Corrosion of Two Iron-Based Aluminaforming Alloys in NaCl-MgCl2 Molten Salts at 600 °C
Abstract
1. Introduction
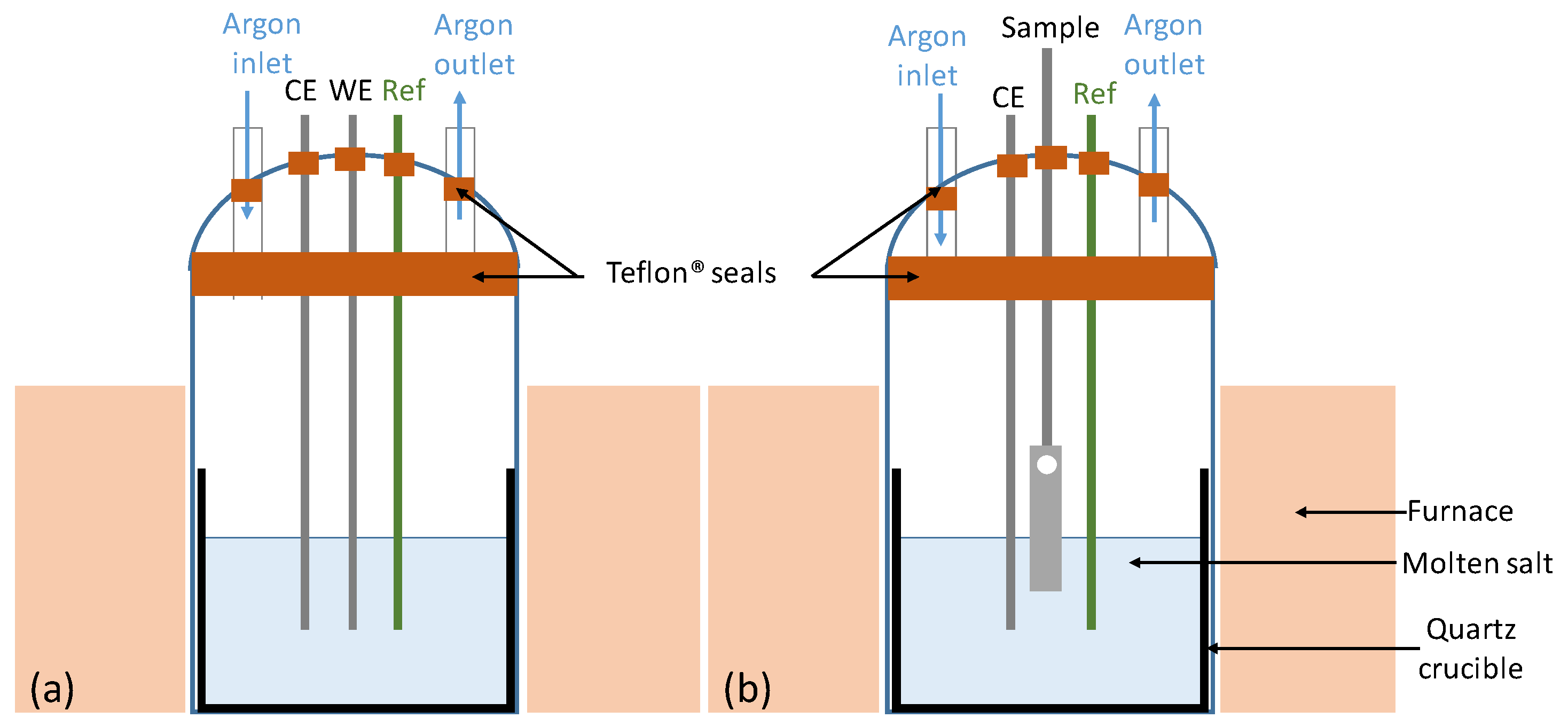
2. Materials and Methods
3. Results
3.1. NaCl-MgCl2 Purification Process
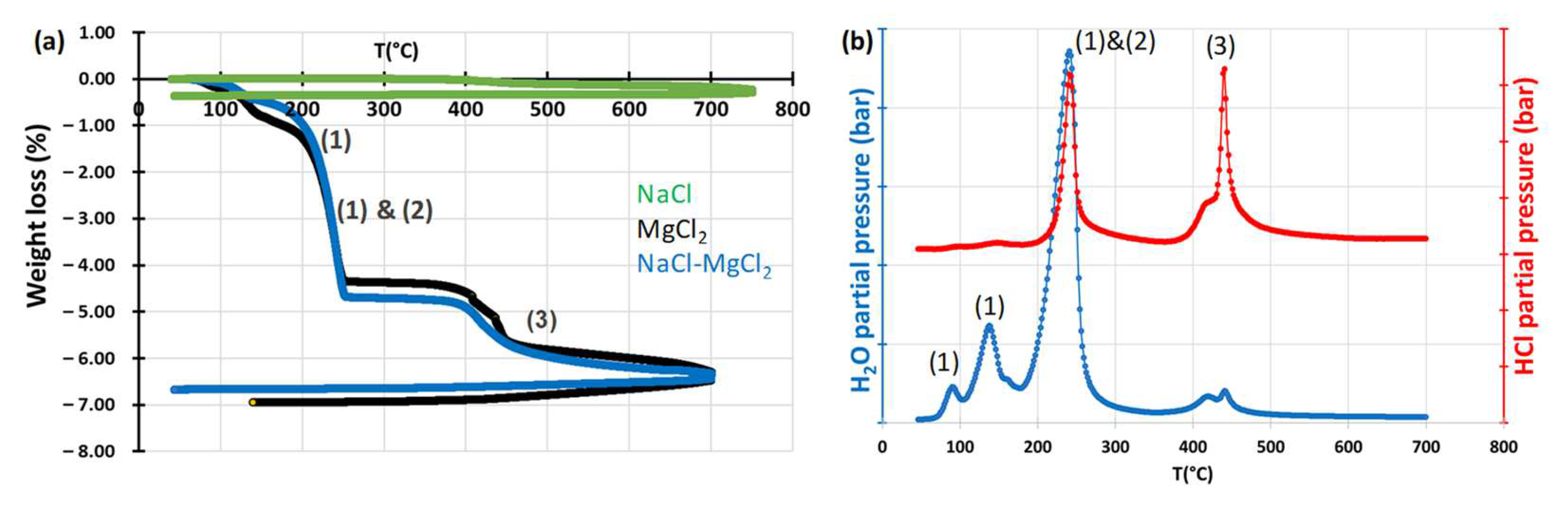
3.1.1. Dehydration of the Salts
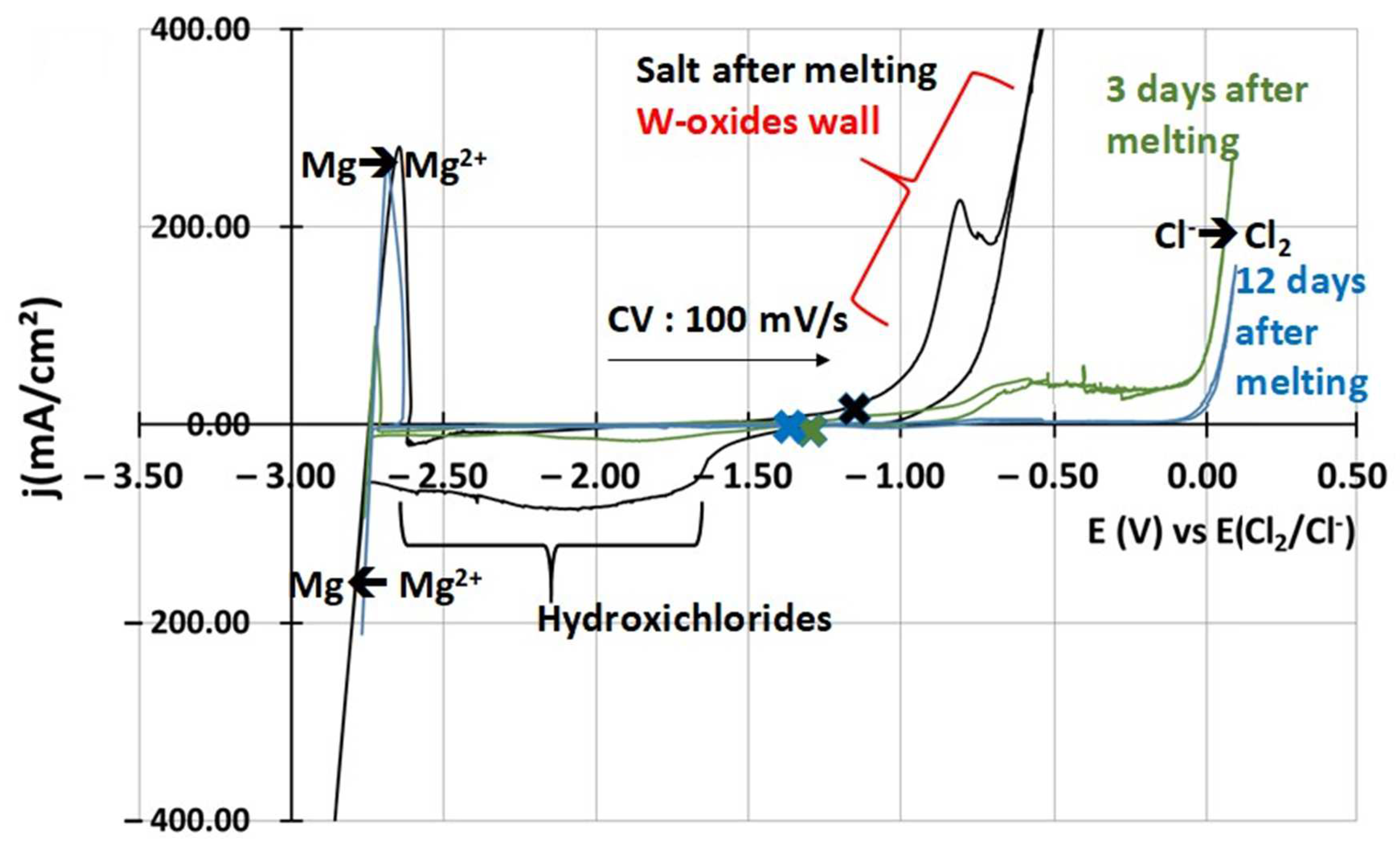
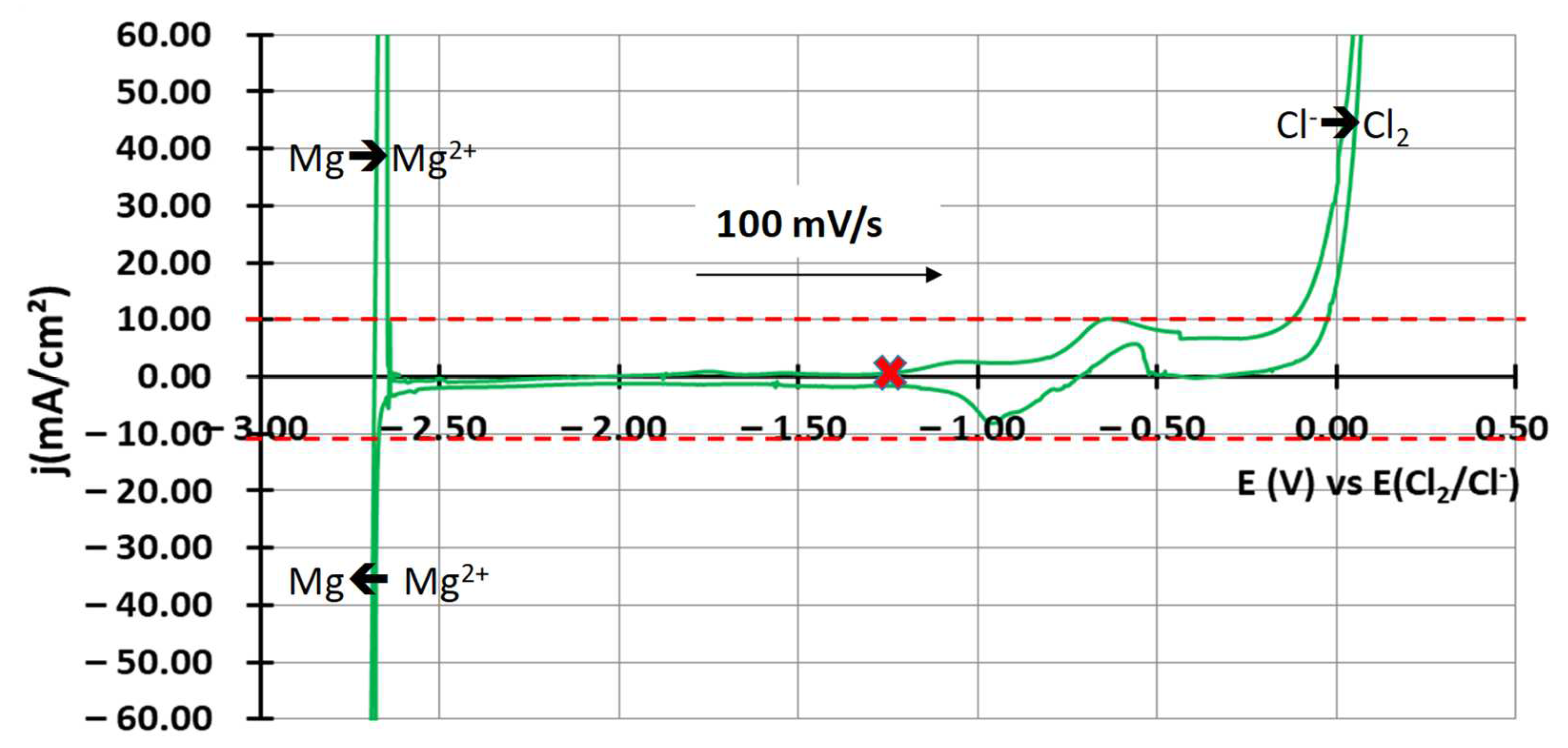
3.1.2. Salt Purification
3.2. OC1 and OC4 Corrosion
3.2.1. Alloys, Microstructures and Preoxidations
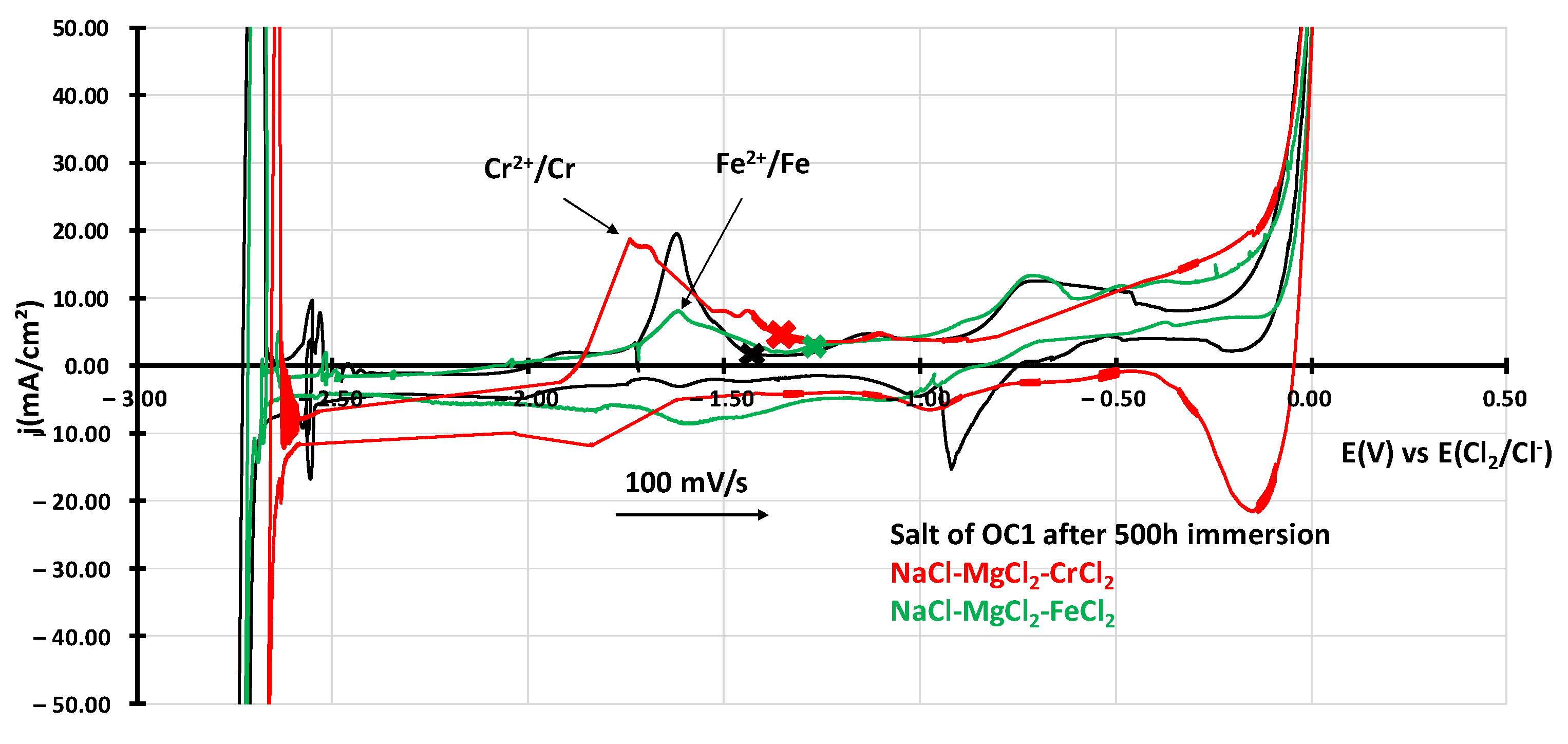
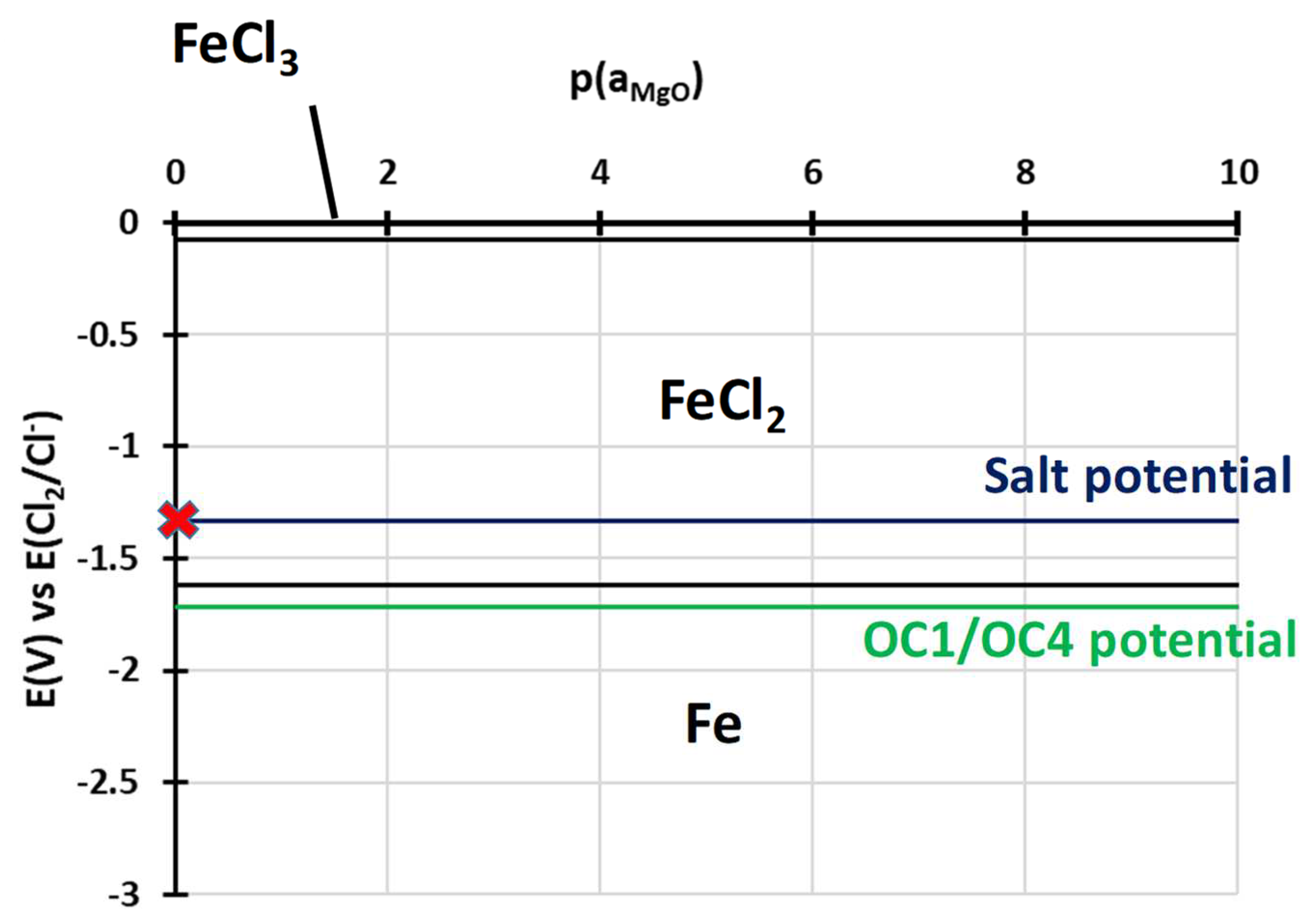
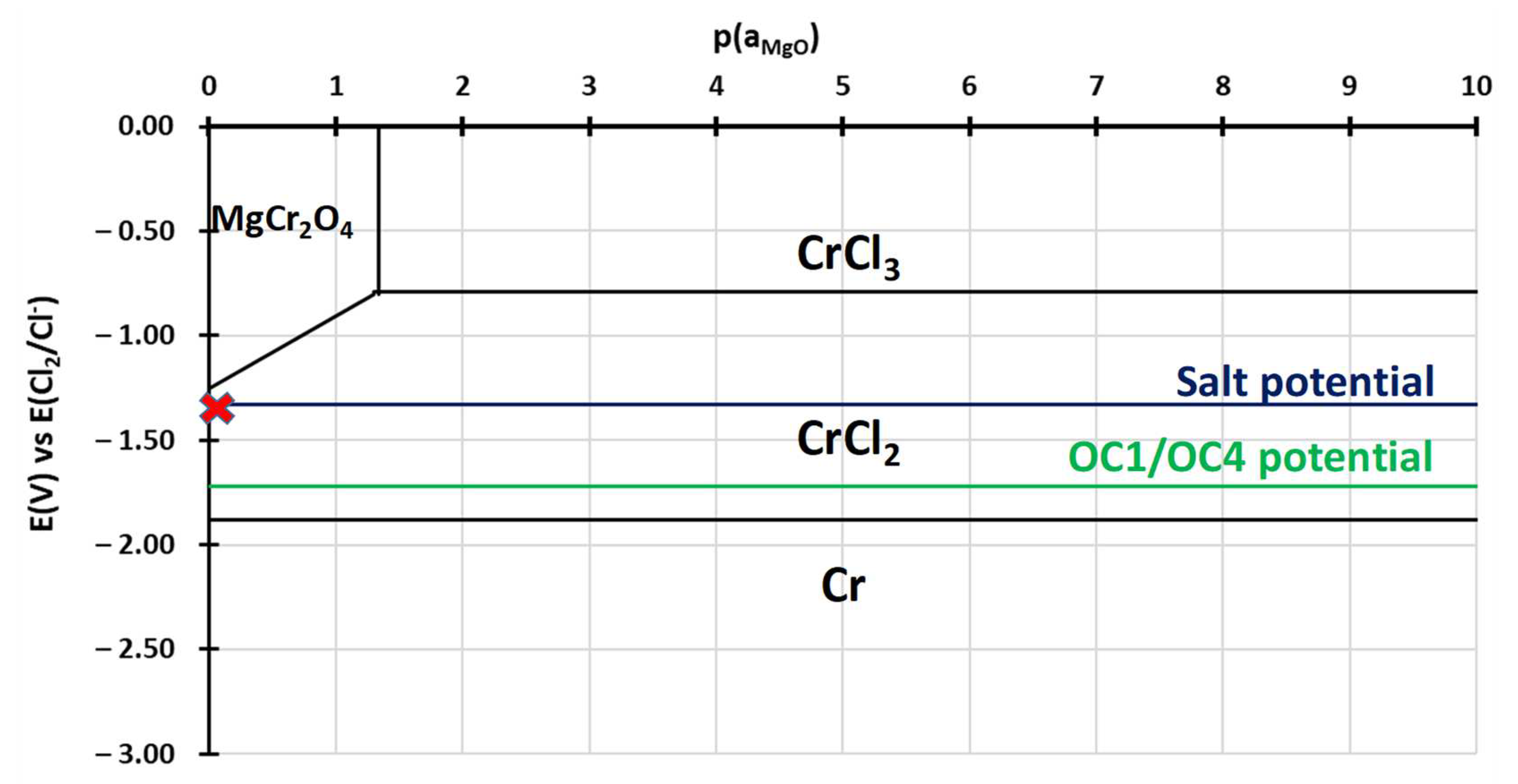
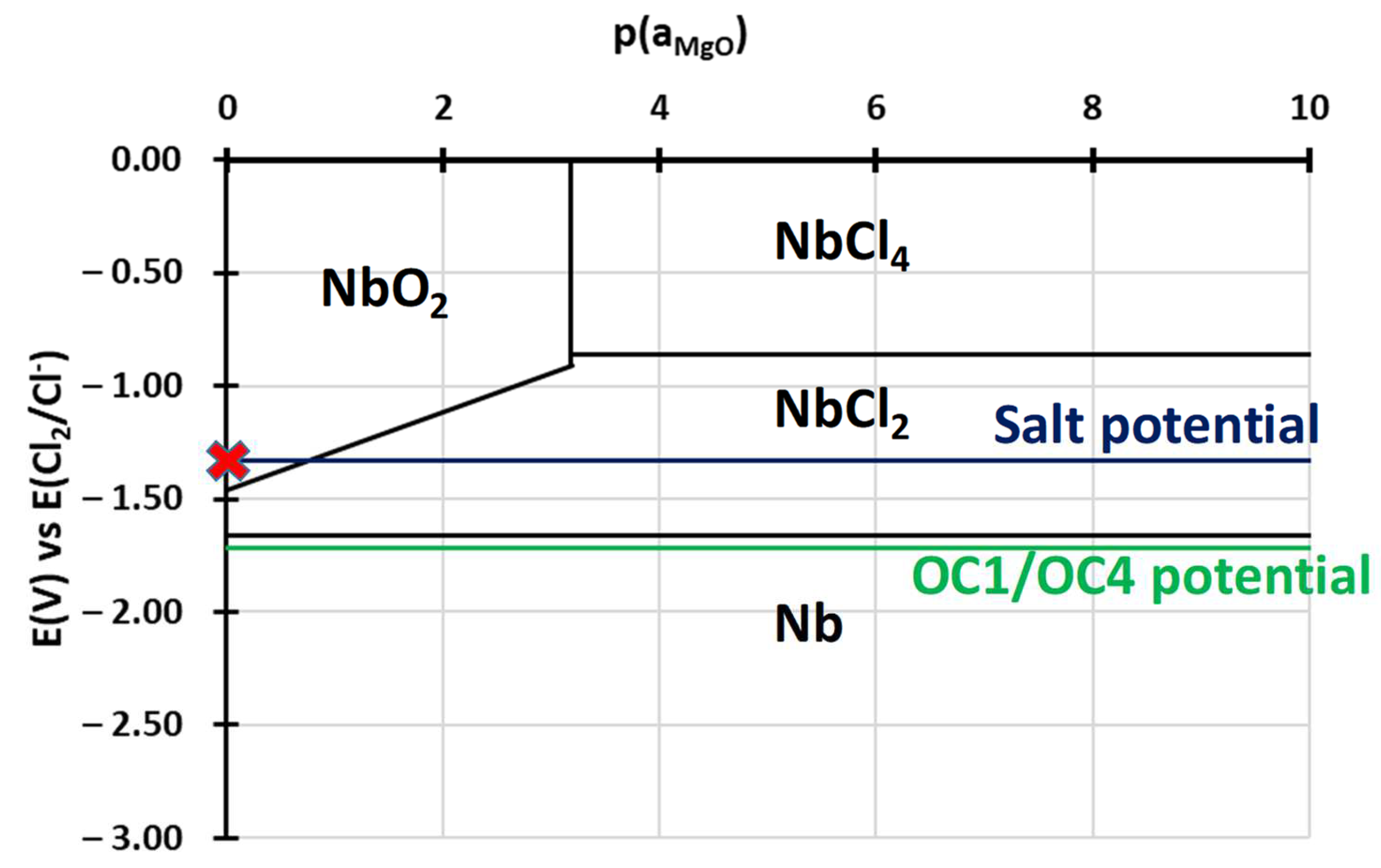
3.2.2. Dissolved Species in NaCl-MgCl2 after Corrosion
3.2.3. Corrosion of OC1 in Liquid Salt
3.2.4. Corrosion of OC1 under Gas Phase
3.2.5. Corrosion of OC4 in Liquid Salt
3.2.6. Corrosion of OC4 under Gas Phase
4. Discussion
5. Conclusions
- The purification protocol allowed us to control the purity of the salt and to obtain reproducible corrosion results. Pre-oxidation of samples in dry air led to alumina formation at the sample surface. For both alloys, Al2O3 formed during the pre-oxidation.
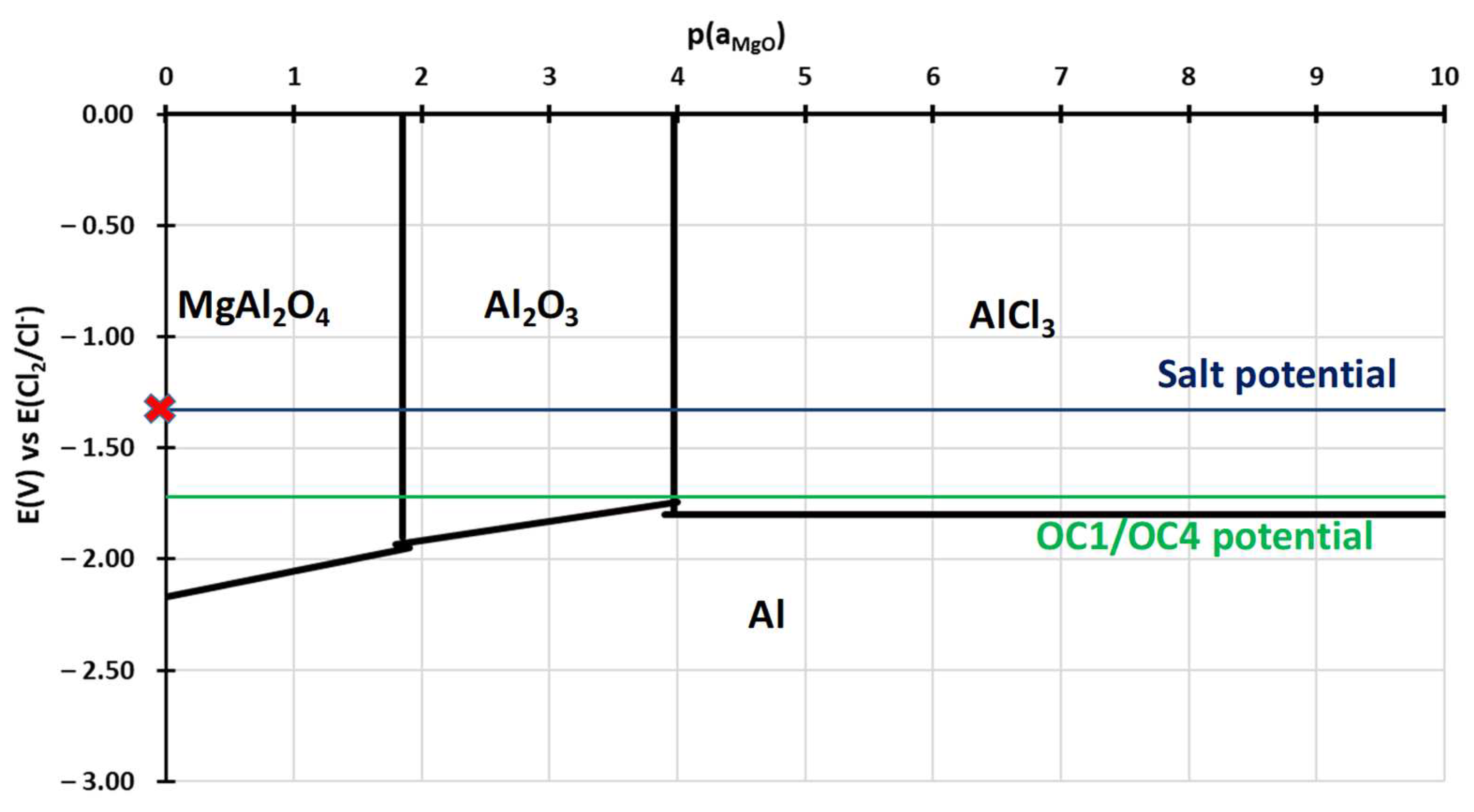
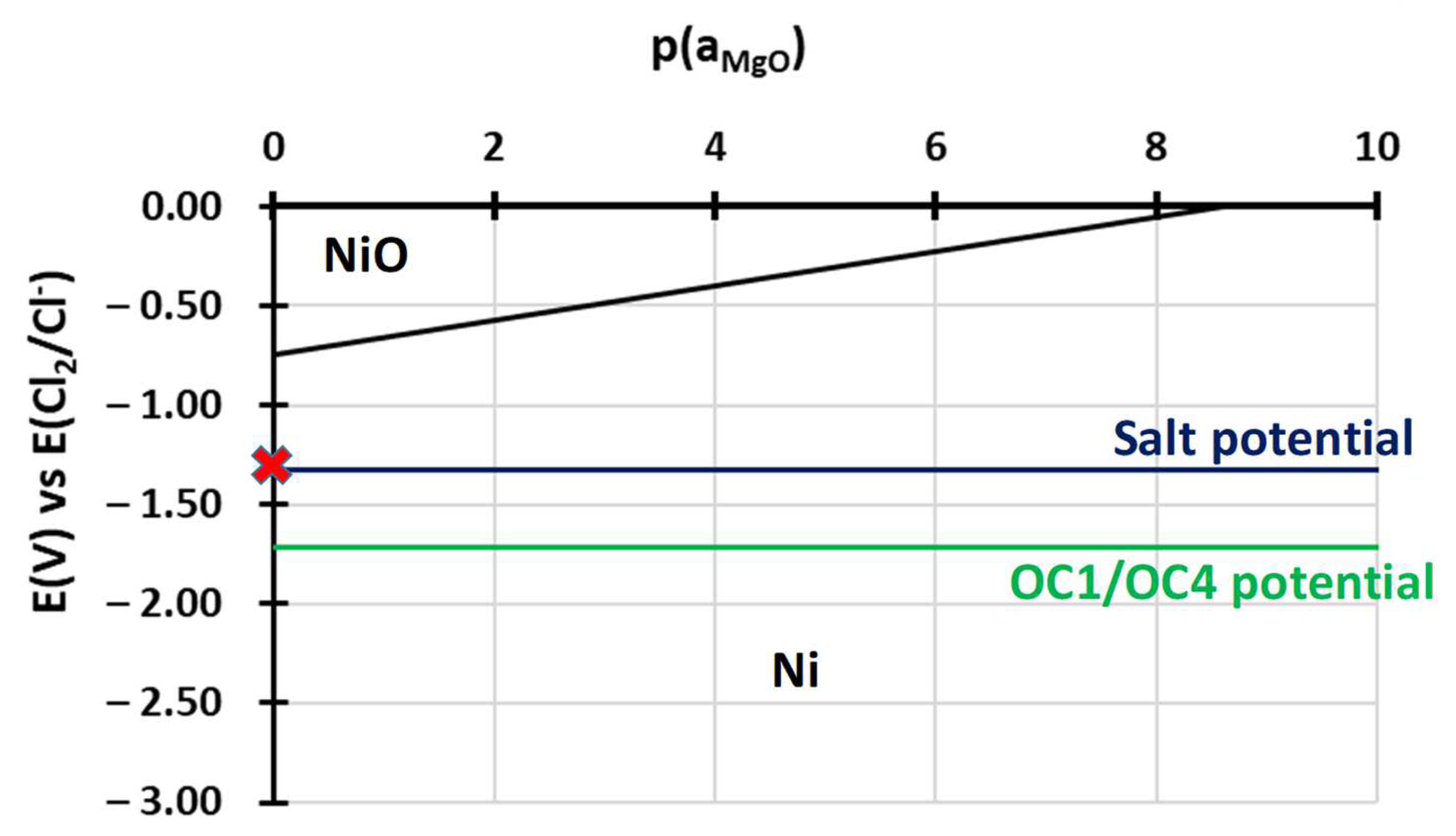
- Alumina seems to be protective in the first steps of oxidation but becomes less efficient as it transforms into MgAl2O4. This behaviour can be explained by the stability diagram calculated considering the activities of elements within the steels. The predictions of the diagrams are in accordance with experimental observations. They could be used to design alloys a priori in order to make them resistant to molten salt corrosion.
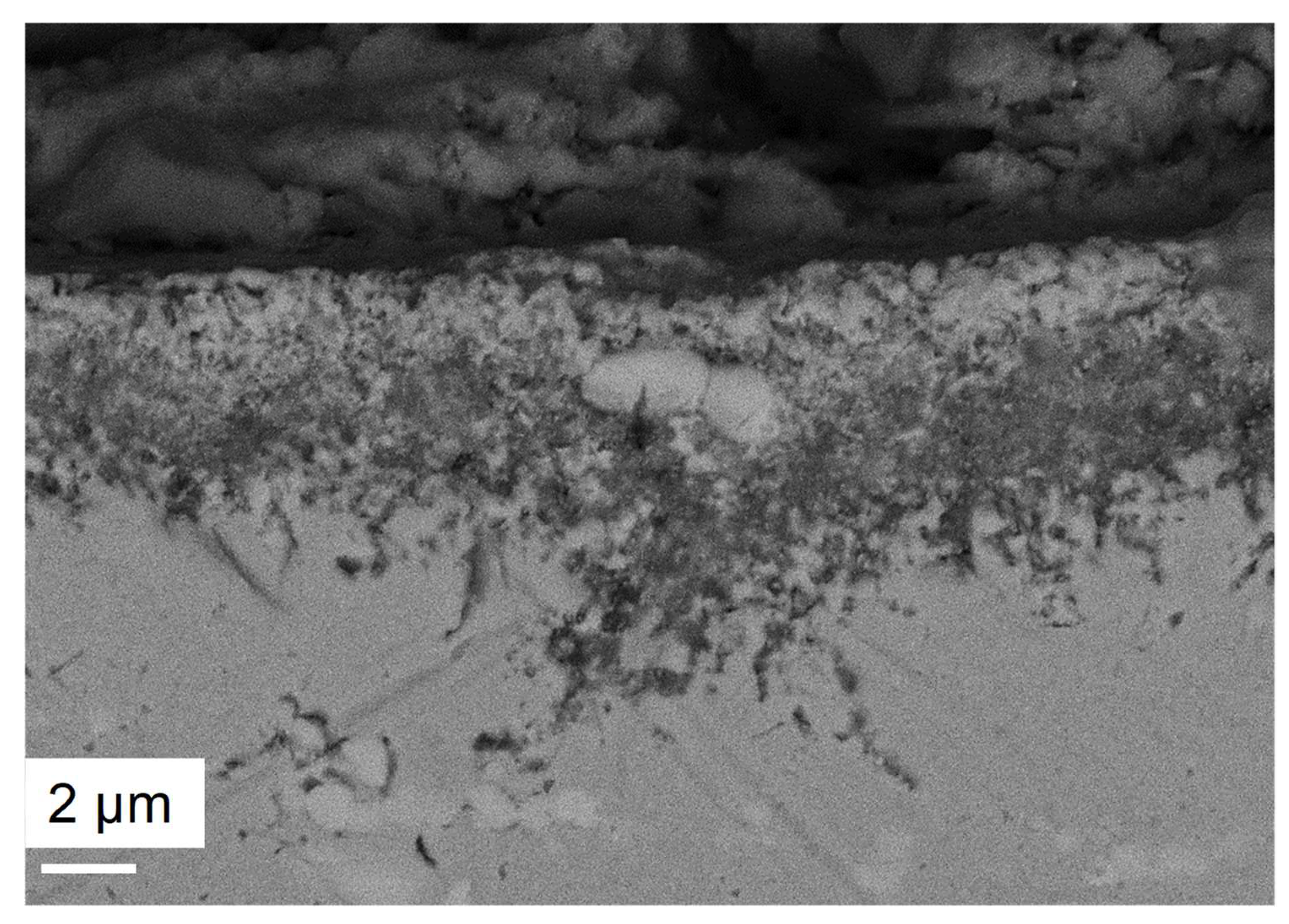
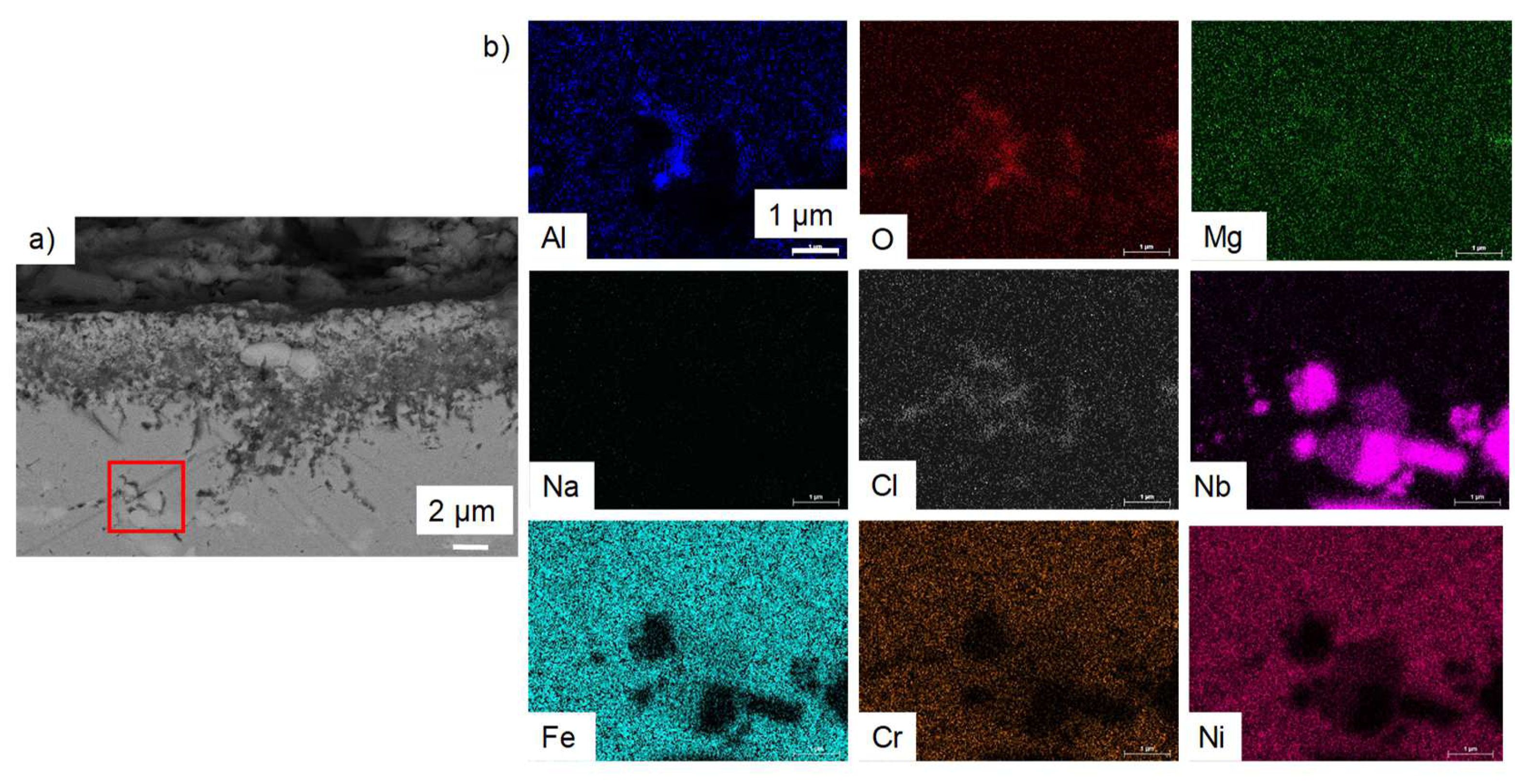
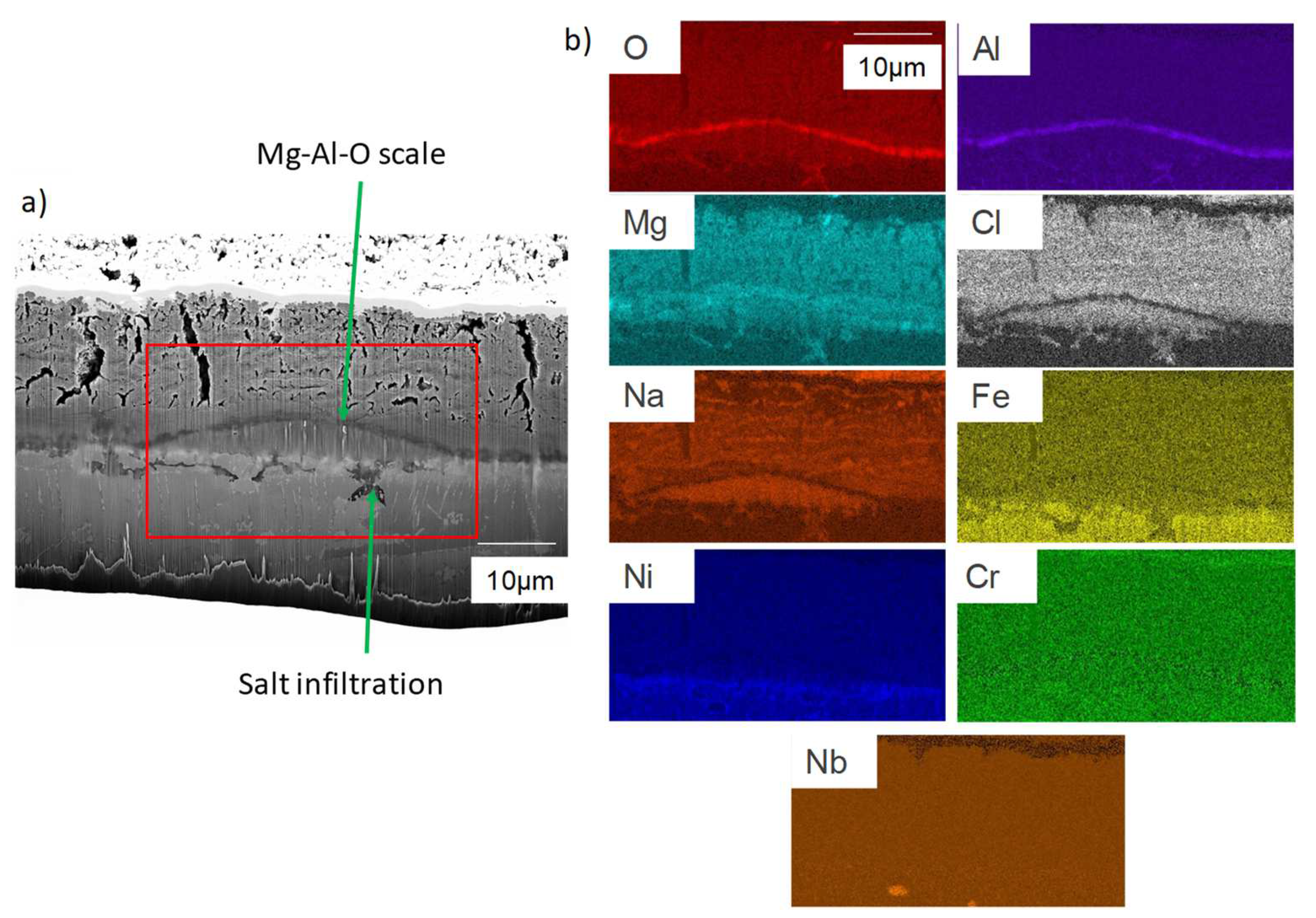
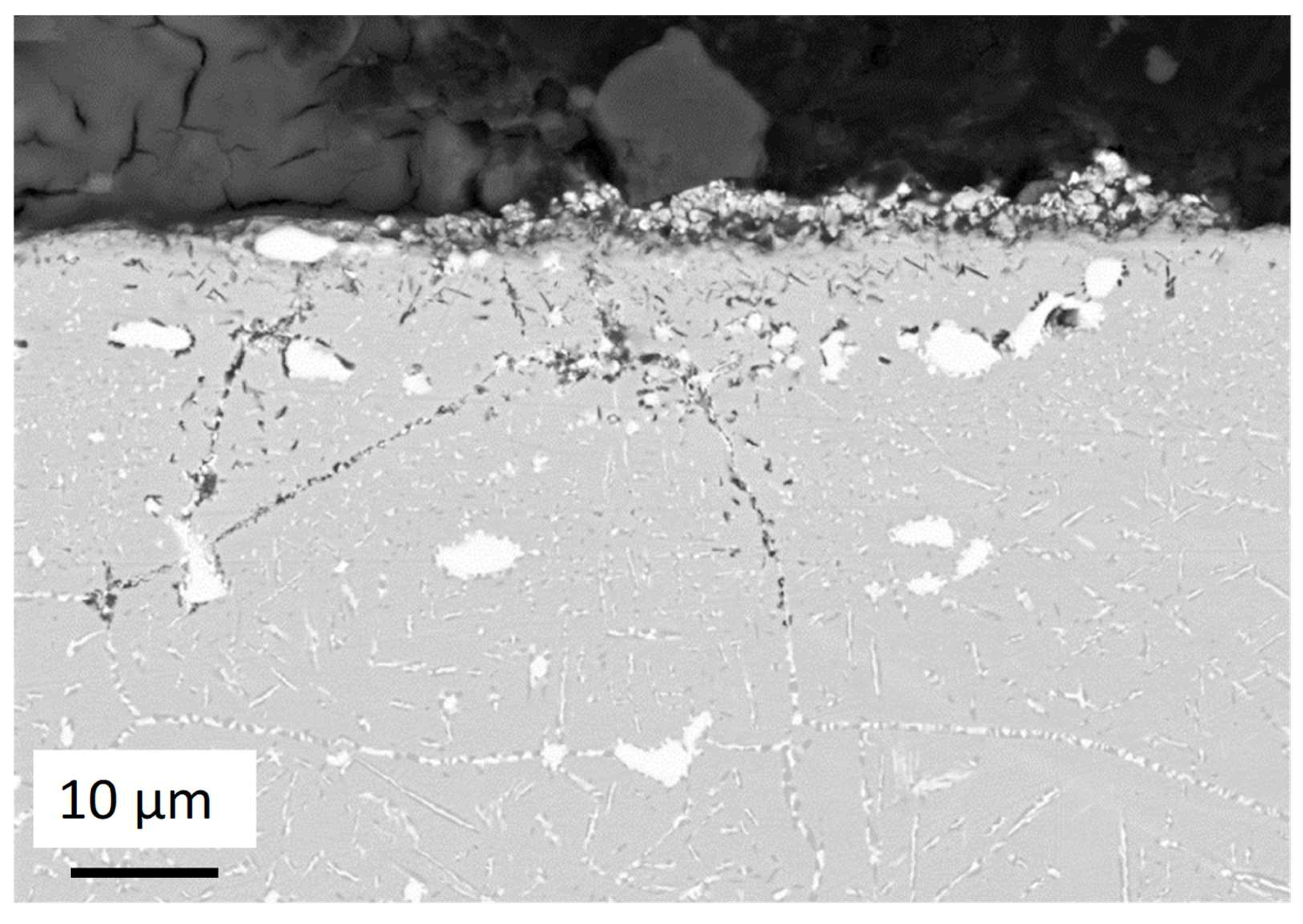
- For both alloys, Al2O3 formed during the pre-oxidation transformed to MgAl2O4, and internal intergranular corrosion occurred leading also to the formation of MgAl2O4 as observed on SEM cross-sections. At the internal corrosion front, Al2O3 precipitates were observed below the MgAl2O4 spinel precipitates, implying that Al2O3 is formed first and then transforms into the spinel. This mechanism occurs for both inter and intragranular corrosion. The internal oxidation depth is consistent with the diffusion of oxygen in the material, both in the matrix and in the grain boundaries, according to Wagner’s model. The diffusion of oxygen could, therefore, be the kinetically limiting factor. To sum it up, oxygen diffuses faster than Mg in the grain boundaries, leading to the formation of Al2O3, which later transforms into MgAl2O4. Due to the formation of a thicker alumina scale during the pre-oxidation, OC4 alloy performed better than OC1 with less intragranular corrosion.
- Electrochemical techniques enable better control of the molten salt environment so that comparable results can be obtained in different batches of salt. They also enable a better understanding of the corrosion mechanism by providing information on the elements dissolved in the salts. Cyclic voltammetry allowed us to observe dissolved chromium.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serp, J.; Allibert, M.; Benes, O.; Delpech, S.; Feynberg, O.; Ghetta, V.; Heuer, D.; Holcomb, D.; Ignatiev, V.; Kloosterman, J.L.; et al. The molten salt reactor (MSR) in generation IV: Overview and perspectives. Prog. Nucl. Energy 2014, 77, 308–319. [Google Scholar] [CrossRef]
- LeBlanc, D. Molten salt reactors: A new beginning for an old idea. Nucl. Eng. Des. 2010, 240, 1644–1656. [Google Scholar] [CrossRef]
- Allibert, M.; Beils, S. Réacteurs à Sels Fondus. 2018. Available online: https://irfu.cea.fr/Meetings/seminaires-MSR/MSR-RapportJourneesMassy_Mars2018.pdf (accessed on 16 November 2021).
- Beneš, O.; Konings, R.J.M. Thermodynamic evaluation of the NaCl–MgCl2–UCl3–PuCl3 system. J. Nucl. Mater. 2008, 375, 202–208. [Google Scholar] [CrossRef]
- Merle, E.; Heuer, D.; Brovchenko, M.; Ghetta, V.; Laureau, A.; Rubiolo, P. Preliminary design assessment of the molten salt fast reactor. In Proceedings of the European Nuclear Conference, Manchester, UK, 9–12 December 2012. [Google Scholar]
- Mortazavi, A.; Zhao, Y.; Esmaily, M.; Allanore, A.; Birbilis, N. High-temperature corrosion of a nickel-based alloy in a molten chloride environment–The effect of thermal and chemical purifications. Sol. Energy Mater. Sol. Cells 2022, 236, 111542. [Google Scholar] [CrossRef]
- Kipouros, G.J.; Sadoway, D.R. A thermochemical analysis of the production of anhydrous MgCl2. J. Light Met. 2001, 1, 111–117. [Google Scholar] [CrossRef]
- Kirsh, Y.; Yariv, S.; Shoval, S. Kinetic analysis of thermal dehydration and hydrolysis of MgCl2-6H2O by DTA and TG. J. Therm. Anal. 1987, 32, 383–408. [Google Scholar] [CrossRef]
- Raiman, S.S.; Lee, S. Aggregation and data analysis of corrosion studies in molten chloride and fluoride salts. Sol. Energy Mater. Sol. Cells 2018, 511, 523–535. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Gussone, J.; Bauer, T. Electrochemical measurement of corrosive impurities in molten chlorides for thermal energy storage. J. Energy Stor. 2018, 15, 408–414. [Google Scholar] [CrossRef]
- Massot, L.; Cassayre, L.; Chamelot, P.; Taxil, P. On the use of electrochemical techniques to monitor free oxide content in molten fluoride media. JEAC 2007, 606, 17–23. [Google Scholar] [CrossRef]
- Skar, R.A. Chemical and Electrochemical Characterisation of Oxide/Hydroxide Impurities in the Electrolyte for Magnesium Production. Ph.D. Thesis, Institutt for Kjemi Norges Teknisk-Naturvitenskapelige Universitet, Gjøvik, Norway, 2001. [Google Scholar]
- Ishitsuka, T.; Nose, K. Stability of protective oxide films in waste incineration environment-solubility measurement of oxides in molten chlorides. CRRSAA 2002, 44, 247–263. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Q.; Yin, H.; Pan, T.J.; Tang, Z. Excellent corrosion resistance of 316 stainless steel in purified NaCl-MgCl2 eutectic salt at high temperature. CRRSAA 2020, 166, 108473. [Google Scholar] [CrossRef]
- Grégoire, B.; Oskay, C.; Meiβner, T.M.; Galetz, M.C. Corrosion mechanisms of ferritic-martensitic P91 steel and Inconel 600 nickel-based alloy in molten chlorides. Part II: NaCl-KCl-MgCl2 ternary system. Sol. Energy Mater. Sol. Cells 2020, 216, 110675. [Google Scholar] [CrossRef]
- Ren, S.; Chen, Y.; Ye, X.X.; Jiang, L.; Yan, S.; Liang, J.; Yang, X.; Leng, B.; Li, Z.; Chen, Z.; et al. Corrosion behaviour of carburized 316 stainless steel in molten chloride salts. Sol. Energy 2021, 223, 1–10. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Li, Z.; Zhang, P.; Su, X. Corrosion behaviour of 316SS and Ni-based alloys in a ternary NaCl-KCl-MgCl2 molten salt. Sol. Energy 2018, 171, 320–329. [Google Scholar] [CrossRef]
- Patel, N.S.; Pavlík, V.; Boca, M. High-Temperature Corrosion Behaviour of Superalloys in Molten Salts–A Review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 83–97. [Google Scholar] [CrossRef]
- Chmakoff, A. Compréhension des Mécanismes de Corrosion en Environnement Réacteurs du Futur à Combustibles et Caloporteur Sels Fondus. Ph.D. Thesis, Université Paris-Saclay, Saclay, France, 2023. [Google Scholar]
- Muránsky, O.; Yang, C.; Zhu, H.; Karatchevtseva, I.; Slama, P.; Novy, Z.; Edwards, L. Molten salt corrosion of Ni-Mo-Cr candidate structural materials for Molten Salt Reactor (MSR) systems. Corros. Sci. 2019, 159, 108087. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Fernandez, A.G.; Tirawat, R.; Turchi, C.; Huddleston, W. Corrosion resistance of alumina-forming alloys against molten chlorides for energy production. I: Pre-oxidation treatment and isothermal corrosion tests. Sol. Energy Mater. Sol. Cells 2017, 166, 222–233. [Google Scholar] [CrossRef]
- Fernández, A.G.; Cabeza, L.F. Anodic Protection Assessment Using Alumina-Forming Alloys in Chloride Molten Salt for CSP Plants. Coatings 2020, 10, 138. [Google Scholar] [CrossRef]
- Gore, P.; Singh, M.P.; Suryateja, D.; Basu, B.; Chattopadhyay, K. Early-stage corrosion of IN 740H alloy in eutectic NaCl-KCl molten salt at high temperatures. Sol. Energy 2023, 252, 330–341. [Google Scholar] [CrossRef]
- Brady, M.P.; Pint, B.A.; Unocic, K.A.; Walker, L.R.; Matthews, W.J. Evaluation of Alumina-Forming Austenitic Stainless Steel Alloys in Microturbines; Oak Ridge National Lab.(ORNL): Oak Ridge, TN, USA, 2010; p. NFE-08-01392. [Google Scholar]
- Gomez-Vidal, J.C.; Fernandez, A.G.; Tirawat, R.; Turchi, C.; Huddleston, W. Corrosion resistance of alumina forming alloys against molten chlorides for energy production. II: Electrochemical impedance spectroscopy under thermal cycling conditions. Sol. Energy Mater. Sol. Cells 2017, 166, 234–245. [Google Scholar] [CrossRef]
- Pint, B.A. (Oak Ridge National Lab. ORNL, Tennessee, USA); Brady, M. (Oak Ridge National Lab. ORNL, Tennessee, USA). E-mail message to author. December 2021.
- Huang, Q.; Lu, G.; Wang, J.; Yu, J. Thermal decomposition mechanisms of MgCl2·6H2O and MgCl2·H2O. J. Anal. Appl. Pyrolysis 2011, 91, 159–164. [Google Scholar] [CrossRef]
- Boghosian, S.; Godø, A.; Mediaas, H.; Ravlo, W.; Ostvold, T. Oxide complexes in alkali-alkaline-earth chloride melts. Acta Chem. Scand. 1991, 45, 145–157. [Google Scholar] [CrossRef][Green Version]
- Skar, R.A.; Mediaas, H.; Ostvold, T. Characterization of oxide and hydroxide in MgCl2-NaCl melts. Can. Metall. Q. 2004, 43, 381–394. [Google Scholar] [CrossRef]
- Kulinkin, A.B.; Feofilov, S.P.; Zakharchenya, R.I. Luminescence of Impurity 3d and 4f Metal Ions in Different Crystalline Forms of Al2O3. Phys. Solid State 2000, 42, 835–838. [Google Scholar] [CrossRef]
- Hanson, K.; Sankar, K.; Weck, P.F.; Startt, J.K.; Dingreville, R.; Deo, C.S.; Sugar, J.D.; Singh, P.M. Effect of excess Mg to control corrosion in molten MgCl2 and KCl eutectic salt mixture. CRRSAA 2022, 194, 109914. [Google Scholar] [CrossRef]
- Delpech, S.; Carrière, C.; Chmakoff, A.; Martinelli, L.; Rodrigues, D.; Cannes, C. Corrosion Mitigation in Molten Salt Environments. Materials 2024, 17, 581. [Google Scholar] [CrossRef]
- Jeong, G.Y.; Sohn, S.; Jeon, Y.; Park, J. Understanding of electrochemical behaviours of niobium in molten LiCl-KCl eutectic for pyrochemical decontamination process. J. Nucl. Mater. 2019, 524, 39–53. [Google Scholar] [CrossRef]
- Prillieux, A. Hydrogen and Water Vapour Effects on Oxygen Solubility and Diffusivity in High Temperature Fe-Ni. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2017. [Google Scholar]
- Sanviemvongsak, T. Oxydation et Corrosion à Haute Température de Superalliages à Base de Nickel Issus de la Fabrication Additive. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2020. [Google Scholar]

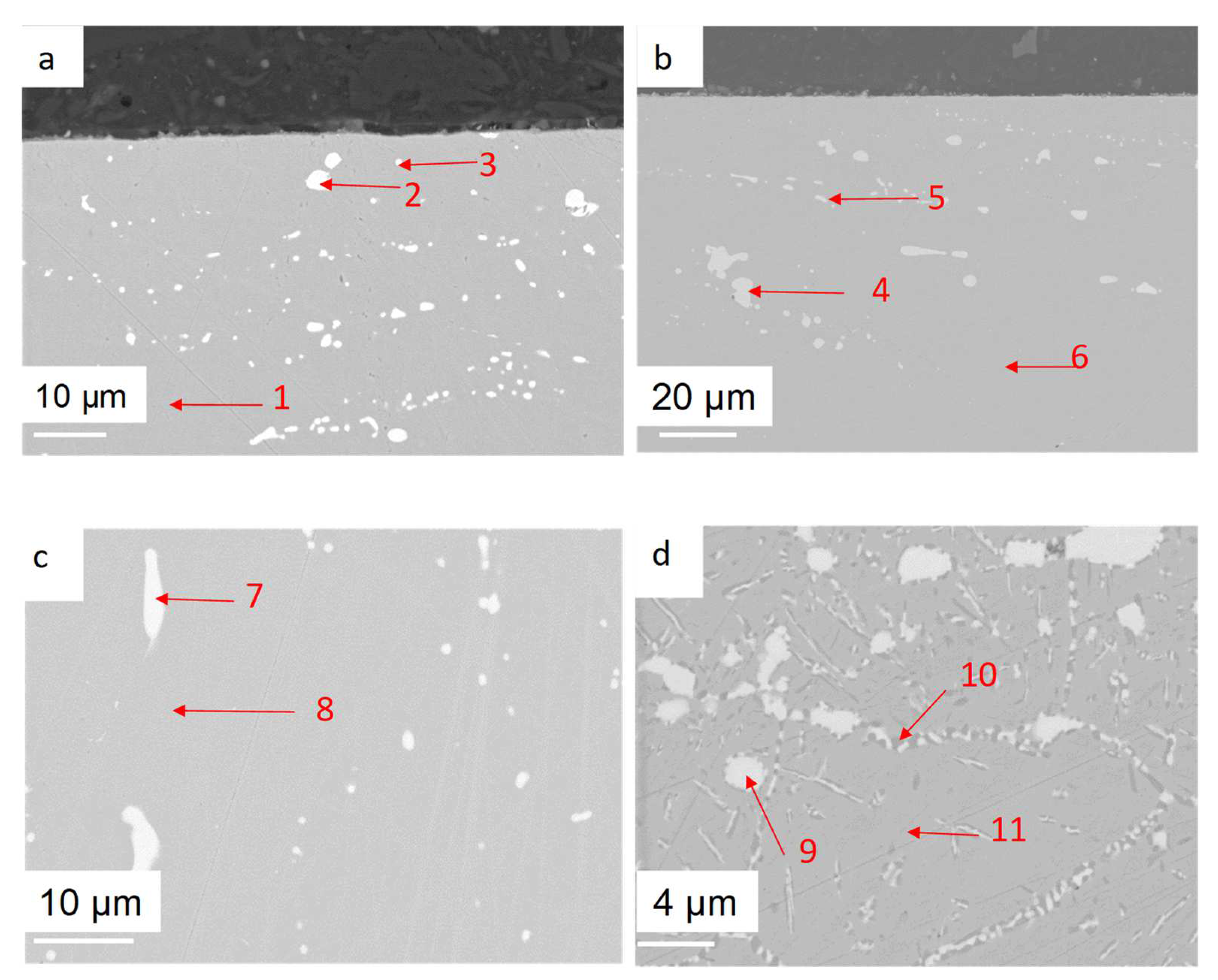
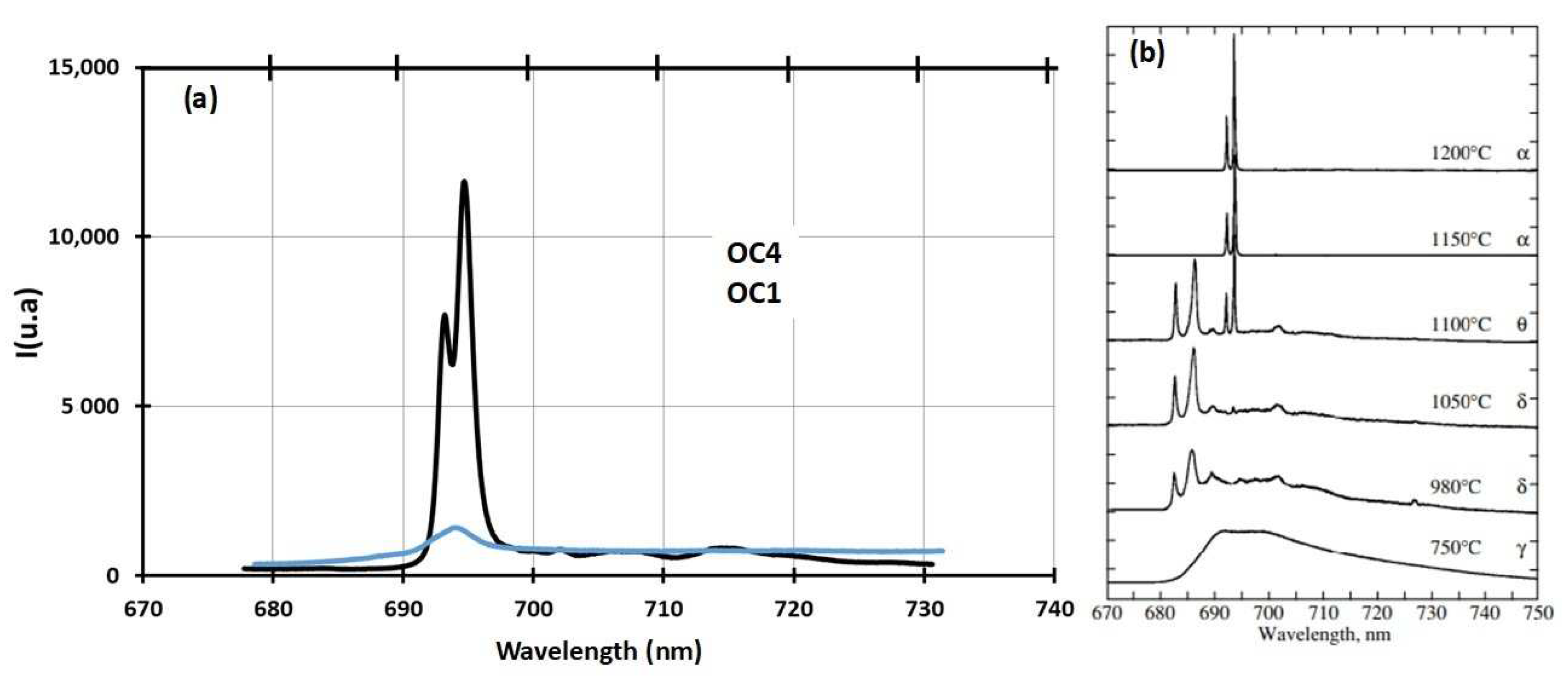
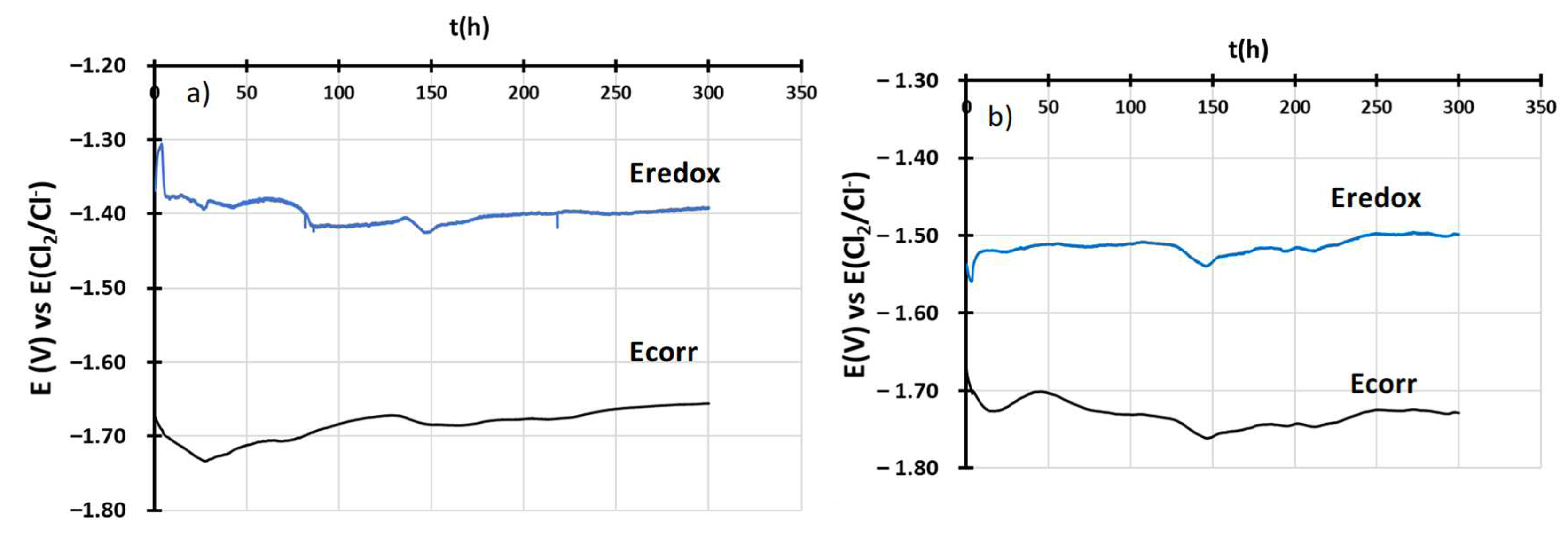
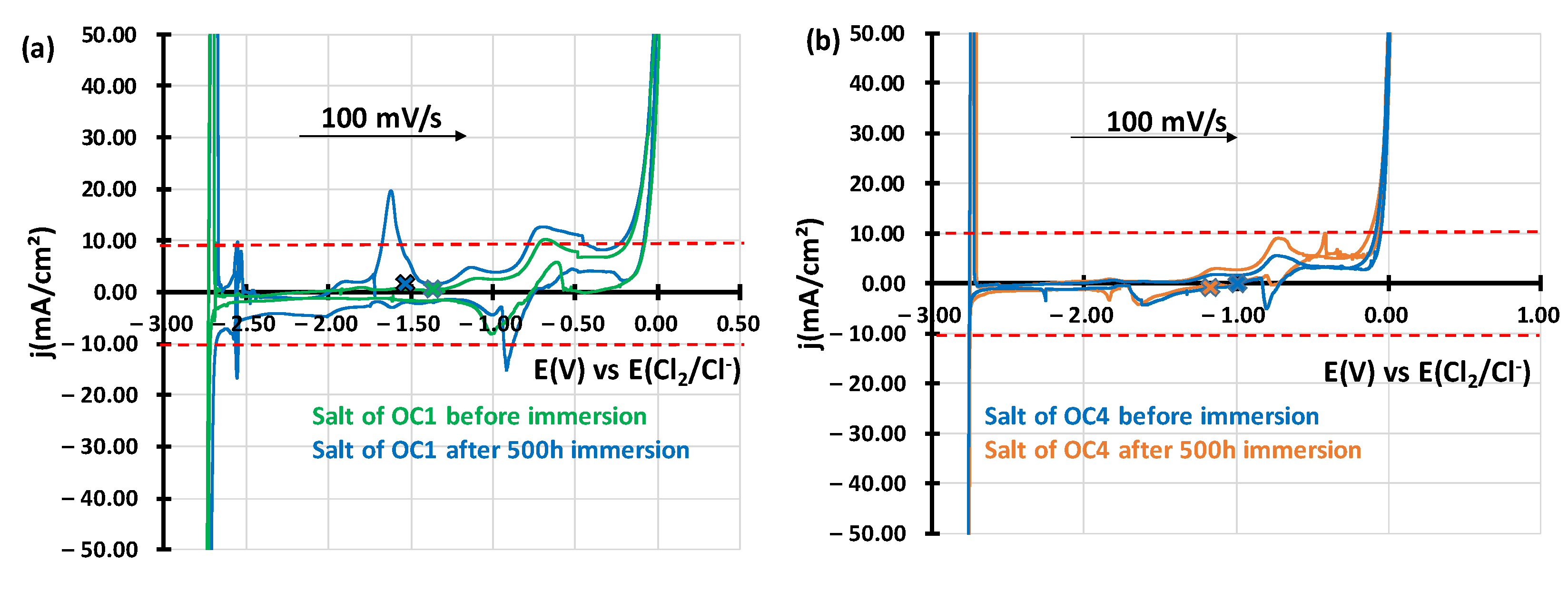
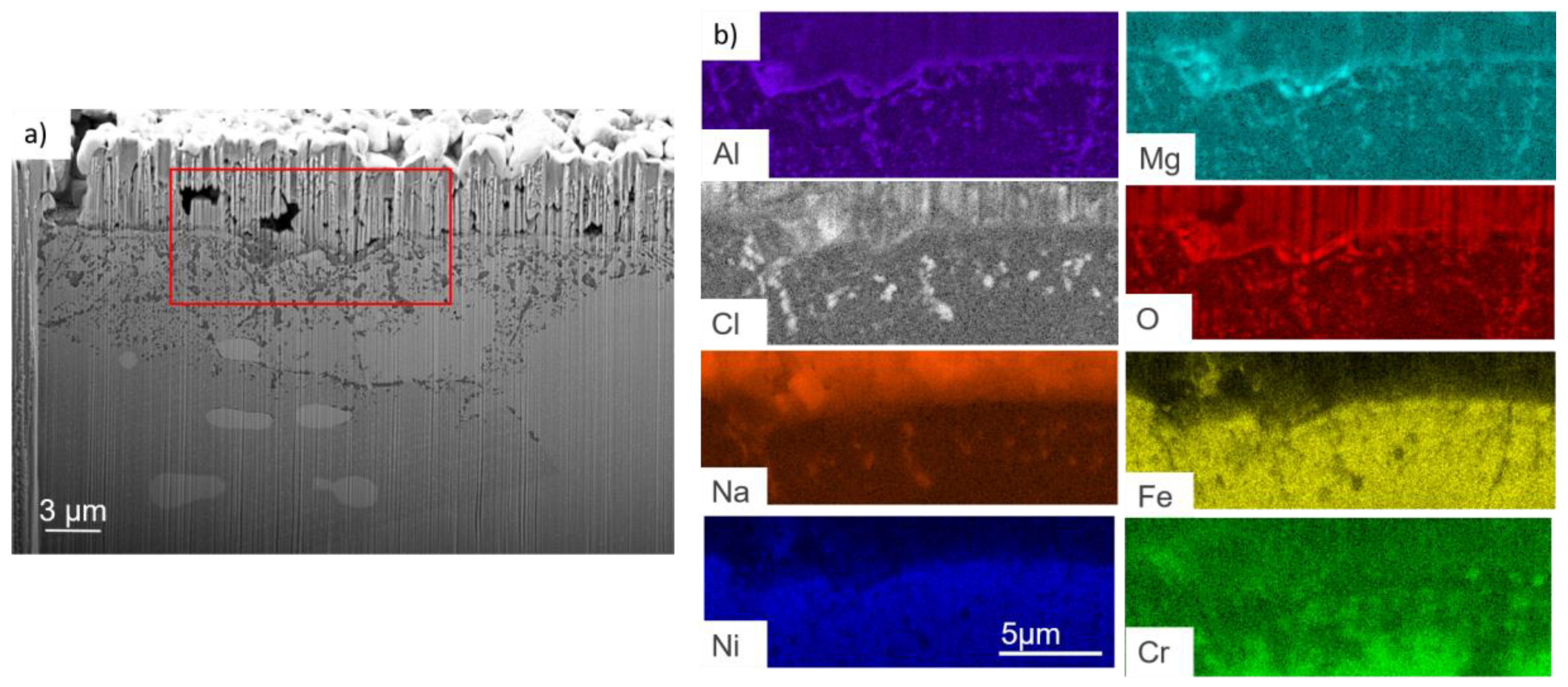
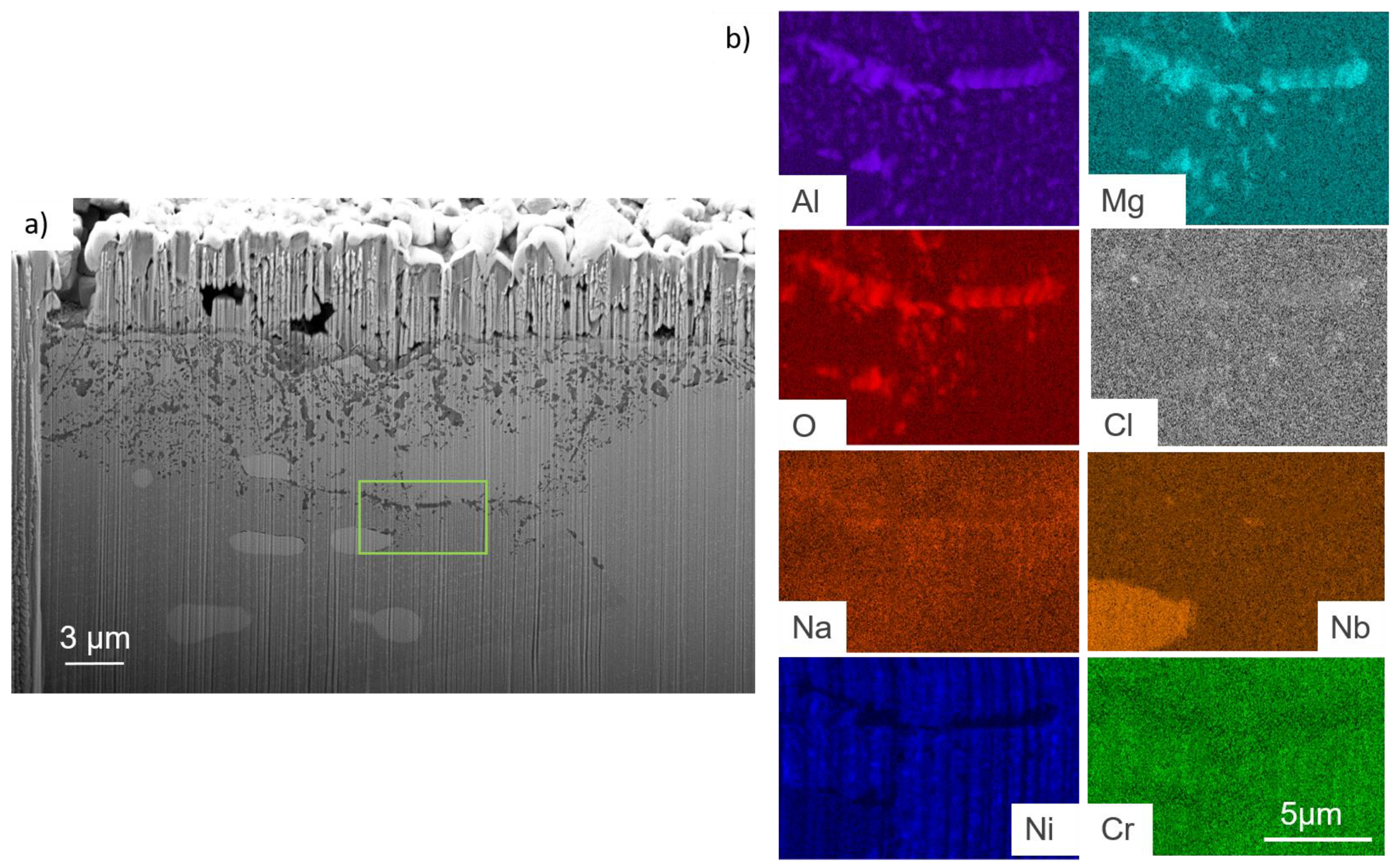
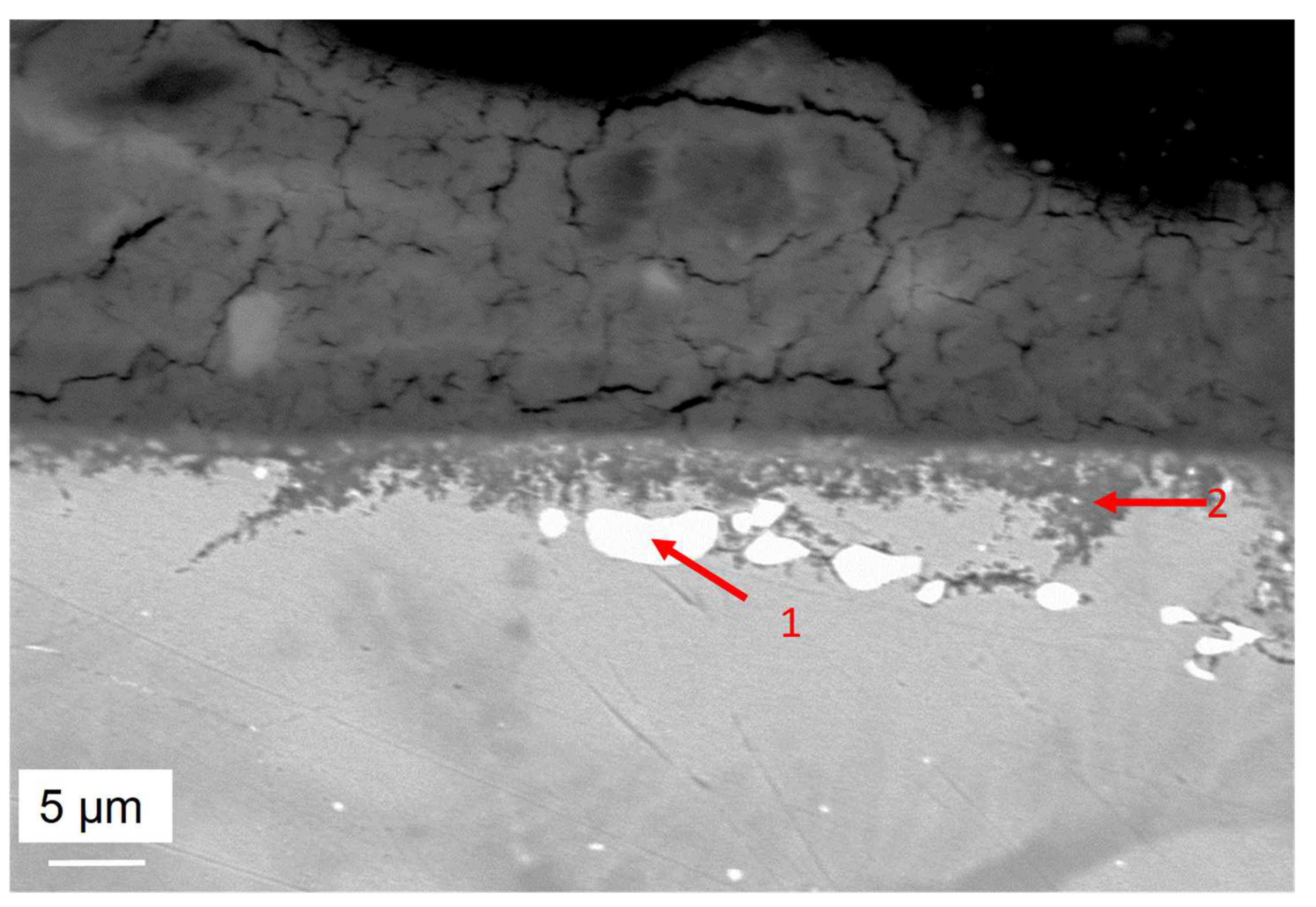

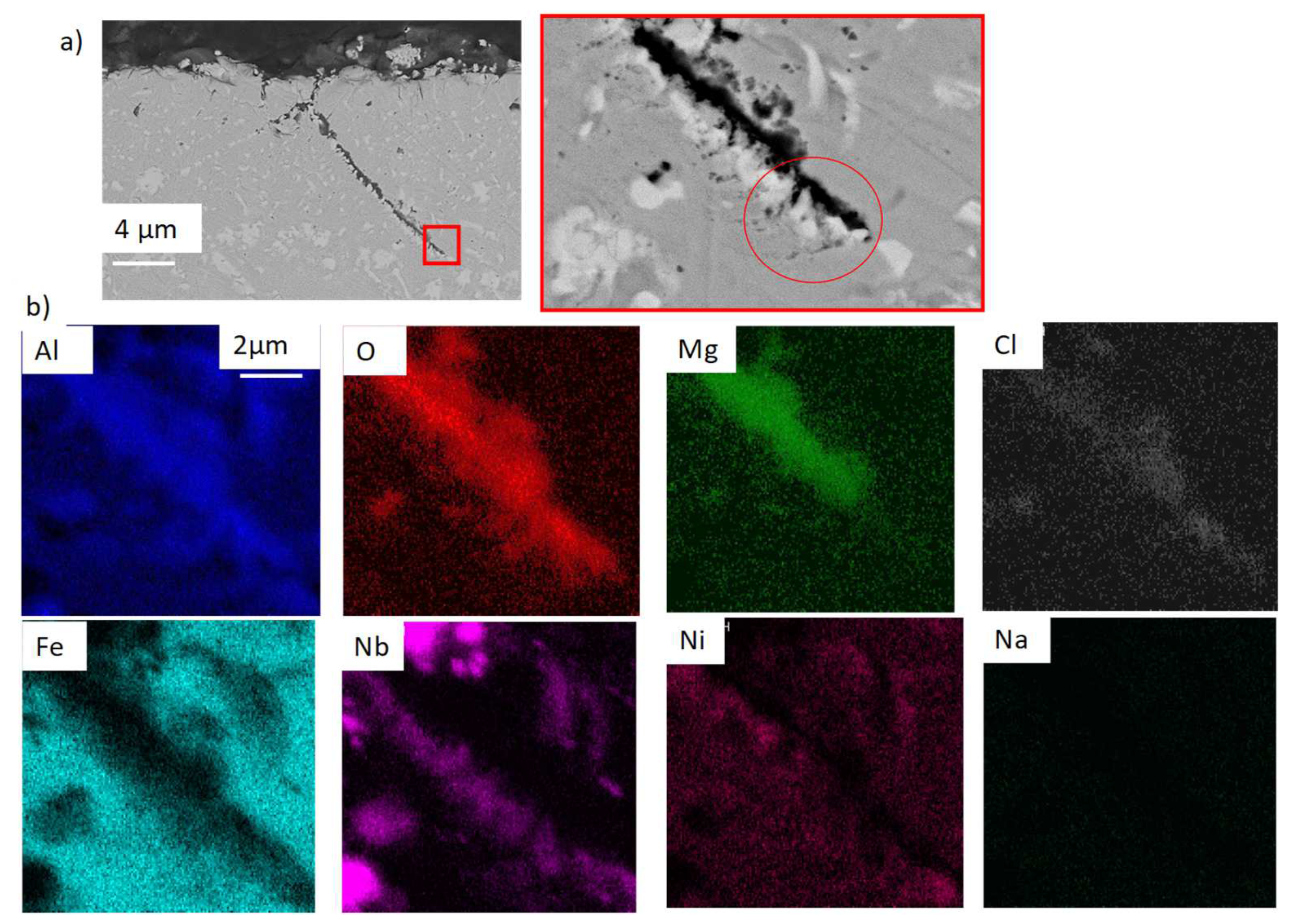
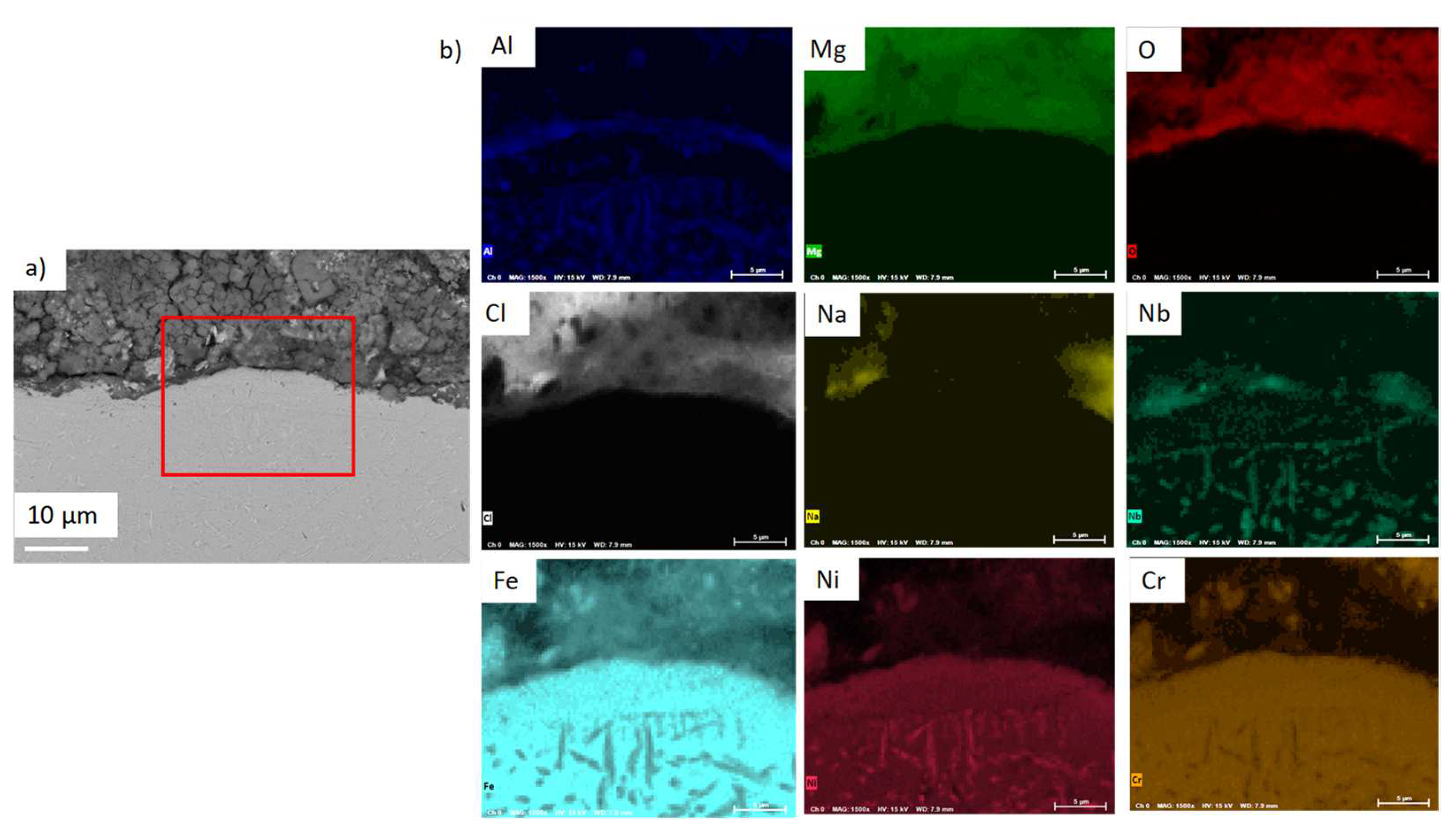
| wt% | C | Ni | Cr | Al | Nb | Mo | Ti | W | Fe |
|---|---|---|---|---|---|---|---|---|---|
| OC4 | 0.1 | 25 | 14 | 3.5 | 2.5 | 2 | 0.05 | 1 | Bal. |
| OC1 | 0.1 | 20 | 14 | 3 | 2.5 | 2 | 0.05 | 1 | Bal. |
| mol% | Fe | Ni | Cr | Nb | Al | Mo | W | C | Sum(%wt) |
|---|---|---|---|---|---|---|---|---|---|
| Spec. 1 | 57.8 | 20.2 | 14.0 | 18.2 | 4.4 | 0.5 | 0.4 | 0 | 103.0 |
| Spec. 2 | 45.7 | 10.1 | 14.0 | 18.2 | 2.2 | 6.0 | 4.0 | 0 | 107.9 |
| Spec. 3 | 12.3 | 3.8 | 7.5 | 72.3 | 0.0 | 3.8 | 0.09 | 0 | 95.8 |
| Spec. 4 | 43.2 | 11.5 | 11.6 | 21.9 | 0.0 | 5.6 | 5.03 | 0 | 100.8 |
| Spec. 5 | 8.5 | 3.0 | 3.6 | 82.8 | 0.0 | 2.0 | 0.2 | 0 | 97.3 |
| Spec. 6 | 57.6 | 18.7 | 11.6 | 0.5 | 5.8 | 0.9 | 1.5 | 0 | 96.9 |
| Spec. 7 | 1.1 | 2.0 | 2.13 | 88.8 | 0.37 | 3.9 | 1.74 | 0 | 99.2 |
| Spec. 8 | 47.2 | 23.2 | 14.0 | 1.4 | 3.0 | 1.7 | 1.6 | 0 | 92.0 |
| Spec. 9 | 1.1 | 2.7 | 2.9 | 57.1 | 0.2 | 2.1 | 0 | 0 | 98.1 |
| Spec. 10 | 28.0 | 34.5 | 8.0 | 1.0 | 24.9 | 0 | 0.7 | 0 | 92.6 |
| Spec. 11 | 55.3 | 22.5 | 17.1 | 0.1 | 3.8 | 1.3 | 0 | 0 | 88.8 |
| OC1 Phases (mol%) | Fe | Ni | Cr | Al | Nb | W | Mo | C |
|---|---|---|---|---|---|---|---|---|
| FCC_A1#1 | 53 | 23 | 14 | 7 | 0.08 | 0.5 | 0.6 | 5 × 10–5 |
| BCC_A2 | 66 | 5 | 22 | 5 | 0.02 | 0.02 | 0.04 | 7 × 10–6 |
| Laves phases C14 | 58 | 0.4 | 6 | 2 × 10–5 | 8 | 9 | 16 | 0 |
| NbNi3 | 0 | 75 | 0 | 0 | 25 | 0 | 0 | 0 |
| Sigma | 45 | 3 | 44 | 6 × 10–5 | 3 | 0.9 | 5 | 0 |
| FCC A1#2 | 0.002 | 0 | 0.04 | 0 | 53 | 3 × 10–4 | 0 | 46 |
| OC4 Phases (mol%) | Fe | Ni | Cr | Al | Nb | W | Mo | C |
|---|---|---|---|---|---|---|---|---|
| FCC_A1#1 | 51 | 24 | 14 | 7 | 0.08 | 0.03 | 0.6 | 7 × 10–5 |
| Laves phases C14 | 55 | 0.4 | 8 | 2 × 10–5 | 5 | 12 | 15 | 0 |
| NbNi3 | 0 | 75 | 0 | 0 | 25 | 0 | 0 | 0 |
| Sigma | 45 | 3 | 45 | 5 × 10–5 | 0.01 | 1 | 5 | 0 |
| FCC A1#2 | 0.002 | 0 | 0.04 | 0 | 53 | 3 × 10–4 | 0 | 46 |
| Sample | Cr (mol·L–1) | Fe (mol·L–1) | Ni (mol·L–1) | Al (mol·L–1) | Nb (mol·L–1) | Mo (mol·L–1) |
|---|---|---|---|---|---|---|
| OC1 | 4.0 × 10–3 | 2.5 × 10–3 | <7.7 × 10–5 | <1.7 × 10–4 | <4.9 × 10–5 | <4.7 × 10–5 |
| OC4 | <2.3 ×10–4 | <2.1 × 10–4 | <2.0 × 10–4 | <4.4 × 10–4 | <1.3 × 10–4 | <1.2 × 10–4 |
| mol% | O | Al | Mg | Cl | Na | Nb | Ni | Cr | Fe | Mo | W | C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spec. 1 | 5.5 | 0.9 | 0.0 | 0.3 | 1.0 | 16.6 | 9.0 | 10.7 | 39.2 | 4.8 | 2.5 | 9.7 |
| Spec. 2 | 44.5 | 12.6 | 7.2 | 7.3 | 0.3 | 1.1 | 5.5 | 2.5 | 9.7 | 1.8 | 0.0 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellicot, L.; Gruet, N.; Serp, J.; Malacarne, R.; Bosonnet, S.; Martinelli, L. Corrosion of Two Iron-Based Aluminaforming Alloys in NaCl-MgCl2 Molten Salts at 600 °C. Materials 2024, 17, 3224. https://doi.org/10.3390/ma17133224
Pellicot L, Gruet N, Serp J, Malacarne R, Bosonnet S, Martinelli L. Corrosion of Two Iron-Based Aluminaforming Alloys in NaCl-MgCl2 Molten Salts at 600 °C. Materials. 2024; 17(13):3224. https://doi.org/10.3390/ma17133224
Chicago/Turabian StylePellicot, Louis, Nathalie Gruet, Jérôme Serp, Romain Malacarne, Sophie Bosonnet, and Laure Martinelli. 2024. "Corrosion of Two Iron-Based Aluminaforming Alloys in NaCl-MgCl2 Molten Salts at 600 °C" Materials 17, no. 13: 3224. https://doi.org/10.3390/ma17133224
APA StylePellicot, L., Gruet, N., Serp, J., Malacarne, R., Bosonnet, S., & Martinelli, L. (2024). Corrosion of Two Iron-Based Aluminaforming Alloys in NaCl-MgCl2 Molten Salts at 600 °C. Materials, 17(13), 3224. https://doi.org/10.3390/ma17133224







