The Effect of Powder Reuse on Electron Beam Melting for Biomedical Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Building Conditions
2.2. Sample Preparation
2.3. Powder Characterization
2.4. Scaffold Characterization
2.5. In Vitro Characterization
2.5.1. THP-1 Cell Expansion and Differentiation
2.5.2. THP-1 Macrophage Seeding
2.5.3. Cell Adhesion and Viability
2.5.4. Cell Attachment and Morphology
3. Results
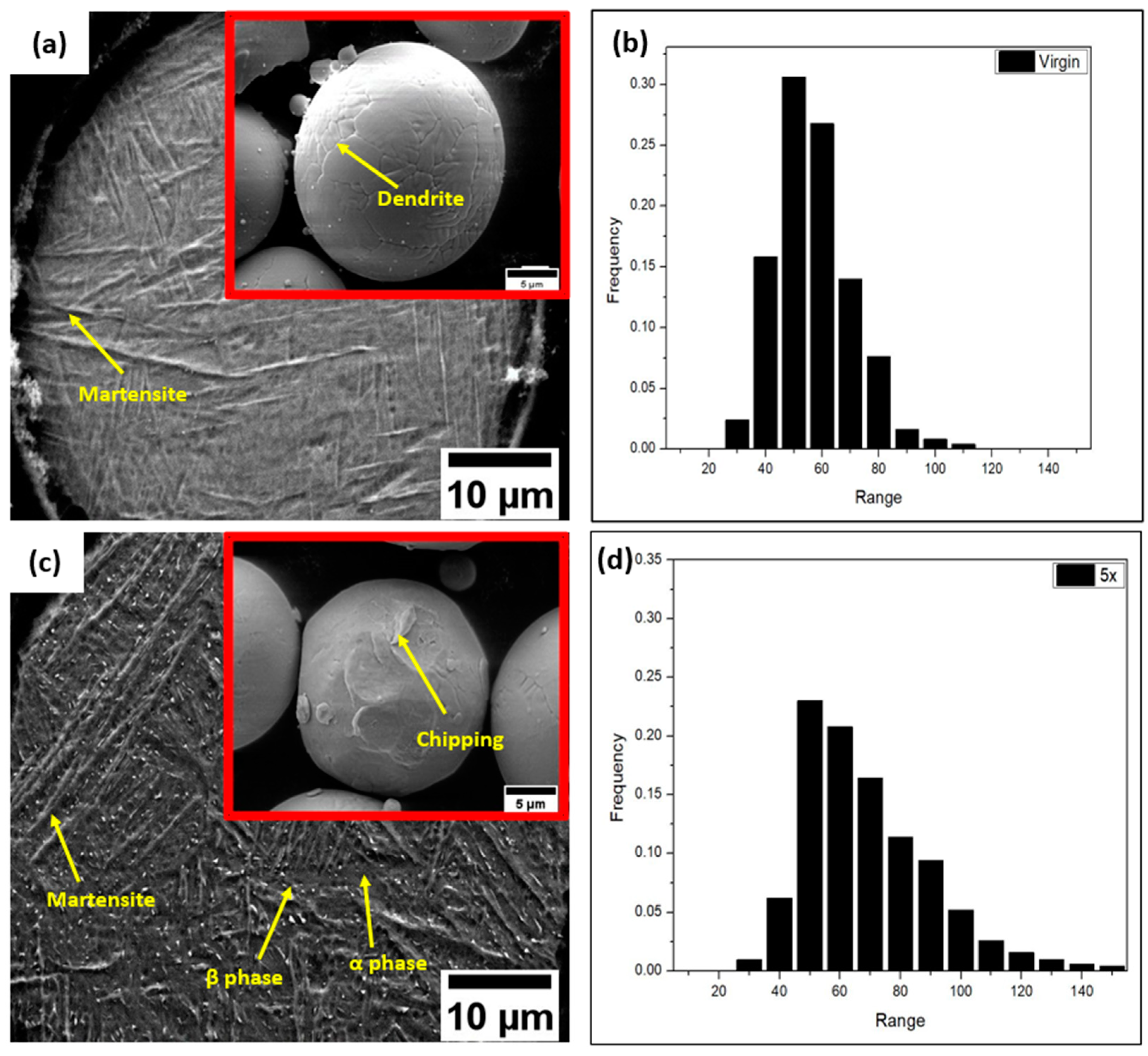
3.1. Powder Samples
3.2. Scaffold Samples
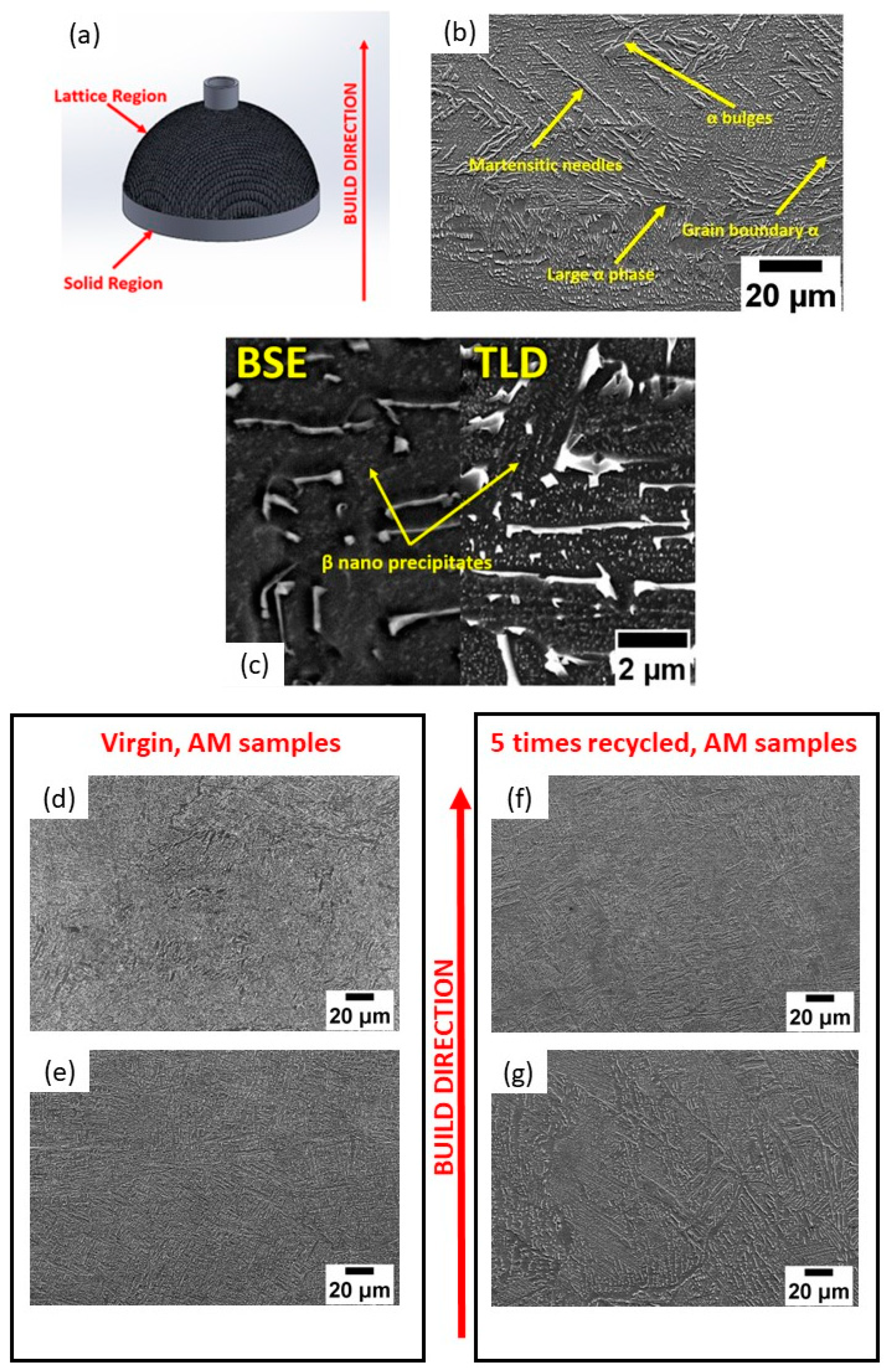
3.2.1. Microstructure and Elemental Composition
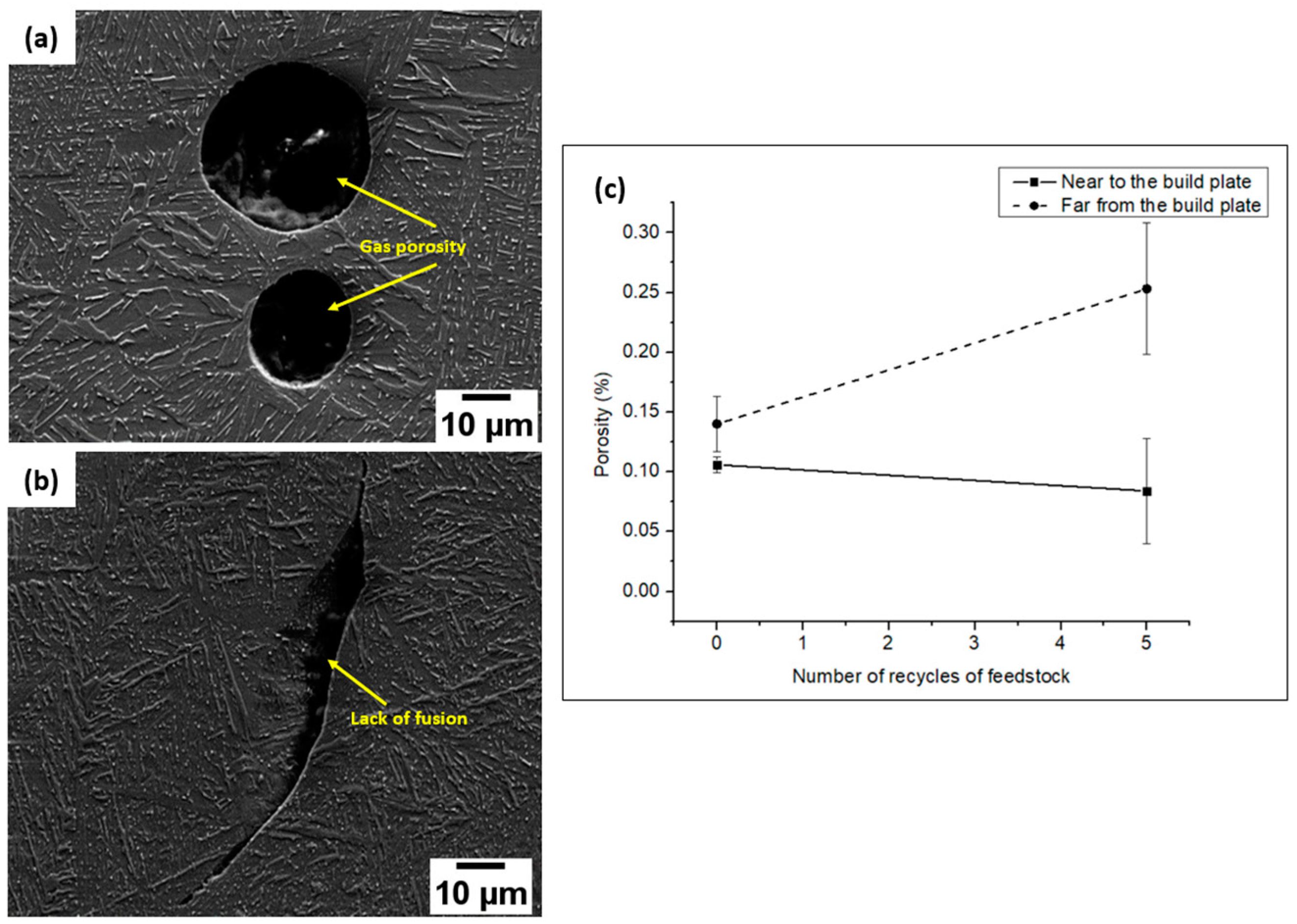
3.2.2. Porosity
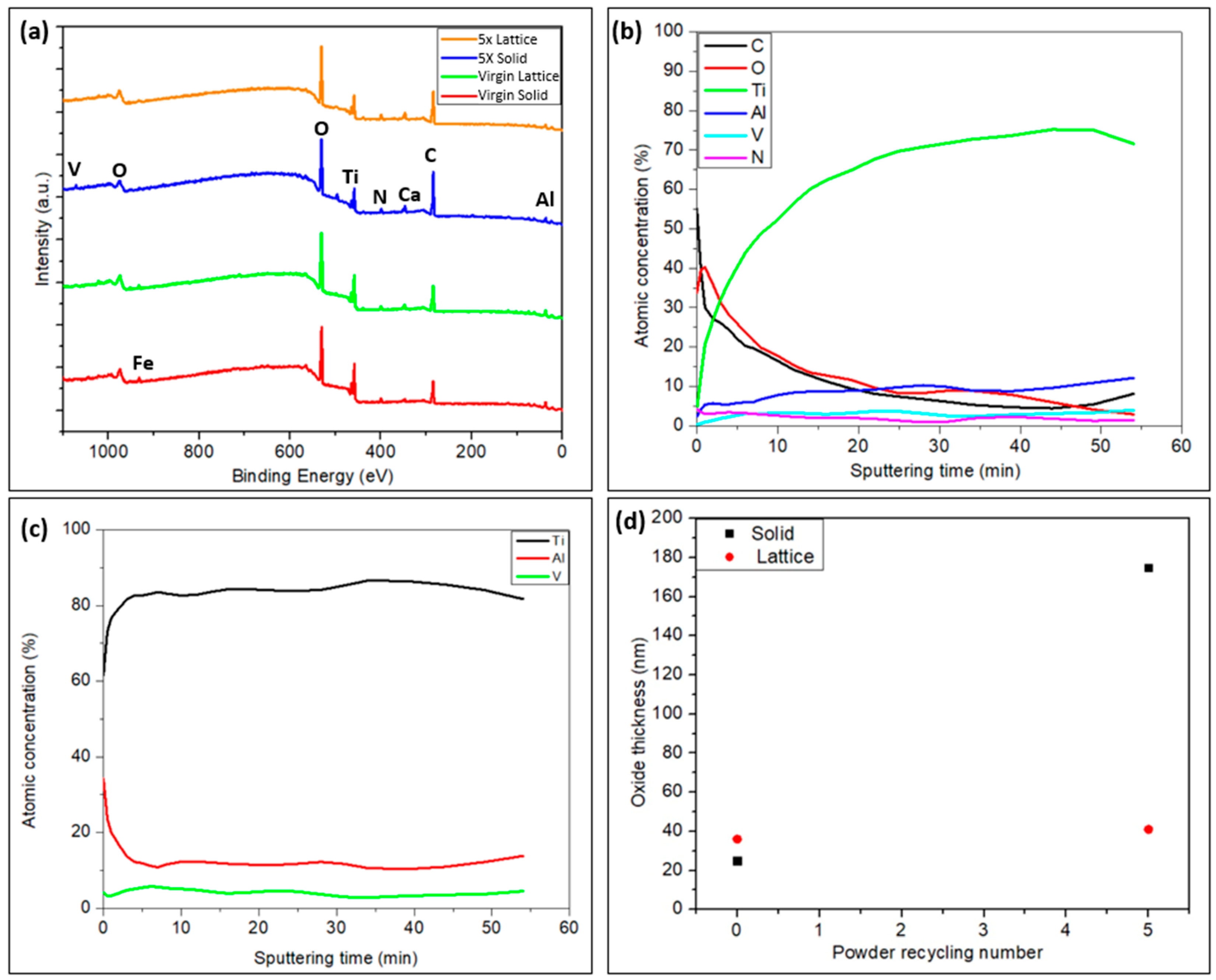
3.2.3. Surface Chemistry of the Solid and Lattice Regions of the Implant Samples
3.2.4. THP-1 Macrophage Viability and Adhesion
4. Discussion
5. Conclusions
- The powder particles were distorted, and the surface quality was found to be degenerated after recycling.
- The martensite percentage in the virgin powder was relatively high. After recycling five times, the powder particles’ microstructure changed visibly. Various microstructural features, like α bulges, a large α phase, and β phases, were discovered in the five-times-recycled powders. Also, the EDS results indicate that the surface area had a greater concentration of Al than the interior region of the powder. The O concentration seemed to be higher with powder recycling.
- Apart from the conventional Ti-6Al-4V phases, unconventional α bulges, large patches, and β nanoparticles were revealed in the microstructure. In addition, the bottom and top sections along the building direction of the AM specimens differed to a certain extent. However, there is a trivial variation with respect to powder recycling.
- The porosity evaluations uncovered the presence of classic EBM flaws, such as a lack of fusion and gas porosities. All the implant samples exhibited a rise in the porosity fractions as the build height rose.
- The implant samples created with recycled powder showed a rise in oxide thickness in both the solid and lattice regions, at least locally, attributed to the higher O concentration observed in the recycled powder due to the repeated recycling process. This seems to be beneficial for implant–tissue interaction. In addition, the enrichment in the Al at the surface points out the possibility of the formation of aluminum oxides at the surface.
- The AM parts fabricated with virgin and recycled powders exhibited cell compatibility. A slight improvement was demonstrated by the parts manufactured with recycled powder due to the increase in surface oxide thickness.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millis, D. Responses of Musculoskeletal Tissues to Disuse and Remobilization. In Canine Rehabilitation & Physical Therapy; Elsevier: Amsterdam, The Netherlands, 2004; pp. 113–159. [Google Scholar] [CrossRef]
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Shah, F.A.; Snis, A.; Matic, A.; Thomsen, P.; Palmquist, A. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater. 2016, 30, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Smoljanić, T.; Milović, L.; Sedmak, S.; Milovanović, A.; Čolić, K.; Radaković, Z.; Sedmak, A. Numerical Investigation of Fatigue Behavior in Ti-6Al-4V Orthopedic Hip Implants Subjected to Different Environments. Materials 2024, 17, 3796. [Google Scholar] [CrossRef] [PubMed]
- Stenlund, P.; Omar, O.; Brohede, U.; Norgren, S.; Norlindh, B.; Johansson, A.; Lausmaa, J.; Thomsen, P.; Palmquist, A. Bone response to a novel Ti–Ta–Nb–Zr alloy. Acta Biomater. 2015, 20, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, S.; Peretyagin, N.; Apelfeld, A.; Smirnov, A.; Yanushevich, O.; Krikheli, N.; Kramar, O.; Kramar, S.; Peretyagin, P. Investigation of MAO Coatings Characteristics on Titanium Products Obtained by EBM Method Using Additive Manufacturing. Materials 2022, 15, 4535. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.P.; Qian, M.; Liu, N.; Zhang, X.Z.; Yang, G.Y.; Wang, J. Effect of Powder Reuse Times on Additive Manufacturing of Ti-6Al-4V by Selective Electron Beam Melting. JOM 2015, 67, 555–563. [Google Scholar] [CrossRef]
- Duda, S. Microstructure Evolution of EBM Fabricated Ti-6Al-4V. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2020. [Google Scholar]
- Shanbhag, G.; Vlasea, M. The effect of reuse cycles on Ti-6Al-4V powder properties processed by electron beam powder bed fusion. Manuf. Lett. 2020, 25, 60–63. [Google Scholar] [CrossRef]
- Toh, W.Q.; Wang, P.; Tan, X.; Nai, M.L.S.; Liu, E.; Tor, S.B. Microstructure and Wear Properties of Electron Beam Melted Ti-6Al-4V Parts: A Comparison Study against As-Cast Form. Metals 2016, 6, 284. [Google Scholar] [CrossRef]
- Ding, R.; Guo, Z. Microstructural evolution of a Ti–6Al–4V alloy during β-phase processing: Experimental and simulative investigations. Mater. Sci. Eng. A 2004, 365, 172–179. [Google Scholar] [CrossRef]
- Lee, D.-G.; Lee, S.; Lee, C.S. Quasi-static and dynamic deformation behavior of Ti–6Al–4V alloy containing fine α2-Ti3Al precipitates. Mater. Sci. Eng. A 2003, 366, 25–37. [Google Scholar] [CrossRef]
- de Formanoir, C.; Martin, G.; Prima, F.; Allain, S.Y.; Dessolier, T.; Sun, F.; Vivès, S.; Hary, B.; Bréchet, Y.; Godet, S. Micromechanical behavior and thermal stability of a dual-phase α+α’ titanium alloy produced by additive manufacturing. Acta Mater. 2018, 162, 149–162. [Google Scholar] [CrossRef]
- Carreon, H.; Carreon, M.; Dueñas, A. Assessment of precipitates of aged Ti-6Al-4V alloy by ultrasonic attenuation. Philos. Mag. 2016, 97, 58–68. [Google Scholar] [CrossRef]
- Tang, H.P.; Zhao, P.; Xiang, C.S.; Liu, N.; Jia, L. Ti-6Al-4V orthopedic implants made by selective electron beam melting. In Titanium in Medical and Dental Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 239–249. [Google Scholar] [CrossRef]
- Ghods, S.; Schultz, E.; Wisdom, C.; Schur, R.; Pahuja, R.; Montelione, A.; Arola, D.; Ramulu, M. Electron beam additive manufacturing of Ti6Al4V: Evolution of powder morphology and part microstructure with powder reuse. Materialia 2020, 9, 100631. [Google Scholar] [CrossRef]
- Sun, Y.; Aindow, M.; Hebert, R.J. The effect of recycling on the oxygen distribution in Ti-6Al-4V powder for additive manufacturing. Mater. High Temp. 2017, 35, 217–224. [Google Scholar] [CrossRef]
- Self, J.; Aiken, C.P.; Petibon, R.; Dahn, J.R. Survey of Gas Expansion in Li-Ion NMC Pouch Cells. J. Electrochem. Soc. 2015, 162, 796–802. [Google Scholar] [CrossRef]
- Cao, Y.; Delin, M.; Kullenberg, F.; Nyborg, L. Surface modification of Ti-6Al-4V powder during recycling in EBM process. Surf. Interface Anal. 2020, 52, 1066–1070. [Google Scholar] [CrossRef]
- Axelsson, S. Surface Characterization of Titanium Powders with X-ray Photoelectron Spectroscopy. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2012. [Google Scholar]
- Tang, H.P.; Wang, J.; Song, C.N.; Liu, N.; Jia, L.; Elambasseril, J.; Qian, M. Microstructure, Mechanical Properties, and Flatness of SEBM Ti-6Al-4V Sheet in As-Built and Hot Isostatically Pressed Conditions. JOM 2017, 69, 466–471. [Google Scholar] [CrossRef]
- Galarraga, H.; Lados, D.A.; Dehoff, R.R.; Kirka, M.M.; Nandwana, P. Effects of the microstructure and porosity on properties of Ti-6Al-4V ELI alloy fabricated by electron beam melting (EBM). Addit. Manuf. 2016, 10, 47–57. [Google Scholar] [CrossRef]
- Goel, S.; Ahlfors, M.; Bahbou, F.; Joshi, S. Effect of Different Post-treatments on the Microstructure of EBM-Built Alloy 718. J. Mater. Eng. Perform. 2018, 28, 673–680. [Google Scholar] [CrossRef]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES, 1st ed.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Yadroitsava, I.; Plessis, A.D.; Yadroitsev, I. Bone regeneration on implants of titanium alloys produced by laser powder bed fusion: A review. In Titanium for Consumer Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–233. [Google Scholar] [CrossRef]
- Ataee, A.; Li, Y.; Fraser, D.; Song, G.; Wen, C. Anisotropic Ti-6Al-4V gyroid scaffolds manufactured by electron beam melting (EBM) for bone implant applications. Mater. Des. 2018, 137, 345–354. [Google Scholar] [CrossRef]
- Larsson, C.; Thomsen, P.; Lausmaa, J.; Rodahl, M.; Kasemo, B.; Ericson, L. Bone response to surface modified titanium implants: Studies on electropolished implants with different oxide thicknesses and morphology. Biomaterials 1994, 15, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.; Thomsen, P.; Aronsson, B.-O.; Rodahl, M.; Lausmaa, J.; Kasemo, B.; Ericson, L. Bone response to surface-modified titanium implants: Studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials 1996, 17, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.V., Jr.; Katz-Demyanetz, A.; Garkun, A.; Bamberger, M. The effect of powder recycling on the mechanical properties and microstructure of electron beam melted Ti-6Al-4 V specimens. Addit. Manuf. 2018, 22, 834–843. [Google Scholar] [CrossRef]
- He, Y.; Burkhalter, D.; Durocher, D.; Gilbert, J.M. Solid-Lattice Hip Prosthesis Design: Applying Topology and Lattice Optimization to Reduce Stress Shielding from Hip Implants. In Proceedings of the 2018 Design of Medical Devices Conference, Minneapolis, MN, USA, 9–12 April 2018; p. V001T03A001. [Google Scholar] [CrossRef]

| Powder/Region | Ti | Al | V | O |
|---|---|---|---|---|
| Virgin/Bulk | 87.67 ± 0.52 | 5.97 ± 0.19 | 3.87 ± 0.36 | 2.19 ± 0.20 |
| Virgin/Surface | 86.95 ± 0.63 | 6.45 ± 0.20 | 3.63 ± 0.28 | 2.64 ± 0.50 |
| 5 times/Bulk | 87.21 ± 0.96 | 5.6 ± 0.21 | 3.85 ± 0.63 | 2.62 ± 1.1 |
| 5 times/Surface | 86.37± 1.51 | 6.72 ± 0.54 | 3.14 ± 0.48 | 3.4 ± 1.13 |
| Sample/Phase | Ti | Al | V | O |
|---|---|---|---|---|
| Virgin/α phase | 88.88 ± 1.07 | 5.55 ± 0.56 | 3.39 ± 1.43 | 2.08 ± 0.31 |
| Virgin/β phase | 74.91 ± 8.10 | 2.89 ± 1.22 | 17.99 ± 8.33 | 1.5 ± 0.78 |
| 5 time/α phase | 86.71 ± 5.39 | 5.89 ± 1.04 | 4.85 ± 5.63 | 1.94 ± 0.27 |
| 5 time/β phase | 72.51 ± 5.50 | 2.74 ± 1.22 | 19.76 ± 6.11 | 1.76 ± 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundayadan Chandroth, A.; Giraldo-Osorno, P.M.; Nyborg, L.; Palmquist, A.; Cao, Y. The Effect of Powder Reuse on Electron Beam Melting for Biomedical Implants. Materials 2024, 17, 4701. https://doi.org/10.3390/ma17194701
Mundayadan Chandroth A, Giraldo-Osorno PM, Nyborg L, Palmquist A, Cao Y. The Effect of Powder Reuse on Electron Beam Melting for Biomedical Implants. Materials. 2024; 17(19):4701. https://doi.org/10.3390/ma17194701
Chicago/Turabian StyleMundayadan Chandroth, Akshay, Paula Milena Giraldo-Osorno, Lars Nyborg, Anders Palmquist, and Yu Cao. 2024. "The Effect of Powder Reuse on Electron Beam Melting for Biomedical Implants" Materials 17, no. 19: 4701. https://doi.org/10.3390/ma17194701
APA StyleMundayadan Chandroth, A., Giraldo-Osorno, P. M., Nyborg, L., Palmquist, A., & Cao, Y. (2024). The Effect of Powder Reuse on Electron Beam Melting for Biomedical Implants. Materials, 17(19), 4701. https://doi.org/10.3390/ma17194701






