Linear and Volumetric Polyethylene Wear Patterns after Primary Cruciate-Retaining Total Knee Arthroplasty Failure: An Analysis Using Optical Scanning and Computer-Aided Design Models
Abstract
1. Introduction
2. Materials and Methods
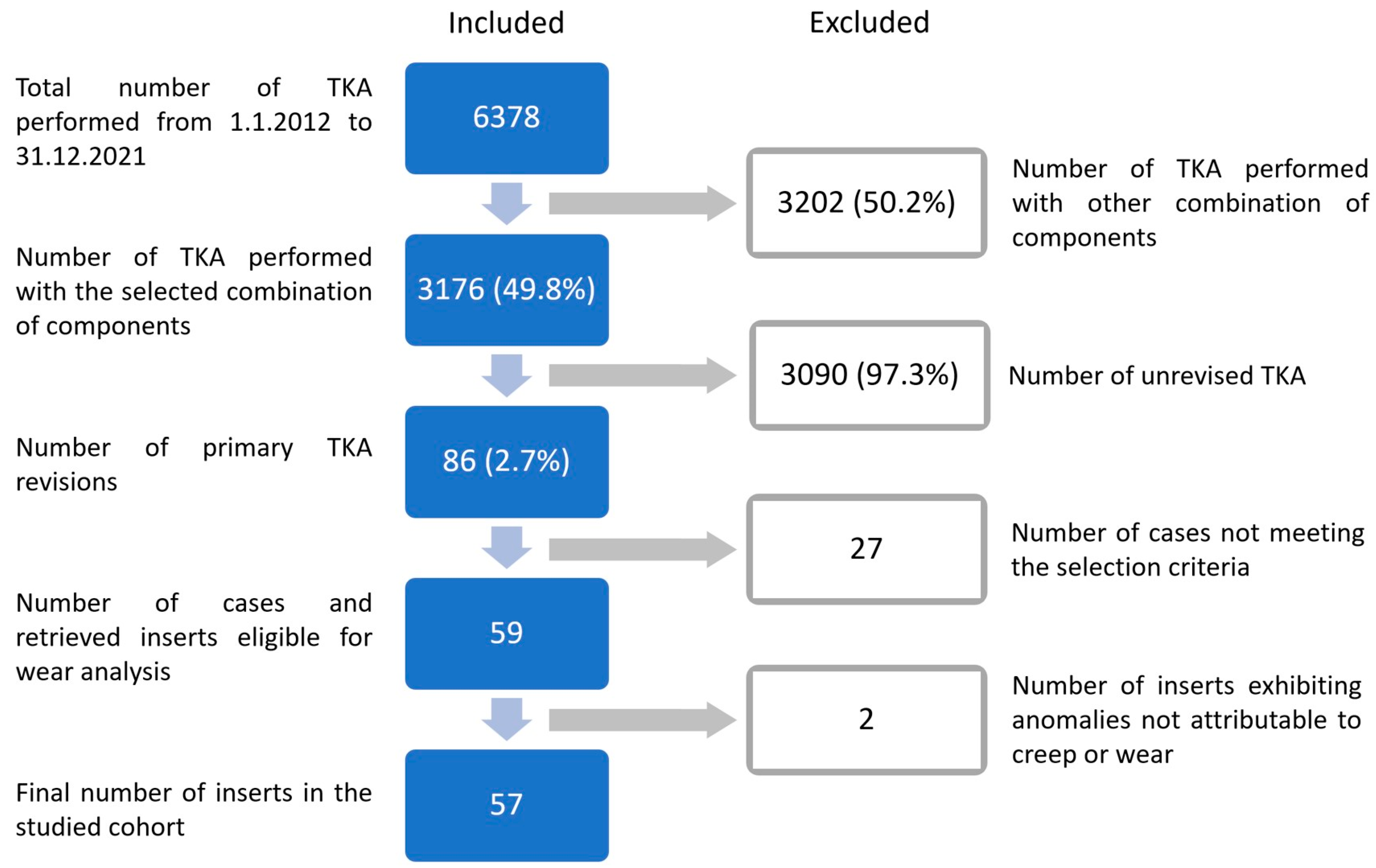
2.1. Retrievals
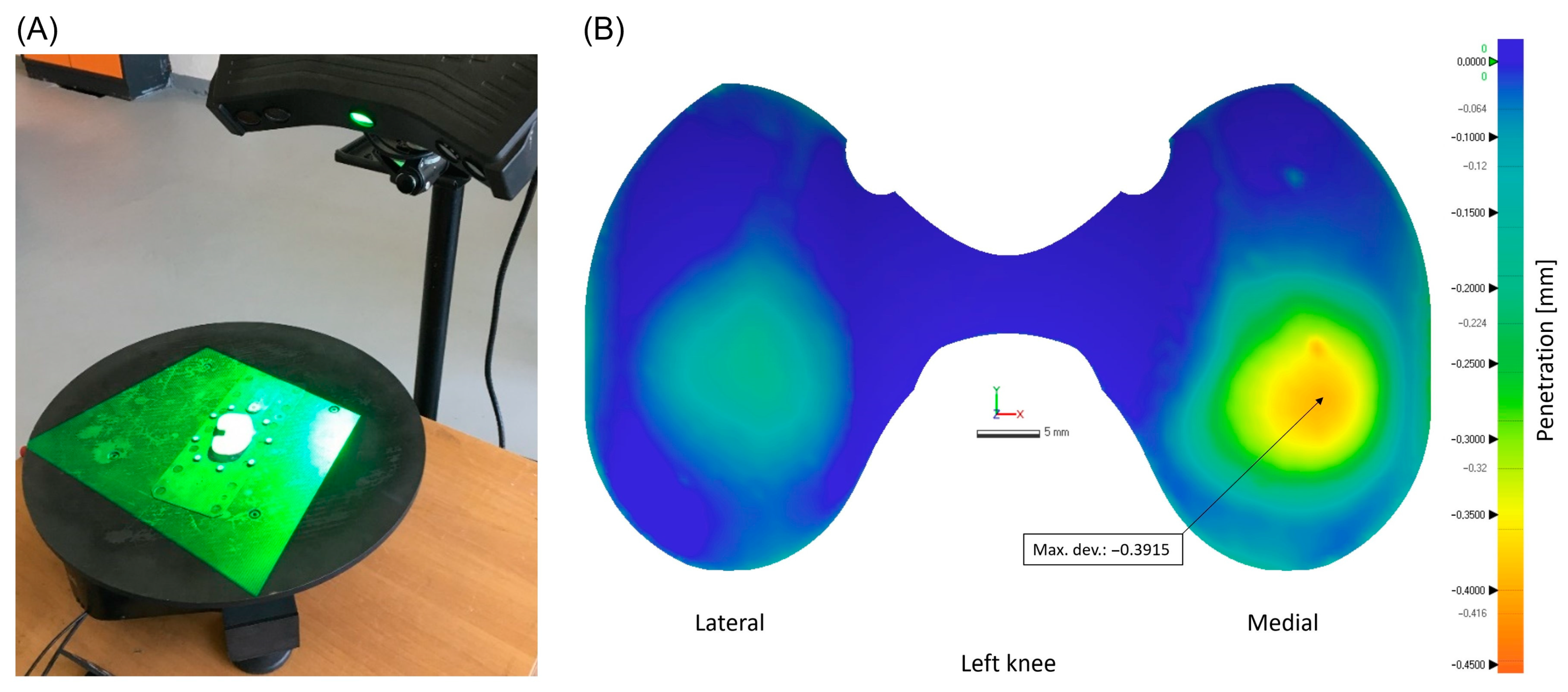
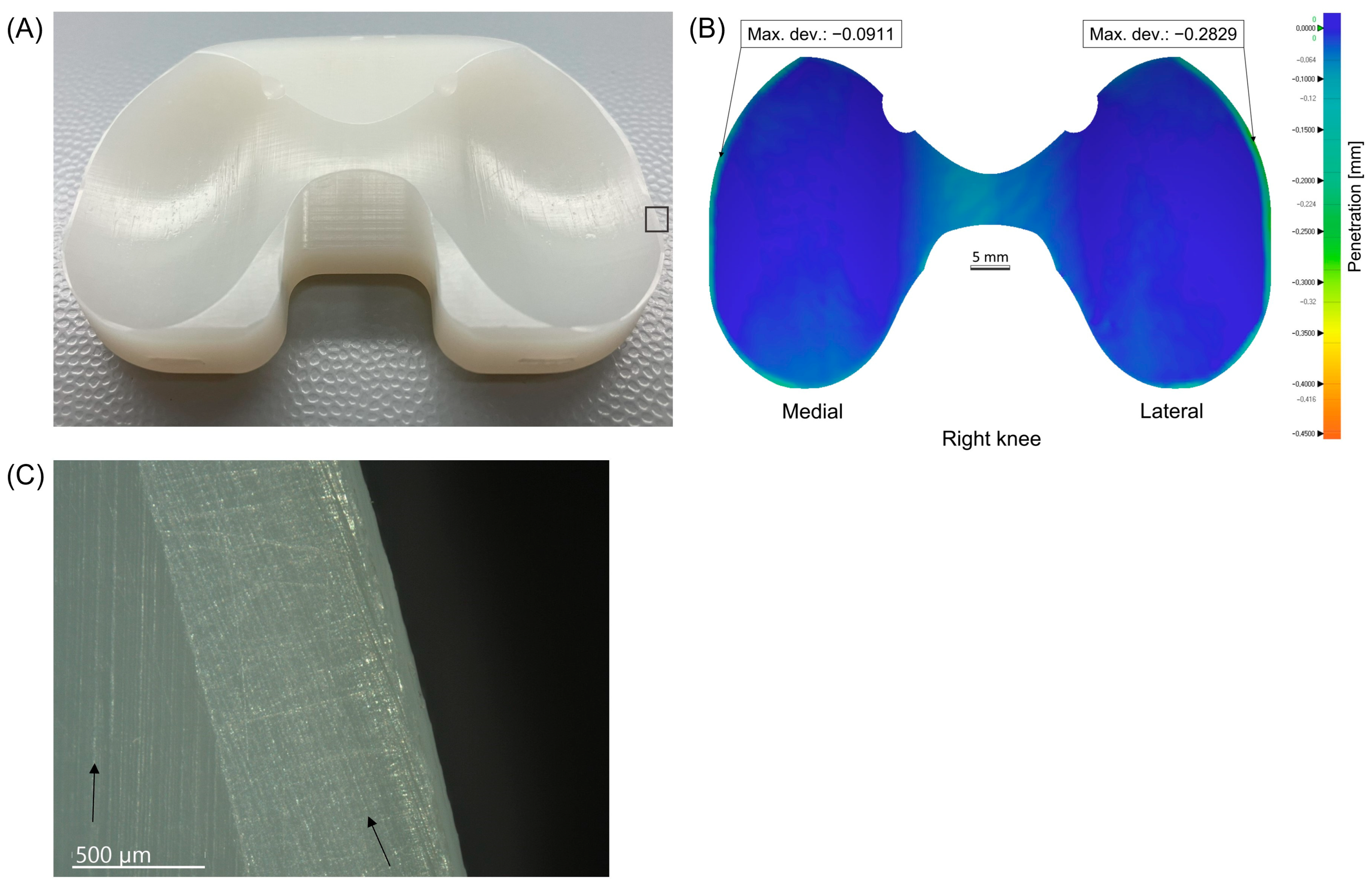
2.2. Scanning Process and Insert Wear Analysis
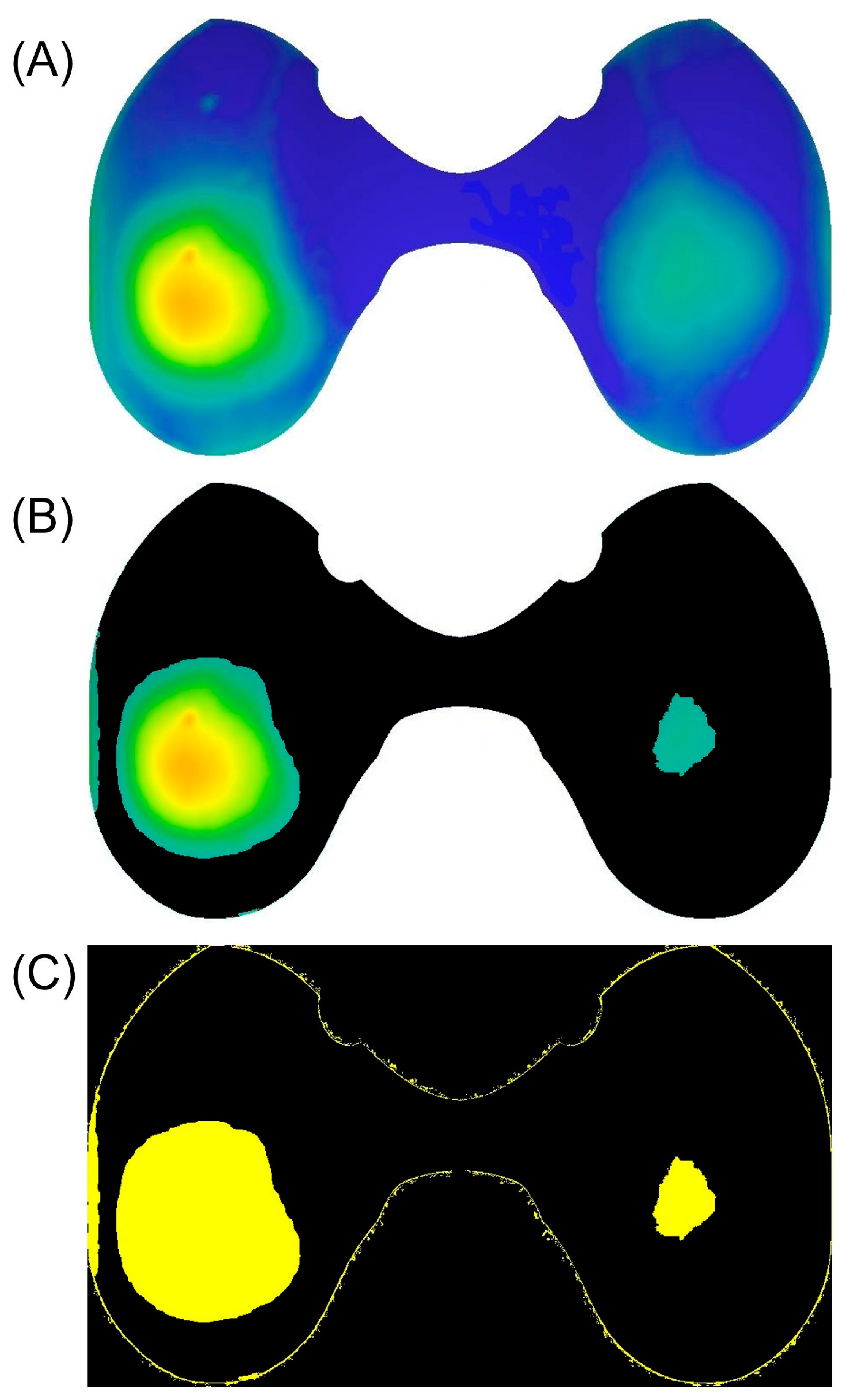
2.3. Determination of Characteristic Wear Patterns
2.4. Identification of All Possible Causes for TKA Failure
- Confirmed PJI according to the current European Bone and Joint Infection Society (EBJIS) definition [34];
- Clinical loosening of the component (intraoperative assessment—evident movement of a prosthetic component within the bone);
- Joint malalignment or component malposition (in the coronal plane: deviation of hip-knee-ankle angle (HKAA) from 180 ± 3° (varus/valgus malalignment), deviation of mechanical lateral distal femoral angle (mLDFA) from 90 ± 3°, deviation of medial proximal tibial angle (MPTA) from 90° to 87°, in the sagittal plane, deviation of distal femoral flexion angle (DFFA) from 90° to 87° and deviation of tibial slope (TS) from 0° to 7°) [37];
- Instability (positive history and clinical examination of abnormal and excessive displacement of a knee prosthesis [38] with intraoperative finding or positive stress radiography);
- Other isolated causes (joint stiffness—flexion contracture greater than 10° and/or flexion limit ranging from 90° [39] and/or pain not classified in any of the previous groups).
2.5. Determination of Groups Based on Nominal Insert Size and Component Size Ratio
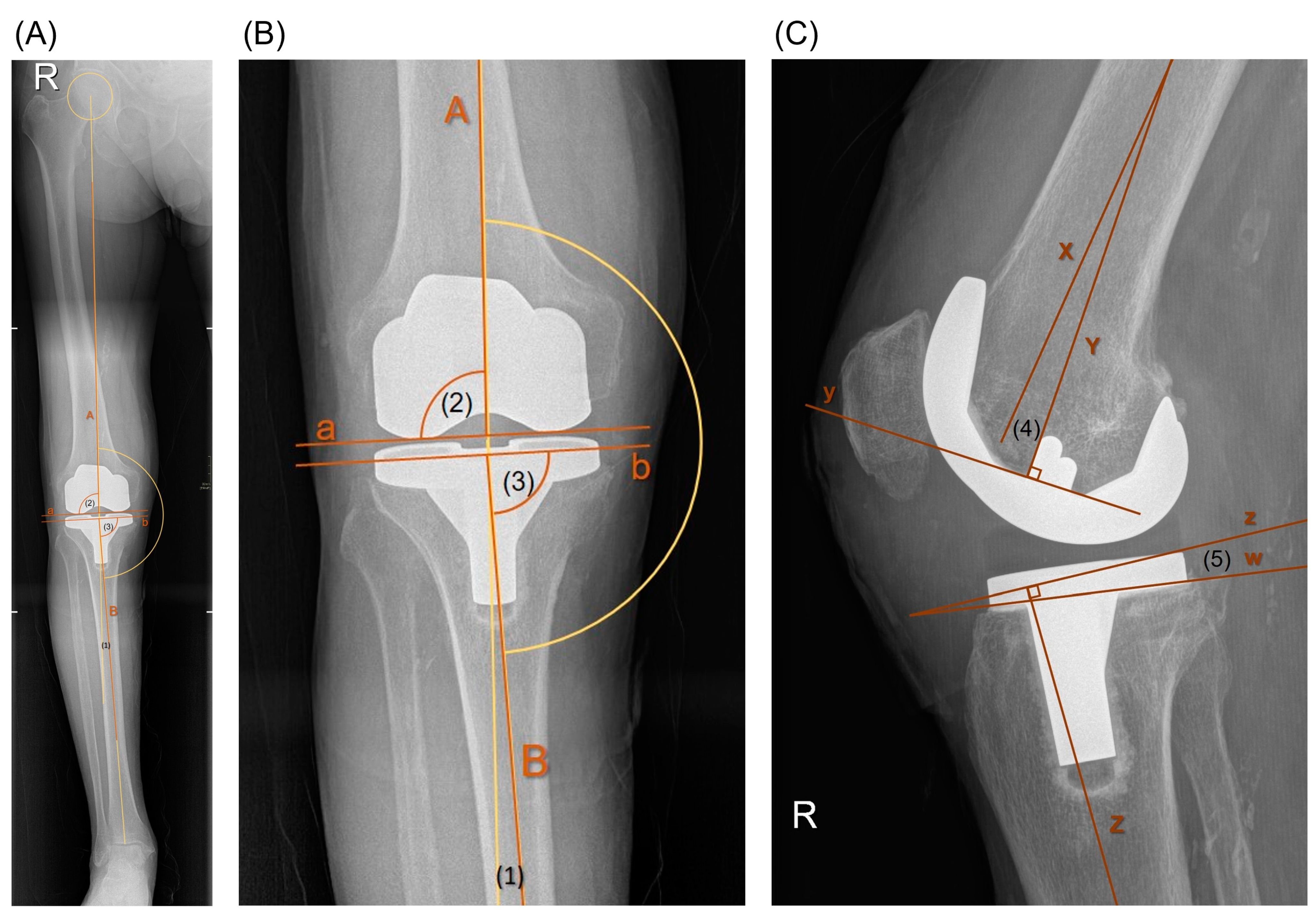
2.6. Radiological Assessment
2.7. Microscopic Analysis of Retrieved Inserts
2.8. Statistical Analysis
3. Results
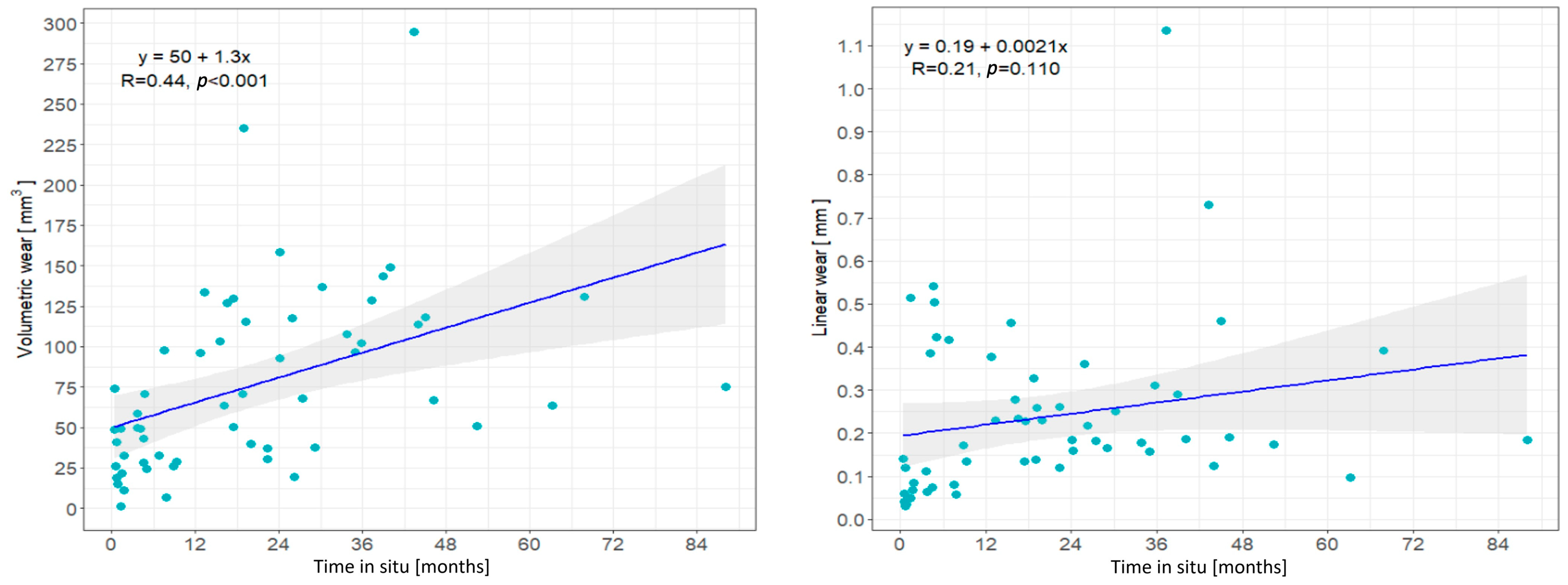
3.1. Patient Demographics, Insert Characteristics and Wear Correlation with Time In Situ
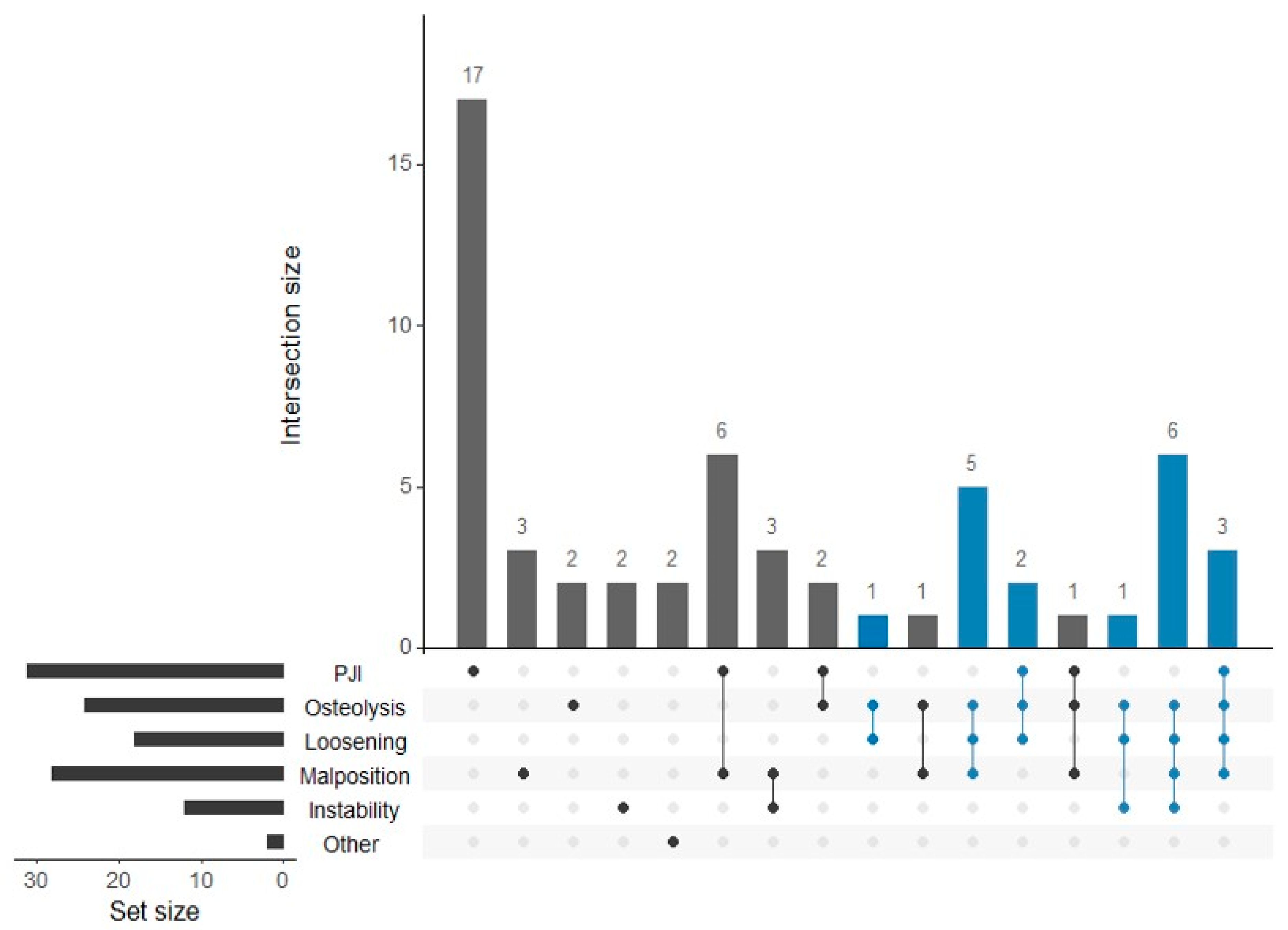
3.2. Distribution of TKA Failure Causes and Their Association with Volumetric Wear
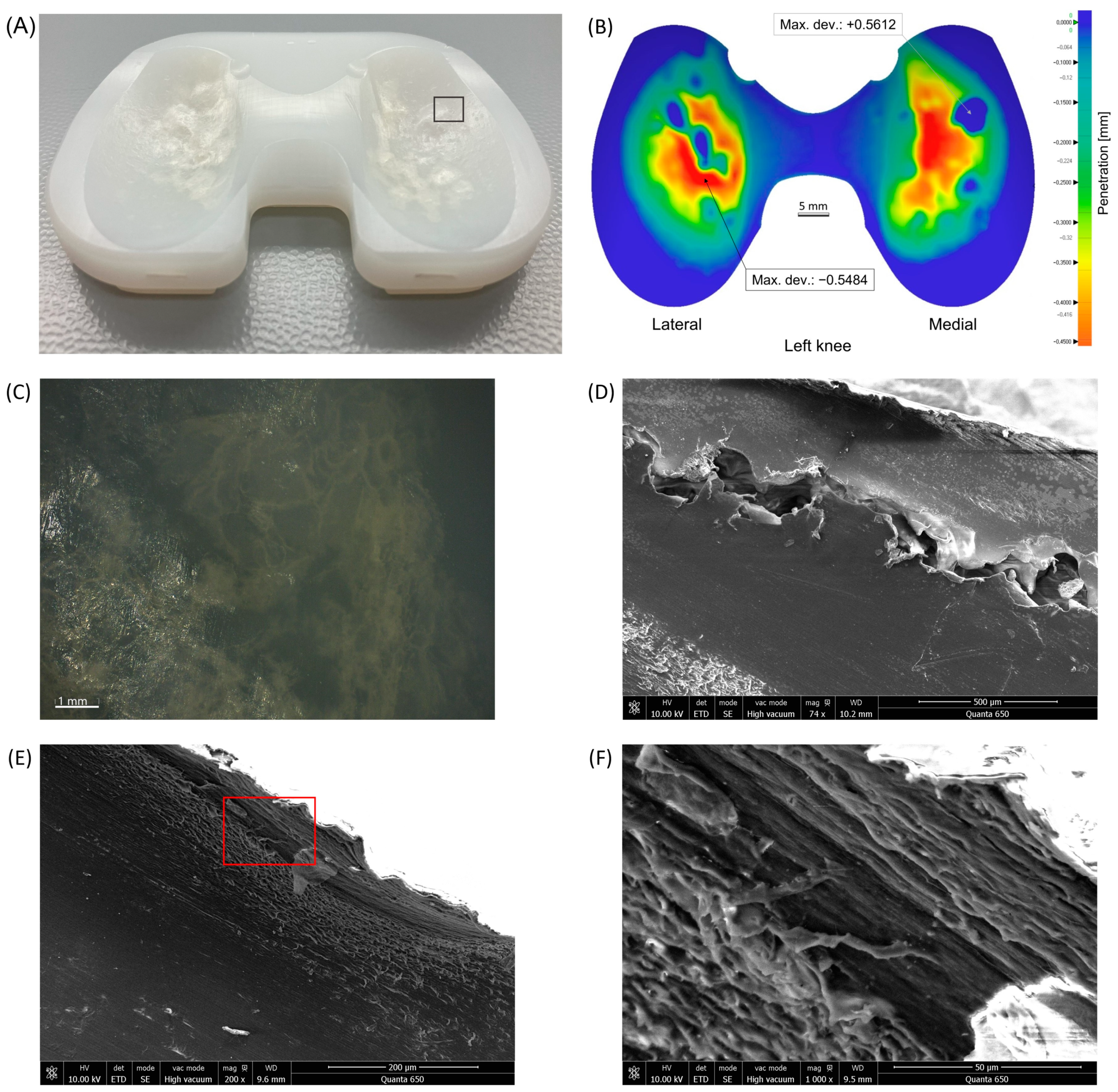
3.3. Wear Distribution Patterns
3.4. Comparison of Wear Rates Based on Insert Size and Component Size Ratio
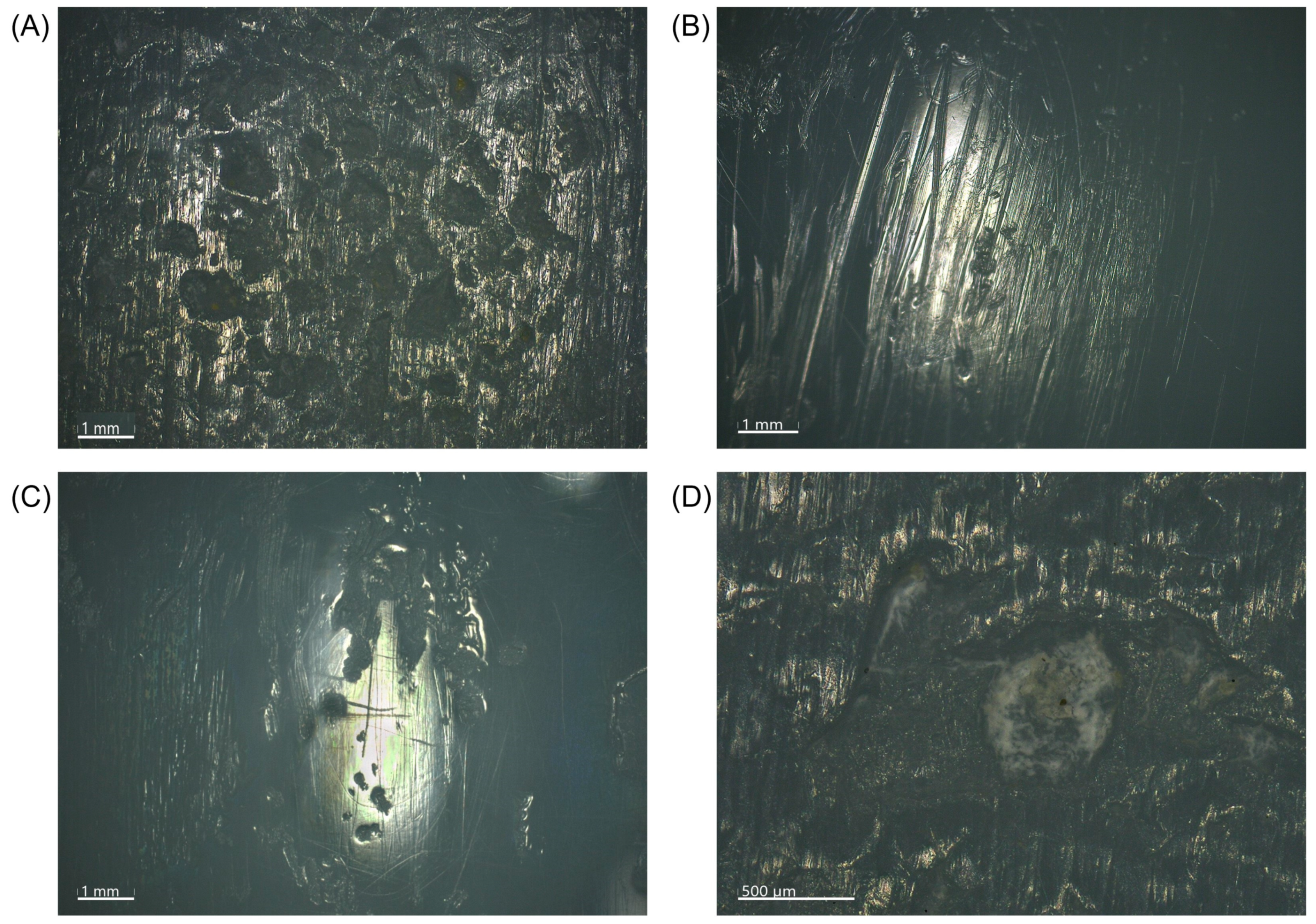
3.5. Microscopic Wear Patterns and Presentation of Excluded Inserts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shichman, I.; Roof, M.; Askew, N.; Nherera, L.; Rozell, J.C.; Seyler, T.M.; Schwarzkopf, R. Projections and Epidemiology of Primary Hip and Knee Arthroplasty in Medicare Patients to 2040–2060. JBJS Open Access 2023, 8, e22. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Ra, H.J. Patient Satisfaction after Total Knee Arthroplasty. Knee Surg. Relat. Res. 2016, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Clement, N.D.; Bardgett, M.; Weir, D.; Holland, J.; Gerrand, C.; Deehan, D.J. The Rate and Predictors of Patient Satisfaction after Total Knee Arthroplasty Are Influenced by the Focus of the Question: A Standard Satisfaction Question Is Required. Bone Jt. J. 2018, 100-B, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Delanois, R.E.; Mistry, J.B.; Gwam, C.U.; Mohamed, N.S.; Choksi, U.S.; Mont, M.A. Current Epidemiology of Revision Total Knee Arthroplasty in the United States. J. Arthroplast. 2017, 32, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Dossett, H.G. Machine Learning: The Future of Total Knee Replacement. Fed. Pract. 2022, 39, 62. [Google Scholar] [CrossRef]
- Khan, M.; Osman, K.; Green, G.; Haddad, F.S. The Epidemiology of Failure in Total Knee Arthroplasty: Avoiding Your next Revision. Bone Jt. J. 2016, 98-B, 105–112. [Google Scholar] [CrossRef]
- Postler, A.; Lützner, C.; Beyer, F.; Tille, E.; Lützner, J. Analysis of Total Knee Arthroplasty Revision Causes. BMC Musculoskelet. Disord. 2018, 19, 55. [Google Scholar] [CrossRef]
- Naudie, D.D.R.; Ammeen, D.J.; Engh, G.A.; Rorabeck, C.H. Wear and Osteolysis Around Total Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 2007, 15, 53–64. [Google Scholar] [CrossRef]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Wimmer, M.A.; Holinka, M. Osteolysis around Total Knee Arthroplasty: A Review of Pathogenetic Mechanisms. Acta Biomater. 2013, 9, 8046–8058. [Google Scholar] [CrossRef]
- Currier, J.H.; Currier, B.H.; Abdel, M.P.; Berry, D.J.; Titus, A.J.; Van Citters, D.W. What Factors Drive Polyethylene Wear in Total Knee Arthroplasty?: Results of a Large Retrieval Series. Bone Jt. J. 2021, 103-B, 1695–1701. [Google Scholar] [CrossRef]
- Li, S.; Scuderi, G.; Furman, B.D.; Bhattacharyya, S.; Schmieg, J.J.; Insall, J.N. Assessment of Backside Wear From the Analysis of 55 Retrieved Tibial Inserts. Clin. Orthop. 2002, 404, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Conditt, M.A.; Thompson, M.T.; Usrey, M.M.; Ismaily, S.K.; Noble, P.C. Backside Wear of Polyethylene Tibial Inserts: Mechanism and Magnitude of Material Loss. J. Bone Jt. Surg. 2005, 87, 326–331. [Google Scholar] [CrossRef]
- Ho, F.-Y.; Ma, H.-M.; Liau, J.-J.; Yeh, C.-R.; Huang, C.-H. Mobile-Bearing Knees Reduce Rotational Asymmetric Wear. Clin. Orthop. 2007, 462, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.M.; Kraay, M.J.; Messerschmitt, P.J.; Goldberg, V.M.; Rimnac, C.M. Analysis of Retrieved Ultra–High-Molecular-Weight Polyethylene Tibial Components From Rotating-Platform Total Knee Arthroplasty. J. Arthroplast. 2009, 24, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Huang, C.-H.; Chang, T.-K.; Ho, F.-Y.; Cheng, C.-K.; Huang, C.-H. Wear-Pattern Analysis in Retrieved Tibial Inserts of Mobile-Bearing and Fixed-Bearing Total Knee Prostheses. J. Bone Jt. Surg. Br. 2010, 92-B, 500–507. [Google Scholar] [CrossRef]
- Srivastava, A.; Lee, G.Y.; Steklov, N.; Colwell, C.W.; Ezzet, K.A.; D’Lima, D.D. Effect of Tibial Component Varus on Wear in Total Knee Arthroplasty. Knee 2012, 19, 560–563. [Google Scholar] [CrossRef]
- Knowlton, C.B.; Wimmer, M.A. An Autonomous Mathematical Reconstruction to Effectively Measure Volume Loss on Retrieved Polyethylene Tibial Inserts. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 101, 449–457. [Google Scholar] [CrossRef]
- Wimmer, M.A.; Laurent, M.P.; Haman, J.D.; Jacobs, J.J.; Galante, J.O. Surface Damage Versus Tibial Polyethylene Insert Conformity: A Retrieval Study. Clin. Orthop. Relat. Res. 2012, 470, 1814–1825. [Google Scholar] [CrossRef]
- Berry, D.J.; Currier, J.H.; Mayor, M.B.; Collier, J.P. Knee Wear Measured in Retrievals: A Polished Tray Reduces Insert Wear. Clin. Orthop. Relat. Res. 2012, 470, 1860–1868. [Google Scholar] [CrossRef]
- Stoner, K.; Jerabek, S.A.; Tow, S.; Wright, T.M.; Padgett, D.E. Rotating-Platform Has No Surface Damage Advantage Over Fixed-Bearing TKA. Clin. Orthop. Relat. Res. 2013, 471, 76–85. [Google Scholar] [CrossRef]
- Paterson, N.R.; Teeter, M.G.; MacDonald, S.J.; McCalden, R.W.; Naudie, D.D.R. The 2012 Mark Coventry Award: A Retrieval Analysis of High Flexion versus Posterior-Stabilized Tibial Inserts. Clin. Orthop. Relat. Res. 2013, 471, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Engh, C.A.; Zimmerman, R.L.; Hopper, R.H.; Engh, G.A. Can Microcomputed Tomography Measure Retrieved Polyethylene Wear? Comparing Fixed-Bearing and Rotating-Platform Knees. Clin. Orthop. Relat. Res. 2013, 471, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.-N.; Jamieson, P.; Teeter, M.G.; McCalden, R.W.; Naudie, D.D.R.; MacDonald, S.J. Retrieval Analysis of Posterior Stabilized Polyethylene Tibial Inserts and Its Clinical Relevance. J. Arthroplast. 2014, 29, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Holleyman, R.J.; Scholes, S.C.; Weir, D.; Jameson, S.S.; Holland, J.; Joyce, T.J.; Deehan, D.J. Changes in Surface Topography at the TKA Backside Articulation Following in Vivo Service: A Retrieval Analysis. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, R.; Scott, R.D.; Carlson, E.M.; Currier, J.H. Does Increased Topside Conformity in Modular Total Knee Arthroplasty Lead to Increased Backside Wear? Clin. Orthop. Relat. Res. 2015, 473, 220–225. [Google Scholar] [CrossRef]
- Knowlton, C.B.; Bhutani, P.; Wimmer, M.A. Relationship of Surface Damage Appearance and Volumetric Wear in Retrieved TKR Polyethylene Liners: RETRIEVED TKR POLYETHYLENE DAMAGE PATTERNS AND VOLUMETRIC WEAR. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2053–2059. [Google Scholar] [CrossRef]
- Li, Z.; Esposito, C.I.; Koch, C.N.; Lee, Y.; Padgett, D.E.; Wright, T.M. Polyethylene Damage Increases With Varus Implant Alignment in Posterior-Stabilized and Constrained Condylar Knee Arthroplasty. Clin. Orthop. Relat. Res. 2017, 475, 2981–2991. [Google Scholar] [CrossRef]
- Łapaj, Ł.; Mróz, A.; Kokoszka, P.; Markuszewski, J.; Wendland, J.; Helak-Łapaj, C.; Kruczyński, J. Peripheral Snap-Fit Locking Mechanisms and Smooth Surface Finish of Tibial Trays Reduce Backside Wear in Fixed-Bearing Total Knee Arthroplasty: A Retrieval Analysis of 102 Inlays. Acta Orthop. 2017, 88, 62–69. [Google Scholar] [CrossRef]
- Sisko, Z.W.; Teeter, M.G.; Lanting, B.A.; Howard, J.L.; McCalden, R.W.; Naudie, D.D.; MacDonald, S.J.; Vasarhelyi, E.M. Current Total Knee Designs: Does Baseplate Roughness or Locking Mechanism Design Affect Polyethylene Backside Wear? Clin. Orthop. 2017, 475, 2970–2980. [Google Scholar] [CrossRef]
- Affatato, S.; Ruzzi, S.; Milosevic, M.; Ruggiero, A. Wear Characterization and Contact Surfaces Analysis of Menisci and Femoral Retrieved Components in Bi-Condylar Knee Prostheses. J. Mech. Behav. Biomed. Mater. 2020, 110, 103959. [Google Scholar] [CrossRef]
- Pourzal, R.; Cip, J.; Rad, E.; Laurent, M.P.; Berger, R.A.; Jacobs, J.J.; Wimmer, M.A. Joint Line Elevation and Tibial Slope Are Associated with Increased Polyethylene Wear in Cruciate-retaining Total Knee Replacement. J. Orthop. Res. 2020, 38, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Tone, S.; Hasegawa, M.; Naito, Y.; Pezzotti, G.; Sudo, A. Raman Spectroscopy Reveals Creep and Wear Rate of E-Beam-Sterilized Conventional UHMWPE Tibial Inserts. J. Mech. Behav. Biomed. Mater. 2020, 110, 103902. [Google Scholar] [CrossRef] [PubMed]
- Levašič, V.; Blas, M.; Kovač, S.; Milošev, I. Valdoltra Knee Arthroplasty Registry Report 2023 (Data 2002–2022); Valdoltra Orthopeadic Hospital: Ankaran, Slovenia, 2024. [Google Scholar]
- McNally, M.; Sousa, R.; Wouthuyzen-Bakker, M.; Chen, A.F.; Soriano, A.; Vogely, H.C.; Clauss, M.; Higuera, C.A.; Trebše, R. The EBJIS Definition of Periprosthetic Joint Infection. Bone Jt. J. 2021, 103-B, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ewald, F.C. The Knee Society Total Knee Arthroplasty Roentgenographic Evaluation and Scoring System. Clin. Orthop. 1989, 248, 9–12. [Google Scholar] [CrossRef]
- Meneghini, R.M.; Mont, M.A.; Backstein, D.B.; Bourne, R.B.; Dennis, D.A.; Scuderi, G.R. Development of a Modern Knee Society Radiographic Evaluation System and Methodology for Total Knee Arthroplasty. J. Arthroplast. 2015, 30, 2311–2314. [Google Scholar] [CrossRef]
- Khalifa, A.A.; Mullaji, A.B.; Mostafa, A.M.; Farouk, O.A. A Protocol to Systematic Radiographic Assessment of Primary Total Knee Arthroplasty. Orthop. Res. Rev. 2021, 13, 95–106. [Google Scholar] [CrossRef]
- Chang, M.J.; Lim, H.; Lee, N.R.; Moon, Y.-W. Diagnosis, Causes and Treatments of Instability Following Total Knee Arthroplasty. Knee Surg. Relat. Res. 2014, 26, 61–67. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. The Stiff Total Knee Arthroplasty: Causes, Treatment Modalities and Results. EFORT Open Rev. 2019, 4, 602–610. [Google Scholar] [CrossRef]
- Saadi, S.B.; Ranjbarzadeh, R.; Kazemi, O.; Amirabadi, A.; Ghoushchi, S.J.; Kazemi, O.; Azadikhah, S.; Bendechache, M. Osteolysis: A Literature Review of Basic Science and Potential Computer-Based Image Processing Detection Methods. Comput. Intell. Neurosci. 2021, 2021, 4196241. [Google Scholar] [CrossRef]
- Yu, B.; Yang, G.; Wang, W.; Zhang, L.; Lin, X. Cross-Linked versus Conventional Polyethylene for Total Knee Arthroplasty: A Meta-Analysis. J. Orthop. Surg. 2016, 11, 39. [Google Scholar] [CrossRef]
- Bistolfi, A.; Giustra, F.; Bosco, F.; Faccenda, C.; Viotto, M.; Sabatini, L.; Berchialla, P.; Sciannameo, V.; Graziano, E.; Massè, A. Comparable Results between Crosslinked Polyethylene and Conventional Ultra-High Molecular Weight Polyethylene Implanted in Total Knee Arthroplasty: Systematic Review and Meta-Analysis of Randomised Clinical Trials. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3120–3130. [Google Scholar] [CrossRef]
- Giustra, F.; Bistolfi, A.; Bosco, F.; Fresia, N.; Sabatini, L.; Berchialla, P.; Sciannameo, V.; Massè, A. Highly Cross-Linked Polyethylene versus Conventional Polyethylene in Primary Total Knee Arthroplasty: Comparable Clinical and Radiological Results at a 10-Year Follow-Up. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Chawla, K.K. Mechanical Behavior of Materials, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-86675-0. [Google Scholar]
- Zhao, D.; Sakoda, H.; Sawyer, W.G.; Banks, S.A.; Fregly, B.J. Predicting Knee Replacement Damage in a Simulator Machine Using a Computational Model with a Consistent Wear Factor. J. Biomech. Eng. 2008, 130, 011004. [Google Scholar] [CrossRef] [PubMed]
- McGloughlin, T.M.; Kavanagh, A.G. Wear of Ultra-High Molecular Weight Polyethylene (UHMWPE) in Total Knee Prostheses: A Review of Key Influences. Proc. Inst. Mech. Eng. [H] 2000, 214, 349–359. [Google Scholar] [CrossRef] [PubMed]
- De Baets, T.; Waelput, W.; Bellemans, J. Analysis of Third Body Particles Generated during Total Knee Arthroplasty: Is Metal Debris an Issue? Knee 2008, 15, 95–97. [Google Scholar] [CrossRef]
- Kulkarni, P.G.; Paudel, N.; Magar, S.; Santilli, M.F.; Kashyap, S.; Baranwal, A.K.; Zamboni, P.; Vasavada, P.; Katiyar, A.; Singh, A.V. Overcoming Challenges and Innovations in Orthopedic Prosthesis Design: An Interdisciplinary Perspective. Biomed. Mater. Devices 2024, 2, 58–69. [Google Scholar] [CrossRef]
- Estok, D.M.; Bragdon, C.R.; Plank, G.R.; Huang, A.; Muratoglu, O.K.; Harris, W.H. The Measurement of Creep in Ultrahigh Molecular Weight Polyethylene. J. Arthroplast. 2005, 20, 239–243. [Google Scholar] [CrossRef]
- Teeter, M.G.; Parikh, A.; Taylor, M.; Sprague, J.; Naudie, D.D. Wear and Creep Behavior of Total Knee Implants Undergoing Wear Testing. J. Arthroplast. 2015, 30, 130–134. [Google Scholar] [CrossRef]
- Valigi, M.; Logozzo, S.; Affatato, S. New Challenges in Tribology: Wear Assessment Using 3D Optical Scanners. Materials 2017, 10, 548. [Google Scholar] [CrossRef]
- Affatato, S.; Valigi, M.; Logozzo, S. Wear Distribution Detection of Knee Joint Prostheses by Means of 3D Optical Scanners. Materials 2017, 10, 364. [Google Scholar] [CrossRef]
- Kornuijt, A.; Kuijer, P.P.F.M.; Van Drumpt, R.A.; Siebelt, M.; Lenssen, A.F.; Van Der Weegen, W. A High Physical Activity Level after Total Knee Arthroplasty Does Not Increase the Risk of Revision Surgery during the First Twelve Years: A Systematic Review with Meta-Analysis and GRADE. Knee 2022, 39, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Ngai, V.; Kunze, J.; Cip, J.; Laurent, M.P.; Jacobs, J.J.; Wimmer, M.A. Backside Wear of Tibial Polyethylene Components Is Affected by Gait Pattern: A Knee Simulator Study Using Rare Earth Tracer Technology. J. Orthop. Res. 2020, 38, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.A.; Crossett, L.S.; Jacobs, J.J.; Rubash, H.E. Malrotation Causing Patellofemoral Complications After Total Knee Arthroplasty. Clin. Orthop. 1998, 356, 144–153. [Google Scholar] [CrossRef] [PubMed]

| Study, Year | Insert Type | Implant | Nu. | Time In Situ (Months) | Assessment/Measuring Method | Wear Rate | Notes/Key Findings |
|---|---|---|---|---|---|---|---|
| Li et al. [11], 2002 | FB | Anatomic Modular Knee (AMK)®, Genesis®, Insall-Burstein II®, PFC® | 55 | (1–73) | Visual assessment of wear patterns, CMM | NA | Backside PE wear results as a significant contributor to the generation of PE debris. |
| Conditt et al. [12], 2005 | CR | AMK® | 15 | (36–146) | Surface laser profilometry | 138 ± 95 mm3/year * | The predicted volume PE loss because of backside wear is substantial and may be sufficient to induce osteolysis. |
| Ho et al. [13], 2007 | MB, FB | New Jersey LCS® RP, Miller Galante I® FB | 51 | 115 (48–162) | Visual assessment of wear patterns | NA | Low-grade wear (burnishing, abrasion, and cold flow) more common in MB knees; high-grade wear (scratching, pitting, metal embedding, and delamination) more common in FB knees. |
| Garcia et al. [14], 2009 | MB | LCS® RP, Sigma® RP | 40 | 30 (0.3–191) | Visual assessment of wear patterns | NA | No significant difference between medial and lateral wear. Wear increases with time in situ, patients’ weight, and BMI. Increased wear in unstable knees. |
| Lu et al. [15], 2010 | MB, FB | LCS®, PCA®, Miller-Galante® | 73 | 121 (48–162) | Visual assessment of wear patterns | NA | Increased articular surface wear in FB knees. Increased backside wear in MB knees. Wear in MB knees not associated with time in situ and BMI. |
| Srivastava et al. [16], 2011 | CR | PFC® | 16 | 92.4 (12–156) | Visual assessment of wear patterns, Laser scanner CMM | 131 ± 78 mm3/year | Varus malalignment of the tibial component (≥3°) increases medial compartment wear and overall insert wear even when the knee is in neutral alignment. |
| Knowlton et al. [17], 2012 | CR | Miller-Galante II® | 9 | (4.1–30.2) | Laser scanner CMM | 39.2 ± 7.2 mm3/year | An autonomous mathematical reconstruction can be used to effectively measure volume loss in retrieved tibial inserts. |
| Wimmer et al. [18], 2012 | CCK/PS, CR | Insall-Burstein II®, Miller-Galante II® | 69 | 18.6 (0.8–59.7), 19.9 (0.4–64.3) | Visual assessment of wear patterns | NA | More conformity (CCK/PS) increase surface fatigue damage in TKA compared to less conform designs (CR). |
| Berry et al. [19], 2012 | MB, FB | a Sigma® RP, b Sigma® with Ti or CoCr trays | 312 | a 36 (0.4–124), b 72 (2–179) | Insert thicknesses measurements using a dial indicator | a 0.04 mm/year; b 0.07 mm/year (total); b 0.02 mm/year *; b 44 mm3/year * | Wear rate lower in RP compared to FB inserts. Backside wear rate lower for FB inserts mated with CoCr trays than for rough Ti trays. Inserts against polished trays (RP or FB) showed no increase in wear rate over time. The wear rate of PS and CR inserts was not different. |
| Stoner et al. [20], 2013 | MB, FB | PFC® Sigma RP, PFC® Sigma | 42 | 24 (5–46) 65 (6–134) | Visual assessment of wear patterns | NA | The increased total damage score on the RP, coupled with increased surface area damaged and the propensity for third-body debris, indicates no damage advantage to MB design. |
| Paterson et al. [21], 2013 | HF, PS | Genesis® II | 40 | 20 (4–43) | Visual assessment of wear patterns, micro-CT | NA | Increased wear of post and backside surface of HF inserts due to ability of higher flexion of the knee. |
| Engh et al. [22], 2013 | MB, FB | LCS®, Sigma® RP, PFC® Sigma | 24 | 52 | Volumetric wear analysis with micro-CT | 43 ± 25 mm3/year; 74 ± 49 mm3/year | Micro-CT can determine the volume and location of wear in retrieved inserts. Significant influence of manufacturer tolerance when short follow-up or low wear present. A more accurate method for greater wear and simulator studies when repeated measurements possible. |
| Pang et al. [23], 2014 | PS | Genesis II® | 83 | 42 (4–124) | Visual assessment of wear patterns | NA | Limb malalignment (varus) and JLE (>5 mm) resulted in more wear. In cases with JLE, an external rotation subluxation wear pattern was found with more damage over the posteromedial aspect of the post. |
| Holleyman et al. [24], 2014 | FB | many different | 30 | 156 (36–240) | Profilometry with a non-contact profilometer | NA | Surface roughness and skewness measured on the insert undersurface and the tibial baseplate of explants. Decreased backside wear found when a polished tibial tray was used compared to an unpolished design. |
| Schwarzkopf et al. [25], 2015 | CR | PFC® | 70 | 142 (15–289) | Insert thicknesses measurements | a 0.0063 mm/year *; 14.2 mm3/year *; b 0.05 mm/year *; 117 mm3/year * | More conforming b (curved) tibial inserts demonstrated more backside-normalized wear than the flatter a (posterior lipped) inserts. |
| Knowlton et al. [26], 2017 | CR | Miller-Galante® II | 64 | 36 (0.4–108) | Visual assessment of wear patterns, video microscopy, low-incidence laser CMM | 12.9 ± 5.97 mm3/year; 0.035 ± 0.017 mm/year medially; 0.034 ± 0.011 mm/year laterally | Although striated patterns were the main contributors to volume loss, visual damage patterns were only moderate predictors. Geometric volume loss provides a more accurate quantification of in vivo wear. |
| Li et al. [27], 2017 | PS, CCK | many different | 156 | >24 | Visual assessment of wear patterns | NA | Higher damage in TKAs with postoperative varus alignment. |
| Łapaj et al. [28], 2017 | CR, PS | many different | 102 | 30 | Visual assessment of wear patterns | NA | A smooth tibial tray surface and a peripheral locking mechanism reduce backside wear in vivo. No significant differences were found between damage scores in CR vs PS inlays or between genders. |
| Sisko et al. [29], 2017 | CR, PS | AMK®, Sigma®, Scorpio®, Triathlon®, Genesis® II | 30 | >48 | Visual assessment of wear patterns, linear wear measurement with micro-CT | Polished designs 0.0102 ± 0.0044 mm/year *; Non-polished designs 0.0224 ± 0.0119 mm/year * | Total backside damage scores and linear wear rates were highest, involving the non-polished design with only a peripheral rim capture. |
| Affatato et al. [30], 2020 | CR, PS | many different | 12 | 70 (12–139) | Visual assessment of wear patterns, topographical analysis | NA | Damage patterns consistent with respect to the main prosthetic components movements. No significant difference found between surface roughness measurements, patient BMI, age at revision, and time in situ. |
| Pourzal et al. [31], 2020 | CR | NexGen® | 59 | 61.2 | Laser scanner CMM | 11.6 mm3/year | Increased wear rates with JLE and increased PTS (>7° vs. <3°). Increased backside wear scores of medium compared to large insert size. |
| Tone et al. [32], 2020 | UC, PS | Inserts form B. Braun Aesculap (Tuttlingen, Germany) | 13 | a (0.75–8.5); b (15.6–97.2) | Raman spectroscopy | a 0.055 ± 0.020 mm/year on med. LD; b 0.041 ± 0.020 mm/year on lat. LD | A strong correlation between the amount of wear and time in situ. Increased insert thickness reduction in medial a compared to lateral b load zone. |
| Currier et al. [10], 2021 | CR, PS | Sigma®, Sigma® RP, NexGen®, Triathlon®, Genesis® II | 1585 | 58 (0–290) | Calculation of wear based on the reference thickness of inserts with survival <16 months | from 0.021 mm/year (PJI group) to 0.067 mm/year (PE wear group) | Wear rate increased with duration in vivo. Lower wear rates associated with older patients, females, polished modular tibial tray surfaces, HXLPE, and constrained TKA designs. |
| n = 57 | |
|---|---|
| Gender, female; n (%) | 33 (57.9%) |
| Age, years; mean ± SD | 69.6 ± 7.8 |
| BMI; mean ± SD | 32.2 ± 5.1 |
| Time in situ, months; median (IQR) | 17.4 (4.4–32.0) |
| Linear wear, mm; median (IQR) | 0.18 (0.11–0.32) |
| Volumetric wear, mm3; median (IQR) | 63.7 (32.6–114.1) |
| TKA side, right; n (%) | 29 (50.9%) |
| Insert size; n (%) | |
| small | 12 (21.1%) |
| medium | 21 (36.8%) |
| large | 24 (42.1%) |
| Nominal component size ratio (F:I), n (%) | |
| ≤1 | 31 (54.4%) |
| >1 | 26 (45.6%) |
| All | Confirmed PJI | Osteolysis | Loosening | Malalignment/Malposition | Instability | Other | |
|---|---|---|---|---|---|---|---|
| One | 26 (45.6%) | 17 (54.8%) | 2 (8.3%) | 0 | 3 (10.7%) | 2 (16.6%) | 2 (100%) |
| Two | 13 (22.8%) | 8 (25.8%) | 4 (16.7%) | 1 (5.6%) | 10 (35.7%) | 3 (25.0%) | 0 |
| Three | 9 (15.8%) | 3 (9.7%) | 9 (37.5%) | 8 (44.4%) | 6 (21.4%) | 1 (8.3%) | 0 |
| Four | 9 (15.8%) | 3 (9.7%) | 9 (37.5%) | 9 (50.0%) | 9 (31.1%) | 6 (50.0%) | 0 |
| Estimate (95% CI) | p-Value | |
|---|---|---|
| Time in situ, months | 0.96 (0.21–1.71) | 0.013 |
| Confirmed PJI | ||
| Yes | 23.96 (−10.9–58.82) | 0.174 |
| No | Ref. | |
| Osteolysis and clinical loosening of a component | ||
| Both causes | 41.16 (10.98–71.34) | 0.009 |
| Osteolysis alone | 52.06 (10.02–94.09) | 0.016 |
| No | Ref. | |
| Joint malalignment or component malposition | ||
| Yes | 25.23 (−2.26–52.72) | 0.071 |
| No | Ref. | |
| Instability | ||
| Yes | 23.16 (−14.38–60.7) | 0.221 |
| No | Ref. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valič, M.; Milošev, I.; Levašič, V.; Blas, M.; Podovšovnik, E.; Koren, J.; Trebše, R. Linear and Volumetric Polyethylene Wear Patterns after Primary Cruciate-Retaining Total Knee Arthroplasty Failure: An Analysis Using Optical Scanning and Computer-Aided Design Models. Materials 2024, 17, 5007. https://doi.org/10.3390/ma17205007
Valič M, Milošev I, Levašič V, Blas M, Podovšovnik E, Koren J, Trebše R. Linear and Volumetric Polyethylene Wear Patterns after Primary Cruciate-Retaining Total Knee Arthroplasty Failure: An Analysis Using Optical Scanning and Computer-Aided Design Models. Materials. 2024; 17(20):5007. https://doi.org/10.3390/ma17205007
Chicago/Turabian StyleValič, Matej, Ingrid Milošev, Vesna Levašič, Mateja Blas, Eva Podovšovnik, Jaka Koren, and Rihard Trebše. 2024. "Linear and Volumetric Polyethylene Wear Patterns after Primary Cruciate-Retaining Total Knee Arthroplasty Failure: An Analysis Using Optical Scanning and Computer-Aided Design Models" Materials 17, no. 20: 5007. https://doi.org/10.3390/ma17205007
APA StyleValič, M., Milošev, I., Levašič, V., Blas, M., Podovšovnik, E., Koren, J., & Trebše, R. (2024). Linear and Volumetric Polyethylene Wear Patterns after Primary Cruciate-Retaining Total Knee Arthroplasty Failure: An Analysis Using Optical Scanning and Computer-Aided Design Models. Materials, 17(20), 5007. https://doi.org/10.3390/ma17205007







