1. Introduction
Meteorites are rocks that originate in outer space and have fallen to the surface of the Earth. They have fascinated humans for thousands of years, even before we could understand their origin. It is interesting to note that the first iron used by humans came from meteorites. Studying meteorites currently enhances our understanding of the solar system and the universe. Similarly, just as it did thousands of years ago, it also drives innovation in materials science and technology. Modern materials science techniques allow us to investigate these samples of materials manufactured in outer space under very different conditions from those known on Earth.
On 20 January 2024 at 21:48 UTC, Hungarian astronomer Krisztián Sárneczky discovered asteroid 2024BX1 [
1]. The bolide was also recorded by the European Fireball Network, IMO/All-Sky7, and the Fireball Recovery and Inter-Planetary Observation Network (FRIPON). A meteoroid, 1 m in size and 140 kg in mass, was predicted to enter the Earth’s atmosphere three hours before its fall [
2]. The impact was predicted to occur west of Berlin, in the Havelland region of Germany. The meteorite fell on 21 January, south of Ribbeck. The first Ribbeck meteorite was found on 25 January: a single stone weighing 171 g, broken into three pieces, was found by Polish hunters. The main mass, weighing 225 g, was found the next day, also by a Polish hunter. About 200 pieces, with a total mass of approximately 1.8 kg, were recovered in a short period of time [
3].
Three weeks after the fall, the Ribbeck meteorite was classified by Dr Ansgar Greshake from the Natural History Museum in Berlin as an aubrite (Meteoritical Bulletin Database). It is described as a heavily brecciated aubrite consisting of 76 ± 3 vol % FeO-free enstatite, 15 ± 2.5 vol % albitic plagioclase, 5.5 ± 1.5 vol % forsterite, and 3.5 ± 1.0 vol % sulfides and metals [
4]. Ribbeck-2024BX1 is the eighth asteroid in contemporary history discovered before impacting Earth and only the twelfth recorded observed fall of an aubrite in history [
1,
4]. Most aubrites are found in cold and hot deserts, with 77 different finds officially listed in the Meteoritical Bulletin.
Aubrites are a rare type of enstatite achondrite meteorite with a unique mineralogical composition. Based on oxygen isotopes, they can be related to enstatite chondrites [
5]. They are mostly composed of Fe-free enstatite (a mineral high in magnesium and low in silicon), minor forsterite, albite, and rare minerals such as sulfides, nitrides, and carbides [
6]. The formation age of aubrites has been measured from 4560 Ma to 4550 Ma, suggesting two distinct groups of aubrites [
5]. Aubrites are most likely related to E-type asteroids, where the spectra of meteorites and asteroids are similar [
5]. Bischoff et al. [
4] indicate that the chemical composition of Ribbeck meteorite is close to the composition of other aubrites, for instance Bishopville, Aubres or Norton County. They also indicate that Ribbeck is not a regolith breccia, but a fragmental breccia. The brecciated texture of Ribbeck clearly indicates the significant impact activities that have affected its parent body. Additional impact events are necessary to account for the formation of small shock-melted areas and shock veins within Ribbeck’s fine-grained clastic matrix.
In recent decades, Raman spectroscopy has gained importance in the study of meteorites [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25]. This technique allows non-destructive analysis of the mineral composition of the meteorite samples. It is highly sensitive to different minerals and provides the information about molecular structure and chemical bonds in the investigated materials. It can also detect both crystalline and amorphous materials, it shows the differences in the composition of different points of the same mineral, and it also allows for an understanding of the distribution of different minerals in the sample. Additionally, the studies using Raman spectroscopy can be performed on small sample sizes and special surface preparation is not required, so the technique is even less invasive.
This paper aims to characterize the chemical and phase compositions of the Ribbeck meteorite shown in
Figure 1. The white and gray fragments were examined using a scanning electron microscope (SEM) with energy dispersive spectroscopy (EDS) and Raman spectrometry. These studies allowed us to identify the minerals that compose the Ribbeck meteorite. In relation to the structure of previously identified meteorites, this will allow for a better understanding of the structure of the solar system. Information about the chemical and phase compositions will inspire the search for new materials for their applications in everyday use.
3. Results and Discussion
After the initial macroscopic assessment of the meteorite, two slivers characterized by white and gray colors lithology (
Figure 1b) were selected for research using the EDS technique coupled with SEM and a Raman spectrometer.
Figure 2 shows their photos taken using SEM. The chemical composition of these slivers was then examined using the EDS technique, with the measurement results (average values from the areas for which the maps were made) presented in
Table 1. For the white sliver, the chemical composition maps indicate homogeneity. In contrast, for the gray sliver the analyzed areas (maps) differed in chemical composition, showing regions with increased concentrations of certain elements. It should be emphasized that the results show a lack of sodium in the chemical composition analyses of the gray sliver, whereas in the white sliver, sodium is present on the surface at a level of ~1.4 at.%.
The results of the EDS analysis (
Table 1) show that the most common element is oxygen, which suggests that the meteorite in question consists of silicon oxides and metals such as Mg, Al, Cr, Fe, Co, Ti, Ni, and Mo. By relating this local information on the chemical composition to the chemical composition of larger fragments of the Ribbeck meteorite [
4], it can be seen that the results presented in
Table 1 well reflect the overall chemical composition. It is worth noting that the local analysis of the chemical composition shows that the high sulfur content (3.09 at. %) is accompanied by a high iron content (9.87 at. %) at the expense of the presence of silicon and other metals (in particular, the absence of aluminum). This indicates that the gray slivers of meteorite will also be characterized by a large diversity in terms of phase composition.
In order to determine the phase composition of these slivers, tests were conducted using a Raman spectrometer. Two lenses, 5× and 50× magnification, were used for the tests. The Raman spectra of the white sliver looked almost identical at randomly selected study points, regardless of the lens used.
Figure 3 illustrates a characteristic spectrum of the sliver, showing Raman lines at 342 cm
−1 (M-O stretch), 665 cm
−1 (Si-O-Si stretch), 687 cm
−1 (Si-O-Si stretch), 1014 cm
−1 (Si-O bridging stretch), and 1033 cm
−1 (Si-O bridging stretch), which are characteristic of the orthopyroxene enstatite [
7,
8,
9].
The EDS analysis of the meteorite slivers (
Table 1) in reference to the general formula of pyroxenes (XY(Si,Al)
2O
6, where X represents Ca, Na, Fe(II), or Mg, and Y represents ions of smaller size, such as Cr, Al, Mg, Co, Ti) confirms this assumption. The Raman spectra of orthopyroxene are very similar to the spectra of clinopyroxene (pyroxenes that crystallize in the monoclinic system). Therefore, it can be assumed that a small contribution of the clinopyroxene phase may also be present in the spectra [
10,
11,
12,
13].
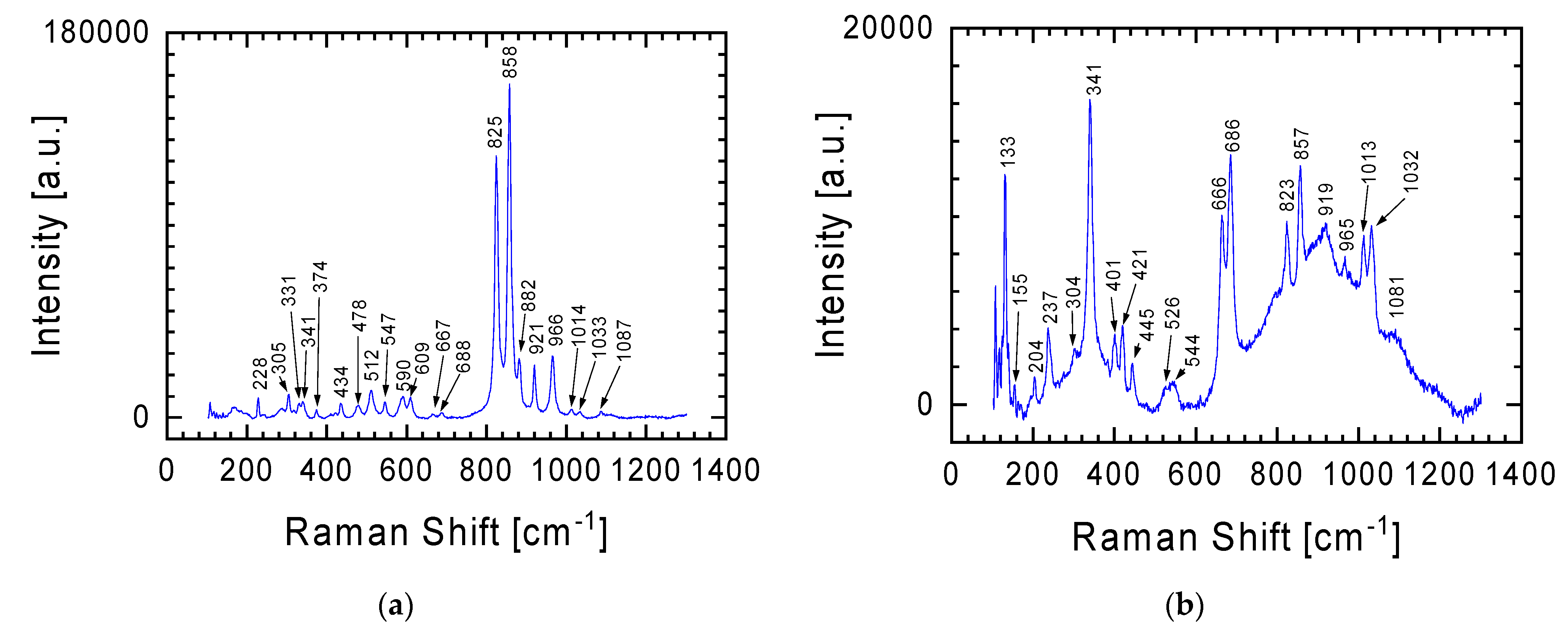
In the case of the gray lithology meteorite fragment, characterized by greater chemical diversity in individual places (see
Table 1), in addition to the spectrum already presented in
Figure 3 for the white lithology fragment, other spectra were recorded, two representative examples of which are presented in
Figure 4. The characteristic Raman lines at 825 and 858 cm
−1 in
Figure 4a are fingerprints of olivine with the chemical formula (Mg,Fe)
2SiO
4 [
14,
15,
16]. The lines are assigned to the Si-O antisymmetric and symmetric stretching vibration of the SiO
4 group (825 and 858 cm
−1, respectively). It should be emphasized here that Raman spectra also allow the differentiation of minerals in the olivine group. From the perspective of the Raman lines presented in
Figure 4, attention should be paid to two members of this group: (i) fayalite (Fe
2SiO
4, black), the iron-rich end-member, and (ii) forsterite (Mg
2SiO
4, light yellow-green), the magnesium-rich end-member of the olivine solid-solution series [
17]. In the case of the spectrum in
Figure 4, it can be said that both of these minerals are present in the tested sample. The higher intensity of the Raman line at 858 cm
−1 than at 825 cm
−1 indicates that the dominant contribution to the spectrum comes from forsterite. This is also evidenced by the presence in the Raman spectrum of lines at 228, 305, 434, 590, 609, 882 and 966 cm
−1, characteristic of magnesium-rich end-member of the olivine solid-solution series. However, Raman lines at 374, 547, and 921 cm
−1 indicate the presence of fayalite.
In the Raman spectrum from
Figure 4a, one can also distinguish lines at 478 and 512 cm
−1, discussed in detail when discussing below the spectrum from Figure 7 (obtained using an objective with 50× magnification), which should be assigned to plagioclase [
13,
18,
19].
The Raman spectrum in
Figure 4b is a specific combination of the spectra from
Figure 3 and
Figure 4a, with the Raman line at 341 cm
−1 becoming more prominent. The Raman line characteristic of orthopyroxene was previously discussed in relation to the spectrum from
Figure 3. Additionally, this spectrum (
Figure 4b) may also suggest an initiated transformation at a moderate shock level of plagioclase to maskelynite. This is indicated by a broad peak with a maximum at 919 cm
−1, which contains characteristic bands for orthopyroxene and plagioclase [
20,
21]. It should also be emphasized that this spectrum was obtained using a 5× magnification objective. Thus, the signal comes from a relatively large area in which a large number of different forms are characterized by short-range order (broad band between 600 and 1200 cm
−1), against which lines from forms with greater order can be seen. The confirmation that the examined place of meteorite could be described as quasi-amorphous is also evidenced by the low intensity of the lines outside this area of spectrum compared with the spectra presented in other figures.
In order to separate the spectra coming from different minerals in the tested sample, tests using a Raman spectrometer were also carried out using a 50× magnification objective. The homogeneity of the chemical composition for the white lithology meteorite sliver (
Table 1) was confirmed by these results. As was the case with measurements with the 5× objective (
Figure 3), measurements with the 50× objective gave the same spectrum (
Figure 5) regardless of the location chosen on the sample for testing. In the case of the gray lithology meteorite sliver, measurements with the 50× objective allowed for the isolation of a larger number of minerals constituting this sliver than in the cases of Raman spectra obtained from measurements with the 5× objective presented above.
The research results presented above show that olivine is one of the minerals identified in the gray lithology meteorite fragment. The analysis of the spectrum in
Figure 4a clearly shows that in the studied meteorite, forsterite and fayalite can be distinguished within the olivine groups.
Figure 6 with spectra obtained at 50× objective magnification allows for a clear distinction between these two elements of the olivine group. Referring to the chemical composition of this meteorite fragment (
Table 1), for places richer in magnesium we have spectra corresponding to forsterite with Raman lines at 220, 306, 438, 589, 610, 826, 858, 883, and 967 cm
−1 (
Figure 6a), and for those richer in iron, spectra correspond to fayalite from Raman lines at 240, 375, 547, 826, 858, and 921 cm
−1 (
Figure 6b). Importantly, in the spectrum assigned to forsterite, the intensity of the Raman line at 858 cm
−1 is higher than that of the Raman line at 826 cm
−1, while in the spectrum assigned to fayalite, Raman lines considered characteristic of olivine have almost identical intensity. Nevertheless, in both spectra (
Figure 6) we can find Raman lines, which should be attributed to second of the considered elements of the olivine group. This means that both forms of the mineral contribute to the individual meteorite crystallites. When examining the chemical composition of the gray meteorite fragment, we see that magnesium and iron are present in every examined place, but their amount is not constant and depends on the place (
Table 1).
Looking at the chemical composition of the gray lithology meteorite fragment (
Table 1), we see that calcium is an important element. This means that in the tested sample we can also expect elements of the olivine group such as monticellite (CaMgSiO
4) and kirschsteinite (CaFeSiO
4), and gray silicate minerals [
19]. Unfortunately, it is difficult to clearly distinguish the Raman lines of these minerals from the presented spectra (
Figure 6) due to the high similarity to the above-discussed elements of the olivine group (forsterite and fayalite).
The difficulty in separating other elements of the olivine group in the spectra presented in
Figure 6, apart from forsterite and fayalite, does not mean that these spectra are devoid of inclusions from the minerals already discussed. Similarly to the spectrum from
Figure 4b (objective magnification 5×), in the spectrum from
Figure 6b we can distinguish Raman lines typical for orthopyroxene: Raman lines at 342, 665, 687, 1014 and 1035 cm
−1.
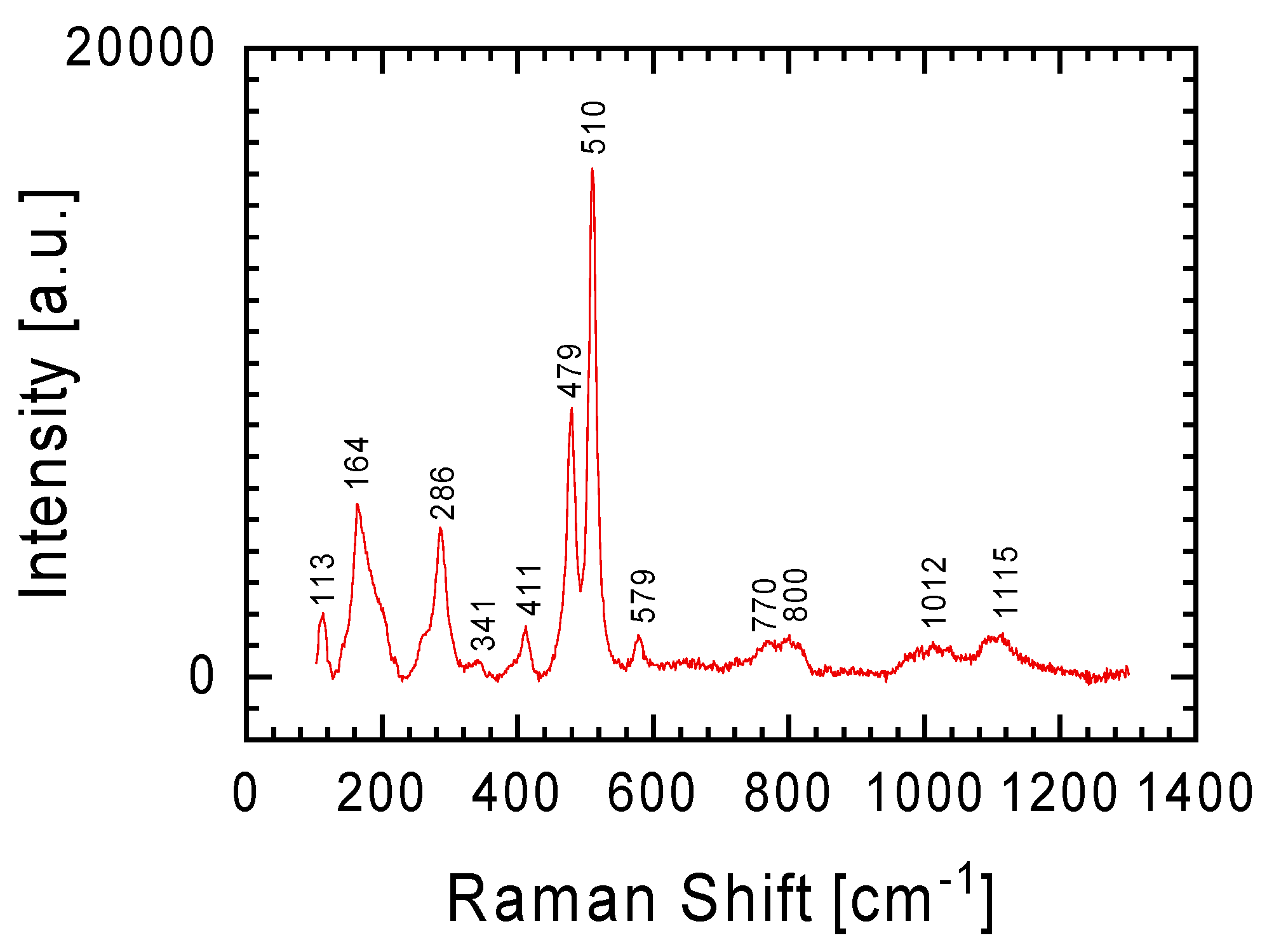
The less intense Raman lines visible in
Figure 4a are the lines at 478 and 512 cm
−1. These bands with a slightly shifted position (lines at 479 and 510 cm
−1, respectively) are clearly visible in the spectrum obtained at 50× objective magnification (
Figure 7). These lines (Si(Al)-O-Si(Al) bend), as well as the weak line at 286 cm
−1 (Si-O-Si bend), should be assigned to plagioclase, a solid solution of the albite Ab (NaAlSi
3O
8) and anorthite An (CaAl
2Si
2O
8) minerals [
13,
18,
19]. The lack of Na in the examined meteorite fragment (
Table 1) indicates that in our case only anorthite contributes to the spectrum in
Figure 7. It is worth mentioning here that in the parallel studies on the Ribbeck meteorite by Bischoff et al. [
4], lines at 284, 326, 463, and 500 cm
−1 were also identified in the Raman spectra, and in the presence of potassium in its chemical composition these lines were considered as characteristic of the K-feldspar-like phase (KAlSi
3O
8). In the case of the discussed meteorite fragments, potassium was not identified (
Table 1).
In the introduction, it was mentioned that the meteorite was found shortly after it fell in January 2024. The finders reported that the found meteorites smelled of sulfur. The tested meteorite fragments showed that a gray lithology fragment may contain up to 3.1 at.% sulfur. It is uncertain whether the sulfur exists in its elemental state or as an oxide, as mentioned by Bischoff et al. [
4]. The Raman spectrum in
Figure 8 displays lines at 152, 219, 246, 438, and 472 cm
−1, indicating the presence of sulfur crystals in the meteorite (lines belonging to stretching vibration of S=S molecules) [
26]. This finding led to a reevaluation of the discussed spectra, suggesting that the presence of sulfur in the meteorite is the source of the less intense Raman lines in the spectra from
Figure 4a and
Figure 6. For example, lines at 155, 196, 228, 240, and 307 cm
−1 for the gray lithology sliver (
Figure 6b), combined with the fact that the sulfur content in the studied fragment increased in parallel with the increase in iron (
Table 1—1st place for gray sliver), may indicate the presence of troilite (FeS) in this sliver [
27]. Additionally, it is noteworthy that the Ribbeck meteorite contains sulfides rich in lithophile elements, which are typically removed during the leaching process. This could explain the sulfur smell at the meteorite fall site.