Reactive Synthesis for Porous (Mo2/3Y1/3)2AlC Ceramics through Mo, Y, Al and Graphite Powders
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Macroscopic Comparison of Samples
3.2. Effects of Sintering Temperature on the Synthesis of Porous (Mo2/3Y1/3)2AlC Ceramics
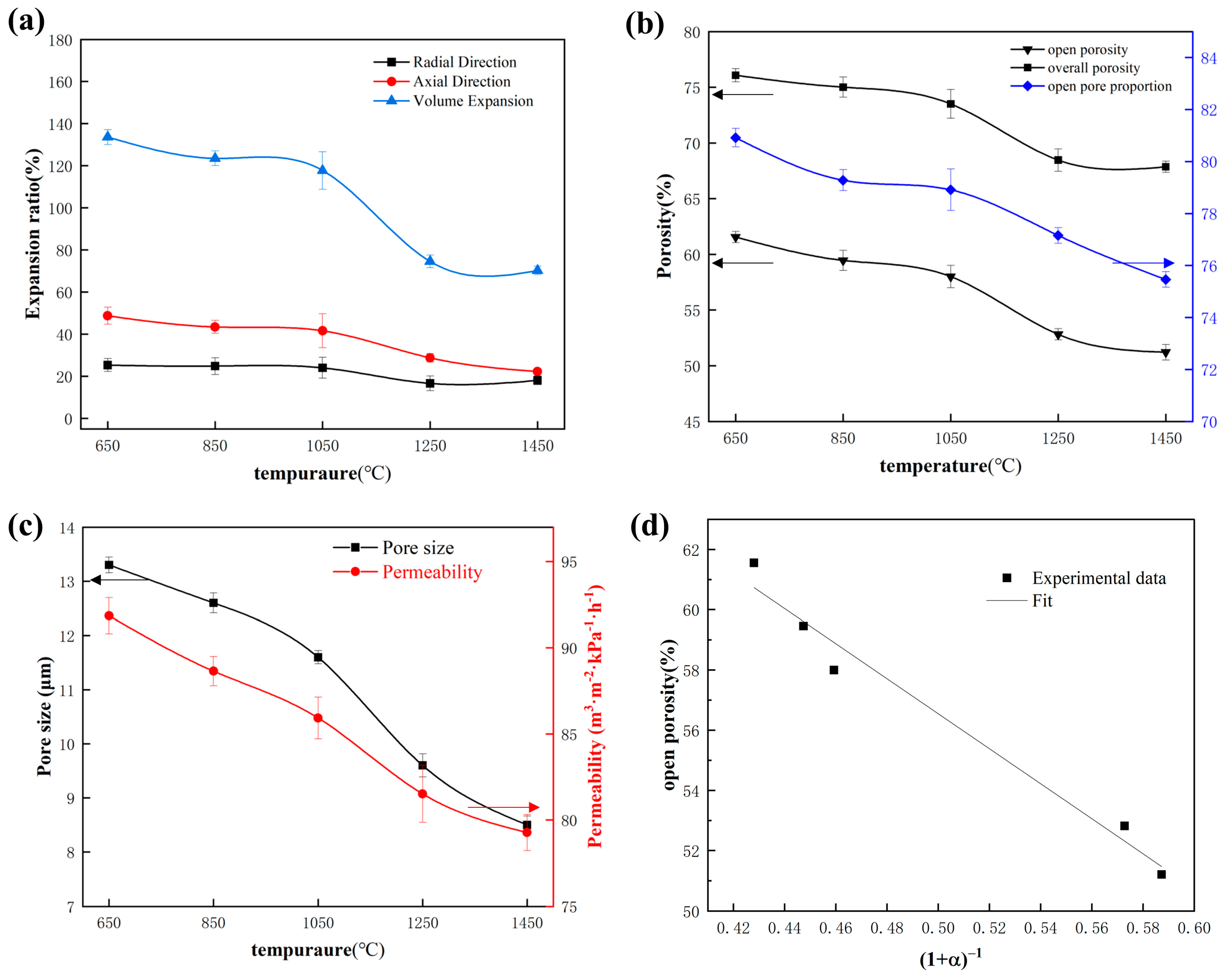
3.3. Effects of Temperature on Volume Evolution and Pore Structure Parameters
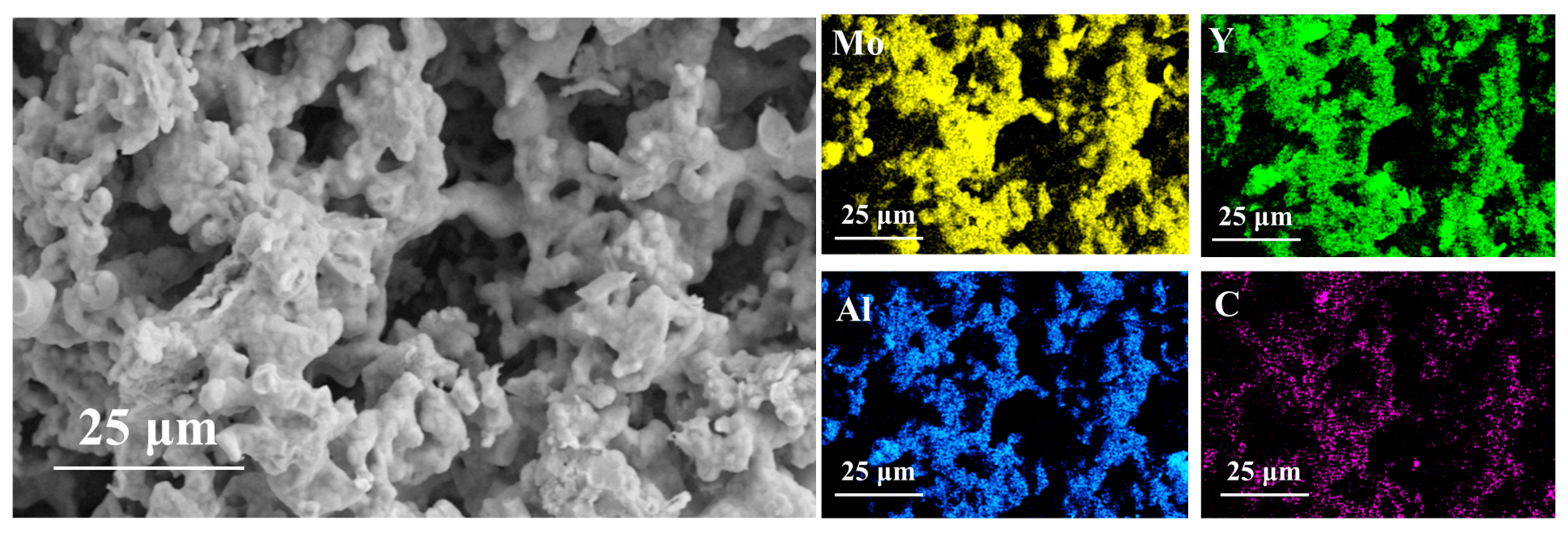
3.4. Micromorphology of Porous (Mo2/3Y1/3)2AlC Ceramics
3.5. Pore-Forming Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barsoum, M.W. The MN+1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Ingason, A.S.; Dahlqvist, M.; Rosen, J. Magnetic MAX phases from theory and experiments; a review. J. Phys. Condens. Matter 2016, 28, 433003. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.M. Progress in research and development on MAX phases: A family of layered ternary compounds. Int. Mater. Rev. 2013, 56, 143–166. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Radovic, M. Elastic and Mechanical Properties of the MAX Phases. Annu. Rev. Mater. Res. 2011, 41, 195–227. [Google Scholar] [CrossRef]
- Ching, W.Y.; Mo, Y.; Aryal, S.; Rulis, P.; Zhou, Y. Intrinsic Mechanical Properties of 20 MAX-Phase Compounds. J. Am. Ceram. Soc. 2013, 96, 2292–2297. [Google Scholar] [CrossRef]
- Haftani, M.; Saeedi Heydari, M.; Baharvandi, H.R.; Ehsani, N. Studying the oxidation of Ti 2AlC MAX phase in atmosphere: A review. Int. J. Refract. Met. Hard Mater. 2016, 61, 51–60. [Google Scholar] [CrossRef]
- Hashimoto, S.; Nishina, N.; Hirao, K.; Zhou, Y.; Hyuga, H.; Honda, S.; Iwamoto, Y. Formation mechanism of Ti2AlC under the self-propagating high-temperature synthesis (SHS) mode. Mater. Res. Bull. 2012, 47, 1164–1168. [Google Scholar] [CrossRef]
- Thomas, T.; Bowen, C.R. Effect of particle size on the formation of Ti2AlC using combustion synthesis. Ceram. Int. 2016, 42, 4150–4157. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Wang, X. Progress in machinable and electrically conductive laminated ternary ceramics (MAX Phases). Adv. Ceram. 2017, 38, 3–20. [Google Scholar] [CrossRef]
- Gorshkov, V.A.; Miloserdov, P.A.; Sachkova, N.V. High-Temperature Synthesis of Cast Materials Based on the MAX Phase Cr2AlC Using CaCrO4 + Al + C Mixtures. Inorg. Mater. 2020, 56, 321–327. [Google Scholar] [CrossRef]
- Kovalev, D.Y.; Luginina, M.A.; Vadchenko, S.G.; Konovalikhin, S.V.; Sychev, A.E.; Shchukin, A.S. Synthesis of a new MAX phase in the Ti–Zr–Al–C system. Mendeleev Commun. 2017, 27, 59–60. [Google Scholar] [CrossRef]
- Zhu, J.F.; Han, N.; Wang, K.; Wang, F. Fabrication of Ti2AlN by Mechanical Alloying and Hot Press Sintering. Adv. Mater. Res. 2011, 194–196, 425–428. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, J.; Jin, Y. Research Progress of Layered Cr2AlC Ternary Ceramic. China Ceram. Ind. 2017, 24, 22–29. [Google Scholar] [CrossRef]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-Assisted Sintering Technology/Spark Plasma Sintering: Mechanisms, Materials, and Technology Developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Oh, H.-C.; Lee, S.-H.; Choi, S.-C. The reaction mechanism for the low temperature synthesis of Cr2AlC under electronic field. J. Alloys Compd. 2014, 587, 296–302. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-based materials for electrochemical energy storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.H.; Xu, N.P.; Zou, J.; Huang, B.Y.; Liu, C.T. Effects of the Al content on pore structures of porous Ti–Al alloys. Intermetallics 2008, 16, 327–332. [Google Scholar] [CrossRef]
- Hernandez, A. Pore size distributions in microporous membranes. A critical analysis of the bubble point extended method. J. Membr. Sci. 1996, 112, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Liao, C.; Wang, J.; Jiang, Y.; He, Y. Reactive synthesis for porous Ti3AlC2 ceramics through TiH2, Al and graphite powders. Ceram. Int. 2014, 40, 6739–6745. [Google Scholar] [CrossRef]
- Aliakbari, A.; Amiri, P.; Dezfuli, A.G. Stability and physical properties of yttrium-based new MAX phases Y2AX (A = Al, Si, Ga, and Ge; X = C and N): A first-principles prediction. Appl. Phys. A 2023, 129, 476. [Google Scholar] [CrossRef]
- ElMelegy, T.A.; Sokol, M.; Barsoum, M.W. Enhanced yield synthesis of bulk dense (M2/3Y1/3)2AlC (M = Cr, W, Mo) in-plane chemically ordered quaternary atomically laminated i-MAX phases and oxidation of (Cr2/3Y1/3)2AlC and (Mo2/3Y1/3)2AlC. J. Alloys Compd. 2021, 867, 158930. [Google Scholar] [CrossRef]
- Watson, R.E.; Weinert, M.; Alatalo, M. Transition-metal aluminide formation: The 4d aluminides. Phys. Rev. B 2001, 65, 014103. [Google Scholar] [CrossRef]
- Chen, M.R.; Jiang, Y.; He, Y.H.; Lin, L.W.; Huang, B.Y.; Liu, C.T. Pore evolution regulation in synthesis of open pore structured Ti–Al intermetallic compounds by solid diffusion. J. Alloys Compd. 2012, 521, 12–15. [Google Scholar] [CrossRef]




| Temperature | Phase | Main Phase |
|---|---|---|
| 650 °C | Mo, Y, Al, C, YAl2 | element powder |
| 850 °C | Mo, Y, Al, C, YAl2, Y3Al, Mo3Al, Y5C6, Mo2C, MoC | element powder |
| 1050 °C | Mo3Al, YAl2, Y3Al, Mo2C, MoC, YAl3C3, (Mo2/3Y1/3)2AlC, C | Mo3Al, YAl2 |
| 1250 °C | (Mo2/3Y1/3)2AlC, YAl, Mo2C, MoC, YAl3C3, Mo3Al2C, Y3Al, Mo3Al | YAl, Mo2C, (Mo2/3Y1/3)2AlC |
| 1450 °C | (Mo2/3Y1/3)2AlC, Mo3Al2C, YAl3C3, Y3Al, YAl, Mo2C, MoC, Mo3Al | (Mo2/3Y1/3)2AlC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.; Xiao, G.; Wang, B.; Yu, K.; Li, J.; Jiang, W.; Zhang, H.; Yang, X.; Yang, J. Reactive Synthesis for Porous (Mo2/3Y1/3)2AlC Ceramics through Mo, Y, Al and Graphite Powders. Materials 2024, 17, 3272. https://doi.org/10.3390/ma17133272
Tan S, Xiao G, Wang B, Yu K, Li J, Jiang W, Zhang H, Yang X, Yang J. Reactive Synthesis for Porous (Mo2/3Y1/3)2AlC Ceramics through Mo, Y, Al and Graphite Powders. Materials. 2024; 17(13):3272. https://doi.org/10.3390/ma17133272
Chicago/Turabian StyleTan, Siwei, Gan Xiao, Baogang Wang, Kui Yu, Jie Li, Wenkai Jiang, Heng Zhang, Xuejin Yang, and Junsheng Yang. 2024. "Reactive Synthesis for Porous (Mo2/3Y1/3)2AlC Ceramics through Mo, Y, Al and Graphite Powders" Materials 17, no. 13: 3272. https://doi.org/10.3390/ma17133272
APA StyleTan, S., Xiao, G., Wang, B., Yu, K., Li, J., Jiang, W., Zhang, H., Yang, X., & Yang, J. (2024). Reactive Synthesis for Porous (Mo2/3Y1/3)2AlC Ceramics through Mo, Y, Al and Graphite Powders. Materials, 17(13), 3272. https://doi.org/10.3390/ma17133272





