Abstract
While particulate bone substitute materials are applied in a variety of augmentation procedures, standardized defects are being used for preclinical testing. This in vitro study evaluated the density and homogeneity of a particulate bone substitute in ridge preservation procedures. Premolars and molars were extracted in ten semimandibles of minipig cadavers. Light body impression material was used for determining the volume of the extraction sites followed by augmentation with particulate material, thereby weighing the graft material needed. Microradiographs and histologic sections were obtained for evaluating the homogeneity and density of the augmentation material. Statistical analyses were based on Shapiro–Wilk tests, Spearman’s rho and one sample Wilcoxon test followed by Bonferroni–Holm correction for multiple testing (α = 0.05). Based on 103 single alveoli evaluated, the mean volume determined was 0.120 cm3 requiring a mean amount of graft material of 0.155 g. With only three exceptions, all parameters (volume, mass of augmentation material, density and homogeneity) correlated significantly (p < 0.020). The apical parts of the alveoli showed reduced density as compared to the middle parts (p < 0.001) and the homogeneity of the augmentation material was also lower as compared to the middle (p < 0.001) and cervical parts (p </= 0.040). The packing of augmentation material is critical when non-standardized defects are treated.
1. Introduction
Prosthetic-driven implant placement frequently requires bone augmentation [1,2,3] or at least the prevention of bone loss following tooth extraction [4,5,6,7]. Similarly, bone augmentation has been described for implants immediately placed in extraction sockets for filling up empty spaces between implants and socket walls [8]. Such situations are comparable to crater-like peri-implantitis lesions [9], which are not only difficult to assess during preoperative imaging [10,11] but also require regenerative treatment following debridement and disinfection [12,13].
While the choice of augmentation technique and biomaterial depends on the defect morphology [2,14], particulate bone substitute materials [1,15] play an important role in implant-related surgeries [14]. Heavily affecting the regenerative potential [16], the shape of the granules [17], pore size within the granules, as well as particle size [18] and interconnectivity of pores have received lots of attention [19,20] in order to optimize the vascularization of the graft [21] and subsequent mechanical bone quality [22,23].
It has been argued based on a clinical study that the complete fill of random-shaped bony defects cannot be achieved predictably [9]. As one of the few options for intervention during augmentation, but at the same time a critical factor, the level of compaction of augmentation material cannot be standardized and depends on the clinical experience of the surgeon [24,25]. In a recent animal study, it was shown that compressive forces in the range of 200 g would facilitate the penetration of particulate graft materials into apical regions of sockets and defects and hence optimize bone formation [24,26].
This basic problem of adapting hard bone filling material to a specific surgical site is already well known from the non-dental field [27]. Using calcium phosphate-based composites in the form of injectable bone substitutes may constitute a convenient alternative, thereby avoiding the problem of incomplete or non-homogenous socket filling. Calcium phosphates have been investigated due to their similarity to the mineral component of bone [27] and various attempts have already been made in order to optimize the handling properties of the material [28]. An interesting approach was described and tested by Klijn et al. [29] adding NaHCO3, Na2HPO4 and NaH2PO4 in specific concentrations and in a strict order to calcium phosphate, which led to the production of CO2 from NaHCO3 [29]. The rationale for this approach was to introduce adequate pores and interconnectivity into the bone substitute.
From a scientific and regulatory point of view, bone augmentation materials are often tested in standardized defects [22,30] in order to allow for a quantitative analysis of healing processes [31,32], which does not reflect reality where, e.g., extraction sockets [26] with variable size and shape are present [5,30], which may affect the regenerative potential.
The primary goal of this animal cadaver study was to evaluate intraalveolar voids being present following augmentation with one specific particulate bone substitute material by determining homogeneity and density. From a methodologic point of view, a secondary endpoint was to compare microradiographic and histologic analysis.
2. Materials and Methods
Ten semi mandibles of adult Aachen minipig cadavers [25,30] were obtained (Heinrichs Tierzucht, Heinsberg, Germany) and the premolars P3 and P4 as well as the first molar M1 were carefully extracted using standard instruments. The premolars had two roots each (mesial and distal) while the molars had four roots (mesiolingual, distolingual, mesiobuccal and distobuccal). The complete extraction of all roots was verified using periapical radiographs (Heliodent, Dentsply Sirona, York, PA, USA/VistaScan image plates, Dürr Dental, Bietigheim-Bissingen, Germany).
Light body silicone impression material (Silasoft, Detax, Ettlingen, Germany; density: 1.17 g/cm3) was subsequently injected into the alveoli and allowed to fully set. After removal, the silicone impressions were weighed for determining the accessible volume. Mean values based on five impressions were noted for statistical analyses.
Particulate bone augmentation material (Creos, Nobel Biocare, Gothenburg, Sweden) was then used for filling the single alveoli, thereby measuring the amount of material needed until the ridge crest is reached. The alveoli were subsequently covered with wax paper mimicking coverage with a membrane in order to avoid the loss of granules during subsequent processing.
All bone specimens were fixed in 10% neutral buffered formalin for 48 h and reduced to small blocks using a diamond band saw (EXAKT 300, EXAKT Advanced Technologies GmbH, Norderstedt, Germany). The specimens were then dehydrated in alcohol solutions of increasing concentrations, clarified in xylene and embedded in polymethylmethacrylate (Technovit 9100, Heraeus Kulzer, Hanau, Germany). One vertical cross-section was obtained per specimen using a cutting and grinding technique [33]. With the sections reduced to a thickness of 120 µm, microradiographs (Faxitron X-ray, Lincolnshire, IL, USA; 14 kV, 0.3 mA, 2.5 min; VistaScan image plates) were made. Following a further reduction of the sections to a thickness of 50–80 µm and staining with toluidine blue O solution after preprocessing in 10% H2O2, the samples were inspected using a microscope (LEICA DM4B, LEICA Mikrosysteme Vertrieb GmbH, Wetzlar, Germany) equipped with a color image analyzing system (LEICA Application Suite, LEICA Phase Expert, LEICA Mikrosysteme Vertrieb GmbH). A representative image of each alveolus was taken depicting its complete outline. Both microradiographs and histologic sections were then evaluated by three independent examiners with the goal of rating the homogeneity (1 = no; 2 = partly; 3 = yes) and density (1 = low; 2 = medium; 3 = high) of the augmentation material in the apical, middle and cervical third of each alveolus.
Statistical analyses were based on Shapiro–Wilk tests on the normal distribution of measurement values and ratings followed by calculating Spearman’s rank correlation coefficients between variables and one-sample Wilcoxon tests (Mann–Whitney tests) for comparisons. Given that ratings in different regions of the alveoli [24] could not be considered as being independent, rating differences (middle-apical; middle-cervical; and apical-cervical) were tested with respect to differing from zero. Correction for multiple testing was performed according to the Bonferroni–Holm method and the level of significance was set at α = 0.05.
3. Results
A total of 103 single alveoli were evaluated and the mean values and standard deviations for the volume of the alveoli (Figure 1) and mass of augmentation material required (Figure 2) as well as for ratings of density and homogeneity (Figure 3) are given in Table 1.
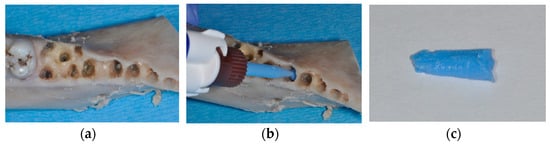
Figure 1.
Hemi-mandible of an Aachen minipig with premolars and first molar extracted (a). Injection of light body silicone into the alveoli for determining the volume available for augmentation (b). Impression material harvested from an alveolus, which was weighed for determining alveolar volume (c).
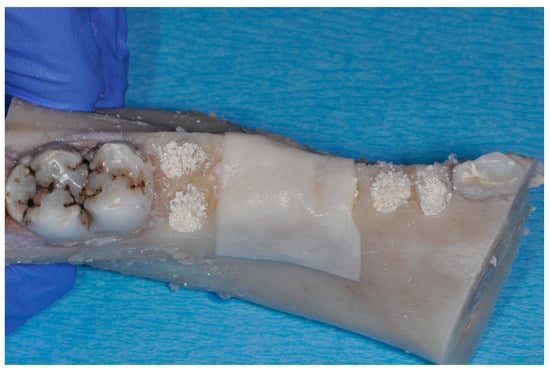
Figure 2.
Particulate bone substitute material was used for filling the single alveoli and the wax paper was adapted and secured in order to avoid particles from falling out during further processing.
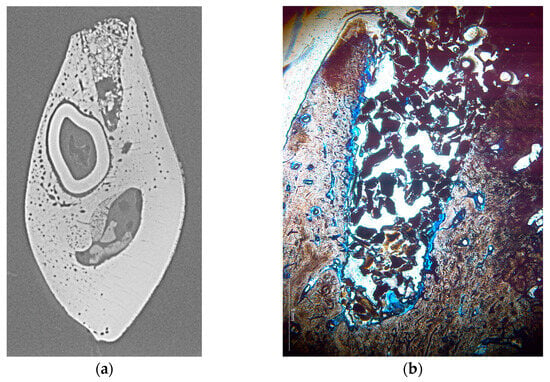
Figure 3.
Representative cross-section of two different alveoli shown as microradiograph (a) and histologic section (b). The microradiograph shows a considerable void within the bone substitute material and high density of the substitute material in the cervical area, while a comparably low density is visible in the histologic section which, however, appears as homogeneous throughout the alveolus.

Table 1.
Mean values and standard deviations for all measurements and ratings.
Shapiro–Wilk tests showed significant values (p < 0.040) for all parameters indicating a non-normal distribution requiring a non-parametric correlation test (Spearman’s rho). While correlation coefficients varied widely (Table 2), significant correlations were found after Bonferroni correction for all combinations of parameters with the following exceptions: Volume/Homogeneity—histology (p = 0.060); Density—microradiograph/Homogeneity—histology (p = 0.060); and Homogeneity—microradiograph/Homogeneity—histology (p = 0.060).

Table 2.
Correlation coefficients (Spearman’s rho) for all parameters evaluated.
Separating the cervical, middle and apical thirds of alveoli, mean values for density and homogeneity were calculated and differences between these regions were expressed as p-values (Table 3). In the apical regions (Figure 4a), significantly lower density of the augmentation material was reached as compared to the middle part of the alveoli (microradiograph p < 0.001; histology p < 0.001). In addition, the middle section showed greater density as compared to the cervical section in histology (p = 0.020) but not in microradiographs (p = 0.200). Similarly, the homogeneity of the augmentation material was significantly lower in the apical region as compared to the middle (microradiograph p < 0.001; histology p < 0.001) and cervical parts (microradiograph p = 0.040; histology p = 0.002). No differences were seen between the cervical and middle regions of the alveoli with respect to homogeneity (Figure 4b).

Table 3.
Mean values, SD and comparisons of apical, middle and cervical thirds of alveoli with respect to density and homogeneity.
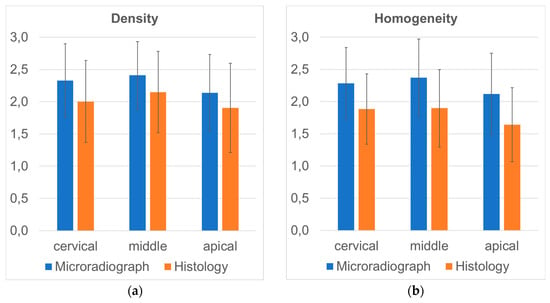
Figure 4.
Mean values for the parameter density (a) and homogeneity (b) of augmentation material recorded in the apical, middle and cervical thirds of alveoli using microradiographs and histology. Note: relative scale used here representing the rating system from 1 to 3.
4. Discussion
Questioning the relevance of standardized but unrealistic defects for evaluating biomaterials during preclinical testing [30], the primary goal of this study was to evaluate the homogeneity and density of augmentation materials in simulated ridge preservation procedures. While manufacturers try to optimize the particle size, pore size and interconnectivity of bone substitute materials [16], the handling of the material by clinicians may alter the overall porosity by applying insufficient or excessive compression [20]. Overall, alveolar ridge preservation has been described as an effective therapy preventing bone resorption [3,4], but the use of a particulate synthetic bone substitute has also been shown to interfere with the normal healing processes of alveolar bone [19] and a certain dependency on the exact grafting material used [34] may exist.
Being in line with a clinical report showing that a complete fill of random-shaped defects cannot be achieved predictably [9], less compaction and less homogeneity of augmentation material was seen in the apical parts of the alveoli. The middle third of the alveoli showed the best values for the parameters density and homogeneity, indicating a certain level of predictability. Taking into account inevitable variations in defect sizes following extractions, standard deviations calculated for the volume and mass of augmentation material did not exceed 35%, while ratings for density and homogeneity showed maximum standard deviations of 25%. The weak but mostly significant correlations found among all parameters studied further indicate the reliability of the data presented as well as the comparability of microradiographic and histologic analysis.
A recent animal study has shown that compressive forces in the range of 200 g acting on the crestal surface of an extraction socket are required for a particulate graft material to penetrate into apical areas [24]. While it is generally argued that voids would compromise bone formation, a calcium phosphate cement with an uneven distribution and shape of bubbles performed better in an animal model as compared to more uniform materials [29]. The authors argued that gas bubble formation for the in situ fabrication of optimal porosity would be hardly controllable as gas bubbles move through the augmentation material leading to greater voids in crestal areas [29]. Based on these findings, it may be argued that the overpacking of bone substitutes hindering access to necessary vasculature may be problematic, too [29]. However, a novel mineral–organic osteoconductive adhesive based on tetracalcium phosphate, phosphoserine and water has shown superior regenerative potential without displaying porosity upon placement [35].
Several limitations have to be considered when interpreting the findings presented. The animal model used here differs morphologically from human patients but is in line with a regularly used animal model for preclinical research [30]. As was pointed out in a previous report [36], the socket volume of human teeth is in the range of 0.5 mL, which is greater as compared to this animal model where, in addition, only single roots of teeth have been considered. Limited access to augmentation sites also plays a role in achieving uniform results, which has not been restricted in this experiment. In addition, working on cadaver bone excludes blood flow, which may be problematic in clinical settings. Also, the bone substitute material was not rehydrated prior to use in order to avoid an uncontrollable variable. As such, the results presented may be seen as best-case scenarios obtained under simplified conditions. Furthermore, conducting this study as a live animal experiment would have allowed us to evaluate the relevance of the void spaces seen with respect to the bone response. While the study at hand was aimed at evaluating the extent of graft compression during augmentation procedures, the limitations of the ex vivo study design hinder the transferal of the results into clinical practice immediately. A potential solution for the problem presented here, i.e., non uniformity of augmentation material, may be the in situ formation of a bone substitute, as previously tried for calcium phosphate-based materials [29]. From a methodologic point of view, the results are limited to this specific bone substitute as the shape of the granules may have an effect on defect filling [17]. As shown in a previous study [22], the material used here shows regenerative performance comparable to a more frequently used particulate bovine material, BioOss. Despite maximum care during processing, artefacts resulting from augmentation material being lost during processing cannot be excluded.
Author Contributions
Conceptualization, M.K. and T.G.-K.; methodology, T.G.-K.; software, S.D.; validation, S.D., V.K.-Q. and A.S.; formal analysis, T.G.-K.; investigation, S.D.; resources, M.K.; data curation, S.D.; writing—original draft preparation, M.K. and T.G.-K.; writing—review and editing, T.G.-K.; visualization, V.K.-Q.; supervision, T.G.-K.; project administration, A.S.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by Nobel Biocare, Gothenburg, Sweden, through the donation of the augmentation material.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the use of cadaver animal bone.
Data Availability Statement
Original data are available from the corresponding author upon reasonable request.
Acknowledgments
The experiments described in this paper were carried out by Samira Dahl in partial fulfillment of the requirements for the degree Dr. med. dent. at Saarland University, Homburg/Saar, Germany. The authors wish to thank Friedrich Graef, Department of Mathematics, University of Erlangen-Nuremberg, for statistical data analysis and Constanze Steiner, Homburg/Saar, for preparing the bone specimens.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ganz, S.D.; Valen, M. Predictable synthetic bone grafting procedures for implant reconstruction: Part two. J. Oral Implantol. 2002, 28, 178–183. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Gubler, M.; Romero-Bustillos, M.; Nicholas, C.L.; Zimmerman, M.B.; Barwacz, C.A. Efficacy of Alveolar Ridge Preservation: A Randomized Controlled Trial. J. Dent. Res. 2020, 99, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 21, 195–223. [Google Scholar] [CrossRef]
- Cardaropoli, G.; Araújo, M.; Hayacibara, R.; Sukekava, F.; Lindhe, J. Healing of extraction sockets and surgically produced—Augmented and non-augmented—Defects in the alveolar ridge. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 435–440. [Google Scholar] [CrossRef]
- Kunert-Keil, C.; Gredes, T.; Heinemann, F.; Dominiak, M.; Botzenhart, U.; Gedrange, T. Socket augmentation using a commercial collagen-based product—An animal study in pigs. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 177–183. [Google Scholar] [CrossRef]
- Pereira, F.P.; Hochuli-Vieira, E.; Maté Sánchez de Val, J.E.; De Santis, E.; Salata, L.A.; Botticelli, D. Bone Ceramic® at Implants Installed Immediately into Extraction Sockets in the Molar Region: An Experimental Study in Dogs. Clin. Implant Dent. Relat. Res. 2016, 18, 360–368. [Google Scholar] [CrossRef]
- Kahnberg, K.E. Immediate implant placement in fresh extraction sockets: A clinical report. Int. J. Oral Maxillofac. Implants 2009, 24, 282–288. [Google Scholar]
- Roccuzzo, M.; Bonino, F.; Bonino, L.; Dalmasso, P. Surgical therapy of peri-implantitis lesions by means of a bovine-derived xenograft: Comparative results of a prospective study on two different implant surfaces. J. Clin. Periodontol. 2011, 38, 738–745. [Google Scholar] [CrossRef]
- Song, D.; Shujaat, S.; de Faria Vasconcelos, K.; Huang, Y.; Politis, C.; Lambrichts, I.; Jacobs, R. Diagnostic accuracy of CBCT versus intraoral imaging for assessment of peri-implant bone defects. BMC Med. Imaging 2021, 21, 23. [Google Scholar] [CrossRef]
- Hilgenfeld, T.; Juerchott, A.; Deisenhofer, U.K.; Krisam, J.; Rammelsberg, P.; Heiland, S.; Bendszus, M.; Schwindling, F.S. Accuracy of cone-beam computed tomography, dental magnetic resonance imaging, and intraoral radiography for detecting peri-implant bone defects at single zirconia implants-An in vitro study. Clin. Oral Implants Res. 2018, 29, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Wang, H.L.; Stumpf, T.; Brodbeck, U.; Bosshardt, D.; Rathe, F. Treatment of Periimplantitis with Electrolytic Cleaning versus Mechanical and Electrolytic Cleaning: 18-Month Results from a Randomized Controlled Clinical Trial. J. Clin. Med. 2021, 10, 3475. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Jepsen, S.; Obreja, K.; Galarraga-Vinueza, M.E.; Ramanauskaite, A. Surgical therapy of peri-implantitis. Periodontol. 2000 2022, 88, 145–181. [Google Scholar] [CrossRef]
- Misch, C.E.; Dietsh, F. Bone-grafting materials in implant dentistry. Implant Dent. 1993, 2, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.C.; Barootchi, S.; Wang, H.L.; Huang, W.X. Non-submerged reconstructive approach for peri-implantitis osseous defect, with removal of implant crowns: 1-year outcomes of a prospective case series study. J. Periodontol. 2022, 93, 1250–1261. [Google Scholar] [CrossRef]
- Amid, R.; Kheiri, A.; Kheiri, L.; Kadkhodazadeh, M.; Ekhlasmandkermani, M. Structural and chemical features of xenograft bone substitutes: A systematic review of in vitro studies. Biotechnol. Appl. Biochem. 2021, 68, 1432–1452. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.; Coimbra, P.; Cabrita, A.; Guerra, F.; Figueiredo, M. Comparison of a xenogeneic and an alloplastic material used in dental implants in terms of physico-chemical characteristics and in vivo inflammatory response. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.N.; Mealey, B.L. Histologic comparison of healing after ridge preservation using human demineralized bone matrix putty with one versus two different-sized bone particles. J. Periodontol. 2012, 83, 174–181. [Google Scholar] [CrossRef]
- De Coster, P.; Browaeys, H.; De Bruyn, H. Healing of extraction sockets filled with BoneCeramic® prior to implant placement: Preliminary histological findings. Clin. Implant Dent. Relat. Res. 2011, 13, 34–45. [Google Scholar] [CrossRef]
- Turco, G.; Porrelli, D.; Marsich, E.; Vecchies, F.; Lombardi, T.; Stacchi, C.; Di Lenarda, R. Three-Dimensional Bone Substitutes for Oral and Maxillofacial Surgery: Biological and Structural Characterization. J. Funct. Biomater. 2018, 9, E62. [Google Scholar] [CrossRef]
- Baheiraei, N.; Nourani, M.R.; Mortazavi, S.M.J.; Movahedin, M.; Eyni, H.; Bagheri, F.; Norahan, M.H. Development of a bioactive porous collagen/β-tricalcium phosphate bone graft assisting rapid vascularization for bone tissue engineering applications. J. Biomed. Mater. Res. A 2018, 106, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.; Palarie, V.; Nacu, V.; Grobecker-Karl, T. A Pilot Animal Study Aimed at Assessing the Mechanical Quality of Regenerated Alveolar Bone. Int. J. Oral Maxillofac. Implants 2020, 35, 313–319. [Google Scholar] [CrossRef]
- Winter, W.; Krafft, T.; Steinmann, P.; Karl, M. Quality of alveolar bone--Structure-dependent material properties and design of a novel measurement technique. J. Mech. Behav. Biomed. Mater. 2011, 4, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.; Romanos, G.E.; Alexandre Gerhke, S.; Gomez-Moreno, G.; Maté-Sánchez de Val, J.E.; Calvo-Guirado, J.L. Biological effects of compressive forces exerted on particulate bone grafts during socket preservation: Animal study. Clin. Oral Implants Res. 2018, 29, 792–801. [Google Scholar] [CrossRef]
- Pawlowsky, K.; Ernst, L.; Steitz, J.; Stopinski, T.; Kögel, B.; Henger, A.; Kluge, R.; Tolba, R. The Aachen Minipig: Phenotype, Genotype, Hematological and Biochemical Characterization, and Comparison to the Göttingen Minipig. Eur. Surg. Res. 2017, 58, 193–203. [Google Scholar] [CrossRef]
- Romanos, G.E.; Delgado-Ruiz, R.A.; Gómez-Moreno, G.; López-López, P.J.; Mate Sanchez de Val, J.E.; Calvo-Guirado, J.L. Role of mechanical compression on bone regeneration around a particulate bone graft material: An experimental study in rabbit calvaria. Clin. Oral Implants Res. 2018, 29, 612–619. [Google Scholar] [CrossRef]
- Low, K.L.; Tan, S.H.; Zein, S.H.; Roether, J.A.; Mouriño, V.; Boccaccini, A.R. Calcium phosphate-based composites as injectable bone substitute materials. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 273–286. [Google Scholar] [CrossRef]
- Bohner, M. Design of ceramic-based cements and putties for bone graft substitution. Eur. Cells Mater. 2010, 20, 1–12. [Google Scholar] [CrossRef]
- Klijn, R.J.; van den Beucken, J.J.; Félix Lanao, R.P.; Veldhuis, G.; Leeuwenburgh, S.C.; Wolke, J.G.; Meijer, G.J.; Jansen, J.A. Three different strategies to obtain porous calcium phosphate cements: Comparison of performance in a rat skull bone augmentation model. Tissue Eng. Part A 2012, 18, 1171–1182. [Google Scholar] [CrossRef]
- Steiner, C.; Karl, M.; Laschke, M.W.; Schupbach, P.; Venturato, A.; Gasser, A. Comparison of extraction sites versus artificial defects with xenogenic bone substitute in minipigs. Clin. Exp. Dent. Res. 2021, 7, 490–501. [Google Scholar] [CrossRef]
- Buser, D.; Hoffmann, B.; Bernard, J.P.; Lussi, A.; Mettler, D.; Schenk, R.K. Evaluation of filling materials in membrane--protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin. Oral Implants Res. 1998, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Broggini, N.; Hjørting-Hansen, E.; Schenk, R.; Buser, D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin. Oral Implants Res. 2006, 17, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Indovina, A., Jr.; Block, M.S. Comparison of 3 bone substitutes in canine extraction sites. J. Oral Maxillofac. Surg. 2002, 60, 53–58. [Google Scholar] [CrossRef]
- Cochran, D.L.; Jones, A.A.; Sugita, R.; Brown, M.C.; Prasad, H.; Kay, G.W. Twelve-month evaluation of a novel mineral-organic adhesive material used to stabilize dental implants placed in oversized osteotomies in vivo in an animal model. Clin. Oral Implants Res. 2022, 33, 391–404. [Google Scholar] [CrossRef]
- Thousand, J.; Datar, J.; Font, K.; Powell, C.A. A root volume study of the adult dentition for ridge preservation purposes. Gen. Dent. 2017, 65, 21–23. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).