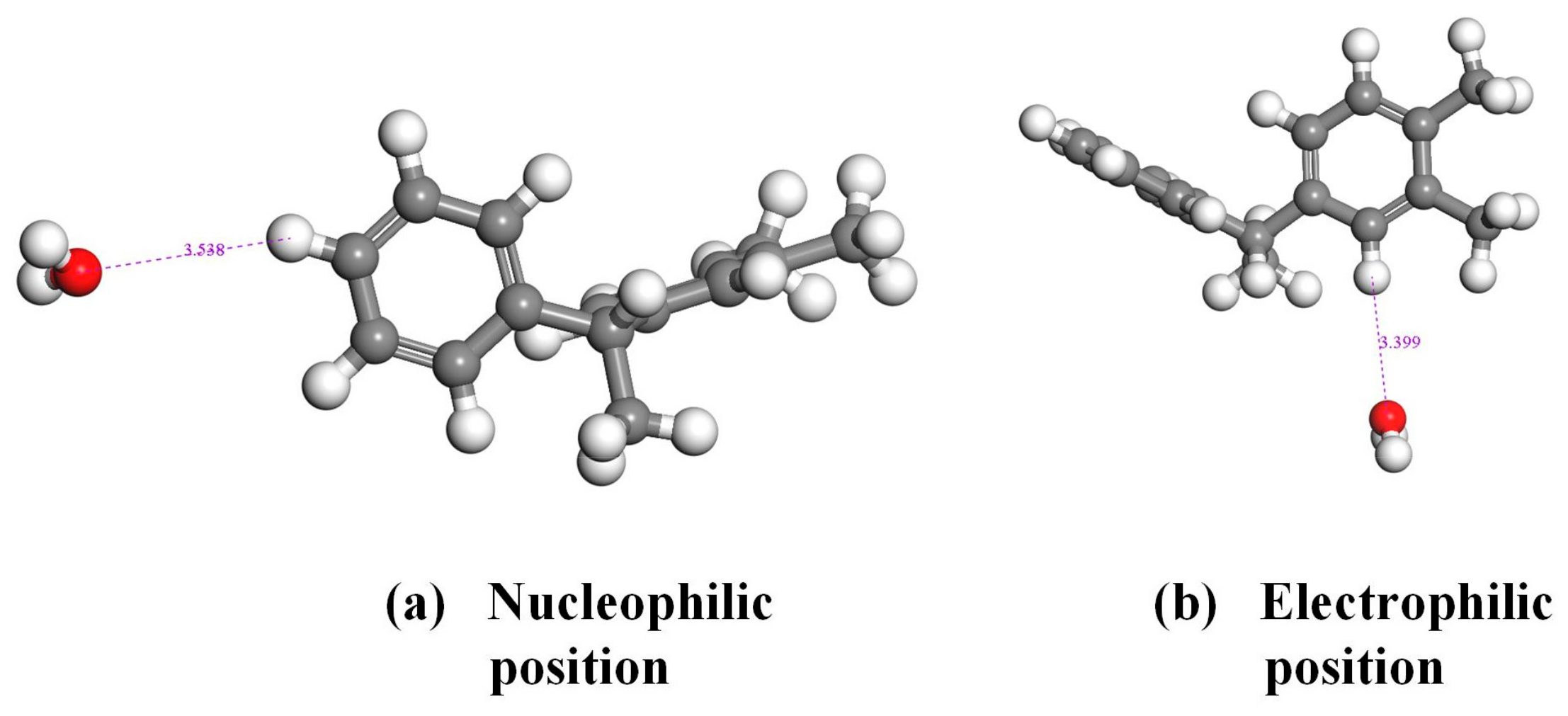
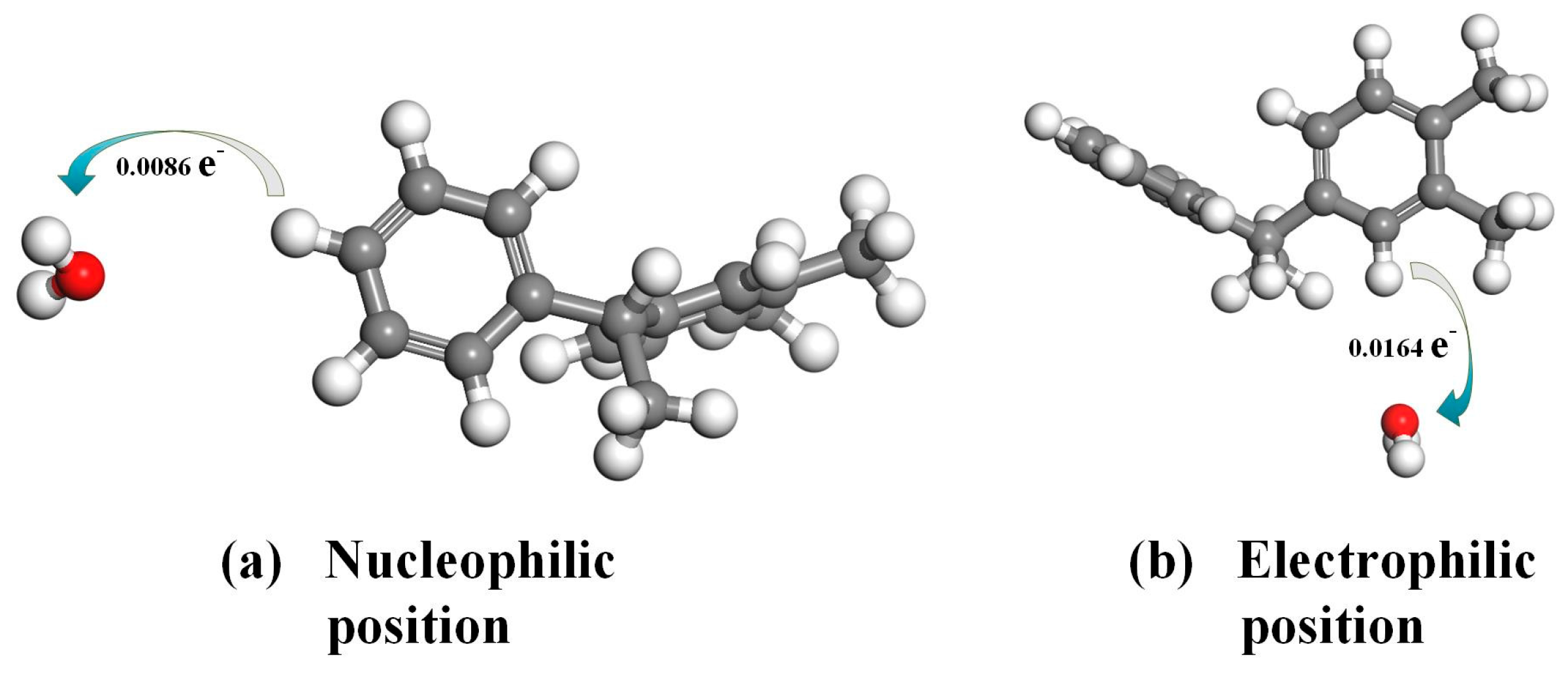
3.2. Frontier Orbit and Fukui Function Analysis of PXE Insulating Oil Molecules
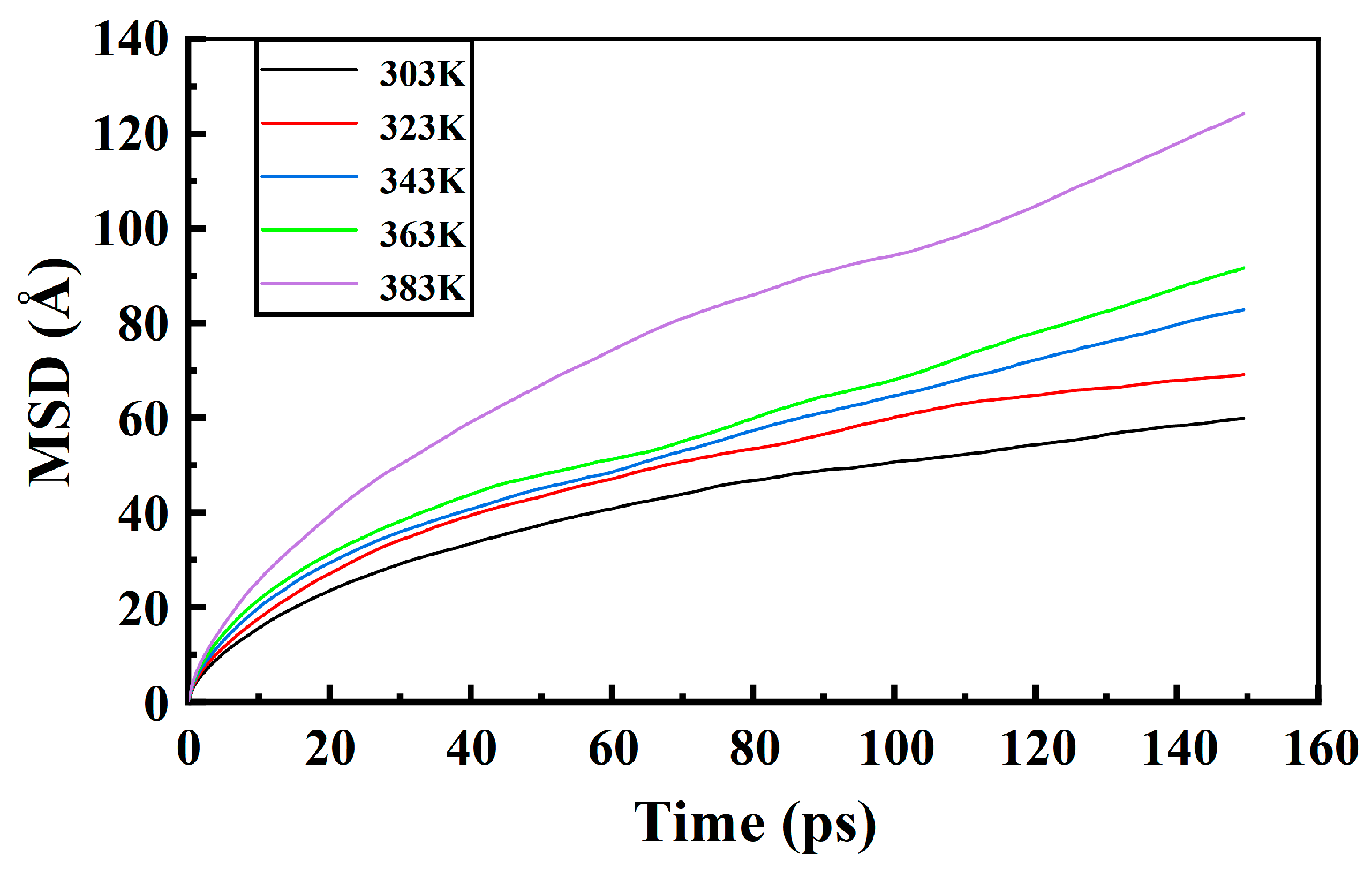
3.3. Diffusion of Moisture in PXE Insulating Oil
3.4. Free Volume of Moisture in PXE Insulating Oil
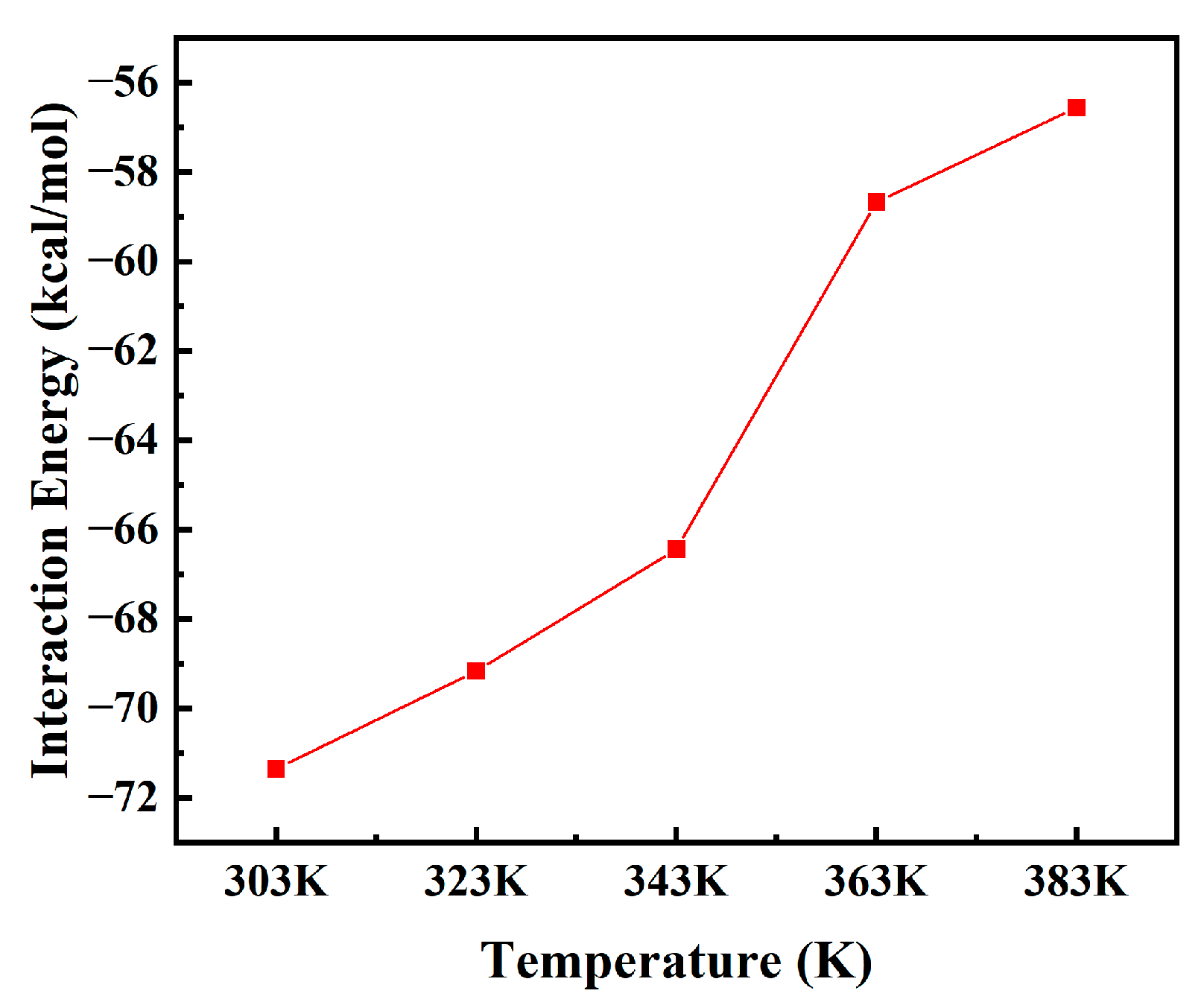
3.5. Interaction Energy Between Moisture and PXE Insulating Oil
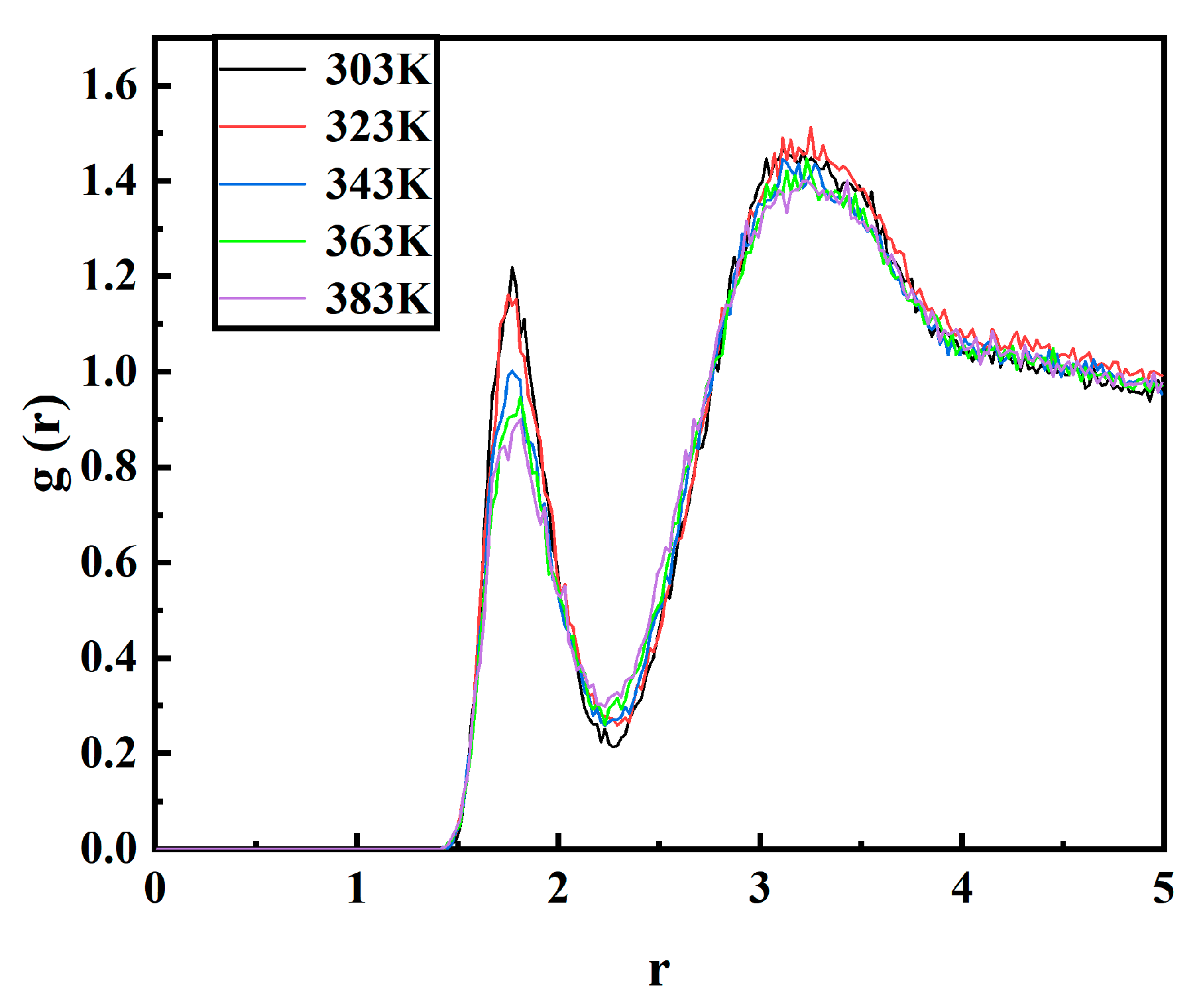
3.6. Radial Distribution Function in PXE Insulating Oil Containing Moisture
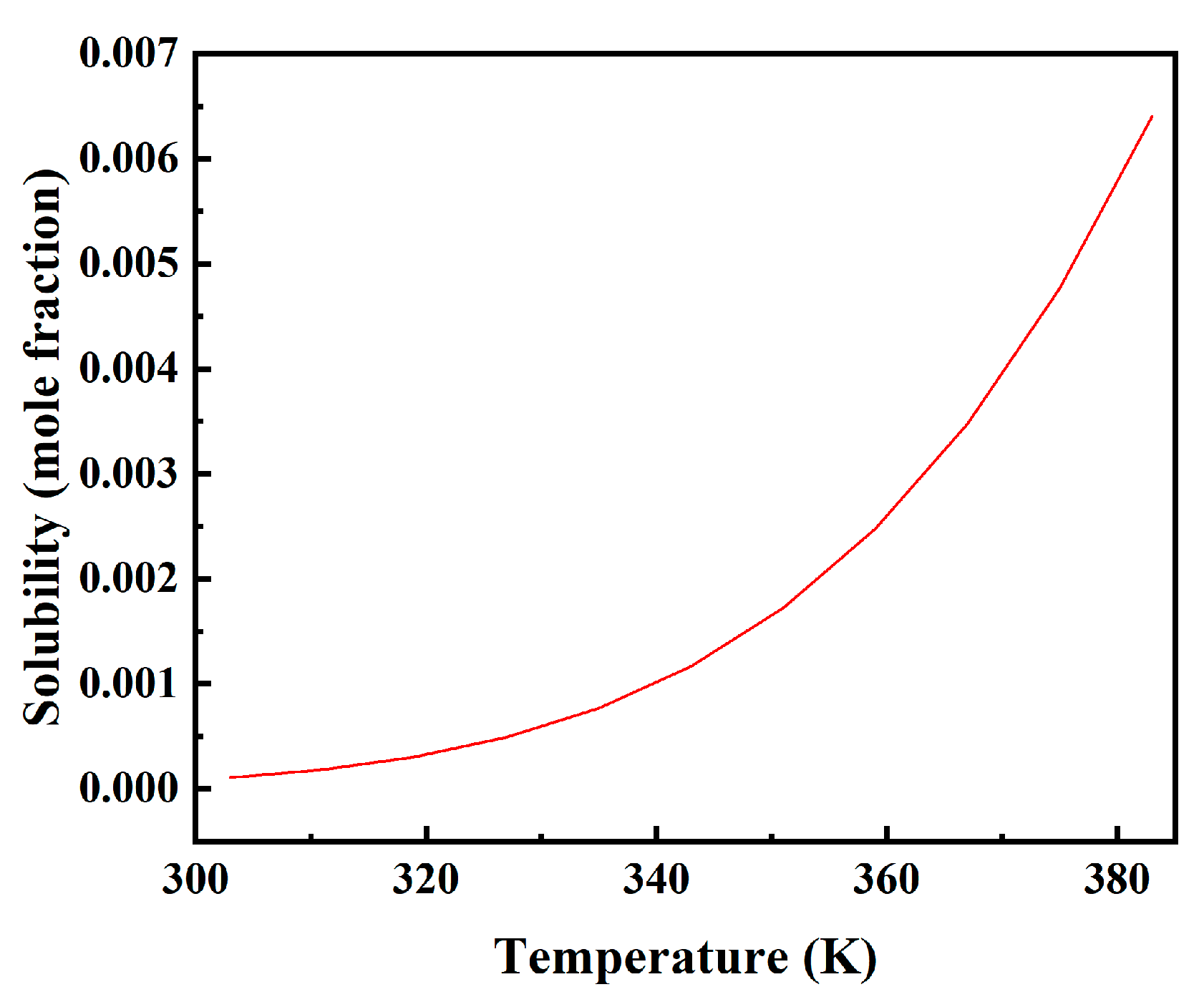
3.7. Prediction of Moisture Solubility in PXE Insulating Oil
4. Conclusions
- (1)
- The hydrogen atoms in the PXE insulating oil molecule exhibit a positive electrostatic potential, with the hydrogen atoms located on the surface of the frontier orbital and Fukui function demonstrating a higher propensity for interaction with water molecules compared with other atoms within the PXE molecule. Notably, hydrogen atoms in nucleophilic and electrophilic positions display an increased likelihood of interacting with water molecules.
- (2)
- The diffusion behavior of water in the insulating oil of the PXE power capacitor is influenced by temperature. As the temperature increases, the affinity between the insulating oil medium and water weakens, leading to a gradual increase in mean square displacement and the free volume fraction of water. Consequently, this enhances the diffusion capability of water within PXE insulating oil.
- (3)
- With the increase in temperature, the radial distribution function and interaction energy exhibit a decrease, while the moisture movement intensity within the insulating oil of the PXE power capacitor shows an increase. Consequently, this results in a reduction in the absolute value of the interaction energy between moisture and the insulating oil of the PXE power capacitor, thereby indicating a weakening of their binding effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gnonhoue, O.G.; Velazquez-Salazar, A.; David, É.; Preda, I. Review of technologies and materials used in high-voltage film capacitors. Polymers 2021, 13, 766. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, Z.; Zhao, L.; Han, S. Power capacitor temperature measurement system using FBG sensors and its validation. IEEE Trans. Instrum. Meas. 2017, 67, 449–458. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, L.; Zhang, X.; Bian, S.; Ran, H.; Yu, C. Study on the failure factors of composite insulation in high-voltage storage capacitors. IEEE Trans. Plasma Sci. 2010, 38, 186–193. [Google Scholar]
- Umran, H.M.; Wang, F.; He, Y. Ageing: Causes and effects on the reliability of polypropylene film used for HVDC capacitor. IEEE Access 2020, 8, 40413–40430. [Google Scholar] [CrossRef]
- Reed, C.; Cichanowskil, S. The fundamentals of aging in HV polymer-film capacitors. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 904–922. [Google Scholar] [CrossRef]
- Shirasaka, Y.; Murase, H.; Okabe, S.; Okubo, H. Cross-sectional comparison of insulation degradation mechanisms and lifetime evaluation of power transmission equipment. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 560–573. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, Q.; Li, H.; See, C.H. Internal insulation condition identification for high-voltage capacitor voltage transformers based on possibilistic fuzzy clustering. Rev. Sci. Instrum. 2020, 91, 014705. [Google Scholar] [CrossRef]
- Sha, X.; Sheng, X.; Zhou, Y.; Wang, B.; Zhu, Z.; Liao, Q.; Liu, Y. Synthesis of P123-Templated and DVB-Cross-linked Meso-macroporous Poly (ionic liquids) with High-Performance Alkylation. Appl. Organomet. Chem. 2020, 34, e5460. [Google Scholar] [CrossRef]
- Shi, M.; Pang, Z.; Li, Y.; Qin, R. Microscopic Properties of Capacitor Insulating Oil under External Electric Field Based on Density Functional Theory. Int. J. Polym. Sci. 2024, 2024, 4942347. [Google Scholar] [CrossRef]
- Fofana, I. 50 years in the development of insulating liquids. IEEE Electr. Insul. Mag. 2013, 29, 13–25. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, H.; Wang, B. Structure–Activity Relationship Models to Predict Properties of the Dielectric Fluids for Transformer Insulation System. Int. J. Mol. Sci. 2024, 25, 6654. [Google Scholar] [CrossRef]
- Liu, H.; Claeys, T.; Pissoort, D.; Vandenbosch, G.A. Prediction of capacitor’s accelerated aging based on advanced measurements and deep neural network techniques. IEEE Trans. Instrum. Meas. 2020, 69, 9019–9027. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Wang, Y.; Lin, F. Dependence of polarization behaviors on electrical and thermal aging for pulse metallized polypropylene film capacitor. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1553–1562. [Google Scholar] [CrossRef]
- Lv, C.; Liu, J.; Zhang, Y.; Yin, J.; Cao, R.; Li, Y.; Liu, X. A method to characterize the shrinking of safe operation area of metallized film capacitor considering electrothermal coupling and aging in power electronics applications. IEEE Trans. Ind. Electron. 2022, 70, 1993–2002. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, B.; Li, L.; Liu, X.; Wen, L.; Song, Y.; Shen, H.; Li, M.; Li, X.; Wu, D. A review of methods for measuring oil moisture. Measurement 2023, 217, 113119. [Google Scholar] [CrossRef]
- Arakelian, V.G.; Fofana, I. Water in oil-filled, high-voltage equipment, Part I: States, solubility, and equilibrium in insulating materials. IEEE Electr. Insul. Mag. 2007, 23, 15–27. [Google Scholar] [CrossRef]
- Li, Y.; Pang, Z.; Zheng, H.; Huang, J.; Huang, B.; Shi, M. Electrical Properties of CF3SO2F Insulating Gas based on Density Functional Theory. IEEE Trans. Dielectr. Electr. Insul. 2023, 31, 297–303. [Google Scholar] [CrossRef]
- Pang, Z.; Li, Y.; Zheng, H.; Qin, R. Microscopic Mechanism of Electrical Aging of PVDF Cable Insulation Material. Polymers 2023, 15, 1286. [Google Scholar] [CrossRef]
- Fu, L.; Li, H.; Deng, Y.; Tang, C. The influence of small molecule products on natural ester impregnated cellulose insulation paper under electric-thermal coupling field. Colloids Surf. A Physicochem. Eng. Asp. 2024, 697, 134426. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Yang, T.; Huang, Z.; Qin, J.; Hu, X.; Tong, X.; Li, J. Investigation on Cold Flow Properties of Natural Ester Insulating Oils Based on Molecular Dynamics. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 1323–1330. [Google Scholar] [CrossRef]
- Liao, R.-J.; Zhu, M.-Z.; Yang, L.-J.; Zhou, X.; Gong, C.-Y. Molecular dynamics study of water molecule diffusion in oil–paper insulation materials. Phys. B Condens. Matter 2011, 406, 1162–1168. [Google Scholar] [CrossRef]
- Tian, W.; Tang, C.; Wang, Q.; Zhang, S.; Yang, Y. The effect and associate mechanism of nano SiO2 particles on the diffusion behavior of water in insulating oil. Materials 2018, 11, 2373. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Tian, W.; Yang, L.; Ding, Z.; Zhang, J.; Tang, C. Simulation effect of SiO2 nanoparticles on the water molecules diffusion inside insulating oil at different temperatures. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125738. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, C.; Qiu, Q.; Yang, L. Effect of water on the diffusion of small molecular weight acids in nano-SiO2 modified insulating oil. J. Mol. Liq. 2020, 314, 113670. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhang, J.; Yang, L.; Zhang, J.; Chen, B.; Tang, C. Simulation of the diffusion behavior of water molecules in palm oil and mineral oil at different temperatures. Renew. Energy 2021, 174, 909–917. [Google Scholar] [CrossRef]
- Zazeri, G.; Povinelli, A.P.R.; Le Duff, C.S.; Tang, B.; Cornelio, M.L.; Jones, A.M. Synthesis and spectroscopic analysis of piperine-and piperlongumine-inspired natural product scaffolds and their molecular docking with IL-1β and NF-κB proteins. Molecules 2020, 25, 2841. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Grzybowski, S.; Liu, Y. Characteristics of moisture diffusion in vegetable oil-paper insulation. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1650–1656. [Google Scholar] [CrossRef]
- Fox, T.G., Jr.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Arab, B. The effect of cross linking density on the mechanical properties and structure of the epoxy polymers: Molecular dynamics simulation. J. Mol. Model. 2013, 19, 3719–3731. [Google Scholar] [CrossRef]



| Temperature | 303 K | 323 K | 343 K | 363 K | 383 K |
|---|---|---|---|---|---|
| D (×10−4 cm2/s) | 0.03172804 | 0.03862912 | 0.06193554 | 0.07656594 | 0.08961657 |
| Temperature | VF | VO | FFV/% |
|---|---|---|---|
| 303 K | 6089.42 | 30,450.09 | 16.67 |
| 323 K | 6112.10 | 30,427.41 | 16.73 |
| 343 K | 6228.34 | 30,311.16 | 17.05 |
| 363 K | 6254.16 | 30,285.35 | 17.12 |
| 383 K | 6316.28 | 30,223.23 | 17.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Pang, Z.; Qin, R.; Huang, J.; Li, Y. Interaction and Diffusion Mechanism of Moisture in Power Capacitor Insulating Oil Based on Molecular Simulation. Materials 2024, 17, 5180. https://doi.org/10.3390/ma17215180
Wei C, Pang Z, Qin R, Huang J, Li Y. Interaction and Diffusion Mechanism of Moisture in Power Capacitor Insulating Oil Based on Molecular Simulation. Materials. 2024; 17(21):5180. https://doi.org/10.3390/ma17215180
Chicago/Turabian StyleWei, Changyou, Zhiyi Pang, Rui Qin, Jiwen Huang, and Yi Li. 2024. "Interaction and Diffusion Mechanism of Moisture in Power Capacitor Insulating Oil Based on Molecular Simulation" Materials 17, no. 21: 5180. https://doi.org/10.3390/ma17215180
APA StyleWei, C., Pang, Z., Qin, R., Huang, J., & Li, Y. (2024). Interaction and Diffusion Mechanism of Moisture in Power Capacitor Insulating Oil Based on Molecular Simulation. Materials, 17(21), 5180. https://doi.org/10.3390/ma17215180






