Abstract
Polyester-based advanced thin films have versatile industrial applications, especially in the fields of textiles, packaging, and electronics. Recent advances in polymer science and engineering have resulted in the development of advanced amorphous and semi-crystalline polyesters with exceptional performance compared to those of conventional polymeric films. Among these, 1,4-cyclohexanedimethanol (CHDM) and cyclic-monomer-based polyesters have gained considerable attention for their exceptional characteristics and potential applications in smart films. This review article provides a comprehensive overview of the recent advances in the synthesis, characterization, and applications of CHDM and cyclic-monomer-based advanced polymers for smart film applications. It discusses the structure–property relationships of these innovative polyesters and highlights their unique characteristics, including thermal, mechanical, and barrier characteristics. Furthermore, this article also emphasizes the solution, melt, and solid-state polymerizations of the polymers. Special emphasis is placed on the influence of the addition of a second diol or second diacid on the performance characteristics of synthesized polyesters/copolyesters to explore their versatile industrial applications. Additionally, the impact of the stereochemistry of the monomers is explored to optimize the characterization of polyesters suitable for industrial applications. Furthermore, this article explores the potential of these advanced polyesters to be considered as materials for smart film applications, especially in the field of flexible electronics. Finally, this article examines the challenges and future recommendations for the development of CHDM and cyclic-monomer-based polyesters for smart film applications. It discusses potential avenues for further research, including in-depth studies for the synthesis and characterization of polyesters, the development of sustainable and biodegradable alternatives to cyclic monomers, alternative green approaches for the synthesis of polymers, etc. This review article provides valuable insight for researchers in academia and industry who are working in the fields of polymer science and materials engineering.
1. Introduction
Soon after the discovery of high-molecular-weight aliphatic polyesters by Carothers and Hill in 1932, polyesters gained the attention of academia and industrial researchers because of their potential applications [1]. Advanced polymers have gained attention because of their potential applications in the fields of catalysis [2,3], sensors [4], flexible electronics [5], medicine [6], wastewater treatment [7], textiles [8], and packaging [9]. Based on their composition, polymers are broadly classified into two groups: aliphatic and aromatic. Aliphatic polymers contain aliphatic diol and aliphatic diacid parts. However, the low thermal, mechanical, and hydrolytic properties of these materials limit their commercial applications. Aromatic polymers contain aromatic diacid and/or aromatic diol parts, and they are well known for their exceptional thermal, mechanical, hydrolysis, and chemical resistance properties [10,11]. Among a broad range of numerous polymers, poly(ethylene terephthalate) (PET) has found widespread applications in the fields of textiles, electronics, packaging, and molded plastic parts [5,9,12,13,14]. Whinfield and Dickinson reported PET as plastic and fiber in 1949 [15]. Because of the wide range of applications of PET, researchers have a keen interest in synthesizing new copolyesters with superior mechanical and barrier properties compared to those of the parent PET. Because of these commercial applications, the performance demands of PET are increasing rapidly. The suitability of PET as a flexible film substrate for electronic devices [5], textile fibers [8], thermoplastic resins [16], transparent and shrinkable films, and elastomers [17,18] has already been explored. However, we cannot use PET at an elevated temperature because of its high crystallization and low glass transition temperature (Tg). The barrier properties, especially the moisture barrier property of PET, drop rapidly above its Tg. So, PET is not suitable for making products that require a moisture barrier at an elevated temperature (above 100 °C). There are two widely used approaches to improve the thermal, mechanical, and barrier properties of PET: first, by introducing some fillers, like graphene [17], silica nanoparticles, or nanotubes [19,20], to the PET resin; second, by controlling the chemical structures of the polyesters themselves. Recently, many efforts have been made to improve the thermal and mechanical properties of copolyesters via the copolymerization and reactive blending of polyesters [21,22,23].
The commercial importance and applications of aromatic polyesters have increased tremendously since the first reported preparation of high-molecular-weight poly(ethylene naphthalene 2,6-dicarboxylate) (PEN) in 1969 [24]. Polyesters with aromatic moieties have attracted attention for decades because of their huge engineering thermoplastic market [25,26,27,28]. PEN is a well-known aromatic polyester with superior barrier and thermal properties (Tg = 120 °C for PEN vs. 81 °C for PET). Because of the presence of a double naphthalene ring in the PEN polymer, PEN has superior thermal stability, excellent mechanical properties, very high chemical resistance, and dimensional stability, which make it an ideal candidate as a high-performance material for applications in the engineering thermoplastic market, biosensors, flexible electronic devices, and a wide range of high-temperature applications [29]. The high thermal stability of PEN makes it suitable for high-temperature applications [28,30]. However, the high birefringence of PEN films, the necking phenomenon that occurs during the biaxial stretching of PEN, and the high cost of the monomer, 2,6-naphthalenedicarbxylic acid (NDA), used for the synthesis of PEN hinder the extensive applications of PEN films in versatile areas. Thus, it has gained the attention of scientists and researchers to find alternative ways to utilize the superior barrier, electrical, thermal, and mechanical properties of PEN at a relatively low cost.
A breakthrough in the polyester industry was the discovery of the poly(1,4-cyclohexanedimethylene terephthalate) (PCT) homopolyester prepared from terephthalic acid (TPA) and 1,4-cyclohexanedimethanol (CHDM) in 1959 [31]. Compared to PET, PCT has a higher Tg (88 vs. 80 °C) and Tm (300 vs. 260 °C), superior chemical resistance, and superior tensile and barrier properties [14]. However, the limited processing window of the PCT homopolymer acts as an obstacle to its commercial applications. The incorporation of varying amounts of CHDM into PET has resulted in the synthesis of a new class of amorphous to highly crystalline copolyesters. These CHDM-based copolyesters rapidly found a strong position in the commercial market of polyesters. Nowadays, CHDM-based copolyesters have a wide range of commercial applications. The performance properties of copolyesters can also be tuned by incorporating the second diacid or second diol.
Numerous pieces of literature are present that emphasize the CHDM diol moiety to enhance the thermal, physical, chemical, and mechanical properties of polymers [14,32,33]. Not only the CHDM content but the stereochemistry of CHDM (cis/trans isomers content) can also improve the resultant properties of the resulting polymers [34,35,36,37]. Trans-CHDM isomers are considered to be more stable than their analogous cis-CHDM isomers [38]. Kibler et al. described that the melting behavior of PCT can be improved by increasing the content of trans-CHDM from 0% to 100% (Tm 248 °C vs. 308 °C) [31]. Not only Tm but Tg of PCT homopolymer is also increased linearly by increasing the trans-CHDM content from 0 to 100% (60 vs. 90 °C). However, the crystallization rate is not similar for different compositions of PCT homopolymer. PCT homopolymer has a limited processing window, which can be controlled by introducing other diacid units into the molecular backbone. When a small amount of isophthalic acid (IPA) is incorporated into the PCT polymer backbone, it widens the processing window at the expense of Tg and Tm [39]. This modified PCT copolymer is called acid-modified PCT (PCTA).
This review provides detailed information on advanced polyesters based on cycloaliphatic CHDM. The effects of second diacid, second diol, and stereochemistry of monomers are discussed in detail. Mainly, PCT homopolymer, glycol-modified PCT, CHDM-modified PET, acid-modified PCT, and the effect of the stereochemistry of monomers and their potential commercial applications are discussed in detail. A new class of biobased PCT copolymers is also discussed in detail in later sections.
2. 1,4-Cyclohexandimethanol (CHDM) and Its Stereoisomers
CHDM is a commercially available diol with a cheaper price, and it is widely used for the synthesis of CHDM-based aliphatic and aromatic polyesters. It modifies the unique properties of synthesized polymers. There are three main isomers of CHDM: 1,2-CHDM, 1,3-CHDM, and 1,4-CHDM. The study of 1,2-CHDM and 1,3-CHDM-based polyesters and copolyesters is beyond the scope of this review. Traditionally, CHDM was synthesized on a commercial scale via the hydrogenation of dimethylene terephthalate (DMT) in a two-step process. The scheme for the synthesis of CHDM from DMT is shown in Figure 1 [40,41,42]. In the first step, DMT is converted into dimethyl cyclohexanedicarboxylate (DMCD) by treating hydrogen in the presence of catalyst (Pd) at the temperature of 180 °C, and in the second step, DMCD is reduced into CHDM in the presence of copper chromite catalyst at the temperature of 200 °C.
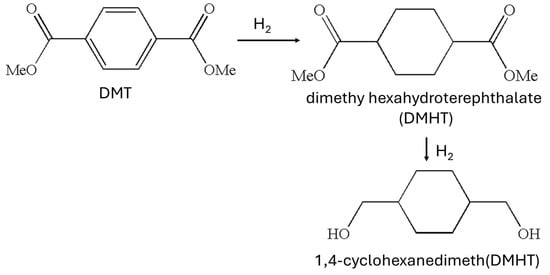
Figure 1.
Synthesis of 1,4-CHDM from DMT [40,41,42].
In the presence of either a bimetallic nano-catalyst or a supported tri-metallic nanocluster, it has also been proposed to prepare CHDM in a single step by hydrogenating DMT (Ru5PtSn). Compared to conventional approaches, these nanocatalysts facilitate the hydrogenation of DMT and allow for a modification reaction with high efficiency in mild conditions (100 °C, 20 bar) [43]. Wei et al. developed a method for preparing DMCD using a continuous hydrogenation process and prepared the CHDM with high activity and selectivity [44]. Recently, PET monomer waste (BHET) has been converted into CHDM in the absence of any kind of solvent utilizing Pd/C and Cu-based metallic catalysts [45]. Yancheng et al. successfully prepared CHDM using bio-based materials. This green synthesis strategy provides a viable alternative to the conventional methods that use hazardous materials [46]. However, some further reports describe the procedure for producing high trans-CHDM [41,47]. Both Eastman Chemical Company USA and TCI Japan are the leading producers of CHDM in the world. CHDM is produced as a cis/trans-isomers 70/30 trans/cis-CHDM isomers combination, and it is used to synthesize all commercial polyesters. The stereochemistry of CHDM has a direct influence on the properties of synthesized polyesters, which will be discussed in detail later. Cis- and trans-CHDM isomers are shown in Figure 2. Furthermore, to support readers’ clarity and ease of understanding, Table 1 highlights the information about the yield rate, catalyst, and summary of the research work, along with relevant references (Table 1).
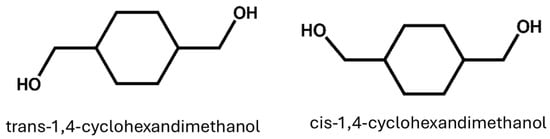
Figure 2.
Trans- and cis-isomers of 1,4-cyclohexanedimethanol (CHDM).

Table 1.
Summary of various preparation methods of 1,4-CHDM.
2.1. Aliphatic Polyesters and Copolyesters Containing CHDM and Their Applications
CHDM has been widely employed for the synthesis of biodegradable aliphatic polyesters and copolyesters with numerous uses. Typically, these copolyesters are synthesized using a two-step melt polymerization or enzymatic polymerization process. Two-step polycondensation of HCDM, sebacoyl chloride, and 1,4-cyclohexane diamine resulted in better mechanical, thermal, and biodegradability properties [57]. Tsai et al. recently synthesized a range of biodegradable aliphatic copolyesters using enzymatic polymerization of 1,3/1,4-CHDM with succinic acid and 1,4-butanediol. They were successful in tuning the characteristics of resultant copolymers by varying the amount of CHDM included [58]. Hansen et al. also reported the enzymatic polymerization of aliphatic copolyesters containing CHDM as a diol moiety and succinic acid, atopic acid, and suberic or sebatic acid as a diacid moiety, using Cutinase from Humicola insolens; however, moderate molecular weights of these polymers limits their applications [59]. Barret et al. effectively synthesized a poly(1,4-cyclohexanedimethanol itaconate) thermoset polymer using single-step enzymatic polymerization and analyzed its mechanical and biocompatibility properties. They discovered that this material could be a strong option for future biomaterials [60]. Nowadays, practically all cycloaliphatic polyesters are synthesized from 1,4-CHDM and 1,4-cyclohexanedicarboxyklic acid (CHDA). Many groups have focused on the polycondensation of CHDM with CHDA in order to synthesize high-molecular-weight cycloaliphatic poly(1,4-cyclohexylene 1,4-cyclohexanedicarboxylate) (PCCD). In comparison to the CHDM diol moiety, the cyclohexane ring structure of CHDA remains stable in both cis- and trans-CHDM configurations. As the trans-CHDA isomer content (mole %) increases, the Tg and Tm of resulting polyesters grow linearly. The trans-CHDA isomers are used to manufacture high-molecular-weight thermoplastic aliphatic polyesters. In contrast to CHDM, cis-/trans-CHDA isomers can easily revert to their equilibrium mixture (68/32%: trans/cis) in the presence of an appropriate catalyst at a high melt polycondensation temperature [39]. Xiaodong et al. synthesized a series of poly(butylene-co1,4-cyclohexanedimethylene carbonate) (PBCC) and investigated the influence of CHDM on the performance properties of synthesized biodegradable PBCC. They observed that the increased CHDM concentration linearly improves the thermal stability and mechanical and heat-distortion properties of PBCC random copolymers [61]. The thermal degradation behavior and other performance attributes of biodegradable aliphatic poly(butylene 1,12-dodecanedioate) random copolyesters were also significantly improved by inserting cycloaliphatic 1,4-cyclohexanedicarboxylic acid units into the molecular backbone. It was discovered that trans-CHDA isomers improve the performance attributes of synthesized aliphatic polymers in a linear manner [62]. Recently, Seul et al. successfully modified the brittle properties of isosorbide (ISB)-based polycarbonate by inserting the second diol. A variety of biodegradable copolycarbonates comprising ISB, cycloaliphatic CHDM, and diphenyl carbonate were synthesized using a two-step melt polymerization technique. A CHDM concentration greater than 50 mole % enhances the ductility of the resulting polyesters. However, when the Tg of the synthesized polymer decreases, we increase the content (mole %) of CHDM because the resultant polymer contains less rigid heterocyclic ISB [63]. Figure 3 depicts the chemical structures of various aliphatic polyesters [64].
Figure 3.
Chemical structure of various aliphatic polyesters [64].
Brunelle et al. filed a patent for the synthesis of PCCD; they successfully synthesized a cycloaliphatic polymer by optimizing the monomer feed ratio and reaction conditions. Later, they were successful in synthesizing the stereoregular polymer by adjusting reaction parameters such as the temperature, catalyst, and time. The reaction conditions were optimized to prevent the isomerization of trans-CHDA isomers, and they successfully generated PCCD polymers with a molecular weight ranging from 75,000 to 80,000 [65]. High-molecular-weight polyoxaesters with acceptable thermal and hydrolysis properties were synthesized by melt polymerization of CHDM with oligo(ethylene glycol) diacid in the presence of a suitable catalyst. The absorbent polyoxaesters may have biomedical applications as suture coverings, and adhesion-prevention barriers have been proposed [66]. Based on the chemical structure, physical performance, and performance characteristics such as structure integrity, adhesion-prevention barriers, UV resistance, and so on, CHDM-based polyesters have prospective applications in the fields of medical, safety protection, and outdoor applications. Because it is feasible to manipulate the structure of the polyesters by incorporating various suitable diacids or diols, academics and industry researchers are investigating the structure–property relationship of homopolyester and copolyesters. Because of this distinct behavior, polyesters have established a prominent position among other performance polymeric materials [67,68,69].
In short, the literature shows that cycloaliphatic polyesters have been synthesized by employing a variety of monomers and methods. Cycloaliphatic polyesters outperform aromatic polyesters in terms of UV stability, optical performance, and good weatherability properties. These cycloaliphatic polymers could be used as weather-able materials and biomaterials. A detailed study is necessary to delve into the diverse applications of these cycloaliphatic polymers.
2.2. Thermally Stable Aromatic Polyesters and Copolyesters Containing CHDM
Thermoplastic polyesters have garnered the attention of academic and industrial researchers because of their vast range of domestic and technical applications [5,9,12,13,14,70]. The synthesis of high-molecular-weight aliphatic polyesters was first reported by Carothers and Hill [1]. However, the inherent poor hydrolytic stability, low glass transition temperature, and melting temperature of aliphatic polyesters have effectively eliminated their commercial applicability. Whinfield and Dickson reported on a new aromatic poly(ethylene terephthalate) (PET) with acceptable Tg and Tm in 1949 [15]. However, PET’s (81 °C) strong crystallinity and low Tg limits its practical applicability at higher temperatures. The mechanical, chemical, and barrier properties of PET can be improved by including rigid cycloaliphatic 1,4-CHDM diol into the backbone of the aliphatic polyester. 1,4-CHDM is accessible commercially in the form of a blend of cis-and trans-CHDM isomers (70/30%). Kibler et al. reported on the synthesis process and thermal characteristics of poly (1,4-cyclohexylene dimethylene terephthalate) (PCT) in 1964. The Eastman Kodak Company successfully synthesized semi-crystalline PCT fiber and marketed it in the fiber industry under the trade name of Kodel for a long period before discontinuing it in 1980 [36]. PCT is now manufactured via two-step melt polymerization with NDA or DMT as a diacid moiety and CHDM as a diol moiety. PCT and its copolyesters have superior thermal, mechanical, chemical, and barrier properties compared to PET [31]. Commercial PCT is highly crystalline with high Tm (295 °C), Tg (about 90 °C), and thermal degradation stability, and it is less expensive than liquid crystalline polymers (LCP). PCT possesses outstanding thermal, mechanical, and hydrolytic stability characteristics but has a similar flow during molding when compared to traditional PET and PBT polymers. Amorphous copolyesters, including rigid and bulky CHDM, have a wide range of commercial applications, including injection-molded polymers for medical and electronic applications [14,40]. However, both a high crystallinity and high melting temperature (295 °C) of PCT (limited processing window) operate as barriers to melt polymerization. As a result, the commercial applications of PCT copolyesters as films have been limited by their properties. For typical plastic applications, the processability of PCT polymer must be increased by adding it with diacid or diol compounds. The distinctive, unique characteristics and applications of several CHDM-based copolyesters are summarized in Table 2.

Table 2.
Unique characteristics and applications of various CHDM-based copolyesters.
3. Preparation of CHDM-Based Advanced Polymers
Typically, CHDM-based homopolyesters and copolyesters are made using a polycondensation process. Depending on the types of polyesters, the chemical structure of CHDM and diacid moieties may be changed to optimize the reaction efficiency. Most high-molecular-weight aromatic polymers are synthesized by polymerizing CHDM with diacid at high temperatures and pressures. There have been numerous research studies on the synthesis of CHDM-based polyesters using different techniques [21,59,80,81]; however, the most common techniques are melt polymerization, solution polymerization, and ring-opening polymerization. The following section provides deeper details about various synthesis strategies
3.1. Solution Polymerization
Almost all polymers are now synthesized using a complicated two-step melt polymerization process. The first process involves the synthesis of pre-polymer by esterification or a transesterification reaction, and in the second step, known as polycondensation, the synthesized prepolymer reacts with diol at relatively high temperatures and pressures. A product removal setup is also included at both stages of polymerization [21,82]. Due to the severe conditions used during melt polymerization, a stoichiometric imbalance is observed due to the degradation and sublimation of monomers, which causes an increase in side reactions and a decrease in reaction efficacy [83,84]. Some of the issues related to melt polymerization are resolved by utilizing appropriate metallic catalysts [85,86]. However, titanium-based catalysts, which are thought to be the most effective of all metallic catalysts, produce a yellow tint in the synthesized product, whilst antimony-based catalysts are involved with some toxicity problems [87,88]. Generally, an extra thermal stabilizer with metallic catalysts is necessary to prevent polymer degradation during polycondensation and the following process, which results in increased cost [89]. It is worth noting that no polymer degradation or product discoloration occurs during the solution polymerization reaction [90,91]. The primary challenge with solution polymerization is the selection of a pure solvent that promotes monomer solubility and the facile recovery of synthesized polymer products. A reproducible one-step solution polycondensation process is widely known for producing pure and well-defined polymers. A schematic diagram of solution polymerization used for the synthesis of cyclic monomers—CHDM, 2,6-naphthalene dicarboxylic and terephtalloyl chloride-based advanced Poly(1-4,cyclohexane dimethylene terephthalate-co-naphthalene dicarboxylate) PCTN copolyester is shown in Figure 4 [92].
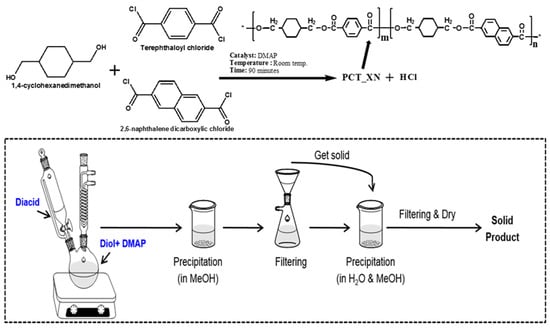
Figure 4.
Schematic diagram for the solution polymerization of PCTN copolyester. * Indicates the open ends of a single monomer of PCTN copolyester ready to react with other monomers to build a long chain polymeric chain.
3.2. Melt Polymerization
CHDM-based aliphatic and aromatic polyesters and copolyesters are preferably synthesized using the two-step melt polymerization technique. Esterification/transesterification is typically the initial step in polymer synthesis, and it is carried out at relatively low temperatures and pressures depending on the monomers utilized. Typically, an excess amount of diol moiety to diacid moiety (1:1.2–2.2) is employed during the synthesis process, resulting in short oligomers during the esterification step. Short oligomers react under high pressure and temperature to form high-molecular-weight polymers, a process known as polycondensation. The byproducts from each process are removed individually.
Two-step melt polymerization techniques have been used to synthesize a wide range of aromatic and aliphatic polyesters and copolyesters [23,32,35,37,49,83]. The optimal reaction conditions, such as temperature, time, and pressure, are chosen based on the polymers to be manufactured. Catalysts and thermal stabilizers are carefully selected because they have a direct influence on the color of the finished product. Figure 5 shows the typical melt polymerization reactor that is used for the melt polymerization of polyesters.
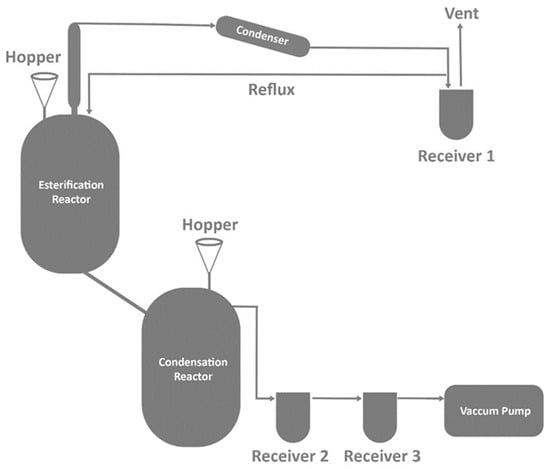
Figure 5.
Pilot-scale melt polymerization reactor [33].
3.3. Ring-Opening Polymerization
Nowadays, cyclic oligoesters’ ring-opening polymerization (ROP) is gaining attention. ROP offers some advantages over conventional melt polymerization. ROP, unlike melt polymerization, occurs at low temperatures and pressures. Furthermore, no by-products, such as water or methanol, are produced during the synthesis of polyesters using ROP [93,94]. A lot of literature exists that focuses on the synthesis of polyesters and copolyesters using ROP [88,93,94]. Aromatic polyesters and copolyesters have been synthesized via ROP [80,95,96]. CHDM-based poly(1,4-cyclhexylenedimethylene terephthalate) cyclic oligomers were synthesized in solution from CHDM and TPC and separated as a mixture of oligomers of various sizes using high-performance chromatography. The ROP of these oligomers was carried out at 310 °C for 30 min in the presence of antimony oxide for the synthesis of a high-molecular-weight PCT homopolymer [96].
Nathalie et al. used ROP to synthesize a series of poly(ethylene-co-1,4-cyclohexanesimethylene terephthalate) copolyesters (coPExCyT), altering the ET/CT monomer ratios ranging from 90/10 to 10/90. Oligomers for the synthesis of coPExCyT copolyesters were synthesized by the copolycondensation of PCT and PET homopolymers. The results indicated that synthesized coPExCyT copolyesters were random copolymers with high molecular weights [80].
3.4. Solid State Polycondensation (SSP) of Polyesters and Copolyesters
The need for high-molecular-weight and low-cost polyesters and copolyesters for engineering plastic applications is growing, indicating the necessity to synthesize tiny particles. Solid state polycondensation (SSP) can be used to produce polyesters with thermally unstable components that can disintegrate under harsh melt polymerization conditions. Isothermal treatment at temperatures ranging between a cold crystallization temperature (Tc) and melting temperature (Tm) in the solid state increases the molecular weight of melt polymerized polyesters and copolyesters. This process is known as solid state polycondensation. The molecular weight of polyesters and copolyesters influences their performance properties, such as thermal stability, tensile strength, fatigue behavior, and hydrolytic stability [97]. SSP allows for the production of high molecular weights of polyesters and copolyesters that would otherwise be impossible to achieve using melt polymerization processes. Melt polymerization produces an intrinsic viscosity (IV) of 0.58–0.68, whereas, for technical applications (such as film, bottle, tire cord, seat belt, airbags, etc.), an IV of typically between 0.70 and 1.20 is required. The SSP process is popular because it eliminates the issues associated with melt polymerization. Some issues arise during the polycondensation of viscous polymers in the melt phase. During melt polycondensation, higher-IV polyesters with increased viscosity are difficult to stir, and thermal degradation occurs at higher temperatures, resulting in low-molecule polymers. It is extremely difficult to extract volatile by-products from highly viscous polymers. This procedure is also carried out at high temperatures, and the vacuum increases the expense of the product. Furthermore, the thermal degradation of the polymer occurs due to the result of undesired side reactions, which hinder the growth of molecular chains. As a result, the melt viscosity and molecular weight of the synthesized product decrease. SSP addresses all of the limitations of melt polycondensation by operating under comparatively mild conditions [98]. SSP is of relevance because of the increasing need for polyesters and copolyesters in widespread areas requiring high molecular weights. Furthermore, certain monomers and polymers demand mild conditions that cannot be met in the melt phase. As a result, the SSP technique is preferred for the production of high-quality homopolyesters and copolyesters with improved performance properties.
On an industrial scale, continuous or discontinuous SSP processes are carried out either in a vacuum or supported with an inert gas flow. Another form of SSP, known as the suspension process in a swollen state, can produce higher-molecular-weight polyesters [99]. Generally, large-scale manufacturing of polyesters or copolyesters suited for high-tech applications is performed in a continuous process. Discontinuous SSP is carried out in a tumble dryer, and it is thought to be versatile and simple. It enables the successful manufacturing of specialty materials on a small scale, particularly for engineering plastics [100]. However, the reactor’s small volume (44 m3) restricts its use. Different SSP parameters, including temperature, residence time, gas type, gas purity, and gas speed, are paid a lot of attention since they have a direct impact on the final quality of SSP [98,101,102]. The prepolymer’s end group concentration, catalyst, molecular weight, homogeneity, and pallet size all have an impact on the final product’s quality [103]. The chemical structures of monomers in polymer synthesis affect the reaction rate, which in turn influences the molecular weight of the synthesized polymer [104]. The SSP reaction follows the classical thermodynamics, second-order chemical kinetics, and diffusion rates. During the process, the tiny chains join together, and then reactive end groups diffuse into the amorphous regions of semi-crystalline polyesters. As a result of SSP, amorphous regions of the result high-quality product are decreased, and the regular arrangement of molecular chains is increased [105,106]. The consequent high product quality and performance properties make SSP an attractive approach for producing high-molecular-weight polymers suited for use as engineering plastics in versatile areas. A summary of the various polymer synthesis techniques, along with pros and cons, are given in Table 3.

Table 3.
Summary of various polymer-synthesis techniques.
4. Synthesis of Cyclic Compound-Based Advanced Hompolyesters and Copolyesters
4.1. Synthesis and Properties of 1,4-Cyclohexanedimethanol (CHDM)-Based Conventional Homopolyesters (PCT and PCN)
The synthesis and application of CHDM-based PCT homopolymers was first discovered by Kibler et al. in 1949 [36]. This polyester was first synthesized by a two-step melt polymerization, and its applicability as a fiber was investigated. On a commercial scale, PCT is synthesized by melt polymerization of NDA or DMT with CHDM in the presence of an appropriate catalyst and stabilizer. Because of the high melting point of PCT homopolymer, polycondensation occurs at a range of temperatures higher than 300 °C [36]. SSP can further boost the molecular weight of the synthesized PCT polyesters [21]. Compared to PET, PCT has greater Tg (88 vs. 80 °C), Tm (300 vs. 260 °C), superior chemical resistance, attractive tensile properties, and barrier properties [14]. Due to its superior thermal characteristics, high clarity, and improved molding characteristics, PCT is used as an injection-molded polyester for developing electronic and automotive parts [39]. However, the limited processing window of the PCT homopolymer makes it unsuitable for a wide range of commercial applications. The addition of various amounts of second diol or second diacid into the PCT homopolymer effectively broadens the processing window, resulting in the synthesis of a new class of amorphous to highly crystalline copolyesters with a strong position as performance materials in the commercial market of polyesters. The replacement of acyclic aliphatic diols with stiff cycloaliphatic CHDM diol leads to the synthesis of polyesters with better thermal properties [39,40]. The chemical structures of PET, PCT, PEN, and PCN are shown in Figure 6a. DSC thermograms of these homopolyesters clearly reveal that stiff cyclohexene units (CHDM) improve the Tg and Tm of the resulting polyesters Figure 6b.
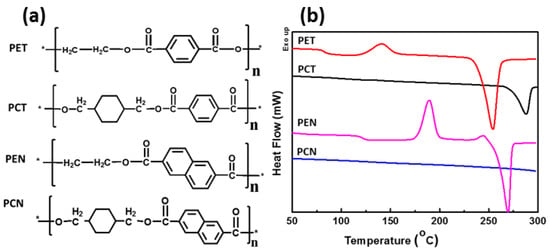
Figure 6.
(a) Chemical structure of PET, PCT, PEN, and PCN homopolyesters; (b) DSC thermograms of PET, PCT, PEN, and PCN homopolyesters. * Indicates the open ends of a single monomer of each homopolyester, ready to react with other monomers to build a long chain polymeric chain.
The type of diol and diacid moieties have a direct impact on polyester performance qualities. A list of comparative properties of PET, PCT, PEN, and PCN is given in Table 4. However, the film properties of PCN polyester are not addressed because the synthesis of PCN resin with such a high melting temperature and limited processing window is unsuitable for industrial usage. The results show that CHDM units enhance the thermal properties of polyesters when compared to those containing EG units. Similarly, rigid and thermally stable naphthalene units improve the mechanical and thermal properties of polyesters when compared to their analogous polyesters containing terephthalate units [24,26,36,39,40].

Table 4.
Chemical composition and comparative properties of PET, PCT, PEN, and PCN homopolymers [24,26,36,39,40].
4.2. Second Diol Modified PCT Copolyesters
Modifying the diol moiety allows for the synthesis of novel polyester-based materials with correct molecular design. Cycloaliphatic 1,4-cyclohexanedimethanol (CHDM) is easily incorporated into the polyesters during melt polymerization. The non-planar ring structure of CHDM enhances the thermal stability and mechanical and barrier properties of the resultant poly(cyclohexane 1,4-dimethylene terephthalate) (PCT) [39]. However, there are significant processing problems associated with the synthesis of PCT that can be addressed by adding a second diol into the polymer backbone itself (Figure 7). Tm of CHDM-modified PET polyester drops initially until it hits the eutectic point around 40 mol% 1,4-CHDM, after which it begins to grow exponentially as the CHDM level increases. At the eutectic point, PET and PCT crystals coexist in the copolyester. The findings revealed that the rigid and cyclic aliphatic CHDM increase the rigidity and regularity of polymer backbones [39].
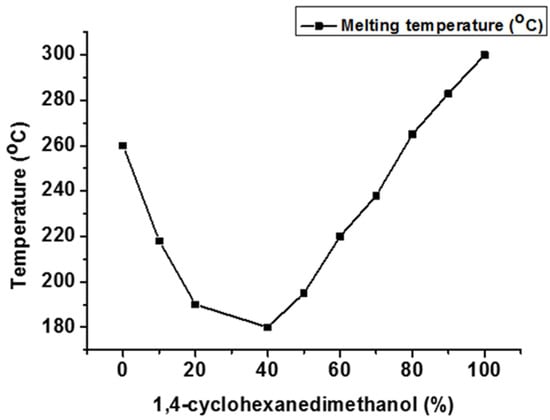
Figure 7.
Effect of 1,4-CHDM on Tm of CHDM-modified PET copolyester [39].
Jo et al. synthesized and investigated the crystallization behavior of poly(m-methylene 2,6-naphthalate-co-1,4-cyclohexanedimthylene 2,6-naphthalate) (m = No. of methylene groups). They discovered that poly(ethylene 2,6-naphthalte-co-1,4-cyclohexanedimethanol 2,6-naphthalate) (PEN-co-CN) had an amorphous structure in the center of copolymer composition, while poly(butylene 2,6-naphthalate-co-1,4-cyclohexanedimthylene 2,6-naphthalate) (PBN-co-CN) and poly(hexamethylene 2,6-naphthalate-co-1,4-cyclohexanedimthylene 2,6-naphthalate) (PHN-co-CN) had very clear melting points and also showed sharp diffraction peaks across the entire range of copolymer composition. Additionally, (PBN-co-CN) exhibited eutectic melting behavior, with Tg increasing linearly as CN % rose (Figure 8) [112]. However, in the case of (PHN-co-CN) copolyester, both Tg and Tm grew linearly as CN units increased, indicating that this copolymer had an isomorphic crystallization nature. These results show that BN and HN units can co-crystallize together while EN and CN cannot. This trend could be explained by the fact that BN and HN units have equivalent densities, volumes, and repeating unit lengths, whereas EN and CN units do not [112].
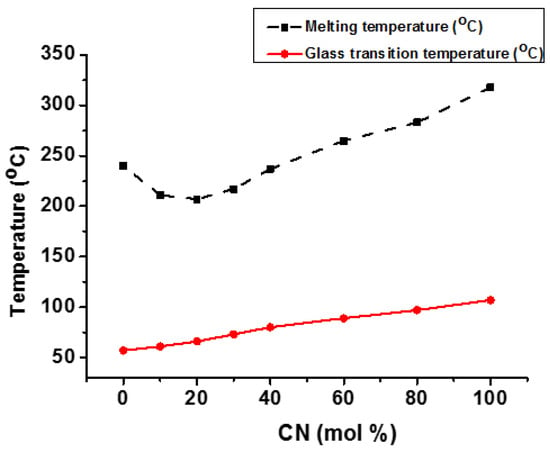
Figure 8.
Effect of composition on Tg and Tm of PBN-co-CN copolyester [112].
Aromatic poly(trimethylene-co-1,4-cyclohexanedimethylene terephthalate) (PTCT) with a random microstructure was synthesized by the two-step melt polymerization of 1,3-propanol, CHDM, and DMT [113]. At 42 mole % of CHDM units, both tri-methylene terephthalate (TT) and cyclohexylene dimethylene terephthalate (CT) coexist in the PTCT copolyesters. At the same time, copolyesters with less than 35 mole % of CT content crystallize in a PTT-type lattice, whereas those with more than 42% CT content crystallize with a PCT-type lattice. The thermal degradation behavior and other thermal parameters of the synthesized copolyesters were improved by increasing the CT content (mol %), as shown in Figure 9.
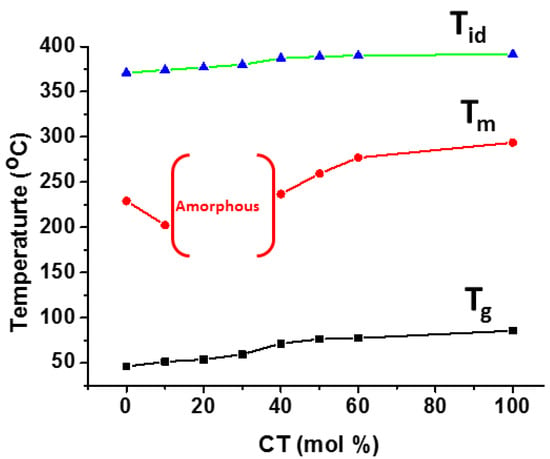
Figure 9.
Effect of composition on Tg and Tm of PTCT copolyester.
A variety of copolyesters derived from PCT and PET homopolymers were identified. Copolyesters were synthesized by polymerizing TPA with different amounts of CHDM and EG, resulting in copolyesters having 12, 31, 32, 61, 70, and 81 mol % CHDM. It was discovered that the free volume and gas permeability of synthesized copolyesters increased linearly with the CHDM concentration [114].
If the EG is replaced with the CHDM, poly(ethylene glycol-co-1,4-cyclihexanedimethanil terephthalate) (PETG) is formed. The inclusion of CT units into the backbone of PET increases the alkali resistance of PETG copolyesters. The amorphous regions of PET and PETG polyesters were more susceptible to attack by the alkali than crystalline regions. Furthermore, hydroxyl anions caused corrosion to crystals without altering the crystalline structure of synthesized polyesters [115].
When the CHDM of PCT is substituted with hexanediol, the resulting poly(1,4-cyclohexylenedimethylene terephthalate-co-hexamethylene terephthalate) [P(CT-co-HT)] are random copolyesters with iso-dimorphic co-crystallization behavior. DSC and WXRD results confirmed that synthesized copolyesters are crystalline in nature with an eutectic point at 80 mol% of HT when a crystal transition from a PCT-type crystal to PHT-type crystal occurs [116].
4.3. Second Diacid-Modified PCT Copolyesters and Their Applications
As previously stated, CHDM and TPA-based PCT homopolymers have a limited processing window, which can be regulated by increasing other diacid units in the molecular backbone. When a small quantity of isophthalic acid (IPA) is added to the PCT polymer backbone, it expands the processing window at the expense of Tg and Tm [39]. This PCT copolymer is known as acid-modified PCT (PCTA). Figure 10 demonstrates that as the IPA content percentage increases in PCT, both Tg and Tm are decreased. However, Tm decreased more than Tg. It was also found that at higher concentrations (more than 40%) of IPA, completely amorphous copolymers were obtained [39,117]. These transparent amorphous copolymers have excellent mechanics, hydrolysis, and chemical resistance. PCTA has several attractive performance features, which can be due to the presence of tough and hydrophobic CHDM units. These copolymers can be melt-processed without pre-drying and hold a very strong position in the plastic industry due to their performance properties. PCT_XA_X30 (30% IPA) copolyesters have good physical, thermal, and mechanical properties equivalent to glycol-modified PCT and CHDM-modified PCT [39].
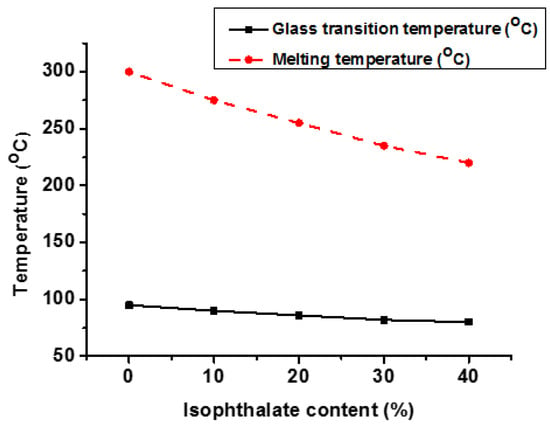
Figure 10.
Effect of isophthalate content (%) on Tg and Tm of PCT copolyester [39].
The thermally stable naphthalene unit (NDA) was identified as a modifier for acid-modified copolyesters (PCTAs). The addition of a modest amount of diacid into the polymer backbone effectively widens the processing window of PCT polyester by lowering its Tm. These semi-crystalline copolyesters (PCTN) are durable and transparent resins. The impact of increasing levels of naphthalene content (mol %) on the thermal characteristics (Tg, Tm, ∆Hm) and degradation behavior of PCT is summarized in Table 5 [21,92]. As indicated, the thermal degradation stability and Tg of the synthesized PCTN copolyesters rose linearly as the number of naphthalene units increased. However, the Tm of copolymers first decreases until it reaches the eutectic point (36 mole % of naphthalene), after which it begins to increase by increasing the naphthalene concentration. At the eutectic point, PCT and PCN crystals coexist in the copolymers, and after this point, the main crystal structure is dominated by the PCN-type crystal that enhances the thermal, mechanical, and physical properties of copolymers [21,82,117].

Table 5.
Thermal properties of PCTN# copolymers after SSP [92].
Many patents have been filed for the synthesis of CHDM-based thermally stable copolyesters containing naphthalene units. These copolyesters demonstrated unusually high Tg, Tm, and thermal stability [36,118,119]. The incorporation of naphthalene units into PCT results in the synthesis of a new class of PCTN copolyesters with remarkable gas barrier properties that are suitable for packaging applications. Sublett demonstrated that the gas barrier properties of PCTN copolyesters synthesized using CHDM and containing about 92/8 cis/trans isomers may be successfully regulated by controlling the amount of incorporated naphthalene units (Figure 11) [120]. In general, PCTN copolyesters outperform ordinary polymers in terms of barrier and thermal properties. It is evident from Figure 11 that the addition of cyclic rings of naphthalene in the backbone of PCT homopolyester resulted in the significant improvement in the barriers characteristics of the resultant PCTN copolyester. It improved behaviour is attributed to more stable structure of the PCTN copolyesters that is caused by the naphthalene rings.
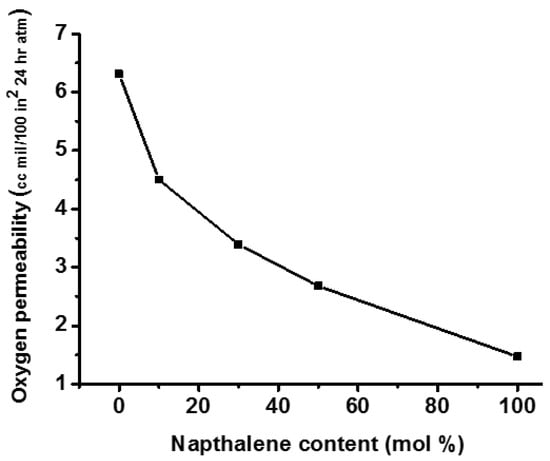
Figure 11.
Effect of naphthalene content on the gas barrier properties of PCTN [120].
Researchers have turned their focus to bio-based polymers in response to serious environmental pollution and the rapid depletion of oil resources. Extensive research has been conducted to develop monomers from renewable resources that could potentially replace the monomers derived from petrochemical resources [121,122,123]. The NDA as a second diacid moiety and biobased isosorbide as a second diol moiety can also be included in the backbone structure of PCT homopolyester, resulting in a quadri-polymer with superior thermal, barrier, and degrading properties [78,124]. The chemical structure of several acid-modified PCT copolyesters is shown in Figure 12.
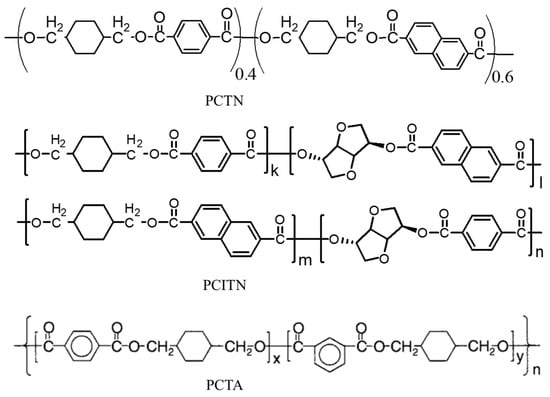
Figure 12.
Chemical structure of acid-modified PCT copolyesters (PCTN, PCITN, and PCTA).
Compared to NDA units, 1,4-cyclohexanedicarboxylic acid (CHDA) (95% trans isomers), as a modifier polyester, retained the toughness of the parent polyester while lowering the Tg. The addition of a modest amount of CHDA units significantly reduces the Tg and Tm of PCT homopolyester. However, high-molecular thermoplastic copolyesters are synthesized by integrating high trans-CHDA isomers, as they improve the thermal characteristics of polyesters [125].
Copolyesters derived from the mixture of EG and CHDM, as well as NDA and SA, have been reported. The heat-distortion temperature, Tm, and degradation behavior of the synthesized copolyesters were discovered to be dependent on the amount of second diol (CHDM) and second diacid (NDA, SA) introduced into the copolyesters. It is crucial to note that the incorporation of 30 mol % or higher amount of CHDM resulted in the synthesis of amorphous copolyesters. The thermal properties (Tg, Tm, and IV) of acid-modified copolyesters are presented in Table 6 [36,82,126].

Table 6.
Thermal properties of acid-modified copolyesters (PCTAs) [36,82,126].
4.4. Effect of Stereochemistry of Monomers on Synthesized Polyesters
There is much literature available that emphasizes the use of CHDM diol moieties to improve the thermal, physical, chemical, and mechanical properties of polymers [14,32,33]. Not only does the CHDM content but also the stereochemistry of CHDM (cis/trans isomers content) improve the overall characteristics of the resulting polymers [34,35,36,37]. Kibler et al. reported that the melting behavior of PCT can be improved by increasing the concentration of trans-CHDM from 0% to 100% (Tm 248 °C vs. 308 °C) [31] (Figure 13). Not only Tm and Tg of PCT homopolymer grow linearly when the trans-CHDM content increases from 0 to 100% (60 vs. 90 °C). However, the crystallization rate is not comparable for different compositions of PCT homopolymer.
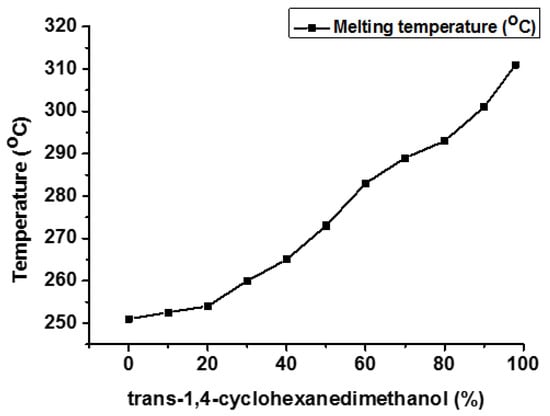
Figure 13.
Effect of trans-1,4-CHDM on Tm of PCT homopolyester [31].
Wang et al. studied the relationship between the stereochemistry of 1,4-CHDM and the performance attributes of bio-based poly(ethylene 2,5-furandicarboxylate) (PECFs) [127]. It was discovered that trans-CHDM isomers significantly improve the Tg, Tm, and Tc. Figure 14 depicts the influence of trans-CHDM on Tg and Tm of PECF copolymers. The polymer crystal structure was changed from amorphous to highly crystalline by increasing the percentage of trans-CHDM isomers from 25% to 98%. The mechanical (tensile strength, tensile modulus) and gas barrier (oxygen and carbon dioxide) properties were dramatically improved by adding trans-CHDM isomers in the synthesized bio-based polymer. Stable and stretched trans-CHDM isomers increase the symmetry of polymeric chains and help to create stable crystals.
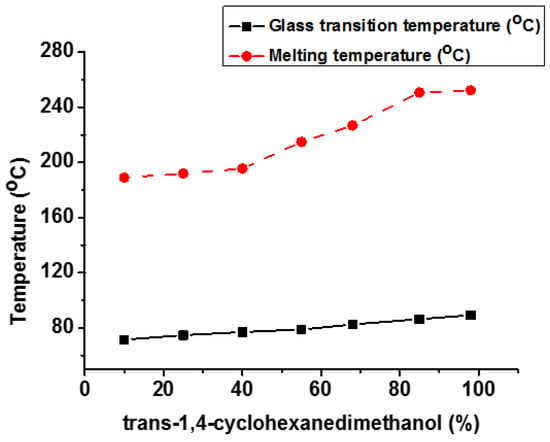
Figure 14.
Effect of trans-CHDM content (%) on Tg and Tm of PECF copolyesters [127].
In a study by Berti et al., it was discovered that by adjusting the cis/trans ratio of the diacid moiety in copolyester, it is possible to control both the thermal properties and crystal structure of the material [128]. Tg, melting behavior, and crystallization behavior of poly(1,4-cyclohexanedimethylene 2,6-naphthalene) (PCN) can be used to tune by controlling the cis/trans configuration of CHDM [129]. Superior thermal, mechanical, and barrier characteristics of copolyesters containing high trans-CHDM content can be attributed to the more symmetrical structure of CHDM, which facilitates the formation of stable crystal structures. Meanwhile, cis-CHDM obstructs the development of stable structures [35,37,125,129,130]. The comparative properties of conventional PET, PCT, PEN, and PCN homopolymers are listed in Table 7 [71,131,132,133,134].

Table 7.
Basic properties of conventional materials used as flexible substrate materials for displays [135,136,137].
5. Polymeric Substrates for Flexible Electronics
PET, PEN, and PCT homopolymers, as well as related copolyesters, are not novel polymers, but they have drawn significant attention from researchers due to their remarkable thermal, chemical, mechanical, gas barrier, and hydrolysis characteristics. There has been a huge effort to introduce the synthesis of new polyester with comprehensive properties of three polyesters stated so far, namely, PET, PCT, and PEN. In this study, a variety of poly(1,4-cyclohexylenedimethylene terephthalate-co-1,4-cyclohexylenedimethylene 2,6-naphthalenedicarboxylate) (PCTN) copolymers having CHDM as a diol and TPA and NDA as diacids were attempted. The importance of the CHDM configuration (cis/trans isomer) on the hydrolytic stability and thermal, mechanical, and barrier properties of copolyesters was investigated in depth. Based on this work, we will be able to develop a polymer with unique applications as a performance material in the field of textiles, the packaging industry, printing and embossing films, and electronics.
Researchers are currently focusing heavily on flexible electronics. These electronics are thin, lightweight, durable, conformable, and rollable. Furthermore, organic light-emitting diode (OLED) materials and the active matrix of thin film transistor (TFT) arrays can be laid down using solution casting and inkjet printing of plastic-based substrate. As a result, adopting roll-to-roll processing in large volumes effectively reduces processing costs. To replace glass, flexible plastic substrate materials exhibit performance properties comparable to glass, such as a smooth surface, chemical resistance, barrier, thermal stability, and very low coefficient of linear thermal expansion (CLTE). However, flexible glass is fragile by nature, and its handling is also very difficult. Until now, no plastic substrate materials have been reported to meet the performance properties required for flexible substrate materials for organic light-emitting displays (OLEDs). The surface roughness, clarity, thermal, thermomechanical, chemical, mechanical, electrical, and magnetic properties are major properties required for flexible substrate materials suitable for displays. In addition to bottom-emitting displays, the substrate materials for OLEDs must have excellent optical properties. The thermal properties of polymeric substrates (CLTE, Tg, and Tm), particularly Tg, must be compatible with the device production process temperature (Tmax). The thermal mismatch between flexible polymeric substrates and device films may cause device breakage. One of the primary concerns with the flexible substrate materials for OLEDs is their dimensional stability. It should not contaminate the device and possess strong barrier properties. It should be inert against the chemicals employed in device manufacture [135,136]. The standard moisture vapor transmission rate and oxygen permeability of flexible substrate materials for displays are 10−6 g/m2/day and 10−5 cm3/m2/day, respectively [138]. The good mechanical qualities of the substrate support the device and strengthen its impact resistance. Eclectically insulating polymeric substrates improve the device efficiency by minimizing coupling capacitances.
Previously, semi-crystalline thermoplastic homopolymers such as PEN and PET; non-crystalline thermoplastic polymers polycarbonate (PC) and polyethersulphone (PES); and high-Tg materials such as polyarylate (PAR), polyimide (PI) and poly cyclic olefin (PCO) were thought to be good candidates for flexible substrates. PC, PES, PAR, and PCO polymers are more transparent and have a higher Tg than PET and PEN. Compared to PET and PEN, these copolyesters have limited chemical resistance and a high coefficient of thermal expansion (CTE). PET, PEN, and PI have desirable performance characteristics. They have reasonably low CTE (15, 13, and 16 ppm/°C, respectively), high mechanical properties, and sufficient chemical resistance for the process. PET and PEN have good optical (transmittance > 85%) and water-absorption (0.014%) properties. However, the low thermal properties of PET and PEN have limited their practical applications in the field of flexible electronics. In contrast to PEN, PI has excellent thermal characteristics, but its yellow color and water absorption properties limit its applicability. At the same time, the water and oxygen permeability rates of conventional materials used as a substrate for flexible displays are 1–10 g/m2/day and 1–10 cm3/m2/day, respectively [136]. So far, no polymer has been reported to meet the rigorous requirements (water and oxygen permeability for organic light-emitting diode (OLED) displays. A comparison of different properties of conventional materials (PET, PEN, glass, steel, and PI) used as base substrates for flexible electronics is summarized in Table 7 [135,136,137].
In recent years, there has been a significant amount of advanced research into the synthesis and development of sophisticated polymer substrates as a smart film for flexible electronics. Currently, PI is the most extensively used polymeric substrate for flexible electronics. The performance parameters, including physical, thermal, mechanical, and barrier characteristics of the randomly oriented, uniaxially oriented, and biaxially oriented advanced polymeric substrates in contrast to conventional PI polymeric substrates, are summarized in Table 8 [33,78,79,124,139,140]. It is crucial to note that there is a potential to build transparent polymeric substrates with good barrier, optical, and thermal characteristics that can replace the yellow PI, which has higher water absorption. Such advanced polymer substrates can be used not only as substrates but also can be used at the top of flexible electronics. Such flexible polymeric smart films with low birefringence and high transmittance also have the potential to replace the brittle glass in flexible displays.

Table 8.
Comparative analysis of the key performance characteristics of advanced polymeric substrates in comparison to conventional PI polymeric substrates [78,79,124,135,140].
6. Future Recommendations for 1,4-Cycloheanedimethanol (CHDM) and Cyclic Monomer-Based Advanced Polyesters
Advanced polymeric materials, including CHDM and cyclic monomers such as TPA, NDA, IPA, ISB, etc., have established a strong position among polymers, laying the groundwork for the creation and characterization of innovative materials with diverse industrial applications. However, in order to further research in this sector, numerous crucial aspects must be investigated. To begin, a thorough study into the synthesis and characterization of novel cyclic monomers with superior characteristics should be conducted in order to expand the library of accessible monomers that serve as the basic building blocks of resulting polymers. It is possible to achieve this by finding alternative synthesis methods, catalysts, and optimized reaction conditions to support the reaction efficiency and yield of the reaction. Additionally, a thorough investigation of the structure–property relationships of the advanced polyesters is required to understand the impact of various monomer structures on the resulting polymers. This would help to design and optimize polyesters with regulated performance characteristics, including mechanical, thermal, and barrier properties for specified applications.
Not only is basic monomer research vital, but research on sustainable and environmentally friendly approaches for the synthesis and processing of CHDM-based polyesters is also very important. The use of biobased raw materials and green synthesis methodologies such as biobased or bio-inspired approaches should be explored. This may include using biobased renewable materials, producing efficient and selective catalysts, and implementing energy-efficient reaction conditions. The development of a recycling procedure, as well as extensive investigation into the biodegradation behavior of these materials, would help to ensure their sustainability and circularity. Collaboration between industry and academia is essential for the commercialization of these materials. Industrial collaborators can provide useful information about the scalability and commercial viability of the produced polymeric materials. The collaborators can also assist in identifying unique industrial requirements and challenges for the development of specialized materials that can establish a strong position in the commercial sector. Furthermore, interdisciplinary collaborations, including researchers and scientists from polymer chemistry, material science, engineering, and industrial design fields, are required. This can lead to holistic approaches in material development, covering not only the synthesis but also the processing, functionalization, and application aspects. Finally, advanced applications of polyesters in a variety of disciplines, including automotive, electronics, textile, packaging, and biomedical sectors, should be pursued in order to fully realize the potential of these advanced polyesters in diverse industries.
7. Conclusions
In conclusion, recent breakthroughs in the development of 1,4-cycloheanedimethanol (CHDM) and cyclic monomer-based advanced polyesters have revealed that these advanced copolyesters have the potential to be employed for smart film production with a wide range of industrial applications. It is also demonstrated that they have the ability to establish a prominent presence among other performance materials. These advanced polyesters have unique structures due to their amorphous and semi-crystalline nature, which results in exceptional performance behavior such as thermal, mechanical, optical, and barrier (water and thermal barrier) characteristics, making them suitable for use in textiles, packaging, and flexible electronics.
This review paper also discusses the role of structure–property relationships in determining the desirable performance characteristics of the resulting polymers. It has been demonstrated that the addition of a second diol or diacid to the primary backbone of the molecular chain considerably improves the performance of synthesized copolyesters, indicating that they are adaptable for various industrial applications. Furthermore, the importance of monomer stereochemistry in maximizing material qualities is emphasized, allowing for bespoke solutions to specific applications.
The exploration of various polymer synthesis approaches, such as solution, melt, and solid-state polymerization, provides significant insight into the synthesis of these innovative polymeric materials. The potential for using sustainable and biodegradable cyclic monomers, as well as green synthesis approaches, represents a promising direction for future research, in line with the increasing focus on environmental sustainability.
Even though cyclic monomer-based polyesters and CHDM have made significant strides, there are still issues that need to be addressed, such as the need for more thorough research on the synthesis and characterization of these materials, the development of more effective and environmentally friendly manufacturing techniques, and the search for novel cyclic monomers with improved properties.
The next generation of smart film applications will benefit greatly from advancements in cyclic monomer-based advanced polyesters and CHDM. Future research should concentrate on enhancing features such as transparency, flexibility, and thermal stability by engineering the polymer backbone structures to establish a balance between amorphous and semi-crystalline phases, as well as investigating environmentally friendly synthesis pathways and sustainable supply.
Overall, this comprehensive review article has provided a complete overview of the current state of CHDM and cyclic monomer-based polyesters for use in smart film applications. It is expected that the knowledge gained from this review will stimulate further study and research in this area, eventually leading to the development of novel materials that can meet the changing needs of diverse sectors, such as construction, electrical and electronics, medical and healthcare, packaging, and textile industries, among others. Academia and industry must work together to overcome difficulties and realize the full promise of these enhanced polyesters in smart film applications [114].
Funding
This work was supported by the Qassim University, Saudi Arabia (QU-APC-2024-9/1).
Acknowledgments
The researcher would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for the financial support (QU-APC-2024-9/1).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CHDM | 1,4-Cycloheanedimethanol | PET | Polyethylene Terephthalate |
| PEN | Poly(ethylene Naphthalene 2,6-dicarboxylate) | NDA | 2,6-Naphthalenedicarbxylic Acid |
| PCT | Poly(1,4-cyclohexanedimethylene terephthalate) | TPA | Terephthalic Acid |
| IPA | Isophthalic Acid | PCTA | acid-modified PCT |
| DMT | Dimethylene Terephthalate | DMCD | Dimethyl Cyclohexanedicarboxylate |
| CHDA | 1,4-Cyclohexanedicarboxyklic Acid | PCCD | Poly(1,4-cyclohexylene 1,4-cyclohexanedicarboxylate) |
| PBCC | Poly(butylene-co1,4-cyclohexanedimethylene carbonate) | ISB | Isosorbide |
| LCP | Liquid Crystalline Polymers | PCTN | Poly(1-4,cyclohexane dimethylene terephthalate-co-naphthalene dicarboxylate) |
| ROP | ring-opening polymerization | coPExCyT | Poly(ethylene-co-1,4-cyclohexanesimethylene terephthalate) copolyesters |
| SSP | solid state polycondensation | Tc | Cold Crystallization Temperature |
| Tm | melting temperature | PCN | Poly(cyclohexane dimethylene naphthalene dicarboxylate) |
| Mw | Weight Average Molecular Weight | Mn | Number Average Molecular Weight. |
| PEN-co-CN | Poly(ethylene 2,6-naphthalte-co-1,4-cyclohexanedimethanol 2,6-naphthalate) | PBN-co-CN | Poly(butylene 2,6-naphthalate-co-1,4-cyclohexanedimthylene 2,6-naphthalate) |
| PHN-co-CN | Poly(hexamethylene 2,6-naphthalate-co-1,4-cyclohexanedimthylene 2,6-naphthalate) | PTCT | Poly(trimethylene-co-1,4-cyclohexanedimethylene terephthalate) |
| PETG | Poly(ethylene glycol-co-1,4-cyclihexanedimethanil terephthalate) | P(CT-co-HT) | Poly(1,4-cyclohexylenedimethylene terephthalate-co-hexamethylene terephthalate) |
| PCTIN | Poly(1,4-cyclohexane dimethylene isosorbide terephthalate naphthalate) | N | 2,6-NDA. |
| S | succinic acid | SA | sebacic acid |
| PECFs | Poly(ethylene 2,5-furandicarboxylate). | PCN | Poly(1,4-cyclohexanedimethylene 2,6-naphthalene) |
| TFTs | Thin Film Transistor. | OLEDs | Organic Light-Emitting Diodes |
| Tmax | Fabrication Process Temperature. | PC | Polycarbonate |
| PES | Polyethersulphone | PAR | Polyarylate |
| PI | Polyimide | PCE | Poly Cyclic Olefin |
| CTE | Coefficient of Thermal Expansion |
References
- Carothers, B.W.H.; Hill, J.W. Studies of polymerization and ring formation. The use of molecular evaporation as a means for propagating chemical reactions. J. Am. Chem. Soc. 1932, 54, 1557–1559. [Google Scholar] [CrossRef]
- Hussain, F.; Shaban, S.M.; Kim, J.; Kim, D.-H. One-pot synthesis of highly stable and concentrated silver nanoparticles with enhanced catalytic activity. Korean J. Chem. Eng. 2019, 36, 988–995. [Google Scholar] [CrossRef]
- Ali, A.; Sattar, M.; Hussain, F.; Tareen, M.H.K.; Militky, J.; Noman, M.T. Single-step green synthesis of highly concentrated and stable colloidal dispersion of core-shell silver nanoparticles and their antimicrobial and ultra-high catalytic properties. Nanomaterials 2021, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-based sensors: A review. Polym. Test. 2018, 67, 342–348. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale 2014, 6, 1622–1628. [Google Scholar] [CrossRef]
- Hussain, F.; Khurshid, M.F.; Masood, R.; Ibrahim, W. Developing antimicrobial calcium alginate fibres from neem and papaya leaves extract. J. Wound Care 2017, 26, 778–783. [Google Scholar] [CrossRef]
- Awan, J.A.; Rehman, S.U.; Bangash, M.K.; Ali, U.; Asad, M.; Hussain, F.; Jaubert, J.-N. Development and characterization of electrospun curcumin-loaded antimicrobial nanofibrous membranes. Text. Res. J. 2021, 91, 1478–1485. [Google Scholar] [CrossRef]
- Bang, H.J.; Kim, H.Y.; Jin, F.L.; Park, S.J. Fibers spun from 1,4-cyclohexanedimethanol-modified polyethylene terephthalate resin. J. Ind. Eng. Chem. 2011, 17, 805–810. [Google Scholar] [CrossRef]
- McIntyre, J.E. The historical development of polyesters. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; Wiley Sussex: Chichester, UK, 2004; pp. 1–28. ISBN 0471498564. [Google Scholar]
- Bier, G. Polyarylates (polyesters from aromatic dicarboxylic acids and bisphenols). Polymer 1974, 15, 527–535. [Google Scholar] [CrossRef]
- Turner, S.R.; Walter, F.; Voit, B.I.; Mourey, T.H. Hyperbranched aromatic polyesters with carboxylic acid terminal groups. Macromolecules 1994, 27, 1611–1616. [Google Scholar] [CrossRef]
- Boland, C.S.; Khan, U.; Ryan, G.; Barwich, S.; Charifou, R.; Harvey, A.; Backes, C.; Li, Z.; Ferreira, M.S.; Mobius, M.E.; et al. Sensitive electromechanical sensors using viscoelastic graphene-polymer nanocomposites. Science 2016, 354, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.K.; Arora, H. Surgical Gown: A Critical Review. J. Ind. Text. 2009, 38, 205–231. [Google Scholar] [CrossRef]
- Turner, S.R. Development of amorphous copolyesters based on 1,4-cyclohexanedimethanol. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5847–5852. [Google Scholar] [CrossRef]
- Rex, J.; Dickson, J.T.; Lothian, E. Polymeric Linear Terephthalic Esters. US Patent 2,465,319, 22 March 1949. [Google Scholar]
- Pech-May, N.W.; Vales-Pinzón, C.; Vega-Flick, A.; Cifuentes, Á.; Oleaga, A.; Salazar, A.; Alvarado-Gil, J.J. Study of the thermal properties of polyester composites loaded with oriented carbon nanofibers using the front-face flash method. Polym. Test. 2016, 50, 255–261. [Google Scholar] [CrossRef]
- Shih, W.K. Shrinkage modeling of polyester shrink film. Polym. Eng. Sci. 1994, 34, 1121–1128. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Qiu, Y.; Wu, D. Mechanical properties of thermoplastic polyester elastomer controlled by blending with poly(butylene terephthalate). Polym. Test. 2016, 55, 152–159. [Google Scholar] [CrossRef]
- Khankrua, R.; Pivsa-Art, S.; Hiroyuki, H.; Suttiruengwong, S. Thermal and mechanical properties of biodegradable polyester/silica nanocomposites. Energy Procedia 2013, 34, 705–713. [Google Scholar] [CrossRef]
- Bae, J.; Lee, S.; Kim, B.C.; Cho, H.H.; Chae, D.W. Polyester-based thermoplastic elastomer/MWNT composites: Rheological, thermal, and electrical properties. Fibers Polym. 2013, 14, 729–735. [Google Scholar] [CrossRef]
- Park, S.; Hussain, F.; Kang, S.; Jeong, J.; Kim, J. Synthesis and properties of copolyesters derived from 1,4-cyclohexanedimethanol, terephthalic acid, and 2,6-naphthalenedicarboxylic acid with enhanced thermal and barrier properties. Polymer 2018, 42, 662–669. [Google Scholar] [CrossRef]
- Zhang, J.-B.; Wang, X.-L.; He, Q.-X.; Zhao, H.-B.; Wang, Y.-Z. A novel phosphorus-containing poly(1,4-cyclohexylenedimethylene terephthalate) copolyester: Synthesis, thermal stability, flammability and pyrolysis behavior. Polym. Degrad. Stab. 2014, 108, 12–22. [Google Scholar] [CrossRef]
- Koo, J.M.; Hwang, S.Y.; Yoon, W.J.; Lee, Y.G.; Kim, S.H.; Im, S.S. Structural and thermal properties of poly(1,4-cyclohexane dimethylene terephthalate) containing isosorbide. Polym. Chem. 2015, 6, 6973–6986. [Google Scholar] [CrossRef]
- Duking, I.N.; Chester, W. Popolyesters. US Patent 3,436,376, 1 April 1969. [Google Scholar]
- Kasmi, N.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Poly(1,4-cyclohexanedimethylene 2,6-naphthalate) polyester with high melting point: Effect of different synthesis methods on molecular weight and properties. Express Polym. Lett. 2018, 12, 227–237. [Google Scholar] [CrossRef]
- Meehan, S.J.; Sankey, S.W.; Jones, S.M.; MacDonald, W.A.; Colquhoun, H.M. Cocrystalline Copolyimides of Poly(ethylene 2,6-naphthalate). ACS Macro Lett. 2014, 3, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Mileva, D.; Portale, G.; Zhang, L.; Balzano, L.; Alfonso, G.C.; Androsch, R. Mesophase-Mediated Crystallization of Poly(butylene-2,6-naphthalate): An Example of Ostwald’s Rule of Stages. ACS Macro Lett. 2012, 1, 1051–1055. [Google Scholar] [CrossRef]
- Hu, B.; Ottenbrite, R.M. Biaxially oriented poly(ethylene 2,6-naphthalene) film: Manufacture, properties and commercial applications. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; Wiley Sussex: Chichester, UK, 2004; pp. 335–359. ISBN 0471498564. [Google Scholar]
- Callander, D.D. Properties and Applications of Poly(Ethylene 2,6-Naphthalene), its Copolyesters and Blends. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; Wiley Sussex: Chichester, UK, 2004; pp. 323–334. ISBN 0471498564. [Google Scholar]
- Wu, W.; Wagner, M.H.; Qian, Q.; Pu, W.; Kheirandish, S. Morphology and barrier mechanism of biaxially oriented poly(ethylene terephthalate)/poly(ethylene 2,6-naphthalate) blends. J. Appl. Polym. Sci. 2006, 101, 1309–1316. [Google Scholar] [CrossRef]
- Kibler, C.J.; Bell, A.; Smith, J.G. Polyesters of 1,4-cyclohexanedimethanol 1. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 2115–2125. [Google Scholar] [CrossRef]
- Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and crystallization of new fully renewable resources-based copolyesters: Poly(1,4-cyclohexanedimethanol-co-isosorbide 2,5-furandicarboxylate). Polym. Degrad. Stab. 2018, 152, 177–190. [Google Scholar] [CrossRef]
- Hussain, F.; Park, S.; Jeong, J.; Kang, S.; Kim, J. Structure–property relationship of poly(cyclohexane 1,4-dimethylene terephthalate) modified with high trans-1,4-cyclohexanedimethanol and 2,6-naphthalene dicarboxylicacid. J. Appl. Polym. Sci. 2020, 137, 48950. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, J.; Wang, J.; Liu, X.; Zhang, R.; Hu, G.; Na, H.; Zhu, J. Soft segment free thermoplastic polyester elastomers with high performance. J. Mater. Chem. A 2015, 3, 13637–13641. [Google Scholar] [CrossRef]
- Celli, A.; Marchese, P.; Sisti, L.; Dumand, D.; Sullalti, S.; Totaro, G. Effect of 1,4-cyclohexylene units on thermal properties of poly(1,4-cyclohexylenedimethylene adipate) and similar aliphatic polyesters. Polym. Int. 2013, 62, 1210–1217. [Google Scholar] [CrossRef]
- Kibler, C.J.; Alan, B.; James, G. Linear Polyesters and Polyester-Amides from 1,4-cyclohexanedimethanol. US Patent 2,901,466, 25 August 1959. [Google Scholar]
- Celli, A.; Marchese, P.; Sullalti, S.; Berti, C.; Barbiroli, G. Eco-friendly poly(butylene 1,4-cyclohexanedicarboxylate): Relationships between stereochemistry and crystallization behavior. Macromol. Chem. Phys. 2011, 212, 1524–1534. [Google Scholar] [CrossRef]
- Rosado, M.T.S.; Maria, T.M.R.; Castro, R.A.E.; Canotilho, J.; Silva, M.R.; Eusébio, M.E.S. Molecular structure and polymorphism of a cyclohexanediol: Trans-1,4-cyclohexanedimethanol. CrystEngComm 2014, 16, 10977–10986. [Google Scholar] [CrossRef]
- Turner, S.R.; Seymour, R.W.; Dombroski, J.R. Amorphous and crystalline polyesters based on 1,4-cyclohexanedimethanol. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; Wiley Sussex: Chichester, UK, 2004; pp. 267–292. ISBN 0471498564. [Google Scholar]
- Martínez de Ilarduya, A.; MuñToz Guerra, S. Polyesters Based on Cyclohexanedimethanol. In Handbook of Engineering and Specialty Thermoplastics; Thomas, S., Visakh, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2004; pp. 181–182. ISBN 978-0-470-63926-9. [Google Scholar]
- Robert, H.H.; Knowles, M.B.; Kingsport, T. Preparation of Trans-1,4-cyclohexanedimethanol. US Patent 2,917,549, 15 December 1949. [Google Scholar]
- Raja, R.; Khimyak, T.; Thomas, J.M.; Hermans, S.; Johnson, B.F.G. Single-Step, Highly Active, and Highly Selective Nanoparticle Catalysts for the Hydrogenation of Key Organic Compounds. Angew. Chemie Int. Ed. 2001, 40, 4638–4642. [Google Scholar] [CrossRef]
- Hungria, A.B.; Raja, R.; Adams, R.D.; Captain, B.; Thomas, J.M.; Midgley, P.A.; Golovko, V.; Johnson, B.F.G. Single-step conversion of dimethyl terephthalate into cyclohexanedimethanol with Ru5PtSn, a trimetallic nanoparticle catalyst. Angew. Chemie Int. Ed. 2006, 45, 4782–4785. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Chao, S.Y.; Lin, H.N. Method for Preparing Dimethyl 1,4-Cyclohexanedicarboxylate and Method for Preparing 1,4-Cyclohexanedimethanol. US Patent 9,550,721,B2, 24 January 2017. [Google Scholar]
- Guo, X.; Xin, J.; Lu, X.; Ren, B.; Zhang, S. Preparation of 1,4-cyclohexanedimethanol by selective hydrogenation of a waste PET monomer bis(2-hydroxyethylene terephthalate). RSC Adv. 2015, 5, 485–492. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Z.; Liu, Y.; Li, G.; Wang, A.; Cong, Y.; Zhang, T.; Wang, F.; Li, N. Synthesis of 1,4-cyclohexanedimethanol, 1,4-cyclohexanedicarboxylic acid and 1,2-cyclohexanedicarboxylates from formaldehyde, crotonaldehyde and acrylate/fumarate. Angew. Chemie Int. Ed. 2018, 57, 6901–6905. [Google Scholar] [CrossRef]
- Scarlett, J.; Michael, A.; Wood, C.R. Process for the Production of Cyclohexanedmethanol. US Patent 5,387,752, 7 February 1995. [Google Scholar]
- Xiao, X.; Xin, H.; Qi, Y.; Zhao, C.; Wu, P.; Li, X. One-pot conversion of dimethyl terephthalate to 1,4-cyclohexanedimethanol. Appl. Catal. A Gen. 2022, 632, 118510. [Google Scholar] [CrossRef]
- Liu, Y.; Turner, S.R. Synthesis and Properties of Cyclic Diester Based Aliphatic Copolyesters. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2162–2169. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, W.; Fang, Y. Kinetics of hydrogenation of dimethyl 1,4-cyclohexanedicarboxylate to 1,4-cyclohexanedimethanol. Int. J. Chem. Kinet. 2023, 55, 455–466. [Google Scholar] [CrossRef]
- Levin, S.; Diner, I.; Gurevich, G. Catalytic Hydrogenation of Dimethyl Production of Dimethyl Hexahydroterephthalate. All-Union Sci. Res. Inst. Pet. Chem. Process. 1962, 2, 566–572. [Google Scholar] [CrossRef]
- Lewandowski, G.; Wroblewska, A.; Milchert, E. Synthesis of 1, 4-cyclohexanedimethanol by hydrogenation of dimethyl terephthalate and its application as a substrate in syntheses of polyesters. Polimery 2007, 52, 39–43. [Google Scholar] [CrossRef]
- Jiang, X.; Chang, Z.; Yang, L.; Du, W.; Hou, Z. Hydrogenation of waste PET degraded bis (2-hydroxyethyl) cyclohexane-1, 4-dicarboxylate to 1, 4-cyclohexanedimethanol over Cu-based catalysts. Fuel 2024, 363, 130944. [Google Scholar] [CrossRef]
- Li, X.; Sun, Z.; Chen, J.; Zhu, Y.; Zhang, F. One-pot conversion of dimethyl terephthalate into 1, 4-cyclohexanedimethanol with supported trimetallic RuPtSn catalysts. Ind. Eng. Chem. Res. 2014, 53, 619–625. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, K.; Lin, Q.; Guo, W.; Chen, M.; Chen, C.; Zhang, C.; Fei, J.; Zhu, Y.; Li, J. Value-added Upcycling of PET to 1, 4-Cyclohexanedimethanol by a Hydrogenation/Hydrogenolysis Relay Catalysis. Angew. Chemie Int. Ed. 2024, 63, e202408561. [Google Scholar] [CrossRef]
- Luo, J.; Qu, E.; Zhou, Y.; Dong, Y.; Liang, C. Re/AC catalysts for selective hydrogenation of dimethyl 1, 4- cyclohexanedicarboxylate to 1, 4-cyclohexanedimethanol: Essential roles of metal dispersion and chemical environment. Appl. Catal. A Gen. 2020, 602, 117669. [Google Scholar] [CrossRef]
- Lecomte, H.A.; Liggat, J.J.; Curtis, A.S.G. Synthesis and characterization of novel biodegradable aliphatic poly(ester amide)s containing cyclohexane units. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1785–1795. [Google Scholar] [CrossRef]
- Tsai, Y.; Jheng, L.C.; Hung, C.Y. Synthesis, properties and enzymatic hydrolysis of biodegradable alicyclic/aliphatic copolyesters based on 1,3/1,4-cyclohexanedimethanol. Polym. Degrad. Stab. 2010, 95, 72–78. [Google Scholar] [CrossRef]
- Hunsen, M.; Azim, A.; Mang, H.; Wallner, S.R.; Ronkvist, A.; Wenchun, X.; Gross, R.A. A cutinase with polyester synthesis activity. Macromolecules 2007, 40, 148–150. [Google Scholar] [CrossRef]
- Barrett, D.G.; Merkel, T.J.; Luft, J.C.; Yousaf, M.N. One-step syntheses of photocurable polyesters based on a renewable resource. Macromolecules 2010, 43, 9660–9667. [Google Scholar] [CrossRef]
- Cai, X.; Yang, X.; Zhang, H.; Wang, G. Modification of biodegradable poly(butylene carbonate) with 1,4-cyclohexanedimethylene to enhance the thermal and mechanical properties. Polym. Degrad. Stab. 2017, 143, 35–41. [Google Scholar] [CrossRef]
- Berti, C.; Celli, A.; Marchese, P.; Barbiroli, G.; Di Credico, F.; Verney, V.; Commereuc, S. Novel copolyesters based on poly(alkylene dicarboxylate)s: 2. Thermal behavior and biodegradation of fully aliphatic random copolymers containing 1,4-cyclohexylene rings. Eur. Polym. J. 2009, 45, 2402–2412. [Google Scholar] [CrossRef]
- Park, S.A.; Choi, J.; Ju, S.; Jegal, J.; Lee, K.M.; Hwang, S.Y.; Oh, D.X.; Park, J. Copolycarbonates of bio-based rigid isosorbide and flexible 1,4-cyclohexanedimethanol: Merits over bisphenol-A based polycarbonates. Polymer 2017, 116, 153–159. [Google Scholar] [CrossRef]
- Arévalo-Alquichire, S.; Valero, M. Castor Oil Polyurethanes as Biomaterials. In Elastomers; IntechOpen: London, UK, 2017; pp. 137–157. [Google Scholar]
- Brunelle, D.J.; Jang, T. Optimization of poly(1,4-cyclohexylidene cyclohexane-1,4-dicarboxylate) (PCCD) preparation for increased crystallinity. Polymer 2006, 47, 4094–4104. [Google Scholar] [CrossRef]
- Pal, R.S.; Pal, Y.; Singh, V. Isolation and characterization of n-octa decanoic acid from whole aerial parts of Centella asiatica Linn. Int. J. Pharm. Technol. 2016, 8, 18989–18994. [Google Scholar]
- Akbari, S.; Root, A.; Skrifvars, M.; Ramamoorthy, S.K.; Åkesson, D. Novel Bio-based Branched Unsaturated Polyester Resins for High-Temperature Applications. J. Polym. Environ. 2024, 32, 2031–2044. [Google Scholar] [CrossRef]
- Hoskins, J.N.; Grayson, S.M. Cyclic polyesters: Synthetic approaches and potential applications. Polym. Chem. 2011, 2, 289–299. [Google Scholar] [CrossRef]
- Yoon, H.N. Strength of fibers from wholly aromatic polyesters. Colloid Polym. Sci. 1990, 268, 230–239. [Google Scholar] [CrossRef]
- Bastioli, C.; Foa, M.; Floridi, G.; Cella, G.; Fernanda, F.; Milizia, T. Use of Polyester Resins for the Production of Articles Having Good Properties as Barriers to Water Vapor. US Patent 6,727,342 B1, 27 April 2004. [Google Scholar]
- Turner, S.R.; Seymour, R.W.; Smith, T.W. Cyclohexanedimethanol Polyesters. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2003; Volume 2, pp. 127–134. ISBN 978-0-470-60955-2. [Google Scholar]
- Ki, H.C.; Park, O.O. Synthesis, characterization and biodegradability of the biodegradable aliphatic—Aromatic random copolyesters. Polymer 2001, 42, 1849–1861. [Google Scholar] [CrossRef]
- Armentano, I.; Gigli, M.; Martino, S. Recent Advances in Nanocomposites Based on Aliphatic Polyesters: Design, Synthesis, and Applications in Regenerative Medicine. Appl. Sci. 2018, 8, 1452. [Google Scholar] [CrossRef]
- Phan, D.-N.; Lee, H.; Choi, D.; Kang, C.-Y.; Im, S.; Kim, I.; Phan, D.-N.; Lee, H.; Choi, D.; Kang, C.-Y.; et al. Fabrication of Two Polyester Nanofiber Types Containing the Biobased Monomer Isosorbide: Poly (Ethylene Glycol 1,4-Cyclohexane Dimethylene Isosorbide Terephthalate) and Poly (1,4-Cyclohexane Dimethylene Isosorbide Terephthalate). Nanomaterials 2018, 8, 56. [Google Scholar] [CrossRef]
- Legrand, S.; Jacquel, N.; Amedro, H.; Saint-Loup, R.; Pascault, J.P.; Rousseau, A.; Fenouillot, F. Synthesis and properties of poly(1,4-cyclohexanedimethylene-co-isosorbide terephthalate), a biobased copolyester with high performances. Eur. Polym. J. 2019, 115, 22–29. [Google Scholar] [CrossRef]
- El-Ghazali, S.; Khatri, M.; Hussain, N.; Khatri, Z.; Yamamoto, T.; Kim, S.H.; Kobayashi, S.; Kim, I.S. Characterization and biocompatibility evaluation of artificial blood vessels prepared from pristine poly (Ethylene-glycol-co-1, 4-cyclohexane dimethylene-co-isosorbide terephthalate), poly (1, 4 cyclohexane di-methylene-co-isosorbide terephthalate) nanofi. Mater. Today Commun. 2021, 26, 102113. [Google Scholar] [CrossRef]
- Bhagia, S.; Bornani, K.; Ozcan, S.; Ragauskas, A.J. Terephthalic Acid Copolyesters Containing Tetramethylcyclobutanediol for High-Performance Plastics. ChemistryOpen 2021, 10, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Hussain, F.; Park, S.; Kang, S.-J.; Kim, J. High Thermal Stability, High Tensile Strength, and Good Water Barrier Property of Terpolyester Containing Biobased Monomer for Next-Generation Smart Film Application: Synthesis and Characterization. Polymers 2020, 12, 2458. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Park, S.; Jeong, J.; Jeong, E.; Kang, S.; Yoon, K.; Kim, J. Fabrication and characterization of poly(1,4-cyclohexanedimthylene terephthalate-co-1,4-cyclohxylenedimethylene 2,6-naphthalenedicarboxylate) (PCTN) copolyester film: A novel copolyester film with exceptional performances for next generation. J. Appl. Polym. Sci. 2021, 138, 49840. [Google Scholar] [CrossRef]
- Gonzalez-Vidal, N.; De Ilarduya, A.M.; Muñoz-Guerra, S. Poly(ethylene-co-1,4-cyclohexylenedimethylene terephthalate) copolyesters obtained by ring opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2009, 19, 5954–5966. [Google Scholar] [CrossRef]
- Velmathi, S.; Nagahata, R.; Takeuchi, K. Extremely Rapid Synthesis of Aliphatic Polyesters by Direct polycondensation of 1:1 mixtures of dicarboxylic acids and diols using microwaves. Polym. J. 2007, 39, 841–844. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jo, W.H.; Lee, S.C. Synthesis and isodimorphic cocrystallization behavior of poly(1,4-cyclohexylenedimethylene terephthalate- co -1,4-cyclohexylenedimethylene 2,6-naphthalate) copolymers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 177–187. [Google Scholar] [CrossRef]
- Hu, L.; Wu, L.; Song, F.; Li, B.G. Kinetics and modeling of melt polycondensation for synthesis of poly[(butylene succinate)-co-(butylene terephthalate)], 1—Esterification. Macromol. React. Eng. 2010, 4, 621–632. [Google Scholar] [CrossRef]
- Lodha, A.; Ghadage, R.S.; Ponrathnam, S. Polycondensation reaction kinetics of wholly aromatic polyesters. Polymer 1997, 38, 6167–6174. [Google Scholar] [CrossRef]
- Shah, T.; Bhatty, J.; Gamlen, G.; Dollimore, D. Aspects of the chemistry of poly(ethylene terephthalate): 5. Polymerization of bis(hydroxyethyl)terephthalate by various metallic catalysts. Polymer 1984, 25, 1333–1336. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Roupakias, C.P.; Bikiaris, D.N.; Achilias, D.S. Study of various catalysts in the synthesis of poly(propylene terephthalate) and mathematical modeling of the esterification reaction. Polymer 2003, 44, 931–942. [Google Scholar] [CrossRef]
- Shotyk, W.; Krachler, M. Contamination of bottled waters with antimony leaching from polyethylene terephthalate (PET) increases upon storage. Environ. Sci. Technol. 2007, 41, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Kotek, R.; Tonelli, A. Review of conventional and novel polymerization processes for polyesters. Prog. Polym. Sci. 2006, 31, 1009–1037. [Google Scholar] [CrossRef]
- Muller, R. Keys to the trematoda. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 124. [Google Scholar] [CrossRef]
- Kwolek, S.L.; Morgan, P.W. Preparation of polyamides, polyurethanes, polysulfonamides, and polyesters by low temperature solution polycondensation. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 2693–2703. [Google Scholar] [CrossRef]
- Giol, E.D.; Van den Brande, N.; Van Mele, B.; Van Vlierberghe, S.; Dubruel, P. Single-step solution polymerization of poly(alkylene terephthalate)s: Synthesis parameters and polymer characterization. Polym. Int. 2018, 67, 292–300. [Google Scholar] [CrossRef]
- Hussain, F.; Jeong, J.; Park, S.; Kang, S.-J.; Kim, J. Single-step solution polymerization and thermal properties of copolyesters based on high trans-1,4-cyclohexanedimethanol, terephthaloyl dichloride, and 2,6-naphthalene dicarboxylic chloride. Polym. 2019, 43, 475–484. [Google Scholar] [CrossRef]
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef]
- Bednarek, M. Branched aliphatic polyesters by ring-opening (co)polymerization. Prog. Polym. Sci. 2016, 58, 27–58. [Google Scholar] [CrossRef]
- Macdonald, J.P.; Shaver, M.P. An aromatic/aliphatic polyester prepared via ring-opening polymerisation and its remarkably selective and cyclable depolymerisation to monomer. Polym. Chem. 2016, 7, 553–559. [Google Scholar] [CrossRef]
- Wan, X.H.; Yang, Y.; Tu, H.L.; Lan, H.; Tan, S.; Zhou, Q.F.; Turner, S.R. Synthesis, characterization, and ring opening polymerization of poly(1,4-cyclohexylenedimethylene terephthalate) cyclic oligomers. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1828–1833. [Google Scholar] [CrossRef]
- Achilias, D.S.; Chondroyiannis, A.; Nerantzaki, M.; Adam, K.V.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Solid State Polymerization of Poly(Ethylene Furanoate) and Its Nanocomposites with SiO2 and TiO2. Macromol. Mater. Eng. 2017, 302, 1700012. [Google Scholar] [CrossRef]
- Culbert, B.; Christel, A. Continuous solid-state polycondensation of polyesters. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; Wiley Sussex: Chichester, UK, 2004; pp. 143–194. ISBN 9780470090688. [Google Scholar]
- Tate, S.; Watanabe, Y.; Chiba, A. Synthesis of ultra-high molecular weight poly(ethylene terephthalate) by swollen-state polymerization. Polymer 1993, 34, 4974–4977. [Google Scholar] [CrossRef]
- Willem, F.; Borman, H.; Pittsfield, M. Solid State Polymerization of Poly (1,4-Butylene Terephthalate). US Patent 3,953,404, 27 April 1976. [Google Scholar]
- Kasmi, N.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-State polymerization of poly(Ethylene Furanoate) biobased Polyester, II: An efficient and facile method to synthesize high molecular weight polyester appropriate for food packaging applications. Polymers 2018, 10, 471. [Google Scholar] [CrossRef]
- Dröscher, M.; Wegner, G. Poly(ethylene terephthalate): A solid state condensation process. Polymer 1978, 19, 43–47. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, X.J.; Zhang, J.; Feng, L.F.; Wang, J.J. Experimental and modeling study of the solid state polymerization of poly(ethylene terephthalate) over a wide range of temperatures and particle sizes. J. Appl. Polym. Sci. 2013, 127, 3814–3822. [Google Scholar] [CrossRef]
- Göltner, W. Solid-State Polycondensation of Polyester Resins: Fundamentals and Industrial Production. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; Wiley Sussex: Chichester, UK, 2004; pp. 195–242. ISBN 9780470090688. [Google Scholar]
- Papaspyrides, C.D.; Vouyiouka, S.N. Solid-state polymerization. Prog. Polym. Sci. 2005, 30, 10–37. [Google Scholar] [CrossRef]
- Steinborn-Rogulska, I.; Rokicki, G. Solid-state polycondensation (SSP) as a method to obtain high molecular weight polymers: Part II. Synthesis of polylactide and polyglycolide via SSP. Polimery 2013, 58, 85–92. [Google Scholar] [CrossRef]
- Youssef, A.-R.M.A. Solution and Bulk Polimerization. High. Technol. Inst. Tenth Ramadan City 2019, 1–7. [Google Scholar] [CrossRef]
- Geeta; Preetam. A Review on Polymer Technology. Int. J. Eng. Technol. Res. 2016, 4, 9–14. [Google Scholar]
- Naeimirad, M.; Krins, B.; Gruter, G.J.M. A Review on Melt-Spun Biodegradable Fibers. Sustainability 2023, 15, 14474. [Google Scholar] [CrossRef]
- Kricheldorf, H. Polycondensation: History and New Results; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Steinborn-Rogulska, I.; Rokicki, G. Solid-state polycondensation (SSP) as a method to obtain high molecular weight polymers. Part I. Parameters influencing the SSP process. Polimery 2013, 58, 3–13. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jo, W.H.; Lee, S.C. Synthesis and Crystallization Behavior of Poly( m -methylene 2,6-naphthalate- c o -1,4-cyclohexylenedimethylene 2,6-naphthalate) Copolymers. Macromolecules 2003, 36, 4051–4059. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Yu, A.; Xi, P.; Huang, X.; Li, S. Sequence distribution, thermal properties, and crystallization studies of poly(trimethylene terephthalate-co-1,4-cyclohexylene dimethylene terephthalate) copolyesters. J. Appl. Polym. Sci. 2008, 111, 2751–2760. [Google Scholar] [CrossRef]
- Hill, A.J.; Weinhold, S.; Stack, G.M.; Tant, M.R. Effect of copolymer composition on free volume and gas permeability in poly(ethylene terephthalate)-poly(1,4 cyclohexylenedimethylene terephthalate) copolyesters. Eur. Polym. J. 1996, 32, 843–849. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, W.; Zhang, J. Alkali resistance of poly(ethylene terephthalate) (PET) and poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate) (PETG) copolyesters: The role of composition. Polym. Degrad. Stab. 2015, 120, 232–243. [Google Scholar] [CrossRef]
- Young, G.J.; Won, H.J.; Sang, C.L. Cocrystallization of poly(1,4-cyclohexylenedimethylene terephthalate-co- hexamethylene terephthalate) copolymers. Macromol. Res. 2004, 12, 459–465. [Google Scholar] [CrossRef]
- Kliesh, H.; Oliver Klein, M.K.; Kuhmann, B. White, Biaxially Oriented Polyester Film with a High Portion of Cyclohexanedimethanol and a Primary and Secondary Dicarboxylic Acid Portion and a Method for Its Production and Its Use. US Patent 2012/0196111 A1, 2 August 2012. [Google Scholar]
- Sublett, B.J. Orientable, Heat Setable Semi-Crystalline Copolyesters. US Patent 5,616,404, 1 April 1997. [Google Scholar]
- Dickerson, J.P.; Brink, A.E.; Oshinski, A.J.; Seo, K.S. Copolyesters Based on 1,4-Cyclohexanedmethanol Having Improved Stability. US Patent 5,656,715, 12 August 1997. [Google Scholar]
- Sublett, B.J. Thermoplastic Copolyesters Having Improved Gas Barrier Properties. US Patent 5,552,512, 3 September 1997. [Google Scholar]
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.F.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Gardossi, L.; Ferrario, V.; Guebitz, G.M. Renewable building blocks for sustainable polyesters: New biotechnological routes for greener plastics. Polym. Int. 2016, 65, 861–871. [Google Scholar] [CrossRef]
- Hussain, F.; Jeong, J.; Park, S.; Kang, S.J.; Khan, W.Q.; Kim, J. Synthesis and unique characteristics of biobased high Tg copolyesters with improved performance properties for flexible electronics and packaging applications. J. Ind. Eng. Chem. 2021, 100, 119–125. [Google Scholar] [CrossRef]
- Vanhaecht, B.; Teerenstra, M.N.; Suwier, D.R.; Willem, R.; Biesemans, M.; Koning, C.E. Controlled stereochemistry of polyamides derived fromcis/trans-1,4-cyclohexanedicarboxylic acid. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 833–840. [Google Scholar] [CrossRef]
- Tsai, Y.; Fan, C.H.; Hung, C.Y.; Tsai, F.J. Amorphous copolyesters based on 1,3/1,4-cyclohexanedimethanol: Synthesis, characterization and properties. J. Appl. Polym. Sci. 2008, 109, 2598–2604. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jia, Z.; Sun, L.; Zhang, Y.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) (PEF) with 1, 4-cyclohexanedimethanol: Influence of stereochemistry of 1,4-cyclohexylene units. Polymer 2018, 137, 173–185. [Google Scholar] [CrossRef]
- Berti, C.; Celli, A.; Marchese, P.; Marianucci, E.; Barbiroli, G.; Di Credico, F. Influence of molecular structure and stereochemistry of the 1,4-cyclohexylene ring on thermal and mechanical behavior of poly(butylene 1,4-cyclohexanedicarboxylate). Macromol. Chem. Phys. 2008, 209, 1333–1344. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jo, W.H.; Lee, S.C. Crystal structure determination of poly(1,4-trans-cylcohexylenedimethylene 2,6-naphthalate) by X-ray diffraction and molecular modeling. Macromolecules 2003, 36, 5201–5207. [Google Scholar] [CrossRef]
- Lyman, D.J. Polyurethanes. II. Effect of cis-trans isomerism on properties of polyurethanes. J. Polym. Sci. 1961, 55, 507–514. [Google Scholar] [CrossRef]
- Sakellarides, S.L. Poly(ethylene naphthalate) (PEN). In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2004; Volume 11, pp. 88–114. [Google Scholar]
- Light, R.R.; Seymour, R.W. Effect of sub-Tg relaxations on the gas transport properties of polyesters. Polym. Eng. Sci. 1982, 22, 857–864. [Google Scholar] [CrossRef]
- Boye, C.A. X-ray diffraction studies of poly (1,4-cyclohexylenedimethylene terephthalate). J. Polym. Sci. 1961, 55, 275–284. [Google Scholar] [CrossRef]
- Sandhya, T.E.; Ramesh, C.; Sivaram, S. Copolyesters Based on Poly(butylene terephthalate)s Containing Cyclohexyl Groups: Synthesis, Structure and Crystallization. Macromol. Symp. 2003, 199, 467–482. [Google Scholar] [CrossRef]
- MacDonald, W.A. Engineered films for display technologies. J. Mater. Chem. 2004, 14, 4–10. [Google Scholar] [CrossRef]
- Cheng, I.C.; Wagner, S. Overview of Flexible Electronics. In Flexible Electronics: Materials and Applications Electronic Materials: Science and Technology; Wong, W.S., Salleo, A., Eds.; Springer: New York, NY, USA, 2009; pp. 1–28. ISBN 0387743626. [Google Scholar]
- MacDonald, W.A.; Looney, M.K.; MacKerron, D.; Eveson, R.; Adam, R.; Hashimoto, K.; Rakos, K. Latest advances in substrates for flexible electronics. J. Soc. Inf. Disp. 2007, 15, 1075. [Google Scholar] [CrossRef]
- Lewis, J.S.; Weaver, M.S. Thin film permeation-barrier technology for flexible organic light-emitting devices. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 45–57. [Google Scholar] [CrossRef]
- Hussain, F.; Jeong, J.; Park, S.; Jeong, E.; Kang, S.-J.; Yoon, K.; Kim, J. Fabrication and characterization of a novel terpolyester film: An alternative substrate polymer for flexible electronic devices. Polymer 2020, 210, 123019–123026. [Google Scholar] [CrossRef]
- Hussain, F.; Fayzan Shakir, H.M.; Ali, A.; Rehan, Z.A.; Zubair, Z. Development and characterization of cyclic compound-based biaxially stretched smart polymeric film for futuristic flexible electronic devices. Eur. Polym. J. 2022, 172, 111243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).