3.1. Corrosion Properties and Microstructures
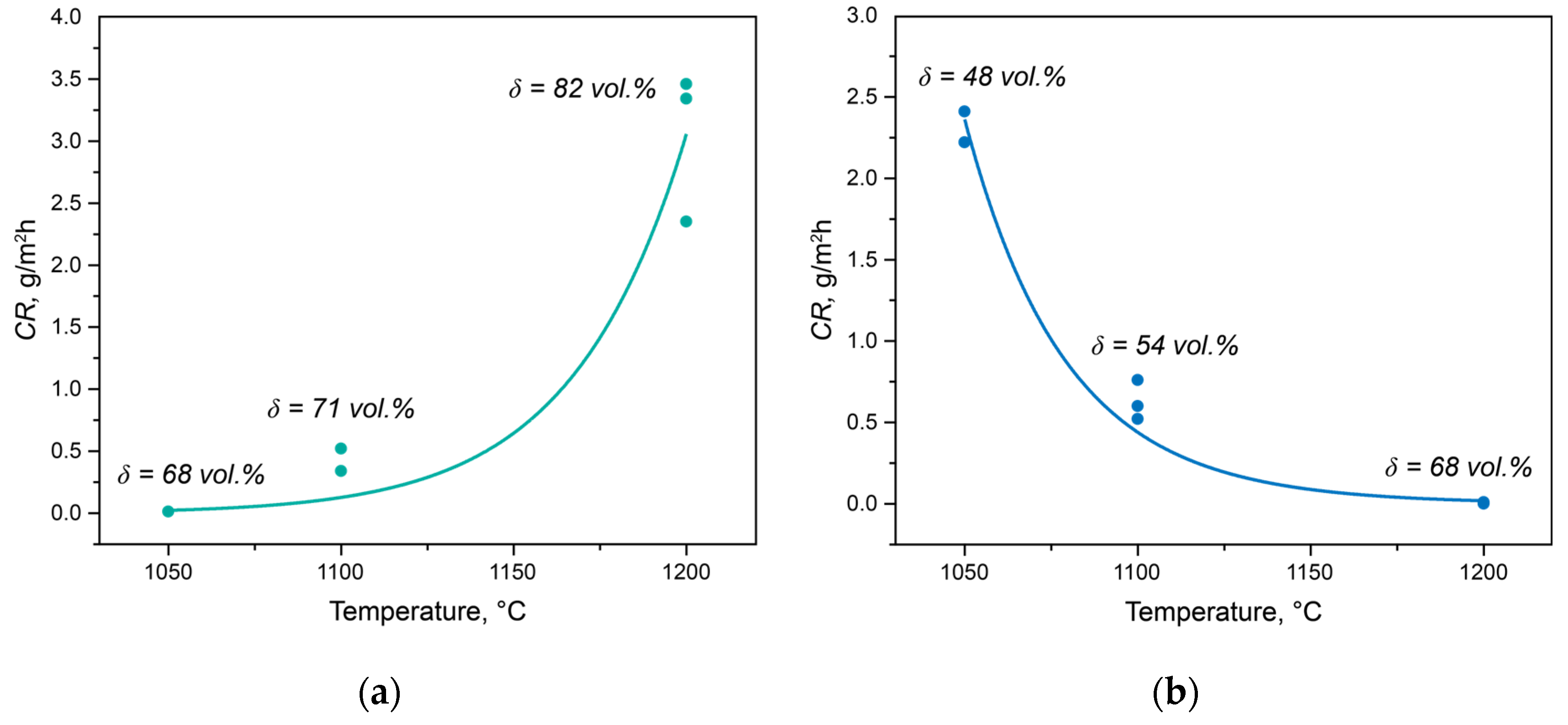
The dependencies of the crevice corrosion rates for the experimental steels subjected to solution heat treatment at different temperatures are shown in
Figure 1. For Steel 1, as the solution heat treatment temperature increased from 1050 to 1200 °C, the corrosion rate significantly rose from 0.01 to 3.40 g/m²·h (
Figure 1a). In the case of Steel 2, the corrosion resistance behavior differed. With the increase in the solution heat treatment temperature, the corrosion resistance, as assessed by the crevice corrosion rate, markedly decreased from 2.32 to 0.00 g/m²·h (
Figure 1b).
These results lead to the conclusion that these two steels with similar PREN values and identical solution heat treatments result in significantly different crevice corrosion resistances. The microstructures of the samples after testing were investigated for the interpretation of this phenomenon (
Figure 2). Additionally, the microstructural analysis included additional samples after solution heat treatment at intermediate temperatures of 1150 and 1250 °C.
After solution annealing at 1050 °C for 60 min in Steel 1, the fraction of δ-ferrite was 68 vol.%. Austenite was represented by individual elongated grains and colonies of small islands. Increasing the solution annealing temperature to 1100 °C raised the δ-ferrite fraction to 71 vol.%, and the morphology of austenite remained unchanged. Further increasing the solution annealing temperature to 1200 °C led to an increase in the δ-ferrite fraction to 82 vol.%. After this treatment, colonies of small austenitic grains completely disappeared, and the remaining grains decreased in size, losing their sharp angularity due to simultaneous processes of dissolution and Ostwald ripening [
20]. With an increase in the solution annealing temperature to 1250 °C, the δ-ferrite fraction increased to 90 vol.%, and the austenite islands further reduced in size, approaching a more rounded shape. Similar trends in structural changes were observed during the solution annealing of Steel 2—the amount of δ-ferrite increased from 48 to 69 vol.%, with the temperature increasing from 1050 to 1200 °C.
Thus, the change in phase balance does not fully explain the change in corrosion properties. To illustrate this, the volume fractions of ferrite for each temperature are plotted in
Figure 1. The behavior of Steel 1 is entirely understandable since in a chloride-ion-containing environment during testing, ferrite acts as the anode and actively dissolves [
21]. Therefore, with an increase in the volume fraction of ferrite, the anode’s surface area increases, leading to an elevated corrosion rate. The behavior of Steel 2 is entirely unpredictable since its inferior corrosion properties correspond to the best phase balance.
The behavior of the corrosion resistance of the two DSSs, seemingly similar in structural changes, is not only due to the change in the quantity of austenite and ferrite but also to other phases that are much more difficult to detect.
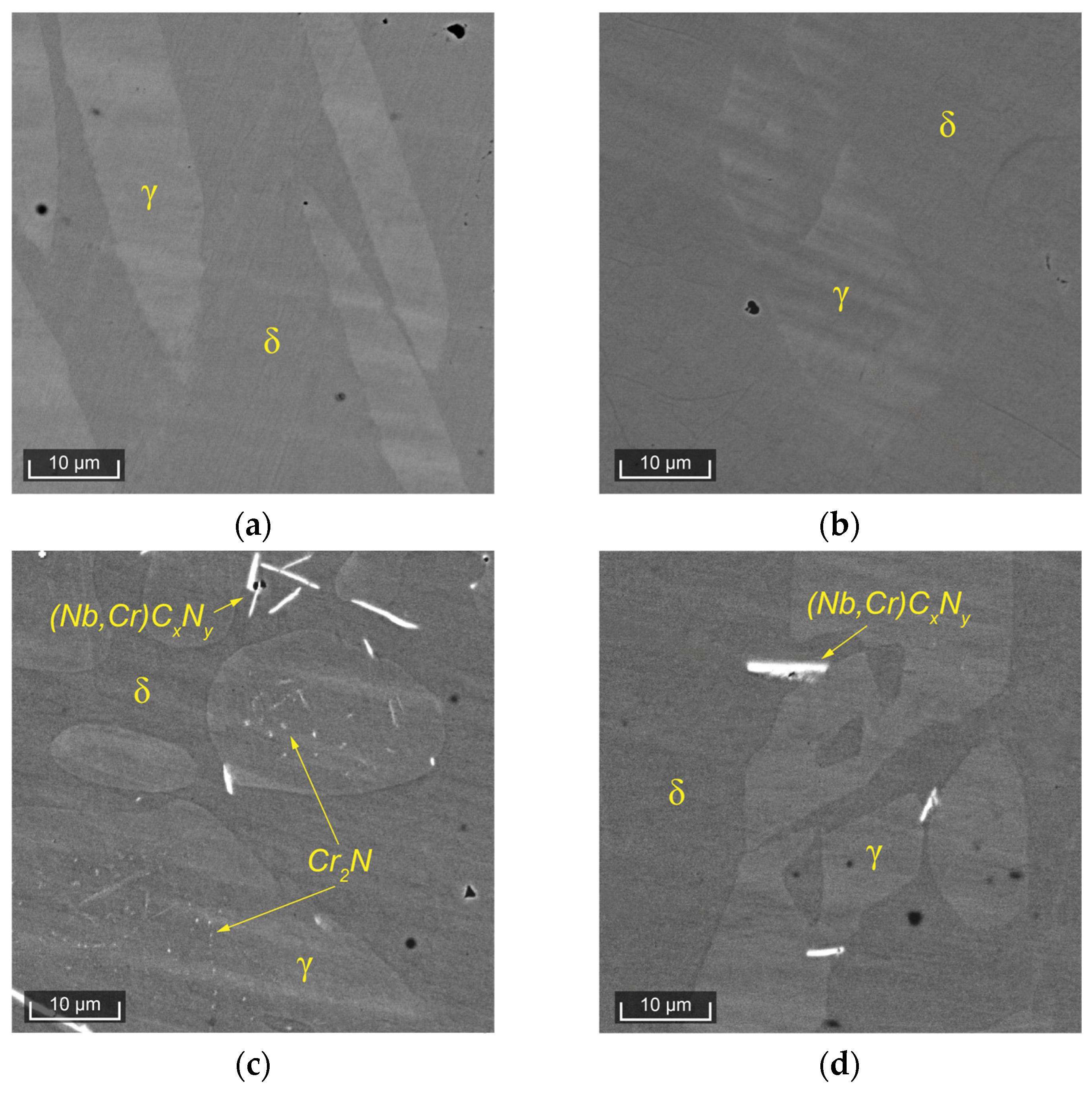
Figure 3 presents microstructural images of the experimental steels obtained using a scanning electron microscope.
In Steel 1, after the solution heat treatment at 1050 °C, a distinct ferritic matrix and islands of austenite are clearly visible (
Figure 3a). After the solution heat treatment at 1250 °C, the microstructure remains the same. In Steel 2, after the solution heat treatment at 1050 °C, in addition to ferrite and austenite, inclusions of niobium carbonitrides and dispersed precipitates, morphologically identifiable as chromium nitrides [
22], are well observed (
Figure 3c). After the solution heat treatment at 1250 °C, in Steel 2, the quantity of niobium carbonitrides slightly decreases, and dispersed chromium nitrides are hardly distinguishable. These inclusions significantly reduce the corrosion resistance of the alloy. Thus, in the considered DSSs with similar PRENs and nearly identical behavior of ferrite and austenite during heat treatment, the behavior of secondary phases differs, leading to radical differences in corrosion resistance.
3.2. Thermodynamic Modeling and Redistribution of Chemical Elements
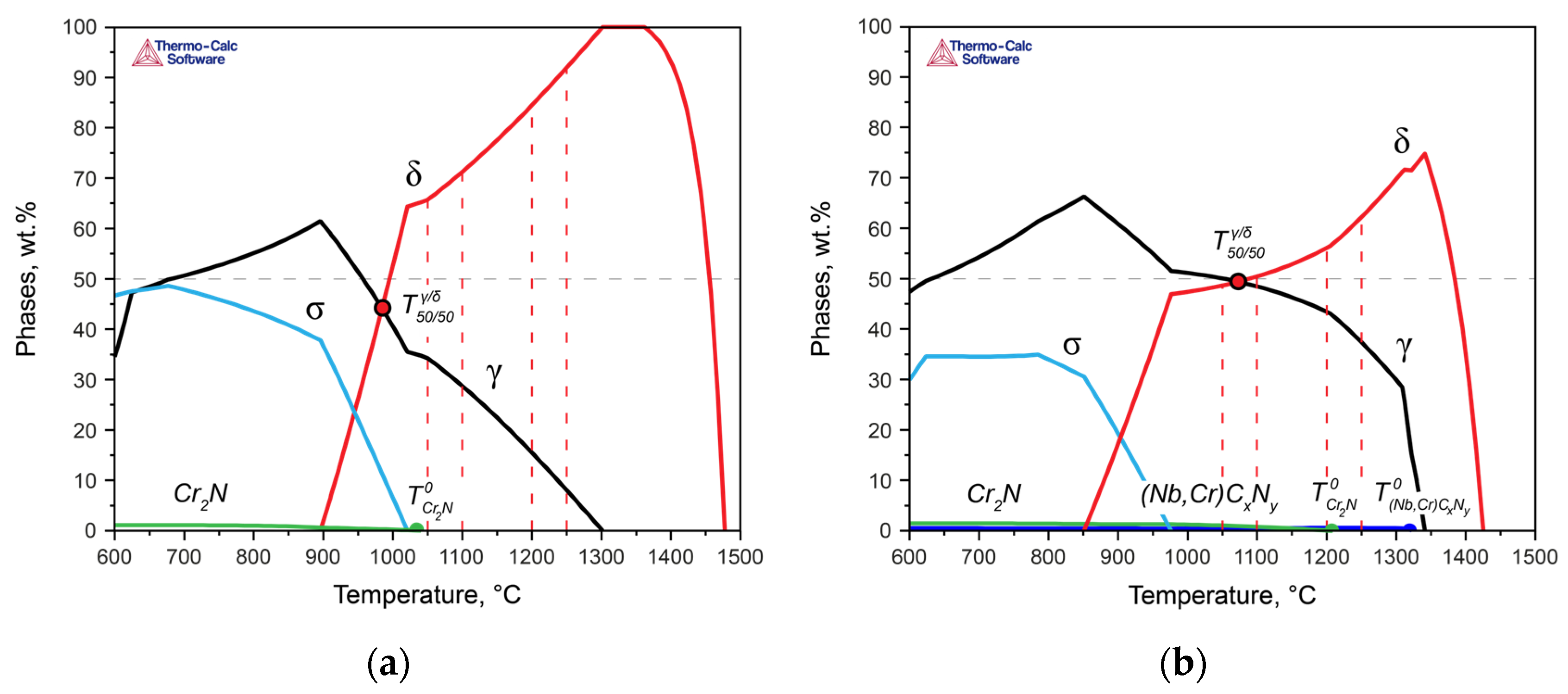
To describe the behavior of phases in the DSSs, thermodynamic modeling of the phase formation processes was carried out (
Figure 4). The modeling considered the liquid phase and utilized the associated solution model as well as the sublattice–regular solution model to describe the austenite and carbonitrides (with the parameters of the face-centered cubic lattice), ferrite (with the parameters of the body-centered cubic lattice), and existing chromium nitrides (with the parameters of the hexagonal close-packed lattice) to characterize the behavior of chromium nitride. In this context, carbon was taken into account, understanding that this compound is a complex carbonitride. However, for simplicity, both here and in subsequent discussions, we will refer to it as chromium nitride (Cr
2N).
According to calculations performed for Steel 1 (
Figure 4a), crystallization began with the formation of δ-ferrite dendrites, and after solidification, a polymorphic transformation to austenite occurred in the solid state. In Steel 2, crystallization similarly proceeded with the formation of primary δ-ferrite dendrites, but at the end of crystallization, some amount of austenite was formed via the peritectic–eutectic reaction (
Figure 4b) [
23]. Upon further cooling, the fraction of austenite increased due to the diffusion growth of γ-crystals and polymorphic transformation of δ → γ. Thus, the results of the thermodynamic modeling fully describe the observed evolution of the structures of the experimental steels during the heat treatment. Additionally, the difference in the crystallization mechanisms, namely, L → δ in Steel 1 and L → δ → δ + γ in Steel 2, explains the different initial morphology of austenite maintained at low heat treatment temperatures (
Figure 2a,e).
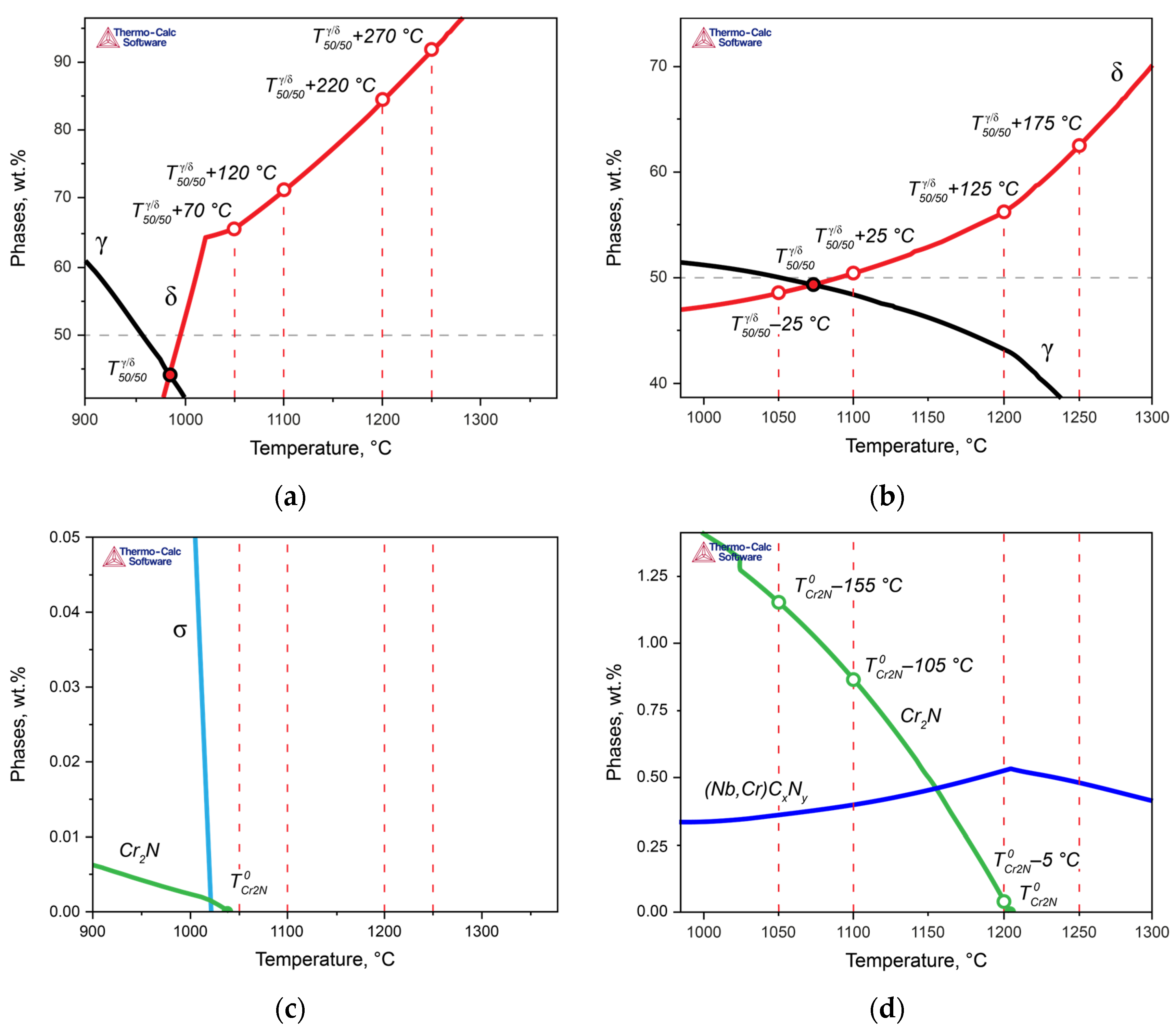
With a further decrease in temperature, in Steel 1, at 1040 °C, the formation of the chromium nitride became possible, and at 1010 °C, the σ-phase was formed. In Steel 2, which contains significantly more nitrogen than Steel 1, along with niobium, niobium carbonitrides formed at the end of the solidification temperatures, and at 1205 °C, chromium nitrides formed. The σ-phase was formed at 975 °C.
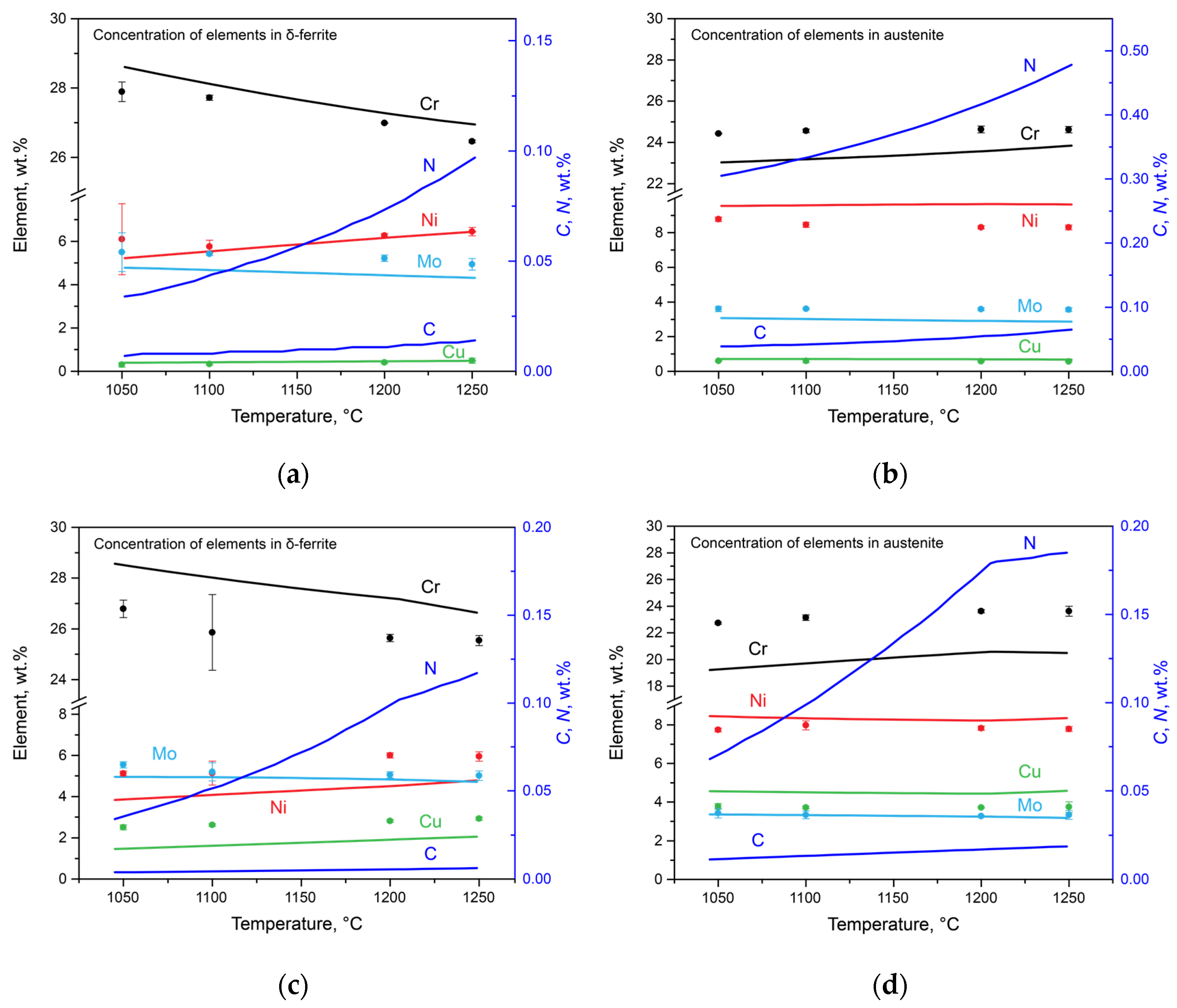
Simultaneous with the change in the phase composition, there was a redistribution of the alloying elements among austenite, ferrite, carbonitrides, and nitrides. This phenomenon was investigated using thermodynamic modeling and direct measurements using the SEM-EDS method. The results are presented in
Figure 5, where the calculated results are represented with lines, and the experimental data are marked by dots. Using SEM-EDS, the chromium, nickel, molybdenum, and copper contents were estimated, and these results are discussed below along with the calculated nitrogen and carbon contents. The data on the concentrations of these elements can only be obtained via calculations.
In Steel 1, with the increase in the temperature of the solution heat treatment from 1050 to 1250 °C, the following changes occurred: in δ-ferrite (
Figure 5a), the chromium and molybdenum contents decreased from 27.89 to 26.46 wt.% and from 5.66 to 4.94 wt.%, respectively; the nickel content increased from 6.10 to 6.45 wt.%; and the copper content increased from 0.30 to 0.49 wt.%. In austenite (
Figure 5b), the chromium content slightly increased from 24.43 to 24.62 wt.%, and the molybdenum content decreased from 3.60 to 3.56 wt.%. The nickel content decreased from 8.78 to 8.30 wt.%, and the copper content remained almost unchanged at 0.59 wt.%. According to the calculation, the nitrogen content in ferrite increased from 0.03 to 0.10 wt.%, and in austenite, it increased from 0.31 to 0.48 wt.%. The carbon content increased from 0.007 to 0.014 wt.% in ferrite and from 0.039 to 0.065 wt.% in austenite.
For Steel 2, in δ-ferrite (
Figure 5c), the concentrations of chromium and molybdenum decreased from 26.53 to 25.44 wt.% and from 5.54 to 5.01 wt.%, respectively, while the amounts of nickel and copper increased from 5.12 to 5.95 wt.% and from 2.50 to 2.92 wt.%, respectively. In austenite (
Figure 5d), the chromium concentration increased from 22.73 to 23.62 wt.%, and the molybdenum concentration decreased from 3.42 to 3.33 wt.%. The nickel concentration remained practically unchanged at 7.77%, while the copper concentration increased from 3.77 to 3.83 wt.%. The calculated nitrogen content increased from 0.03 to 0.12 wt.% in ferrite and from 0.07 to 0.18 wt.% in austenite. The carbon content increased from 0.004 to 0.006 wt.% in ferrite and from 0.011 to 0.019 wt.% in austenite.
Despite some differences between the experimental and calculated data, which arose due to the incomplete diffusion processes during transformations [
24], the similarity between the results is good. The largest mistake was observed for chromium, which was associated with its lower diffusion coefficient [
25].
In Steel 2, in addition to ferrite and austenite, the formation of niobium carbonitrides and chromium nitrides occurs at processing temperatures, contributing to the redistribution of nitrogen. Due to these particles, a significant depletion of the solid solution in nitrogen occurs (
Figure 6a). The same applies to carbon, which is part of the complex carbonitrides (
Figure 6b). However, the situation with carbon is more contradictory. While there is a depletion of ferrite and austenite in carbon, formally increasing the PREN of the phases, the formed particles will degrade the corrosion resistance of the entire alloy due to the emergence of new interphase boundaries. Nevertheless, since these effects are difficult to formally account for, at this stage, it was considered that the contribution of particles to the reduction in corrosion properties was primarily due to the depletion of the solution.
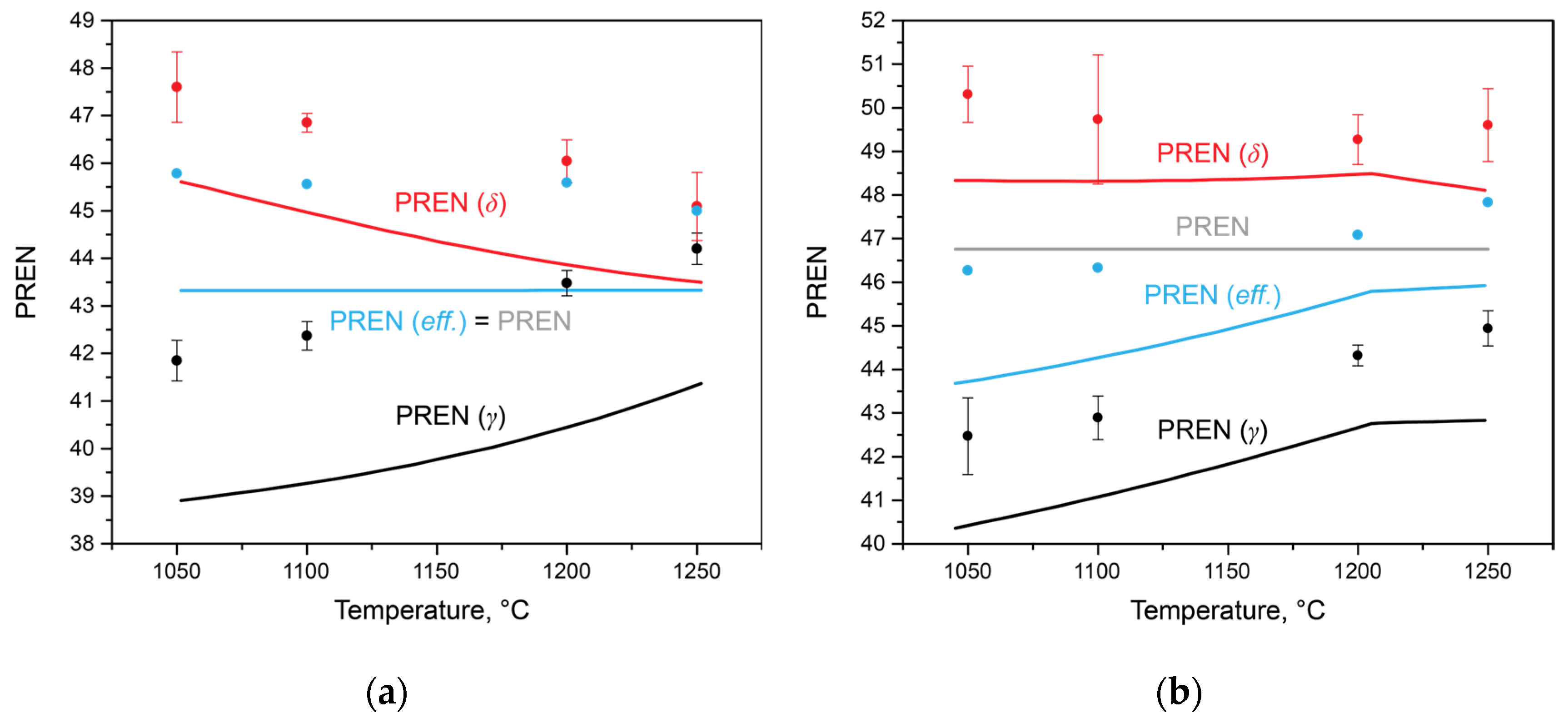
Using the data on the chemical compositions of austenite and ferrite in each steel at each temperature, it is possible to estimate the PREN for each phase, taking into account the redistribution of elements, including between secondary phases. The amounts of nitrogen and carbon were considered, utilizing the values of the limiting solubilities for each phase at the corresponding temperature, estimated using Thermo-Calc calculations (
Figure 5). Additionally, to assess the integral characteristics of the entire alloy, this study proposes calculating the effective PREN value for the alloy, considering the volume fractions of austenite and ferrite (3):
where
PRENδ is the PREN in δ-ferrite;
Vδ is the volume fraction of δ-ferrite;
PRENγ is the PREN in austenite; and
Vγ is the volume fraction of austenite.
This calculation allows for the consideration of phase balance, as well as the formation of secondary phases. The results of these calculations are presented in
Figure 7. In Steel 1 (
Figure 7a), as the solution heat treatment temperature increased and the amount of δ-ferrite increased from 68 to 90 wt.% (1050 and 1250 °C, respectively), the PREN values calculated for austenite and ferrite changed monotonically. The
PRENδ decreased, primarily due to a decrease in the chromium concentration, while the
PRENγ increased due to an increase in chromium and nitrogen. Since no secondary phases are present in this steel within the temperature range of the considered heat treatments, the
PRENeff remains constant and coincides with the PREN calculated according to Equation (1) for the average composition of the steel. Considering the results of the corrosion tests, it can be concluded that in this steel, the processes of the anodic dissolution of ferrite in a chloride-containing environment play a key role. Therefore, the
PRENδ more effectively describes the overall steel properties than the PREN.
In Steel 2, there is a bend in the curves of changes in the PREN of austenite and ferrite at a temperature of 1205 °C, above which the formation of chromium nitrides is impossible. The
PRENδ value remained constant in the temperature range from 1050 to 1205 °C and then decreased. At the same time, the value of
PRENeff, calculated using thermo-calc, also increased from 43.79 to 46.08. However, in all heat treatment intervals, it did not reach the PREN value calculated for the average composition of the steel due to the redistribution of nitrogen and carbon between austenite, ferrite, chromium nitride, and niobium-based carbonitride. Analyzing the data in
Figure 7b in conjunction with the corrosion rate values in
Figure 1b, it can be concluded that in this steel, with similar PREN values, the processes of precipitate formation play a crucial role in determining corrosion properties. The PREN values calculated for the experimental data are also plotted on the same figure. Considering the measurement error and taking into account the incompleteness of diffusion processes in elemental redistribution, it can be concluded that the trends in their changes correspond well with the calculated data.
3.3. Determining Thermodynamic Criteria
The above analysis demonstrated that for steels with the same PREN level, formally belonging to the same class of corrosion resistance, this indicator does not predict their behavior when assessing their corrosion resistance. Therefore, let us now consider the properties of the experimental steels while taking into account the critical temperatures of the steels:
,
, and
. Since all solution heat treatments were conducted at temperatures above
, we will not consider this critical point here. For convenience,
Figure 8 presents more detailed fragments of
Figure 3, in which the critical temperatures of the two steels are marked, and the heat treatment temperatures are indicated as the difference between the actual solution heat treatment temperature and the temperatures
(
Figure 8a,b) and
(
Figure 8c,d), respectively. In
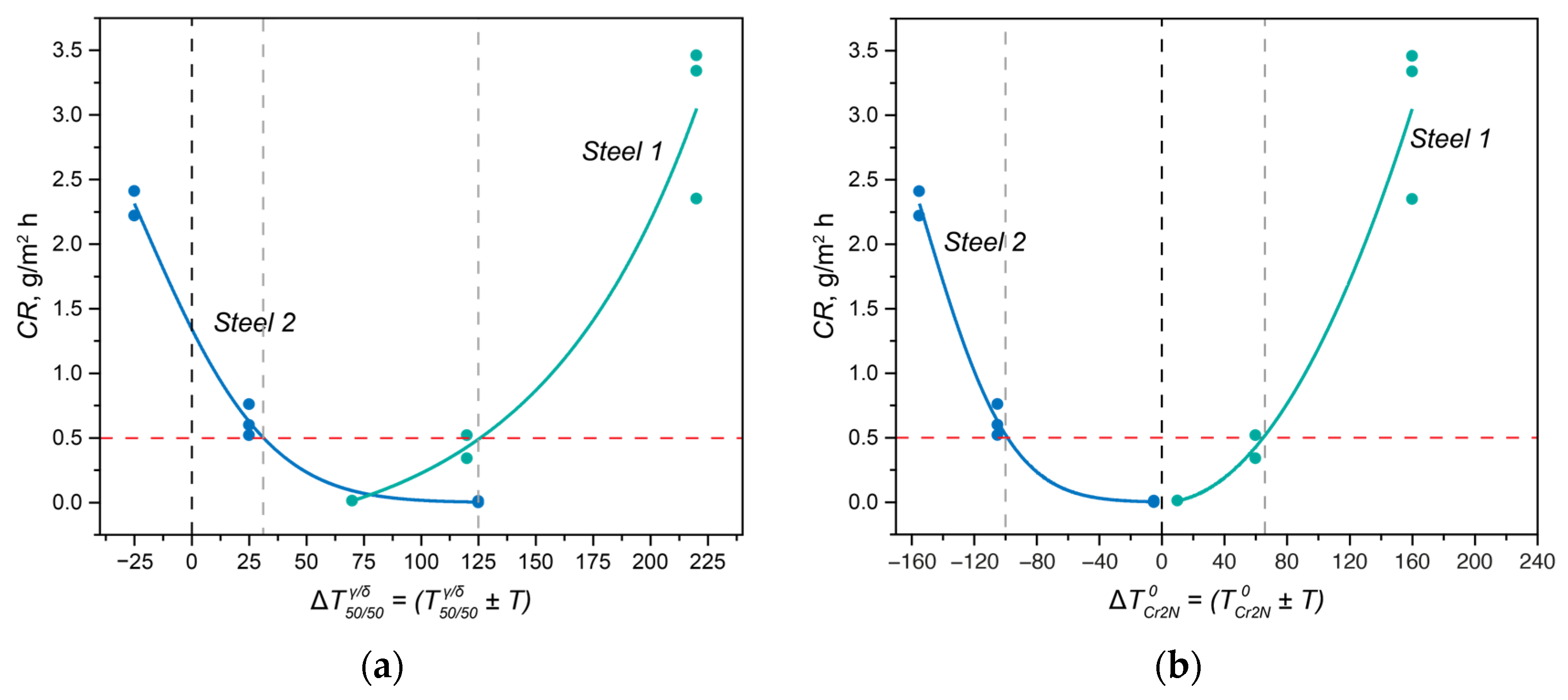
Figure 9, the dependence of the crevice corrosion rate for the two studied steels is shown, depending on the deviations in the heat treatment temperatures from the critical thermodynamic points.
In Steel 1, at 1050 °C, the solution heat treatment temperature was higher than
by Δ
T = 70 °C (
Figure 8a,c and green curves in
Figure 9). As the solution heat treatment temperature increased, it moved further away from
with an even larger Δ
T:
+ 120 °C at 1100 °C,
+ 220 °C at 1200 °C, and
+ 270 °C at 1250 °C. Moreover, all solution heat treatment temperatures were above
, so for all treatment temperatures, Δ
T was greater than zero, indicating the phase diagram region where chromium nitride was dissolved. Consequently, to ensure high corrosion resistance for this steel, it is essential to primarily maintain a small deviation in the solution heat treatment temperature from
.
In Steel 2, the situation was different: as the distance from
(
+ Δ
T) increased, the corrosion rate of the steel decreased (
Figure 8b,d and blue curves in
Figure 9a). However, due to the high temperature of
during annealing at 1050 and 1100 °C, the difference between the actual solution heat treatment temperature and
was less than zero, meaning chromium nitride formed and depleted the solid solution of nitrogen. However, when the annealing temperature reached 1200 °C, the ΔT with respect to
became approximately –5 °C, practically approaching the region where chromium nitride should dissolve. As a result, the corrosion resistance increased.
The conclusions drawn from these considerations have direct practical applications. When formulating requirements for the corrosion resistance of the developed DSSs, it is necessary to take into account the critical points of the phase diagrams and select chemical compositions in such a way that the actual heat treatment temperatures do not deviate from them by a critical Δ
T. For example, if the required crevice corrosion rate of the steel should not exceed 0.5 g/m
2·h for centrifugal pumps used in the petroleum and petrochemical industries, the composition should be chosen with consideration of the
PRENeff so that the following inequalities (4)–(6) are simultaneously satisfied (data from
Figure 9):
In the given inequalities, the difference between the solution annealing temperature and should be calculated as an absolute value since there may be some fluctuation in the phase composition, toward an increase in either the ferrite fraction or the austenite fraction. The difference between the solution heat treatment temperature and is strictly defined, as it represents the maximum possible undercooling below the temperature of chromium nitride formation, at which intensive nitride formation still does not occur within the actual annealing times. In the case of strongly negative values of ΔT concerning the temperature of , it is necessary to maximize not the PRENeff but the PREN of the phase acting as the anode in the specific test environment (specifically, for chloride-containing solutions, it is the PRENδ). Additionally, it is crucial to consider inequality (6) to exclude the formation of the σ-phase.
The proposed approach can be further extended to other grades of DSSs. Via such analysis, it is possible to more accurately assess the values of ΔT for each critical temperature and obtain more universal criteria for selecting DSS compositions, considering the peculiarities of alloying in each specific case, as well as the real heating rates, holding times, and cooling rates characteristic of specific industrial equipment.