Investigation of Structural, Elastic and Magnetic Properties of CoCr2−xZrxO4 Nanoparticles
Abstract
1. Introduction
2. Experimental Techniques
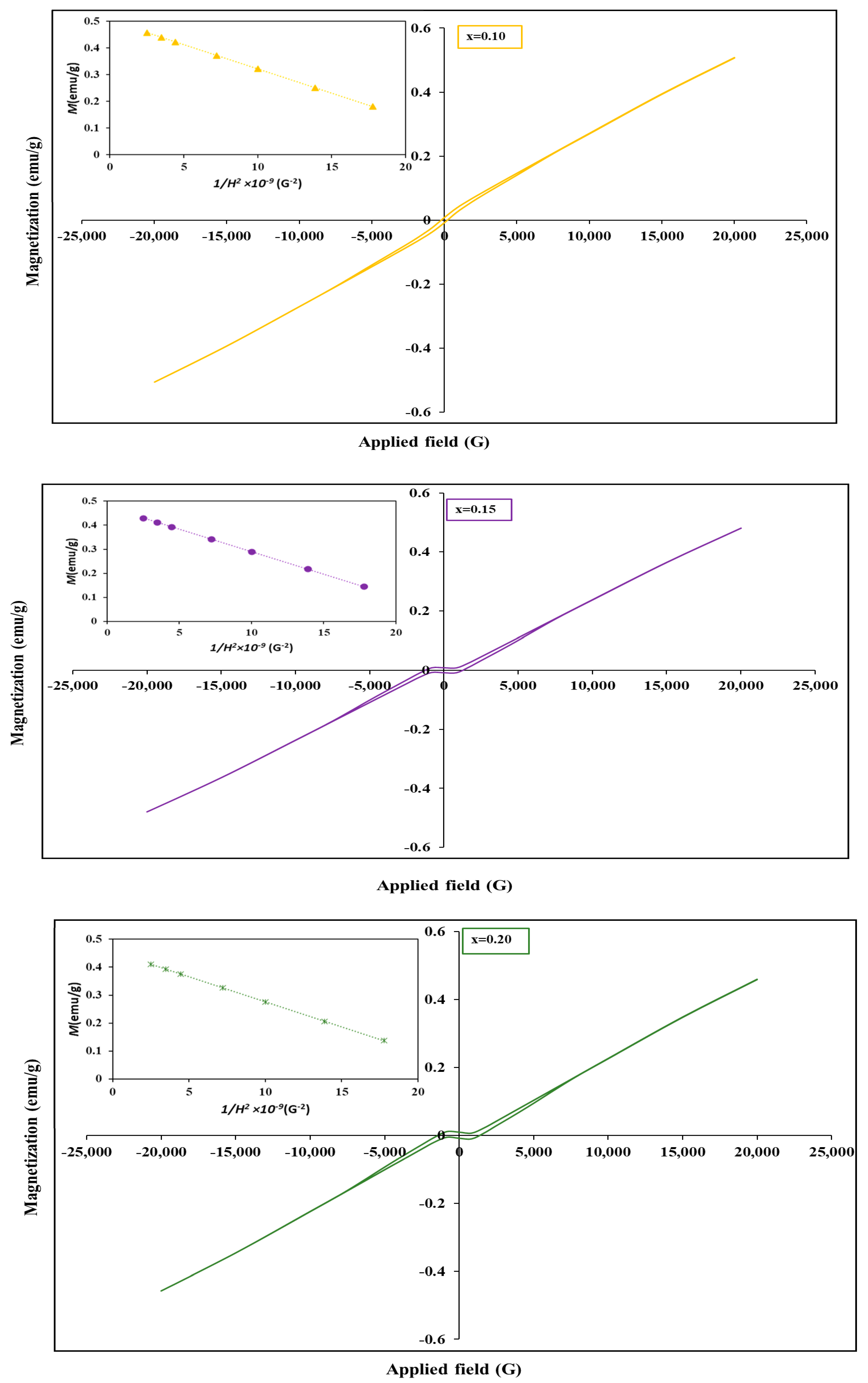
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyons, D.H.; Kaplan, T.A.; Dwight, K.; Menyuk, N. Classical theory of the ground spin-state in cubic spinels. Phys. Rev. B 1962, 126, 540–555. [Google Scholar] [CrossRef]
- van Groenou, A.B.; Bongers, P.F.; Stuyts, A.L. Magnetism, microstructure and crystal chemistry of spinel ferrites. Mater. Sci. Eng. 1969, 3, 317–392. [Google Scholar] [CrossRef]
- Tomiyasu, K.; Fukunaga, J.; Suzuki, H. Magnetic short-range order and reentrant-spin-glass-like behavior in CoCr2O4 and MnCr2O4 by means of neutron scattering and magnetization measurements. Phys. Rev. B—Condens. Matter Mater. Phys. 2004, 70, 214434. [Google Scholar] [CrossRef]
- Lawes, G.; Melot, B.; Page, K.; Ederer, C.; Hayward, M.A.; Proffen, T.; Seshadri, R. Dielectric anomalies and spiral magnetic order in CoCr2O4. Phys. Rev. B—Condens. Matter Mater. Phys. 2006, 74, 024413. [Google Scholar] [CrossRef]
- Ishibashi, H.; Yasumi, T. Structural transition of spinel compound NiCr2O4 at ferrimagnetic transition temperature. J. Magn. Magn. Mater. 2007, 310, e610–e612. [Google Scholar] [CrossRef]
- Bush, A.A.; Shkuratov, V.Y.; Kamentsev, K.E.; Cherepanov, V.M. Preparation and X-Ray diffraction, dielectric, and Mössbauer characterization of Co1−xNix Cr2O4 solid solutions. Inorg. Mater. 2013, 49, 296–302. [Google Scholar] [CrossRef]
- Kim, I.; Oh, Y.S.; Liu, Y.; Chun, S.H.; Lee, J.-S.; Ko, K.-T.; Park, J.-H.; Chung, J.-H.; Kim, K.H. Electric polarization enhancement in multiferroic CoCr2O4 crystals with Cr-site mixing. Appl. Phys. Lett. 2009, 94, 042505. [Google Scholar] [CrossRef]
- Choi, Y.J.; Okamoto, J.; Huang, D.J.; Chao, K.S.; Lin, H.J.; Chen, C.T.; Van Veenendaal, M.; Kaplan, F.T.; Cheong, S.W. Thermally or magnetically induced polarization reversal in the multiferroic CoCr2O4. Phys. Rev. Lett. 2009, 102, 067601. [Google Scholar] [CrossRef] [PubMed]
- Köseoğlu, Y.; Baykal, A.; Toprak, M.S.; Gözüak, F.; Başaran, A.C.; Aktaş, B. Synthesis and characterization of ZnFe2O4 magnetic nanoparticles via a PEG-assisted route. J. Alloys Compd. 2008, 462, 209–213. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Siddiquah, M.R. Electrical and magnetic properties of chromium-substituted cobalt ferrite nanomaterials. J. Alloys Compd. 2008, 453, 513–518. [Google Scholar] [CrossRef]
- He, H.Y. Structural and Magnetic Property of Co1−x Nix Fe2O4 Nanoparticles Synthesized by Hydrothermal Method. Int. J. Appl. Ceram. Technol. 2014, 11, 626–636. [Google Scholar] [CrossRef]
- Zakrzewska, K. Mixed oxides as gas sensors. Thin Solid Films 2001, 391, 229–238. [Google Scholar] [CrossRef]
- Kim, B.-N.; Hiraga, K.; Morita, K.; Sakka, Y. A high-strain-rate superplastic ceramic. Nature 2001, 413, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Galdikas, A.; Martūnas, Z.; Šetkus, A. SnInO-based chlorine gas sensor. Sens. Actuators B Chem. 1992, 7, 633–636. [Google Scholar] [CrossRef]
- Reddy CV, G.; Manorama, S.V.; Rao, V.J. Semiconducting gas sensor for chlorine based on inverse spinel nickel ferrite. Sens. Actuators B Chem. 1999, 55, 90–95. [Google Scholar] [CrossRef]
- Durrani, S.K.; Hussain, S.Z.; Saeed, K.; Khan, Y.; Arif, M.; Ahmed, N. Hydrothermal synthesis and characterization of nanosized transition metal chromite spinels. Turk. J. Chem. 2012, 36, 111–120. [Google Scholar] [CrossRef]
- Dutta, D.P.; Manjanna, J.; Tyagi, A.K. Magnetic properties of sonochemically synthesized CoCr2O4 nanoparticles. J. Appl. Phys. 2009, 106, 043915. [Google Scholar] [CrossRef]
- Li, S.; Zhao, G.; Bi, H.; Huang, Z.; Lai, H.; Gai, R.; Du, Y. Synthesis and anomalous magnetic properties of CoCr2O4 nanocrystallites with lattice distortion. J. Magn. Magn. Mater. 2006, 305, 448–451. [Google Scholar] [CrossRef]
- Li, S.; Bi, H.; Tian, Z.; Xu, F.; Gu, B.; Lu, M.; Du, Y. Surface spin pinning effect of polymer decomposition residues in CoCr2O4 nanocrystallites system. J. Magn. Magn. Mater. 2004, 281, 11–16. [Google Scholar] [CrossRef]
- Rath, C.; Mohanty, P. Magnetic phase transitions in cobalt chromite nanoparticles. J. Supercond. Nov. Magn. 2011, 24, 629–633. [Google Scholar] [CrossRef]
- Hu, D.-S.; Han, A.-J.; Ye, M.-Q.; Chen, H.-H.; Zhang, W. Preparation and Spectroscopy Analysis of Spinel CoCr2−xAlxO4 by Low-temperature Combustion Synthesis. J. Inorg. Mater. 2011, 26, 285–289. [Google Scholar] [CrossRef]
- Singh, R.K.; Yadav, A.; Narayan, A.; Singh, A.K.; Verma, L.; Verma, R.K. Thermal, structural and magnetic studies on chromite spinel synthesized using citrate precursor method and annealed at 450 and 650 °C. J. Therm. Anal. Calorim. 2012, 107, 197–204. [Google Scholar] [CrossRef]
- Cui, H.; Zayat, M.; Levy, D. Sol-gel synthesis of nanoscaled spinels using propylene oxide as a gelation agent. J. Sol-Gel Sci. Technol. 2005, 35, 175–181. [Google Scholar] [CrossRef]
- Das, D.; Biswas, R.; Ghosh, S. Systematic analysis of structural and magnetic properties of spinel CoB2O4 (B = Cr, Mn and Fe) compounds from their electronic structures. J. Phys. Condens. Matter 2016, 28, 446001. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Ullah, A.; Rahman, S.; Tahir, A.; Nadeem, K.; ur Rehman, M.A.; Hussain, S. Structural, magnetic, and dielectric properties of multiferroic Co1−xMgxCr2O4 nanoparticles. J. Magn. Magn. Mater. 2017, 433, 178–186. [Google Scholar] [CrossRef]
- Kumar, N.; Sundaresan, A. On the observation of negative magnetization under zero-field-cooled process. Solid State Commun. 2010, 150, 1162–1164. [Google Scholar] [CrossRef]
- Suchomski, C.; Reitz, C.; Brezesinski, K.; de Sousa, C.T.; Rohnke, M.; Iimura, K.-I.; de Araujo, J.P.E.; Brezesinski, T. Structural, Optical, and Magnetic Properties of Highly Ordered Mesoporous MCr2O4 and MCr2–xFexO4 (M = Co, Zn) Spinel Thin Films with Uniform 15 nm Diameter Pores and Tunable Nanocrystalline Domain Sizes. Chem. Mater. 2012, 24, 155–165. [Google Scholar] [CrossRef]
- Nadeem, K.; Rehman, H.U.; Zeb, F.; Ali, E.; Kamran, M.; Noshahi, N.; Abbas, H. Magnetic phase diagram and dielectric properties of Mn doped CoCr2O4 nanoparticles. J. Alloys Compd. 2020, 832, 155031. [Google Scholar] [CrossRef]
- Kamran, M.; Nadeem, K.; Mumtaz, M. Negative and anomalous T-dependent magnetization trend in CoCr2O4 nanoparticles. Solid State Sci. 2017, 72, 21–27. [Google Scholar] [CrossRef]
- Kumar, G.J.; Rath, C. Study of exchange bias and memory effect in core-shell CoCr2O4 nanoparticles. J. Magn. Magn. Mater. 2018, 466, 69–74. [Google Scholar] [CrossRef]
- Tian, Z.; Zhu, C.; Wang, J.; Xia, Z.; Liu, Y.; Yuan, S. Size dependence of structure and magnetic properties of CoCr2O4 nanoparticles synthesized by hydrothermal technique. J. Magn. Magn. Mater. 2015, 377, 176–182. [Google Scholar] [CrossRef]
- Kavitha, S.; Kurian, M. Effect of zirconium doping in the microstructure, magnetic and dielectric properties of cobalt ferrite nanoparticles. J. Alloys Compd. 2019, 799, 147–159. [Google Scholar] [CrossRef]
- Naik, E.I.; Naik, H.B.; Viswanath, R.; Kirthan, B.R.; Prabhakara, M.C. Effect of zirconium doping on the structural, optical, electrochemical and antibacterial properties of ZnO nanoparticles prepared by sol-gel method. Chem. Data Collect. 2020, 29, 100505. [Google Scholar] [CrossRef]
- Monaji, V.R.; Indla, S.; Rayaprol, S.; Sowmya, S.; Srinivas, A.; Das, D. Temperature dependent magnetic properties of Co1+xTxFe2−2xO4 (T = Zr, Ti). J. Alloys Compd. 2017, 700, 92–97. [Google Scholar] [CrossRef]
- Xu, Q.; Zhan, D.; Huang, D.-P.; Liu, H.-X.; Chen, W.; Zhang, F. Dielectric inspection of BaZr0.2Ti0.8O3 ceramics under bias electric field: A survey of polar nano-regions. Mater. Res. Bull. 2012, 47, 1674–1679. [Google Scholar] [CrossRef]
- Faraz, A. Effect of Concentration of Zr4+ and Ni2+ Dopants on Electrical, Magnetic and Y–K Angle of Mg–Cu Complex Spinel Nanoferrites. J. Supercond. Nov. Magn. 2012, 25, 1055–1063. [Google Scholar] [CrossRef]
- Reda, M.; El-Dek, S.I.; Arman, M.M. Improvement of ferroelectric properties via Zr doping in barium titanate nanoparticles. J. Mater. Sci. Mater. Electron. 2022, 33, 16753–16776. [Google Scholar] [CrossRef]
- Purnamasari, I.; Triyono, D. Effect of zirconium substitution on structural and optical properties of lanthanum orthoferrite. IOP Conf. Ser. Mater. Sci. Eng. 2020, 902, 012031. [Google Scholar] [CrossRef]
- Hashim, M.; Kumar, S.; Shirsath, S.E.; Kotnala, R.K.; Shah, J.; Kumar, R. Influence of Cr3+ ion on the structural, ac conductivity and magnetic properties of nanocrystalline Ni–Mg ferrite. Ceram. Int. 2013, 39, 1807–1819. [Google Scholar] [CrossRef]
- Dawood, M.S.; Elmosalami, T.; Desoky, W. Enhancement of elastic, optical and opto-electrical properties of Ni-Substituted CoFe2O4 nanoparticles with different concentrations. Opt. Mater. 2021, 117, 111101. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Shkir, M.; AlFaify, S. A structural, elastic, mechanical, spectroscopic, thermodynamic, and magnetic properties of polymer coated CoFe2O4 nanostructures for various applications. J. Mol. Struct. 2020, 1205, 127681. [Google Scholar] [CrossRef]
- Babu, B.R.; Tatarchuk, T. Elastic properties and antistructural modeling for nickel-zinc ferrite-aluminates. Mater. Chem. Phys. 2018, 207, 534–541. [Google Scholar] [CrossRef]
- Manjunatha, K.; Jagadeesha Angadi, V.; Srinivasamurthy, K.M.; Matteppanavar, S.; Pattar, V.K.; Mahaboob Pasha, U. Exploring the structural, dielectric and magnetic properties of 5 Mol% Bi3+-substituted CoCr2O4 nanoparticles. J. Supercond. Nov. Magn. 2020, 33, 1747–1757. [Google Scholar] [CrossRef]
- Patil, V.; Shirsath, S.E.; More, S.; Shukla, S.; Jadhav, K. Effect of zinc substitution on structural and elastic properties of cobalt ferrite. J. Alloys Compd. 2009, 488, 199–203. [Google Scholar] [CrossRef]
- Debnath, S.; Das, R. Cobalt doping on nickel ferrite nanocrystals enhances the micro-structural and magnetic properties: Shows a correlation between them. J. Alloys Compd. 2021, 852, 156884. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161. [Google Scholar] [CrossRef]
- Safaan, S.; El Ata, A.A.; El Messeery, M. Study of some structural and magnetic properties of Mn-substituted SrCu hexagonal ferrites. J. Magn. Magn. Mater. 2006, 302, 362–367. [Google Scholar] [CrossRef]
- Akyol, M.; Adanur, I.; Ayaş, A.O.; Ekicibil, A. Magnetic field dependence of magnetic coupling in CoCr2O4 nanoparticles. Phys. B Condens. Matter 2017, 525, 144–148. [Google Scholar] [CrossRef]
- Islam, M.U.; Abbas, T.; Niazi, S.B.; Ahmad, Z.; Sabeen, S.; Chaudhry, M.A. Electrical behaviour of fine particle, co-precipitation prepared Ni–Zn ferrites. Solid State Commun. 2004, 130, 353–356. [Google Scholar] [CrossRef]
- Deraz, N.; Alarifi, A. Preparation and characterization of nano-magnetic Mn0.5Zn0.5Fe2O4 system. Int. J. Electrochem. Sci. 2012, 7, 5828–5836. [Google Scholar] [CrossRef]
- Choudhary, P.; Saxena, P.; Yadav, A.; Sinha, A.K.; Rai, V.N.; Varshney, M.D.; Mishra, A. Weak ferroelectricity and leakage current behavior of multiferroic CoCr2O4 nanomaterials. J. Supercond. Nov. Magn. 2019, 32, 2639–2645. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Fernández, C.D.J.; Sangregorio, C. Exploring the magnetic properties of cobalt-ferrite nanoparticles for the development of a rare-earth-free permanent magnet. Chem. Mater. 2015, 27, 4048–4056. [Google Scholar] [CrossRef]
- Nair, D.S.; Kurian, M. Highly selective synthesis of diphenyl methane via liquid phase benzylation of benzene over cobalt doped zinc nanoferrite catalysts at mild conditions. J. Saudi Chem. Soc. 2019, 23, 127–132. [Google Scholar] [CrossRef]
- El-Said Bakeer, D. Investigation on Optical, Dielectric, and Magnetic Properties of CoAl2−x FexO4 Nanoparticles. J. Supercond. Nov. Magn. 2020, 33, 1789–1801. [Google Scholar] [CrossRef]
- Rikamukti, N.; Purnama, B. Effect of doping Strontium ions in co-precipitated cobalt ferrite. J. Phys. Conf. Ser. 2017, 909, 012012. [Google Scholar] [CrossRef]
- Bitar, Z.; Isber, S.; Noureddine, S.; Bakeer, D.E.-S.; Awad, R. Synthesis, Characterization, Optical Properties, and Electron Paramagnetic Resonance for Nano Zn0.5Co0.5Fe2−xPrxO4. J. Supercond. Nov. Magn. 2017, 30, 3603–3609. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, W.; Hu, R.; Chang, G.; Li, C.; Wang, L. Catalytic activity of spinel oxides MgCr2O4 and CoCr2O4 for methane combustion. Mater. Res. Bull. 2014, 57, 268–273. [Google Scholar] [CrossRef]
- Choudhary, P.; Saxena, P.; Yadav, A.; Rai, V.N.; Mishra, A. Dielectric and ferroelectric properties of CoCr2O4 nanoceramics. J. Adv. Dielectr. 2019, 9, 1950015. [Google Scholar] [CrossRef]
- Khattab, R.; Sadek, H.; Gaber, A. Synthesis of CoxMg1−xAl2O4 nanospinel pigments by microwave combustion method. Ceram. Int. 2017, 43, 234–243. [Google Scholar] [CrossRef]
- Deepty, M.; Srinivas, C.; Kumar, E.R.; Mohan, N.K.; Prajapat, C.L.; Rao, T.C.; Meena, S.S.; Verma, A.K.; Sastry, D.L. XRD, EDX, FTIR and ESR spectroscopic studies of co-precipitated Mn–substituted Zn–ferrite nanoparticles. Ceram. Int. 2019, 45, 8037–8044. [Google Scholar] [CrossRef]
- El-Said Bakeer, D. Elastic study and optical dispersion characterization of Fe-substituted cobalt aluminate nanoparticles. Appl. Phys. A 2020, 126, 443. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Paliychuk, N.; Bououdina, M.; Al-Najar, B.; Pacia, M.; Macyk, W.; Shyichuk, A. Effect of cobalt substitution on structural, elastic, magnetic and optical properties of zinc ferrite nanoparticles. J. Alloys Compd. 2018, 731, 1256–1266. [Google Scholar] [CrossRef]
- El-Ghazzawy, E.H.; Amer, M.A. Structural, elastic and magnetic studies of the as-synthesized Co1−xSrxFe2O4 nanoparticles. J. Alloys Compd. 2017, 690, 293–303. [Google Scholar] [CrossRef]
- Frantsevich, S.A.B.I.N.; Voronov, F.F.; Frant, I.N. Elastic Constants and Elastic Moduli of Metals and Insulators Handbook; Naukova Dumka: Kiev, Ukraine, 1983. [Google Scholar]
- Patange, S.; Shirsath, S.E.; Lohar, K.; Algude, S.; Kamble, S.; Kulkarni, N.; Mane, D.; Jadhav, K. Infrared spectral and elastic moduli study of NiFe2−xCrxO4 nanocrystalline ferrites. J. Magn. Magn. Mater. 2013, 325, 107–111. [Google Scholar] [CrossRef]
- Modi, K.B.; Gajera, J.D.; Pandya, M.P.; Vora, G.; Joshi, H.H. Far-infrared spectral studies of magnesium and aluminum co-substituted lithium ferrites. Pramana 2004, 62, 1173–1180. [Google Scholar] [CrossRef]
- Patange, S.; Shirsath, S.E.; Jadhav, S.; Hogade, V.; Kamble, S.; Jadhav, K. Elastic properties of nanocrystalline aluminum substituted nickel ferrites prepared by co-precipitation method. J. Mol. Struct. 2013, 1038, 40–44. [Google Scholar] [CrossRef]
- Waldron, R.D. Infrared spectra of ferrites. Phys. Rev. B 1955, 99, 1727–1735. [Google Scholar] [CrossRef]
- Anderson, O.L.; Mason, W.P. (Eds.) Physics Acoustics; Academic Press: New York, NY, USA, 1965; Volume 3BC. [Google Scholar]
- Amir; Gungunes, H.; Slimani, Y.; Tashkandi, N.; El Sayed, H.S.; Aldakheel, F.; Sertkol, M.; Sozeri, H.; Manikandan, A.; Ercan, I.; et al. Mössbauer studies and magnetic properties of cubic CuFe2O4 nanoparticles. J. Supercond. Nov. Magn. 2019, 32, 557–564. [Google Scholar] [CrossRef]
- Almessiere, M.; Slimani, Y.; Sertkol, M.; Nawaz, M.; Baykal, A.; Ercan, I. The impact of Zr substituted Sr hexaferrite: Investigation on structure, optic and magnetic properties. Results Phys. 2019, 13, 102244. [Google Scholar] [CrossRef]
- Arora, M.; Chauhan, S.; Sati, P.C.; Kumar, M. Effect of non-magnetic ions substitution on structural, magnetic and optical properties of BiFeO3 nanoparticles. J. Supercond. Nov. Magn. 2014, 27, 1867–1871. [Google Scholar] [CrossRef]
- Culity, B.D.; Graham, C.D. Introduction to Magnetic Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
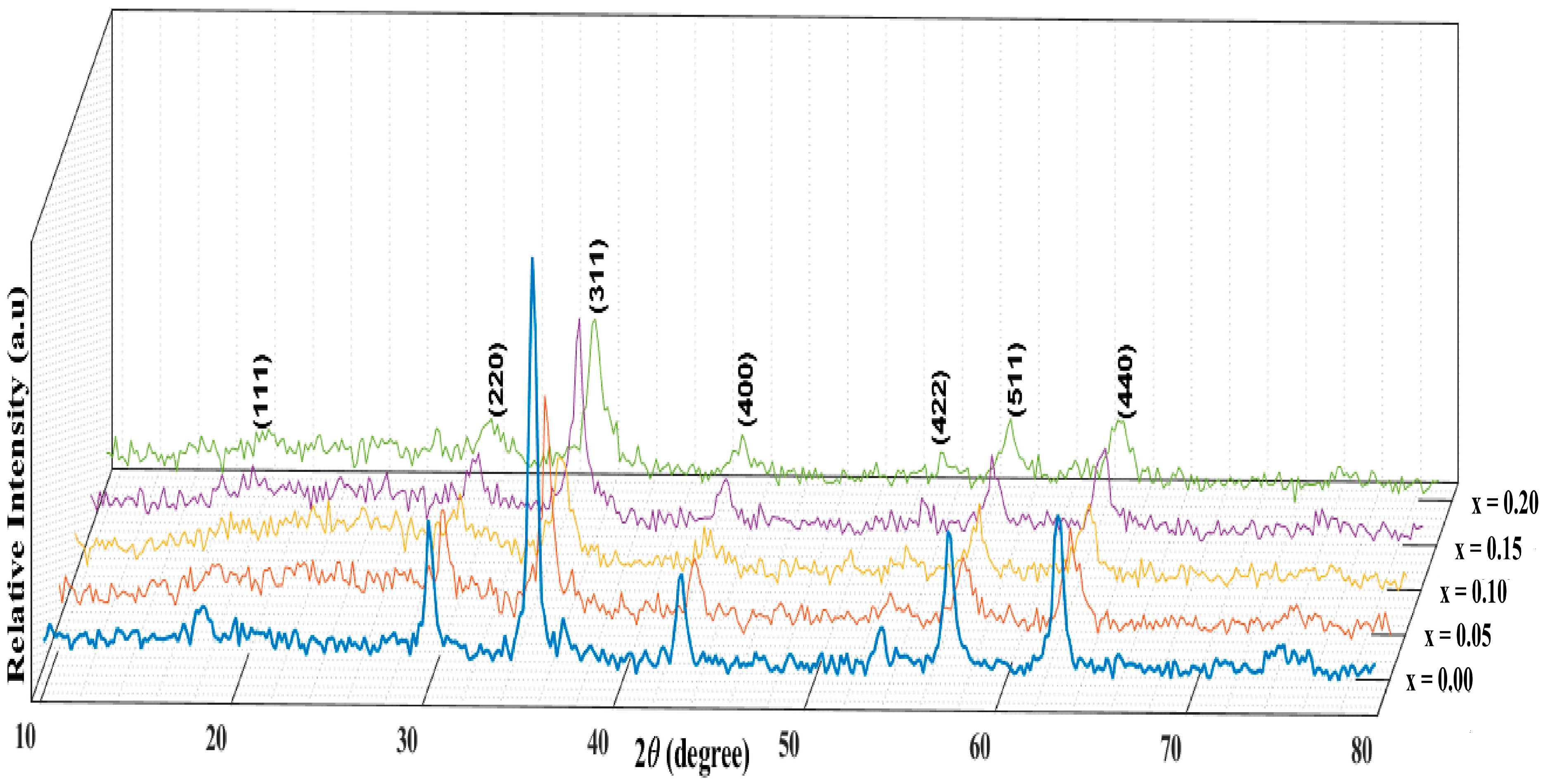
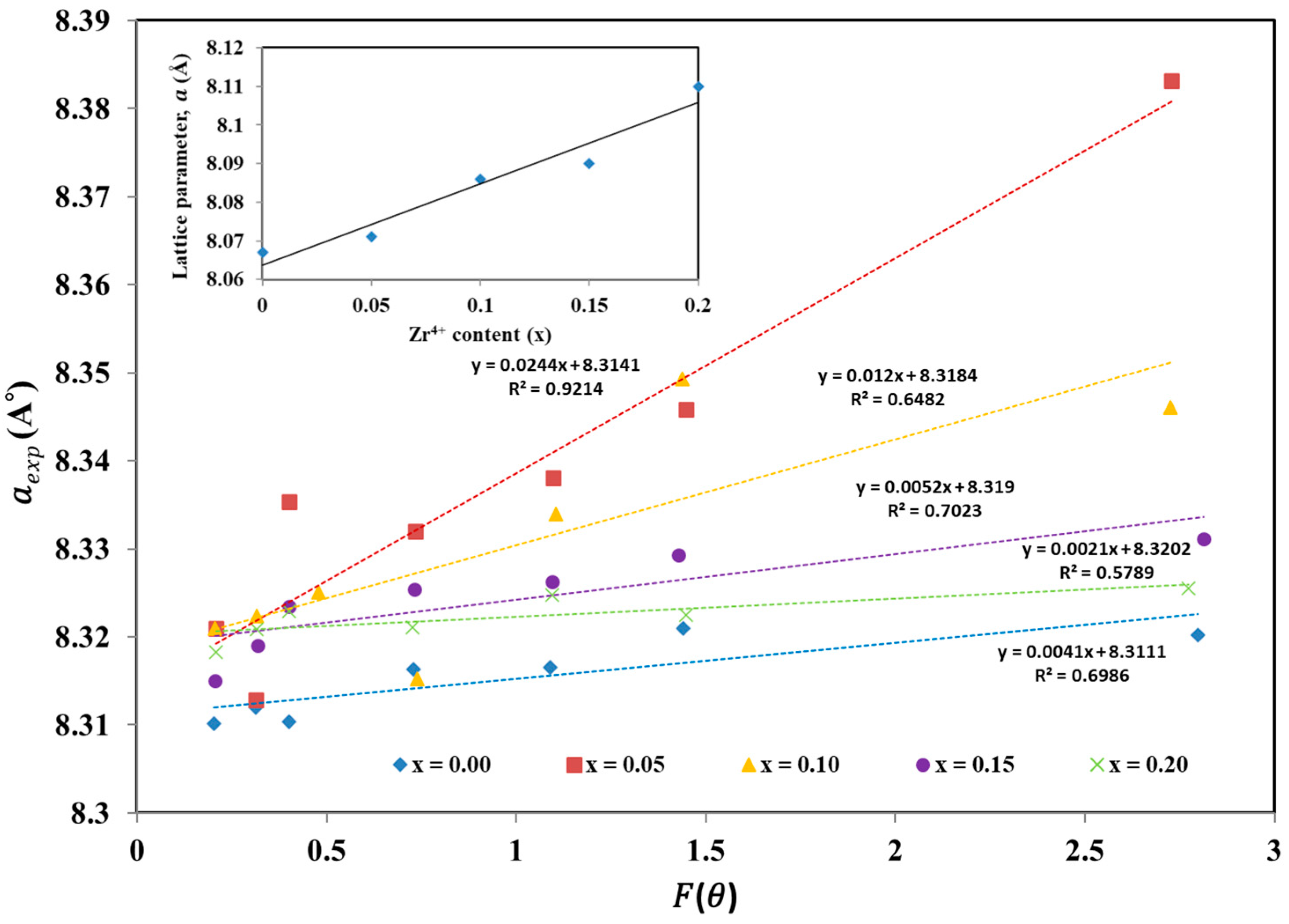
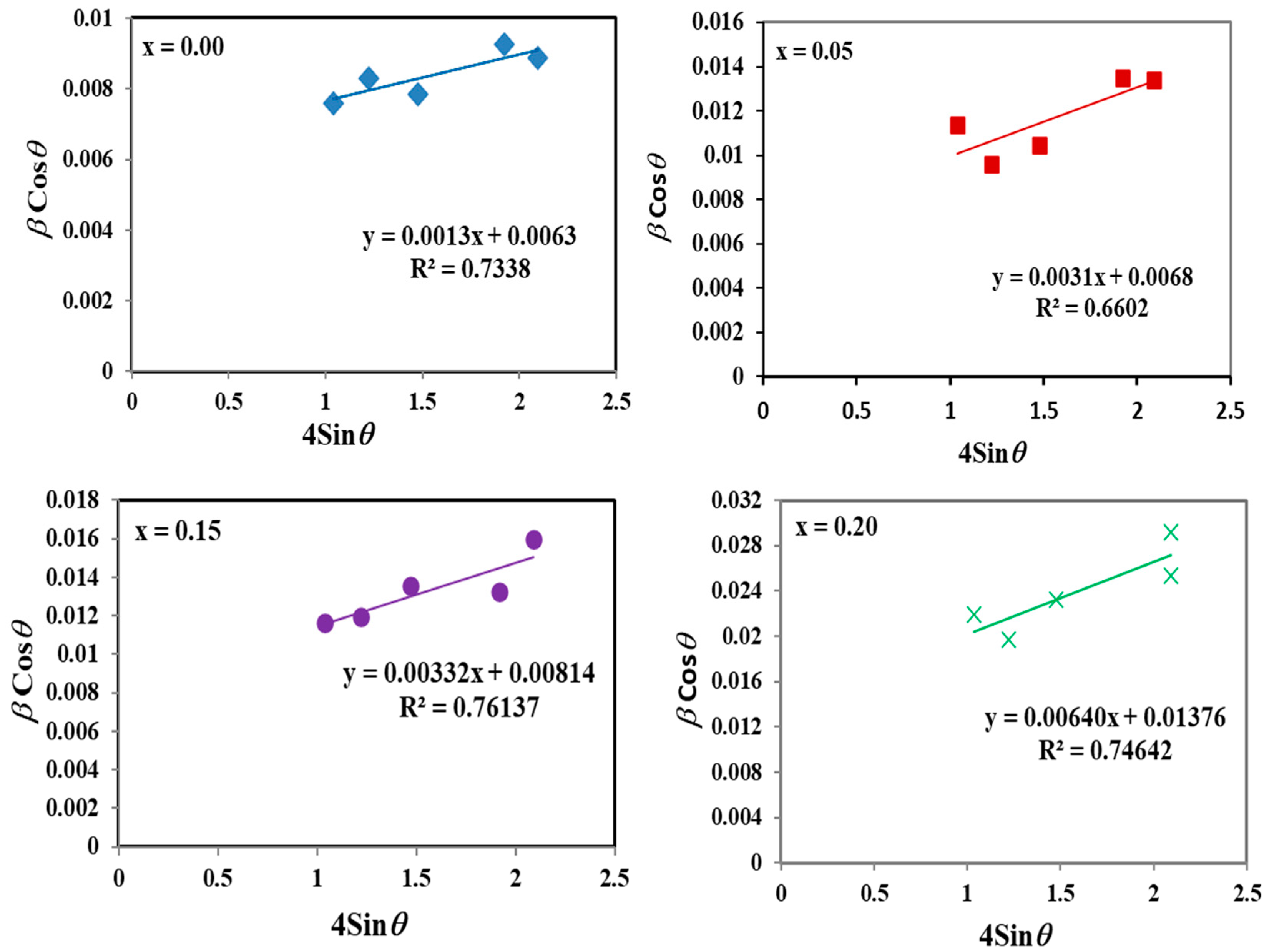
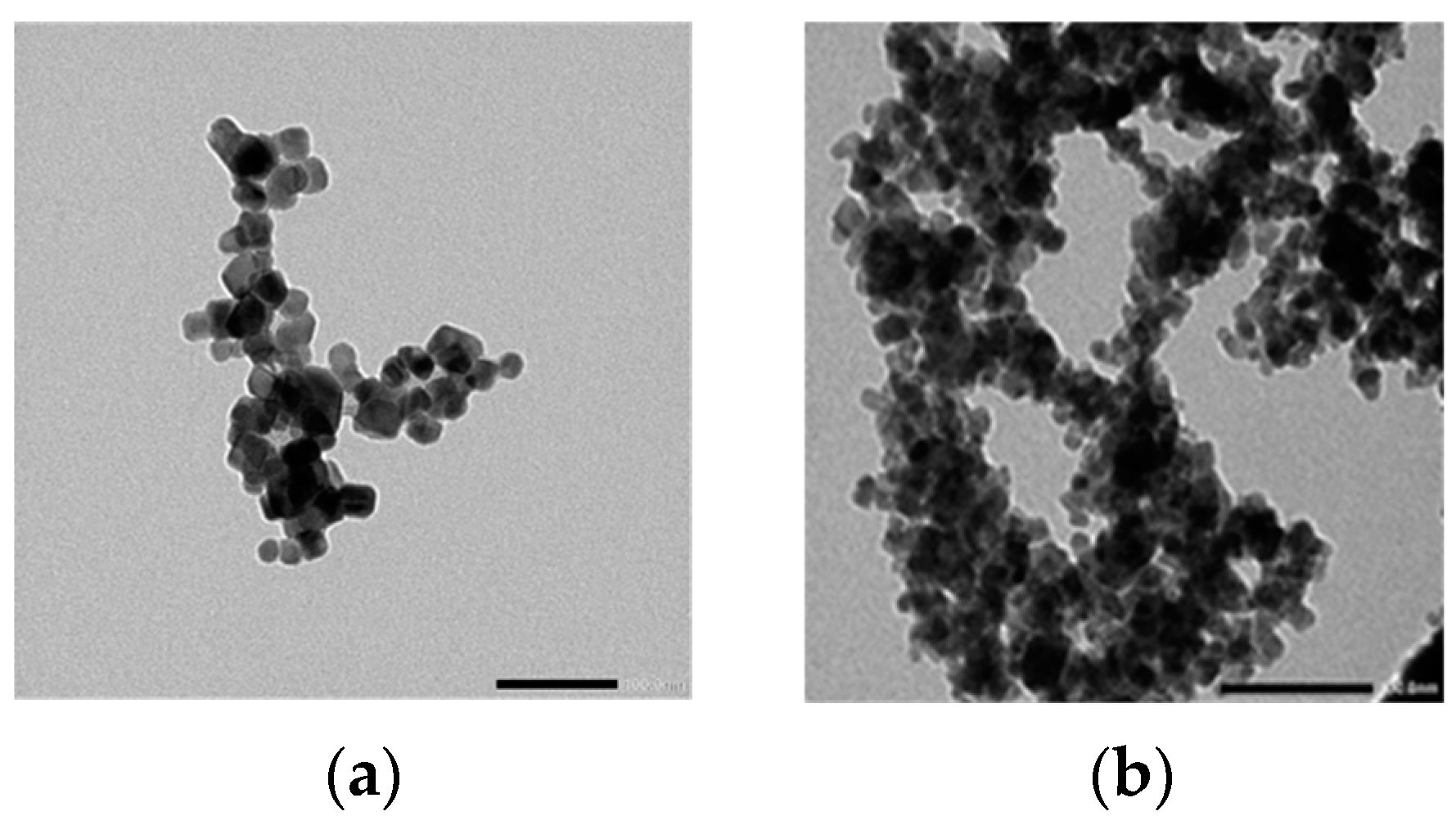

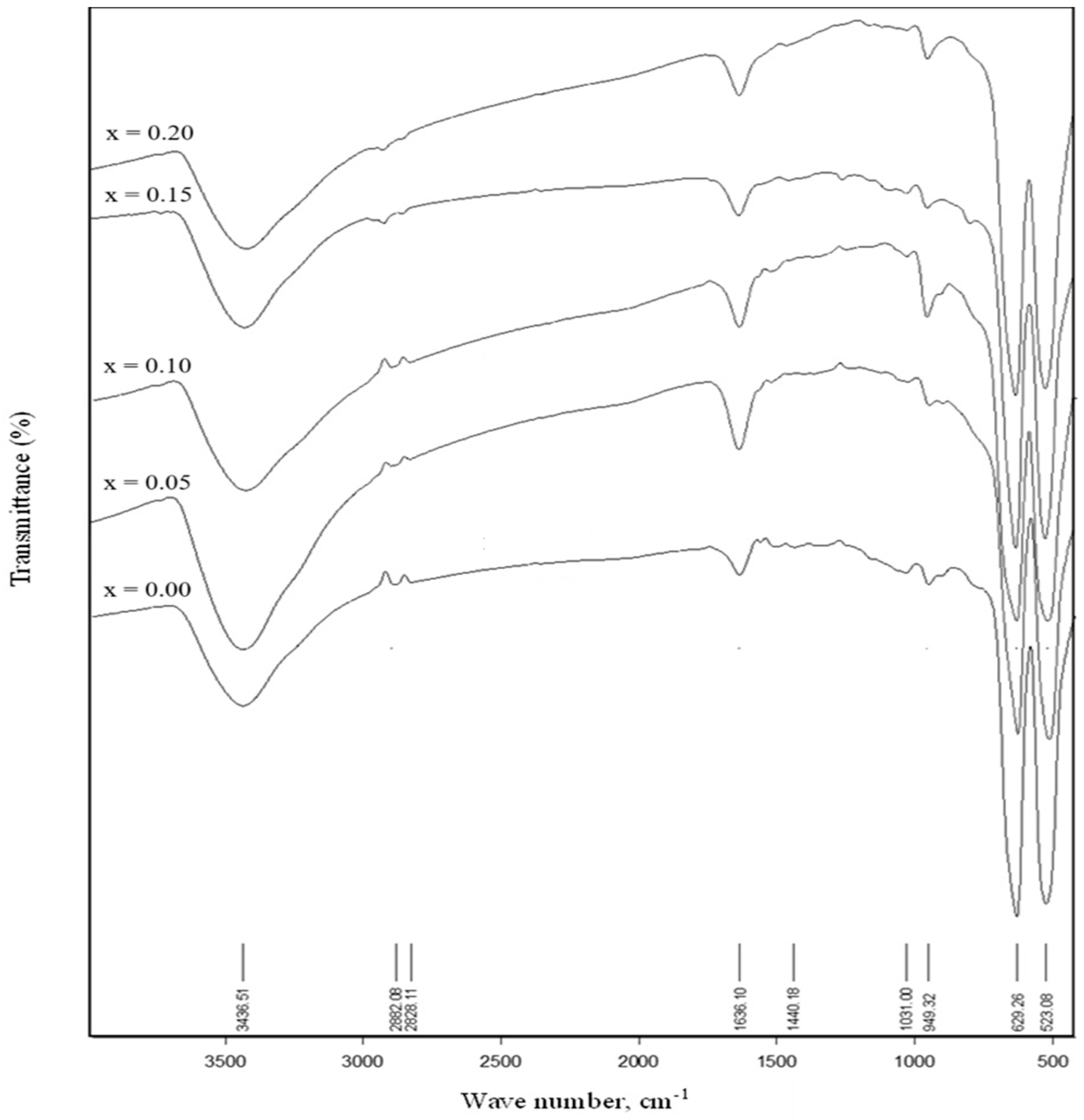

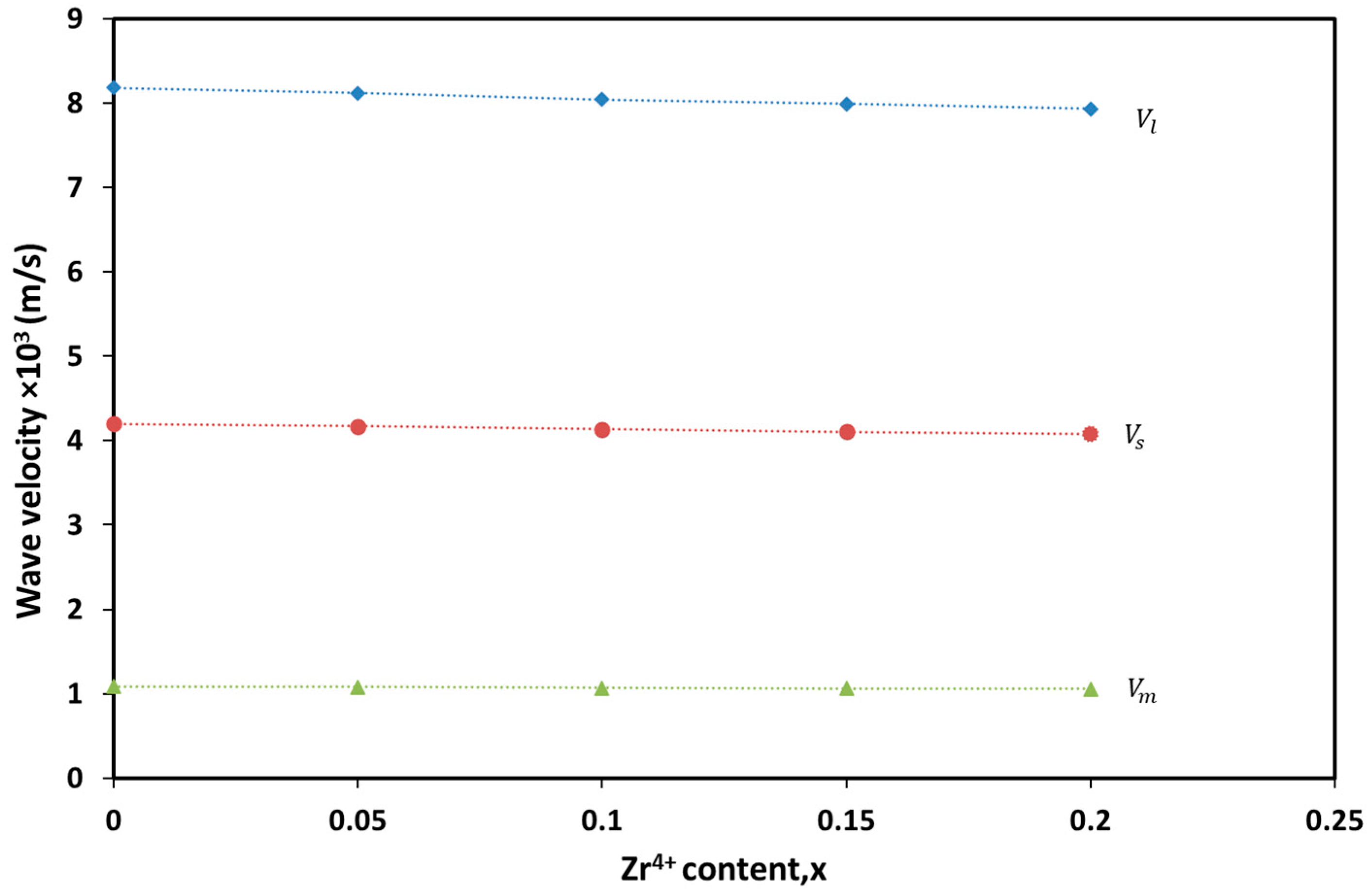
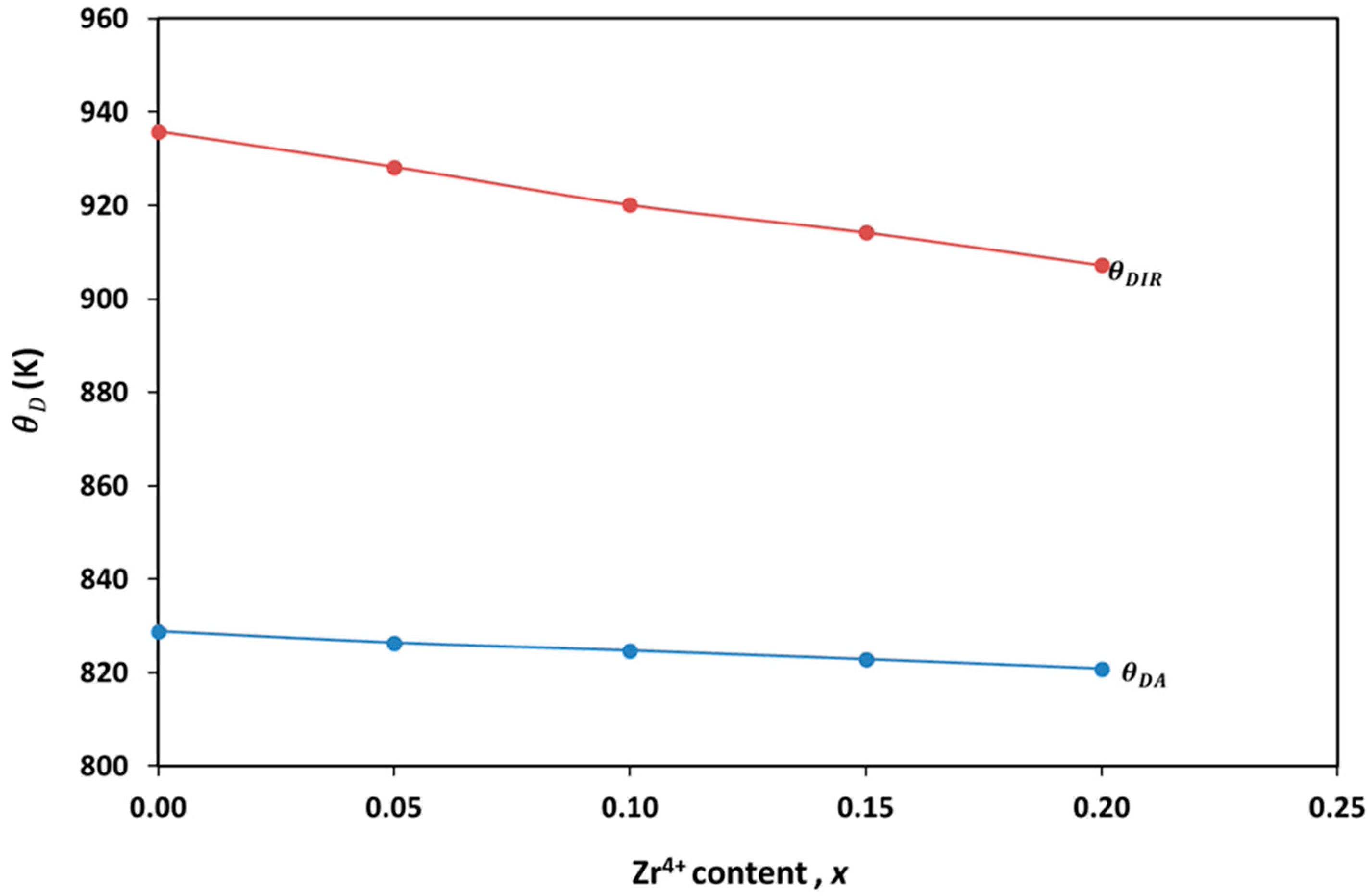
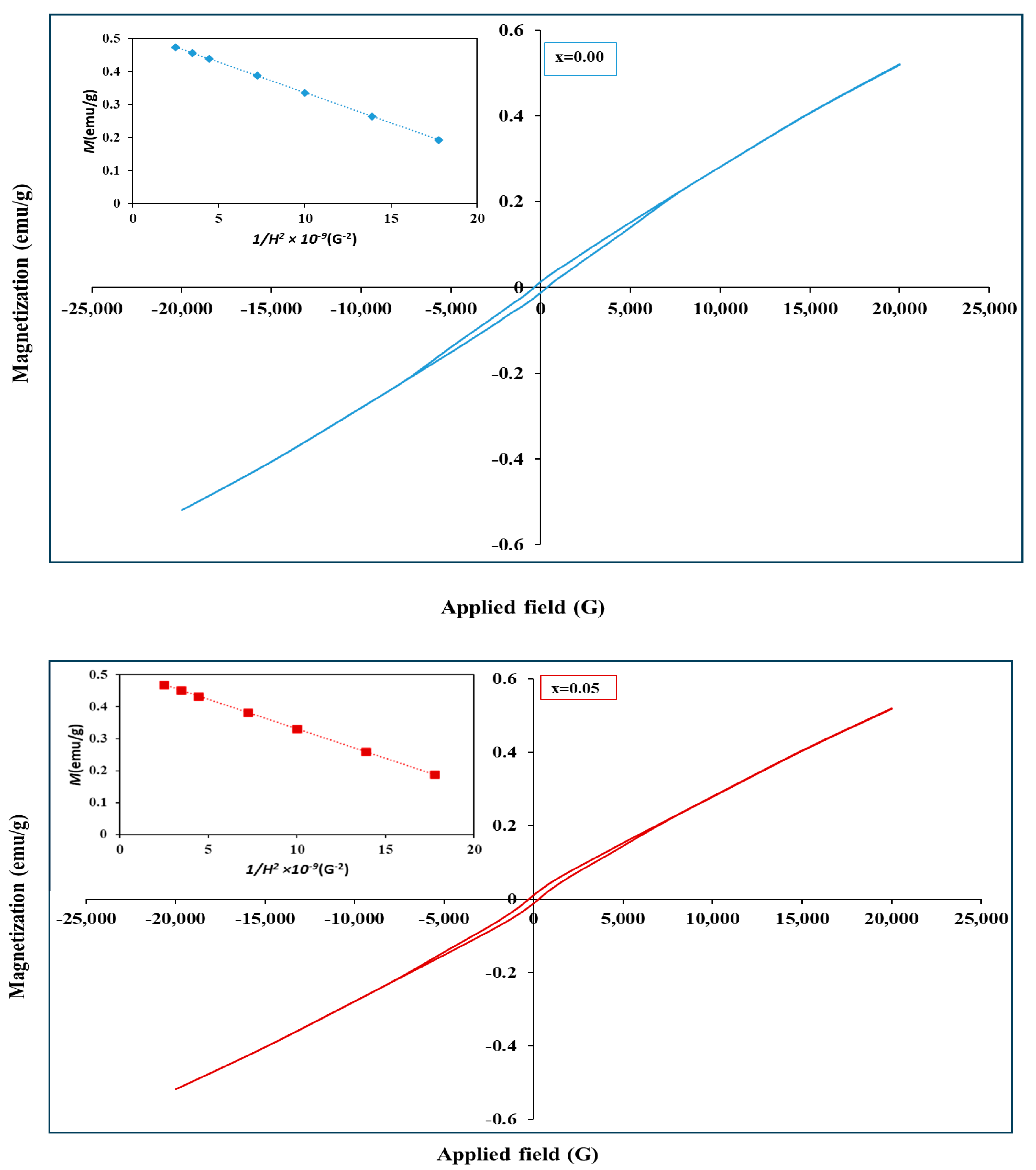
| Zr4+ Content, x | (Å) | (Å) | (g/cm3) | (g/cm3) | P% | Hopping Lengths | Scherrer Equation | W-H Method | TEM | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
(Å) | (Å) | D (nm) | D (nm) | × 10−3 | L (nm) | ||||||
| 0.00 | 8.311 | 8.781 | 5.251 | 4.218 | 19.672 | 3.598 | 2.938 | 18.500 | 22.01 | 1.30 | 16.998 |
| 0.05 | 8.314 | 8.788 | 5.291 | 4.299 | 18.740 | 3.600 | 2.939 | 14.999 | 20.42 | 3.14 | 11.172 |
| 0.10 | 8.318 | 8.795 | 5.328 | 4.357 | 18.224 | 3.602 | 2.941 | 14.294 | 19.53 | 3.21 | 9.346 |
| 0.15 | 8.319 | 8.806 | 5.372 | 4.488 | 16.462 | 3.602 | 2.941 | 11.179 | 17.03 | 3.32 | 7.073 |
| 0.20 | 8.320 | 8.810 | 5.415 | 4.649 | 14.151 | 3.603 | 2.942 | 9.265 | 11.91 | 7.71 | 6.120 |
| x | ν1 (cm−1) | ν2 (cm−1) | (dyne/cm) | (dyne/cm) | GPa | GPa | |
|---|---|---|---|---|---|---|---|
| 0.00 | 629.66 | 523.08 | 2.837 | 1.958 | 288.538 | 103.298 | 0.2636 |
| 0.05 | 626.56 | 522.79 | 2.809 | 1.956 | 286.627 | 102.844 | 0.2641 |
| 0.10 | 625.47 | 521.57 | 2.800 | 1.947 | 285.3545 | 103.320 | 0.2658 |
| 0.15 | 624.81 | 519.64 | 2.794 | 1.932 | 284.101 | 102.874 | 0.2659 |
| 0.20 | 623.74 | 517.92 | 2.784 | 1.919 | 282.724 | 102.775 | 0.2666 |
| x | (emu/g) | × 10−2 (emu/g) | (G) | |
|---|---|---|---|---|
| 0.00 | 0.520 | 1.36 | 362.07 | 0.0209 |
| 0.05 | 0.515 | 1.10 | 278.92 | 0.0211 |
| 0.10 | 0.506 | 0.82 | 213.25 | 0.0209 |
| 0.15 | 0.466 | 0.85 | 1209.2 | 0.0194 |
| 0.20 | 0.432 | 0.93 | 1357.1 | 0.0181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, M.M.E.; El-Said Bakeer, D. Investigation of Structural, Elastic and Magnetic Properties of CoCr2−xZrxO4 Nanoparticles. Materials 2024, 17, 5149. https://doi.org/10.3390/ma17215149
Barakat MME, El-Said Bakeer D. Investigation of Structural, Elastic and Magnetic Properties of CoCr2−xZrxO4 Nanoparticles. Materials. 2024; 17(21):5149. https://doi.org/10.3390/ma17215149
Chicago/Turabian StyleBarakat, Mai M. E., and Doaa El-Said Bakeer. 2024. "Investigation of Structural, Elastic and Magnetic Properties of CoCr2−xZrxO4 Nanoparticles" Materials 17, no. 21: 5149. https://doi.org/10.3390/ma17215149
APA StyleBarakat, M. M. E., & El-Said Bakeer, D. (2024). Investigation of Structural, Elastic and Magnetic Properties of CoCr2−xZrxO4 Nanoparticles. Materials, 17(21), 5149. https://doi.org/10.3390/ma17215149






