Interpenetrated Polymer Network Systems (PEG/PNIPAAm) Using Gamma Irradiation: Biological Evaluation for Potential Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterization
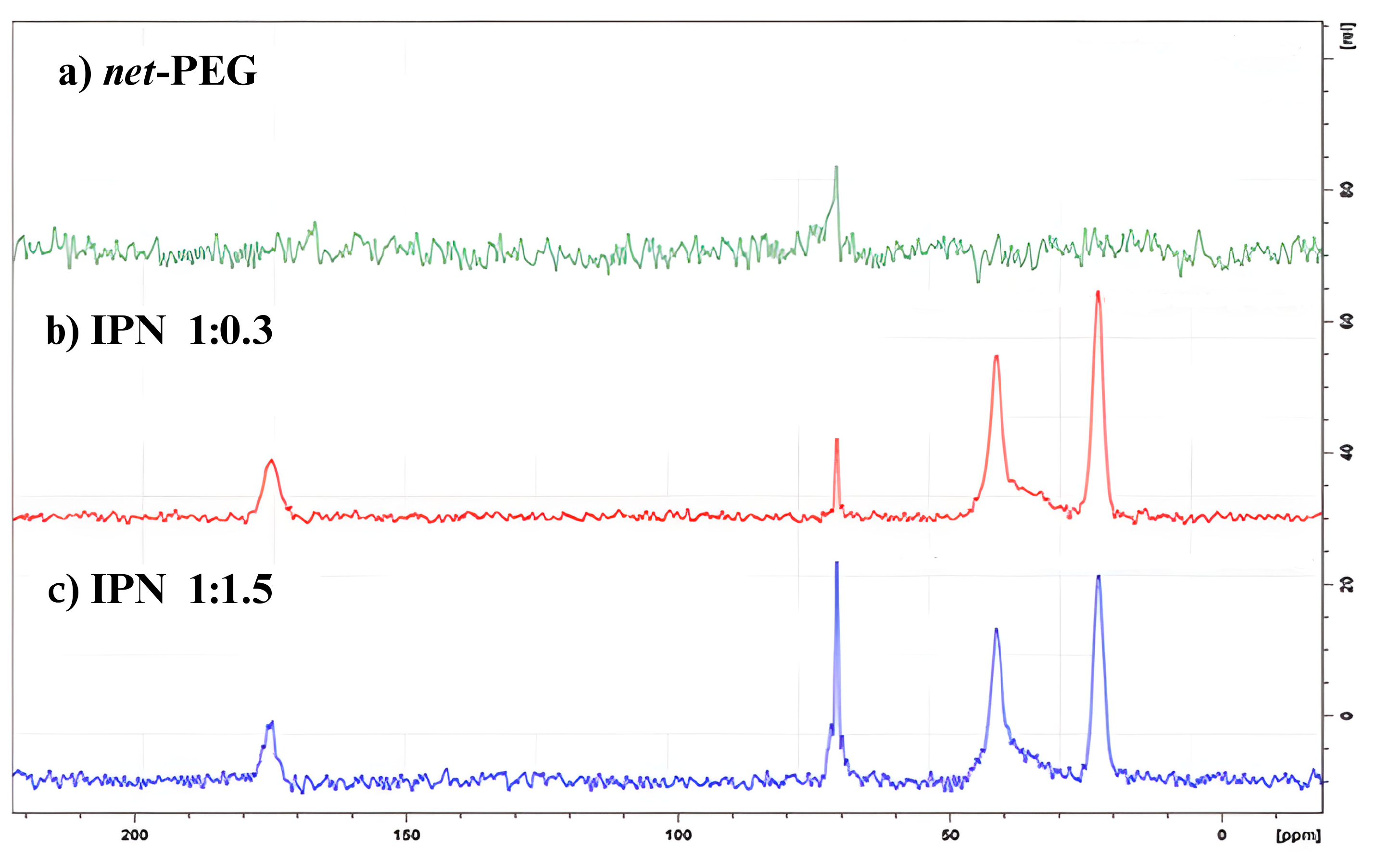
13C Solid-State Nuclear Magnetic Resonance
2.4. Mechanical Tests
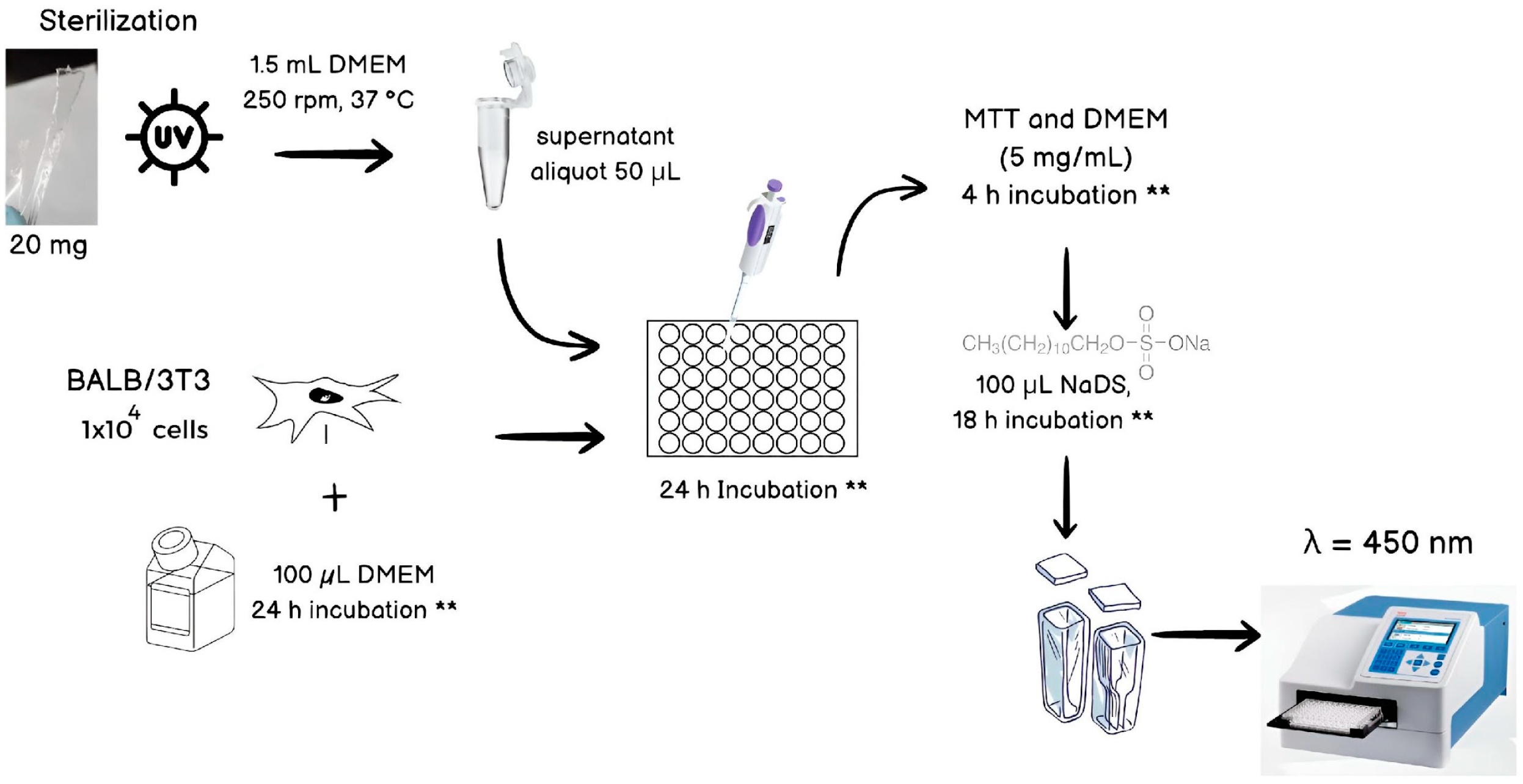
2.5. Biological Tests
2.5.1. Antimicrobial Test
2.5.2. Antibiofouling Tests (Ovalbumin Adsorption)
2.5.3. Cytotoxicity Test
3. Results
3.1. Characterization
13C Solid-State Nuclear Magnetic Resonance (CP/TOSS NMR)
3.2. Mechanical Tests
3.3. Biological Tests
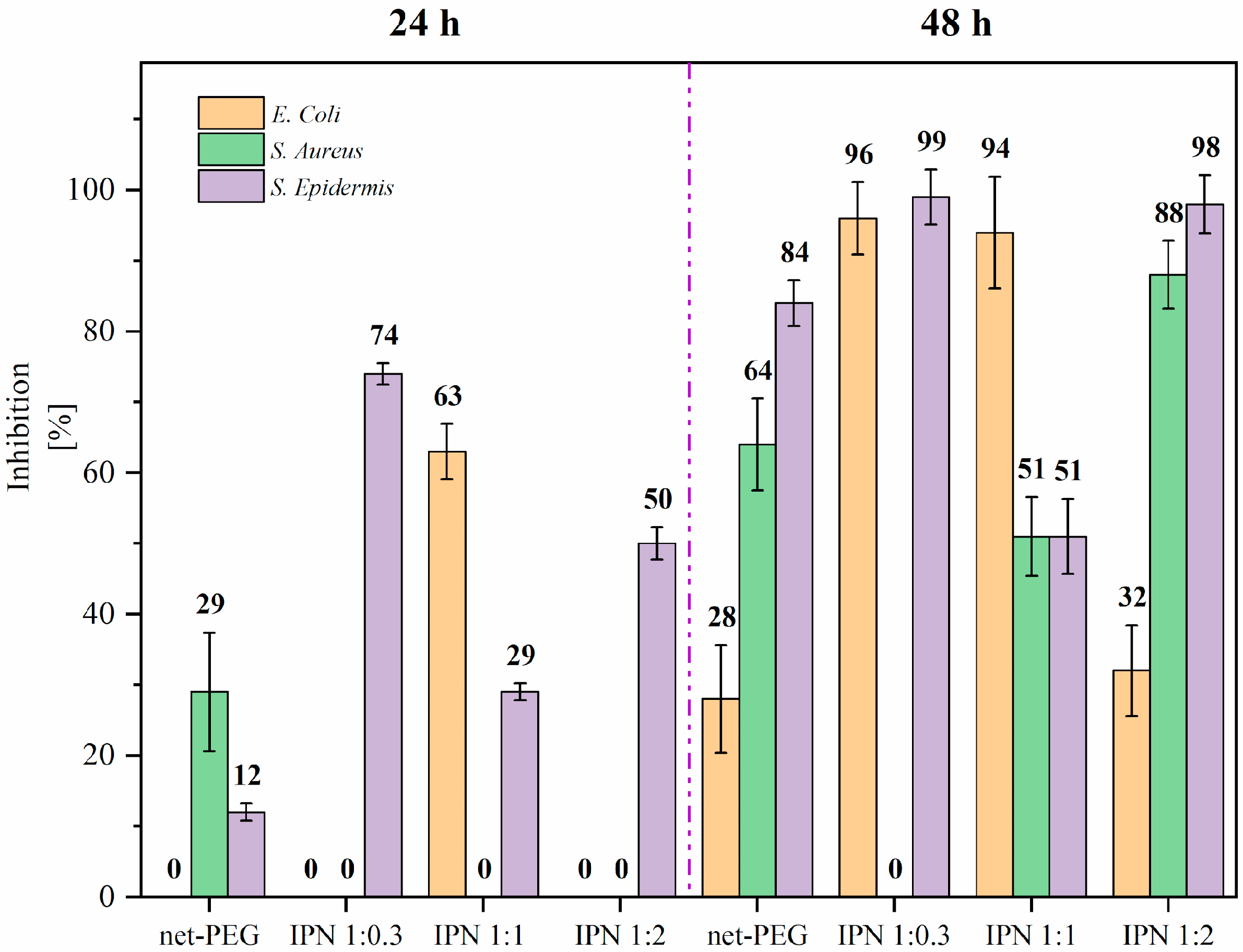
3.3.1. Antimicrobial Test
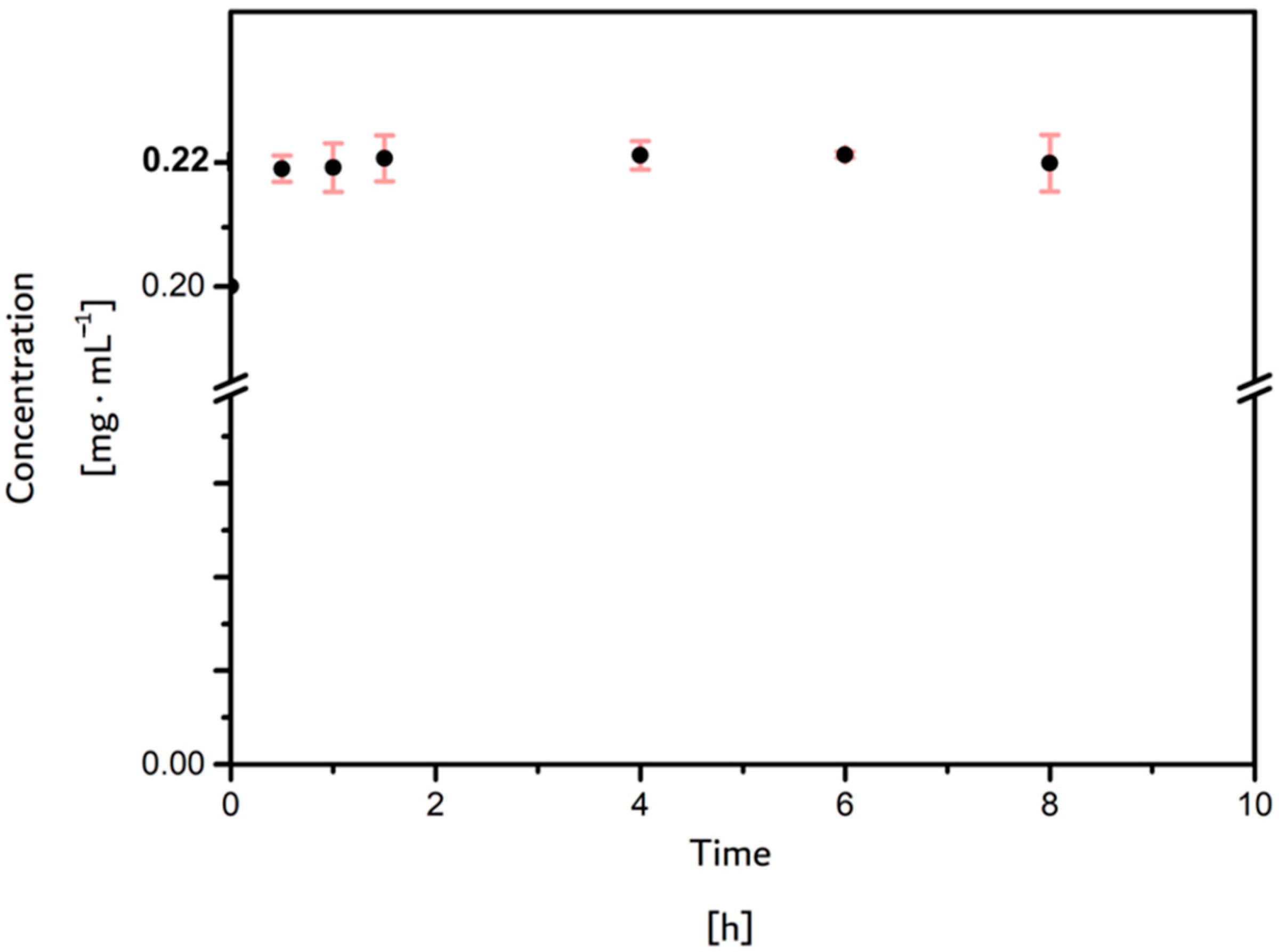
3.3.2. Antibiofouling Tests (Ovalbumin Adsorption)
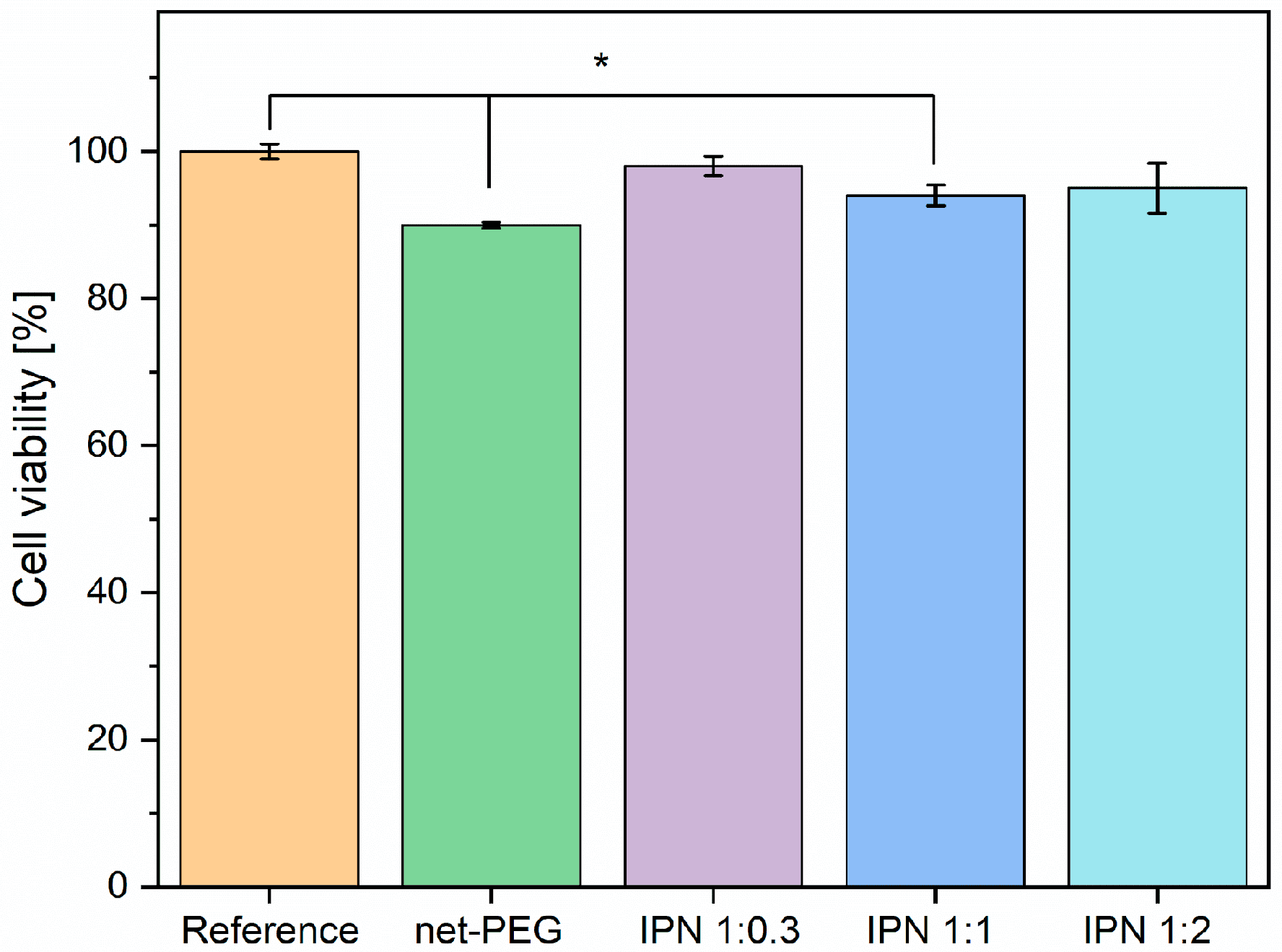
3.3.3. Cytotoxicity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and Biocompatibility: An Historical Overview. J. Biomed. Mater. Res. Part A 2020, 108, 1617–1633. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M. Standardization and Regulation of Biomaterials; LTD: Hongkong, China, 2020; ISBN 9780081029671. [Google Scholar]
- Alós, J.I. Antibiotic Resistance: A Global Crisis. Enferm. Infecc. Microbiol. Clin. 2015, 33, 692–699. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 10 September 2024).
- Malheiro, J.; Simões, M. Antimicrobial Resistance of Biofilms in Medical Devices; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780081003985. [Google Scholar]
- Almalki, M.A.; Varghese, R. Prevalence of Catheter Associated Biofilm Producing Bacteria and Their Antibiotic Sensitivity Pattern. J. King Saud Univ. Sci. 2020, 32, 1427–1433. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of Healthcare-Associated Infections Connected to Medical Devices—An Update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Parsek, M.R. New Insight into the Early Stages of Biofilm Formation. Proc. Natl. Acad. Sci. USA 2018, 115, 4317–4319. [Google Scholar] [CrossRef]
- Floyd, K.A.; Eberly, A.R.; Hadjifrangiskou, M. Adhesion of Bacteria to Surfaces and Biofilm Formation on Medical Devices; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081003985. [Google Scholar]
- Arunasri, K.; Mohan, S.V. Biofilms: Microbial Life on the Electrode Surface; Elsevier B.V.: Amsterdam, The Netherlands, 2018; ISBN 9780444640529. [Google Scholar]
- Tathe, A.; Ghodke, M.; Nikalje, A.P. A Brief Review: Biomaterials and Their Apllication. Int. J. Pharm. Pharm. Sci. 2010, 2, 19–23. [Google Scholar]
- Lanzalaco, S.; Armelin, E. Poly(N-Isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Schultz, T.W.; Yarbrough, J.W.; Koss, S.K. Identification of Reactive Toxicants: Structure-Activity Relationships for Amides. Cell Biol. Toxicol. 2006, 22, 339–349. [Google Scholar] [CrossRef]
- Wadajkar, A.S.; Koppolu, B.; Rahimi, M.; Nguyen, K.T. Cytotoxic Evaluation of N-Isopropylacrylamide Monomers and Temperature-Sensitive Poly(N-Isopropylacrylamide) Nanoparticles. J. Nanoparticle Res. 2009, 11, 1375–1382. [Google Scholar] [CrossRef]
- Malonne, H.; Eeckman, F.; Fontaine, D.; Otto, A.; De Vos, L.; Moës, A.; Fontaine, J.; Amighi, K. Preparation of Poly(N-Isopropylacrylamide) Copolymers and Preliminary Assessment of Their Acute and Subacute Toxicity in Mice. Eur. J. Pharm. Biopharm. 2005, 61, 188–194. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or Not to PEGylate: Immunological Properties of Nanomedicine’s Most Popular Component, Polyethylene Glycol and Its Alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, D.; Gu, J.; Zhang, H.; Wu, X.; Cao, C.; Zhang, X.; Liu, R. The Impact of Antifouling Layers in Fabricating Bioactive Surfaces. Acta Biomater. 2021, 126, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of the Mechanical Properties of Biomaterials on Degradability, Cell Behaviors and Signaling Pathways: Current Progress and Challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Polyethylene Glycol (PEG)-Poly(N-Isopropylacrylamide) (PNIPAAm) Based Thermosensitive Injectable Hydrogels for Biomedical Applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef]
- Ahmadkhani, L.; Abbasian, M.; Akbarzadeh, A. Synthesis of Sharply Thermo and PH Responsive PMA-b-PNIPAM-b-PEGB-PNIPAM-b-PMA by RAFT Radical Polymerization and Its Schizophrenic Micellization in Aqueous Solutions. Des. Monomers Polym. 2017, 20, 406–418. [Google Scholar] [CrossRef]
- Ramos-Ballesteros, A.; Pino-Ramos, V.H.; López-Saucedo, F.; Flores-Rojas, G.G.; Bucio, E. γ-Rays and Ions Irradiation. Surf. Modif. Polym. 2019, 185–209. [Google Scholar] [CrossRef]
- Rana, M.M.; De La Hoz Siegler, H. Tuning the Properties of Pnipam-Based Hydrogel Scaffolds for Cartilage Tissue Engineering. Polymers 2021, 13, 3154. [Google Scholar] [CrossRef]
- Cruz-Gómez, A.; Pérez-Calixto, M.; Velazco-Medel, M.A.; Burillo, G. Antifouling IPNs Made of Poly(Ethylene Glycol)/Poly(N-Isopropyl Acrylamide) Using Gamma Radiation. MRS Commun. 2022, 12, 272–278. [Google Scholar] [CrossRef]
- Pérez-Calixto, D.; Amat-Shapiro, S.; Zamarrón-Hernández, D.; Vázquez-Victorio, G.; Puech, P.H.; Hautefeuille, M. Determination by Relaxation Tests of the Mechanical Properties of Soft Polyacrylamide Gels Made for Mechanobiology Studies. Polymers 2021, 13, 629. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Y.; Song, X.; Zhang, Z.; Xiao, C.; He, C.; Chen, X. PH- and Thermo-Responsive Poly(N-Isopropylacrylamide-Co-Acrylic Acid Derivative) Copolymers and Hydrogels with LCST Dependent on PH and Alkyl Side Groups. J. Mater. Chem. B 2013, 1, 5578–5587. [Google Scholar] [CrossRef] [PubMed]
- Nalawade, T.; Bhat, K.; Sogi, S.P. Bactericidal Activity of Propylene Glycol, Glycerine, Polyethylene Glycol 400, and Polyethylene Glycol 1000 against Selected Microorganisms. J. Int. Soc. Prev. Community Dent. 2015, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Teixeira, P.; Oliveira, R. Mini-Review: Staphylococcus Epidermidis as the Most Frequent Cause of Nosocomial Infections: Old and New Fighting Strategies. Biofouling 2014, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Kambara, M.; Matsumura, H.; Norde, W. Protein Adsorption at Polymer-Grafted Surfaces: Comparison between a Mixture of Saliva Proteins and Some Well-Defined Model Proteins. Biofouling 2003, 19, 355–363. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.

| Sample | PEG:NiPAAm Relation | E [kPa] | E∞ [kPa] |
|---|---|---|---|
| net-PEG | 1:0 | 56.76 ± 11.77 | 26.61 ± 14.57 |
| (net-PEG)-inter-(net-PNiPAAm) | 1:0.3 | 12.16 ± 5.95 | 9.28 ± 4.55 |
| 1:1.5 | 14.05 ± 4.64 | 10.97 ± 5.09 | |
| 1:3 | 52.43 ± 10.72 | 46.00 ± 9.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Gómez, A.; Burillo, G.; Perez-Calixto, D.; Palomino, K.; Magaña, H. Interpenetrated Polymer Network Systems (PEG/PNIPAAm) Using Gamma Irradiation: Biological Evaluation for Potential Biomedical Applications. Materials 2024, 17, 4998. https://doi.org/10.3390/ma17204998
Cruz-Gómez A, Burillo G, Perez-Calixto D, Palomino K, Magaña H. Interpenetrated Polymer Network Systems (PEG/PNIPAAm) Using Gamma Irradiation: Biological Evaluation for Potential Biomedical Applications. Materials. 2024; 17(20):4998. https://doi.org/10.3390/ma17204998
Chicago/Turabian StyleCruz-Gómez, Angélica, Guillermina Burillo, Daniel Perez-Calixto, Kenia Palomino, and Héctor Magaña. 2024. "Interpenetrated Polymer Network Systems (PEG/PNIPAAm) Using Gamma Irradiation: Biological Evaluation for Potential Biomedical Applications" Materials 17, no. 20: 4998. https://doi.org/10.3390/ma17204998
APA StyleCruz-Gómez, A., Burillo, G., Perez-Calixto, D., Palomino, K., & Magaña, H. (2024). Interpenetrated Polymer Network Systems (PEG/PNIPAAm) Using Gamma Irradiation: Biological Evaluation for Potential Biomedical Applications. Materials, 17(20), 4998. https://doi.org/10.3390/ma17204998







