Effects of Different Heating and Cooling Rates during Solution Treatment on Microstructure and Properties of AA7050 Alloy Wires
Abstract
1. Introduction
2. Materials and Methods
3. Results
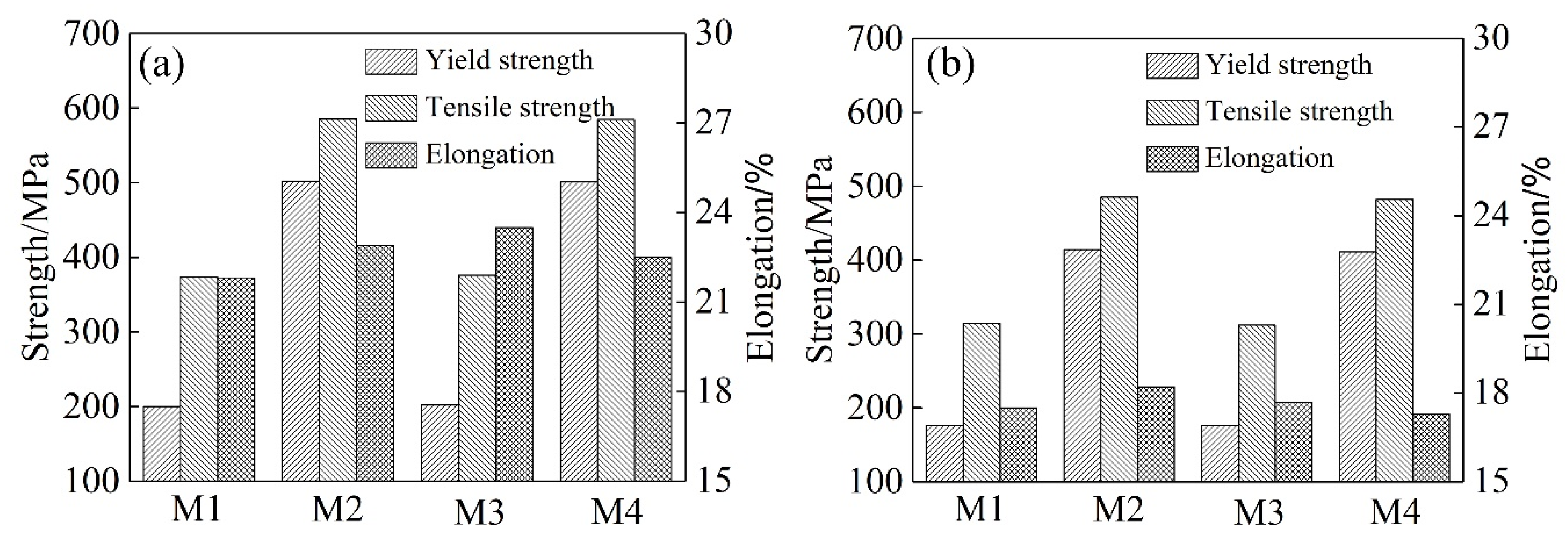
3.1. Mechanical Properties
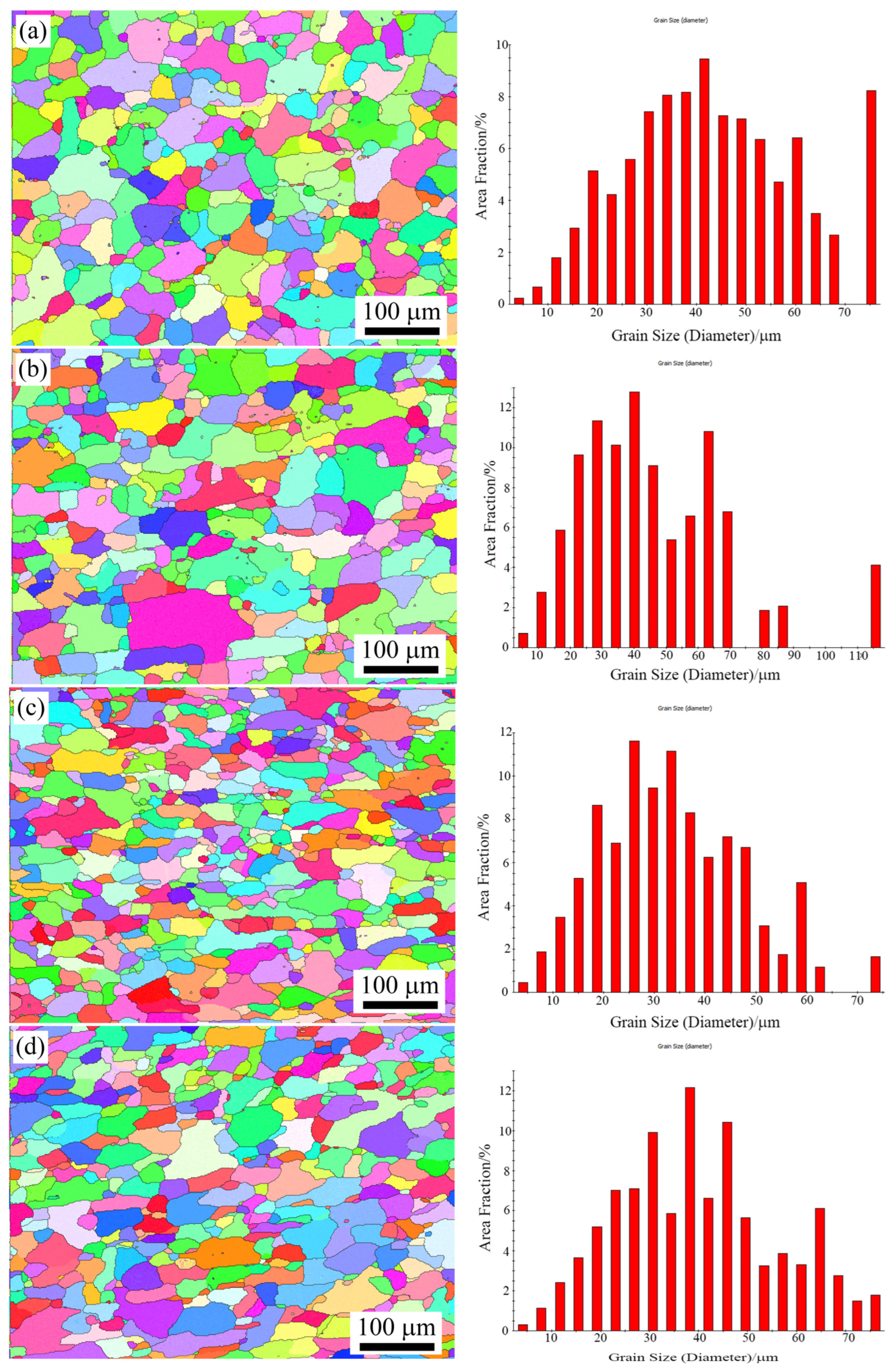
3.2. Recrystallized Grains
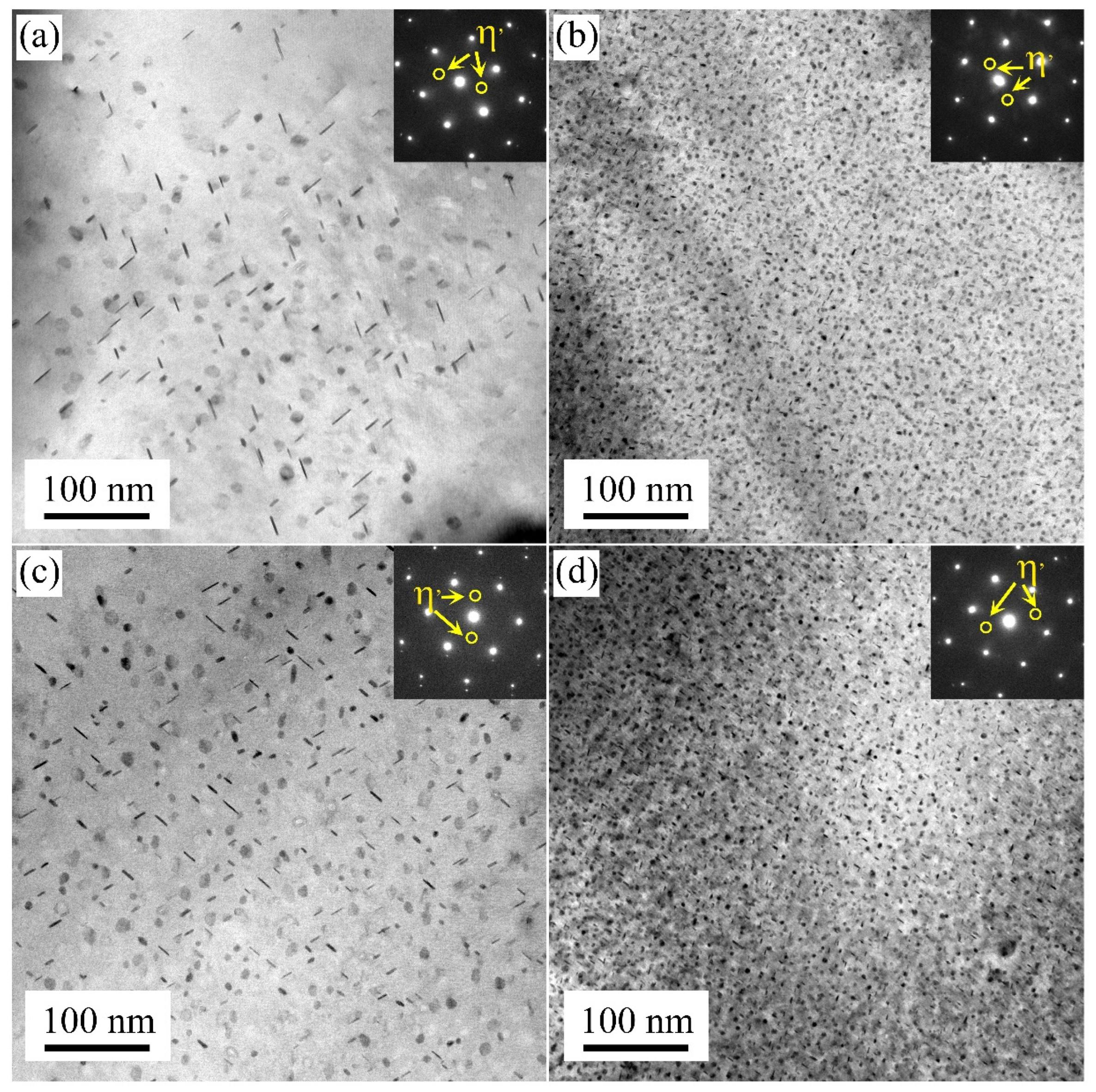
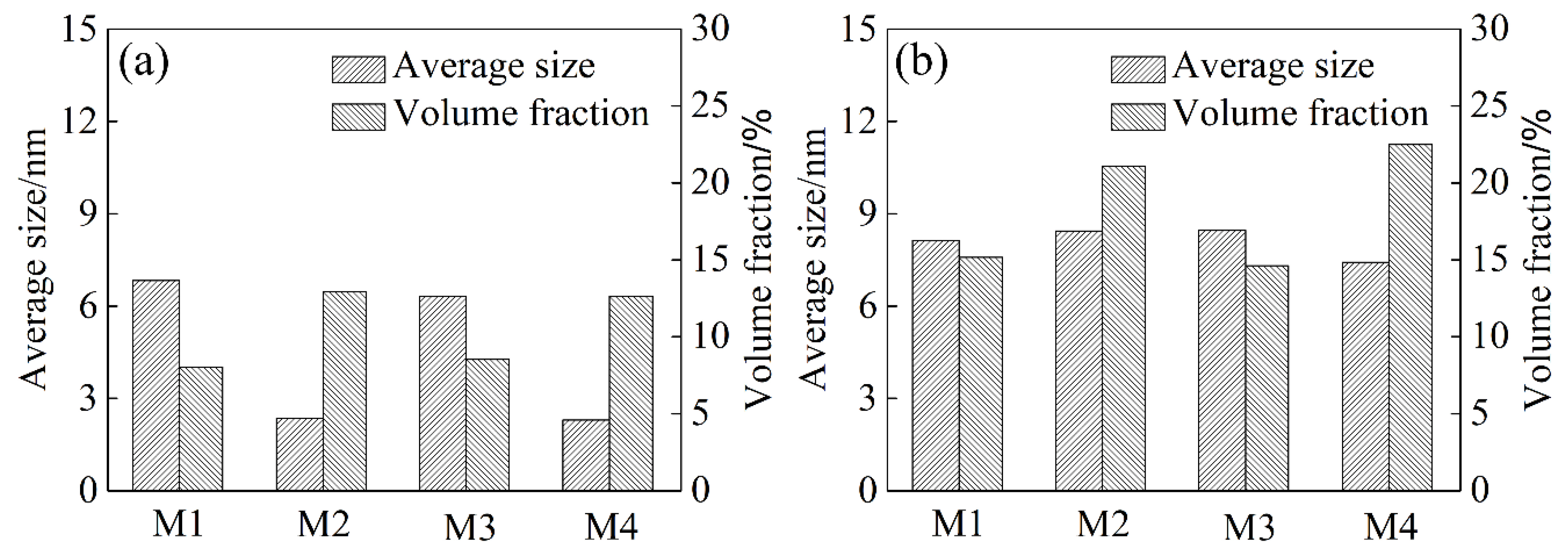
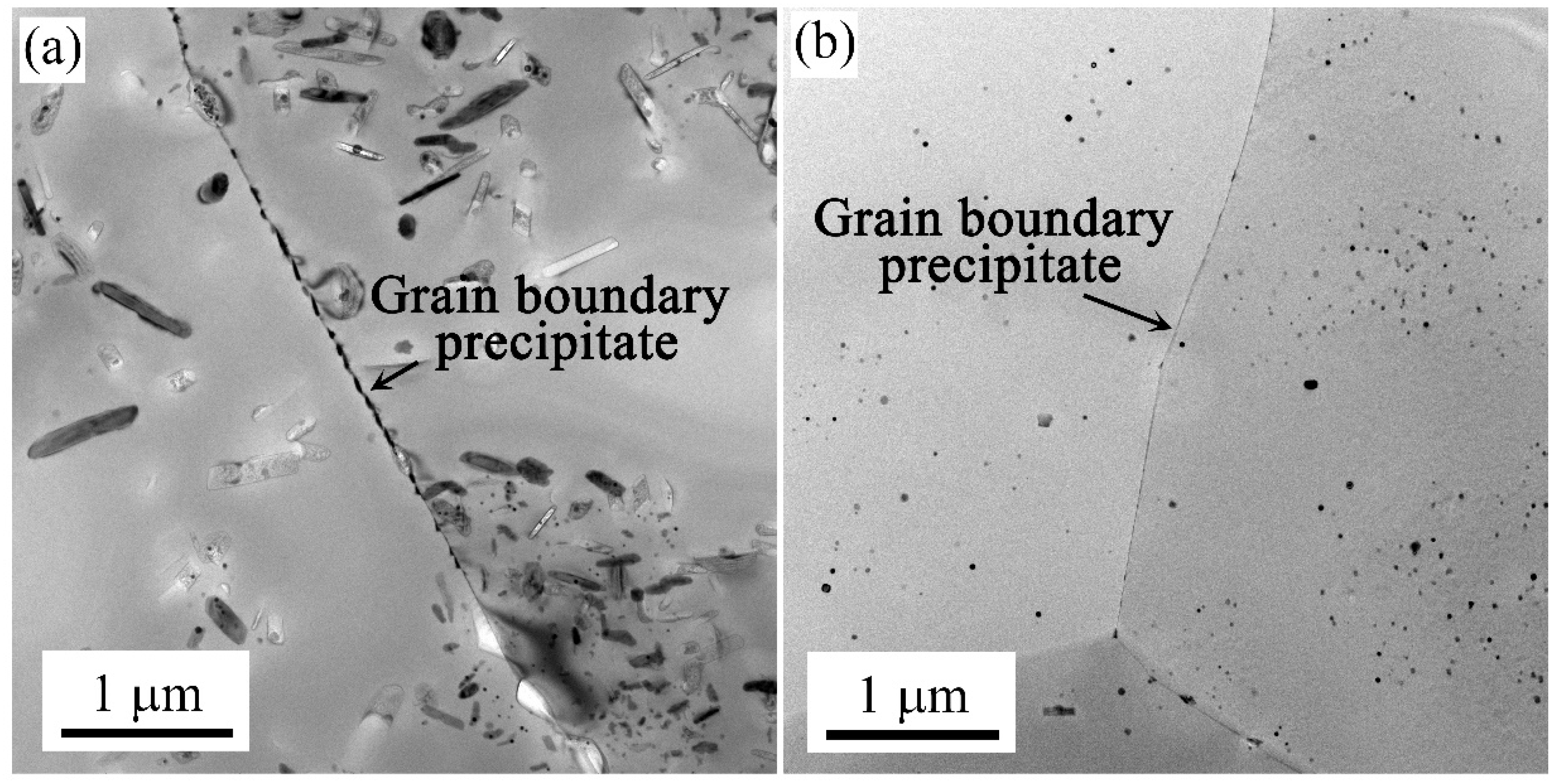
3.3. Intragranular and Grain Boundary Precipitation
3.4. Precipitation in Natural Aging State
4. Discussion
5. Conclusions
- (1)
- The size and uniformity of recrystallization microstructure were greatly affected by the heating rate during the solution treatment. The recrystallized grain of the samples with rapid heating was smaller and more homogeneous than that of slow heating.
- (2)
- When the sample was cooled to RT using the air cooling method, the low density of the η′ strengthening phases formed after artificial aging treatment. While, using the water quenching method, sufficient solute atoms and numerous nucleation sites were provided, resulting in a large number of η′ strengthening phases being formed.
- (3)
- The different heating and cooling methods affected the precipitation in the grain. And, the strength of the alloy wires is dominated by the precipitates. For the alloy in the T6 state, the tensile strength of alloy under the air cooling method reached ~375 MPa, and with the water quenching method it was ~585 MPa. For the alloy in the T73 state, the tensile strength of the former solution method was only ~310 MPa, while the strength of the latter increased to ~485 MPa.
- (4)
- The intermittent precipitation and large widths of PFZ were detected along the grain boundaries. Coarse precipitates formed in the grain and at the grain boundaries under the air-cooling method. Less clusters were observed in the matrix during the following natural aging process, while high density nanoscale clusters and no continuous precipitation are formed when using the water quenching method.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, R.; Liu, B.; Zhang, J.; Zhang, S. Microstructure evolution and properties of 7075 aluminum alloy recycled from scrap aircraft aluminum alloys. J. Mater. Res. Technol. 2022, 19, 354–367. [Google Scholar] [CrossRef]
- Miller, W.S.; Zhuang, L.; Bottema, J.; Wittebrood, A.J.; De Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Atxaga, G.; Arroyo, A.; Canflanca, B. Hot stamping of aerospace aluminium alloys: Automotive technologies for the aeronautics industry. J. Manuf. Process. 2022, 81, 817–827. [Google Scholar] [CrossRef]
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W.S. Recent development in aluminum alloys for aerospace applications. Mater. Sci. Eng. A 2000, 280, 102–107. [Google Scholar] [CrossRef]
- Miramontes, J.C.; Gaona Tiburcio, C.; García Mata, E.; Esneider Alcála, M.Á.; Maldonado-Bandala, E.; Lara-Banda, M.; Nieves-Mendoza, D.; Olguín-Coca, J.; Zambrano-Robledo, P.; López-León, L.D.; et al. Corrosion Resistance of Aluminum Alloy AA2024 with Hard Anodizing in Sulfuric Acid-Free Solution. Materials 2022, 15, 6401. [Google Scholar] [CrossRef]
- Dixit, M.; Mishra, R.S.; Sankaran, K.K. Structure–property correlations in Al 7050 and Al 7055 high-strength aluminum alloys. Mater. Sci. Eng. A 2008, 478, 163–172. [Google Scholar] [CrossRef]
- Vorob’ev, I.A.; Galiakhmetov, T.S. Titanium Alloy Fasteners for Aerospace Engineering. Russ. Eng. Res. 2023, 43, 771–776. [Google Scholar] [CrossRef]
- Sampaio, R.F.V.; Pragana, J.P.M.; Clara, R.G.; Bragança, I.M.F.; Silva, C.M.A.; Martins, P.A.F. New Self-Clinching Fasteners for Electric Conductive Connections. J. Manuf. Mater. Process. 2022, 6, 159. [Google Scholar] [CrossRef]
- Sundaram, S.K.; Rahul, K.; Dayal, R.R.D.; Survesh, S.; Swamenathan, R. Fatigue life estimation of preloaded Aluminium (Al2024-T6) aircraft fasteners using finite element method. Mater. Today 2022, 62, 1369–1374. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, L.; Xia, Y.; Jiang, Y. Hot deformation and processing map of a typical Al–Zn–Mg–Cu alloy. J. Alloys Compd. 2013, 550, 438–445. [Google Scholar] [CrossRef]
- Hadjadj, L.; Amira, R.; Hamana, D.; Mosbah, A. Characterization of precipitation and phase transformations in Al–Zn–Mg alloy by the differential dilatometry. J. Alloys Compd. 2008, 462, 279–283. [Google Scholar] [CrossRef]
- Carvalho, A.L.M.; Renaudin, L.B.; Zara, A.J.; Martins, J.P. Microstructure analysis of 7050 aluminum alloy processed by multistage aging treatments. J. Alloys Compd. 2022, 907, 164400. [Google Scholar] [CrossRef]
- Graf, G.; Spoerk-Erdely, P.; Staron, P.; Stark, A.; Martin, F.M.; Clemens, H.; Klein, T. Quench rate sensitivity of age-hardenable Al-Zn-Mg-Cu alloys with respect to the Zn/Mg ratio: An in situ SAXS and HEXRD study. Acta Mater. 2022, 227, 117727. [Google Scholar] [CrossRef]
- Thronsen, E.; Shah, S.; Hatzoglou, C.; Marioara, C.D.; Wenner, S.; Andersen, S.J.; Holmedal, B.; Holmestad, R. The evolution of precipitates in an Al–Zn–Mg alloy. J. Mater. Res. Technol. 2023, 23, 5666–5680. [Google Scholar] [CrossRef]
- Wang, X.; Guo, M.; Chapuis, A.; Luo, J.; Zhang, J.; Zhuang, L. Effect of solution time on microstructure, texture and mechanical properties of Al–Mg–Si–Cu alloys. Mater. Sci. Eng. A 2015, 644, 137–151. [Google Scholar] [CrossRef]
- Gao, G.; Li, Y.; Wang, Z.; Di, H.; Li, J.; Xu, G. Effects of the Quenching Rate on the Microstructure, Mechanical Properties and Paint Bake-Hardening Response of Al–Mg–Si Automotive Sheets. Materials 2019, 12, 3587. [Google Scholar] [CrossRef]
- Dumont, D.; Deschamps, A.; Bréchet, Y.; Sigli, C.; Ehrström, J.C. Characterisation of precipitation microstructures in aluminium alloys 7040 and 7050 and their relationship to mechanical behaviour. Mater. Sci. Technol. 2013, 20, 567–576. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, B.; Liu, S.; Zhu, B.; Zuo, Y. Changes of microstructure of different quench sensitivity 7,000 aluminum alloy after end quenching. Rare Met. 2014, 33, 270–275. [Google Scholar] [CrossRef]
- GB/T 228-2002; Metallic Materials—Tensile Testing at Ambient Temperature. National Standard of the People’s Republic of China: Beijing, China, 2002.
- Shah, V.; Krugla, M.; Offerman, S.E.; Sietsma, J.; Hanlon, D.N. Effect of Silicon, Manganese and Heating Rate on the Ferrite Recrystallization Kinetics. ISIJ Int. 2020, 60, 1312–1323. [Google Scholar] [CrossRef]
- Nicolaÿ, A.; Franchet, J.M.; Cormier, J.; Logé, R.E.; Fiorucci, G.; Fausty, J.; Van Der Meer, M.; Bozzolo, N. Influence of Joule Effect Heating on Recrystallization Phenomena in Inconel 718. Metall. Mater. Trans. A 2021, 52, 4572–4596. [Google Scholar] [CrossRef]
- Bandi, B.; Van Krevel, J.; Srirangam, P. Interaction Between Ferrite Recrystallization and Austenite Formation in Dual-Phase Steel Manufacture. Metall. Mater. Trans. A 2022, 53, 1379–1393. [Google Scholar] [CrossRef]
- Paul, A.; Zhang, Y.; Knight, S. Heat treatment of 7xxx series aluminium alloys—Some recent developments. Trans. Nonferrous Met. Soc. China 2014, 24, 2003–2017. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Safyari, M.; Hojo, T. Effect of solution treatment temperature on grain boundary composition and environmental hydrogen embrittlement of an Al–Zn–Mg–Cu alloy. Vacuum 2021, 184, 109937. [Google Scholar] [CrossRef]
- Wang, F.; Xiong, B.; Zhang, Y.; Zhu, B.; Liu, H.; He, X. Effect of heat treatment on the microstructure and mechanical properties of the spray-deposited Al–10.8Zn–2.8Mg–1.9Cu alloy. Mater. Sci. Eng. A 2008, 486, 648–652. [Google Scholar] [CrossRef]
- Yang, W.; Ji, S.; Wang, M.; Li, Z. Precipitation behaviour of Al–Zn–Mg–Cu alloy and diffraction analysis from η′ precipitates in four variants. J. Alloys Compd. 2014, 610, 623–629. [Google Scholar] [CrossRef]
- Dumont, M.; Lefebvre, W.; Doisneau-Cottignies, B.; Deschamps, A. Characterisation of the composition and volume fraction of η′ and η precipitates in an Al–Zn–Mg alloy by a combination of atom probe, small-angle X-ray scattering and transmission electron microscopy. Acta Mater. 2005, 53, 2881–2892. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, W.; Berghammer, R.; Gottstein, G. Microstructure evolution and deformation behavior of ultrafine-grained Al–Zn–Mg alloys with fine η′ precipitates. Acta Mater. 2010, 58, 6695–6705. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.; Tang, S.; Zhu, Q.; Song, H.; Cao, L. Co-precipitation of T′ and η′ phase in Al−Zn−Mg−Cu alloys. Mater. Charact. 2020, 169, 110610. [Google Scholar] [CrossRef]
- Chen, J.H.; Costan, E.; van Huis, M.A.; Xu, Q.; Zandbergen, H.W. Atomic Pillar-Based Nanoprecipitates Strengthen AlMgSi Alloys. Science 2006, 312, 416–419. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, Z.; Geng, J.; Zhang, C.; Li, Y.; Yang, Q.; Wang, M.; Chen, D.; Li, Z.; Wang, H. Precipitation and crystallographic relationships of nanosized η/η’ precipitates at S-Al interface in Al−Zn−Mg−Cu alloy. Scr. Mater. 2022, 214, 114643. [Google Scholar] [CrossRef]
- Lee, S.; Jung, J.; Baik, S.; Seidman, D.N.; Kim, M.; Lee, Y.; Euh, K. Precipitation strengthening in naturally aged Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2021, 803, 140719. [Google Scholar] [CrossRef]
- Duchaussoy, A.; Sauvage, X.; Deschamps, A.; Geuser, F.D.; Renou, G.; Horita, Z. Complex interactions between precipitation, grain growth and recrystallization in a severely deformed Al-Zn-Mg-Cu alloy and consequences on the mechanical behavior. Materialia 2021, 15, 101028. [Google Scholar] [CrossRef]
- Li a, X.Z.; Hansen, V.; GjØnnes, J.; Wallenberg, L.R. HREM study and structure modeling of the η′ phase, the hardening precipitates in commercial Al–Zn–Mg alloys. Acta Mater. 1999, 47, 2651–2659. [Google Scholar] [CrossRef]
- Yao, Y.; Euesden, R.; Curd, M.E.; Liu, C.; Garner, A.; Burnett, T.L.; Shanthraj, P.; Prangnell, P.B. Effect of cooling rate on the composition and chemical heterogeneity of quench-induced grain boundary η-phase precipitates in 7xxx aluminium alloys. Acta Mater. 2024, 262, 119443. [Google Scholar] [CrossRef]
- Dong, Q.; Howells, A.; Lloyd, D.J.; Gallerneault, M.; Fallah, V. Effect of solidification cooling rate on kinetics of continuous/discontinuous Al3(Sc,Zr) precipitation and the subsequent age-hardening response in cold-rolled AlMgSc(Zr) sheets. Mater. Sci. Eng. A 2020, 772, 138693. [Google Scholar] [CrossRef]
- Farkoosh, A.R.; Dunand, D.C.; Seidman, D.N. Solute-induced strengthening during creep of an aged-hardened Al-Mn-Zr alloy. Acta Mater. 2021, 219, 117268. [Google Scholar] [CrossRef]
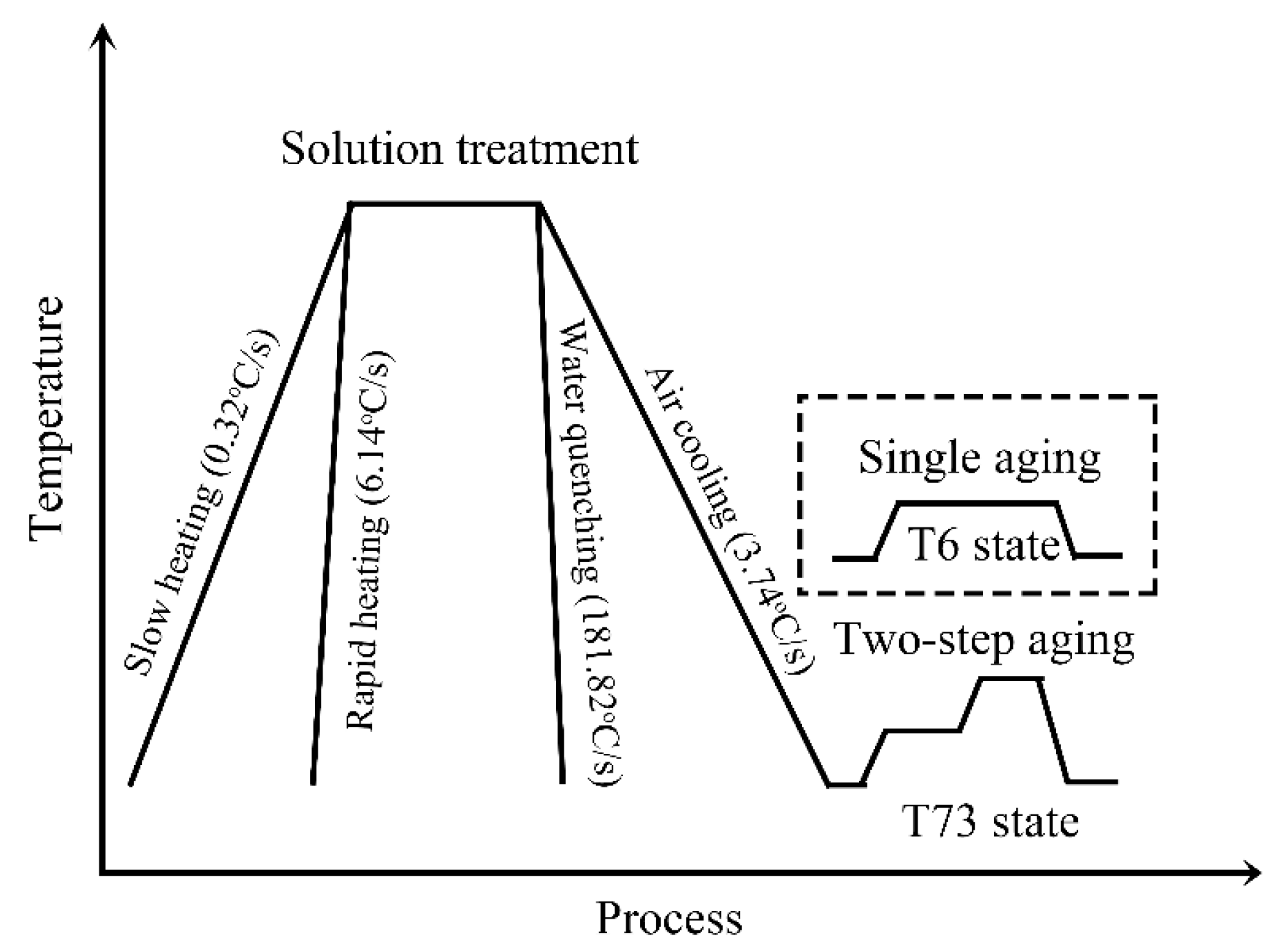



| Wire Samples | Solution Treatment | Aging Conditions |
|---|---|---|
| M1 | Slow heating + Air cooling | T6 state |
| M2 | Slow heating + Water quenching | |
| M3 | Rapid heating + Air cooling | T73 state |
| M4 | Rapid heating + Water quenching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Gao, G.; Li, Z.; Li, X.; Yan, L.; Zhang, Y.; Xiong, B. Effects of Different Heating and Cooling Rates during Solution Treatment on Microstructure and Properties of AA7050 Alloy Wires. Materials 2024, 17, 310. https://doi.org/10.3390/ma17020310
Gao X, Gao G, Li Z, Li X, Yan L, Zhang Y, Xiong B. Effects of Different Heating and Cooling Rates during Solution Treatment on Microstructure and Properties of AA7050 Alloy Wires. Materials. 2024; 17(2):310. https://doi.org/10.3390/ma17020310
Chicago/Turabian StyleGao, Xinyu, Guanjun Gao, Zhihui Li, Xiwu Li, Lizhen Yan, Yongan Zhang, and Baiqing Xiong. 2024. "Effects of Different Heating and Cooling Rates during Solution Treatment on Microstructure and Properties of AA7050 Alloy Wires" Materials 17, no. 2: 310. https://doi.org/10.3390/ma17020310
APA StyleGao, X., Gao, G., Li, Z., Li, X., Yan, L., Zhang, Y., & Xiong, B. (2024). Effects of Different Heating and Cooling Rates during Solution Treatment on Microstructure and Properties of AA7050 Alloy Wires. Materials, 17(2), 310. https://doi.org/10.3390/ma17020310





