Effect of Si and Holding Time on Ti2Al20La Phase in Al-Ti-La Intermediate Alloy
Abstract
1. Introduction
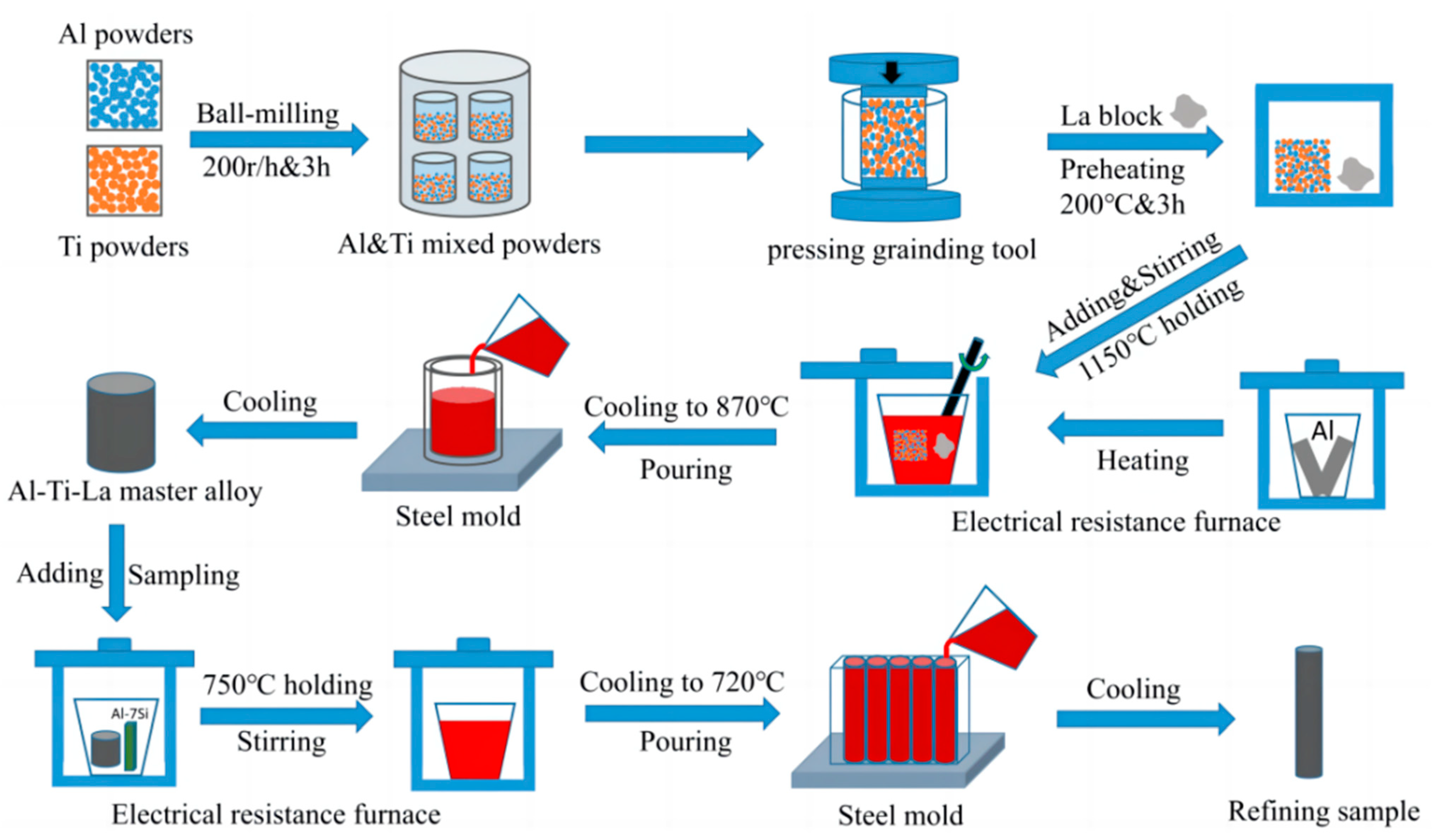
2. Experiment
3. Results and Discussion
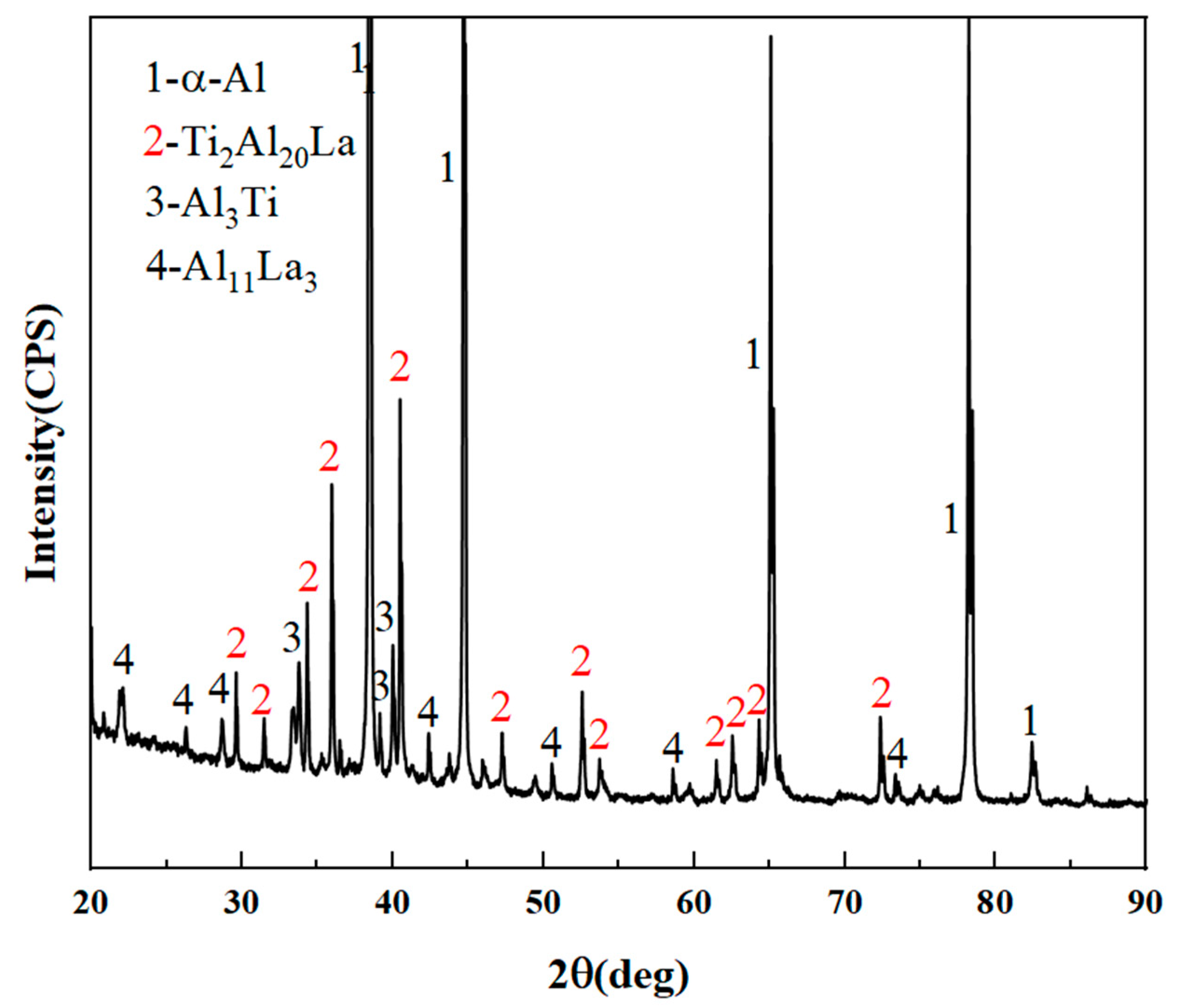
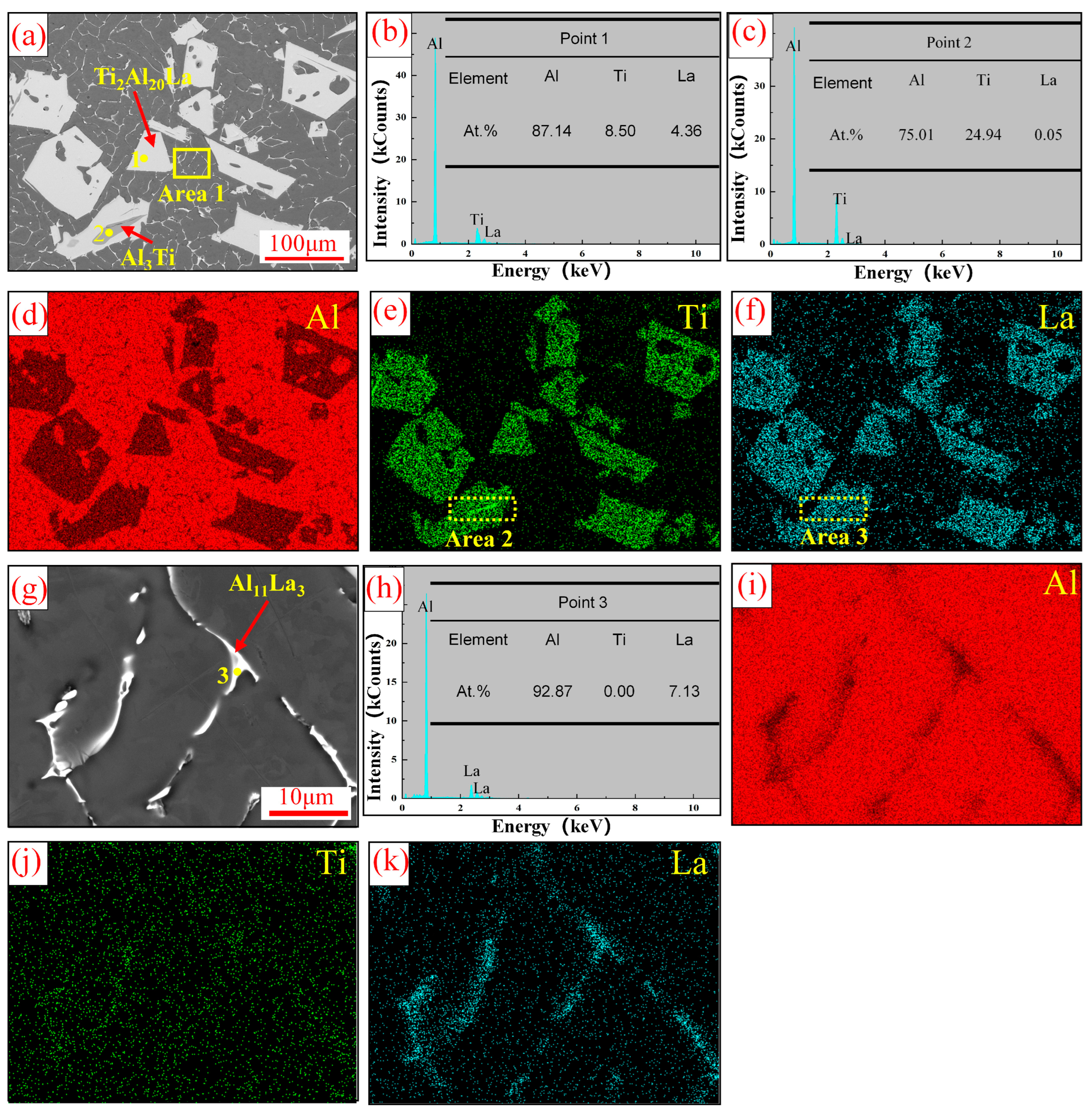
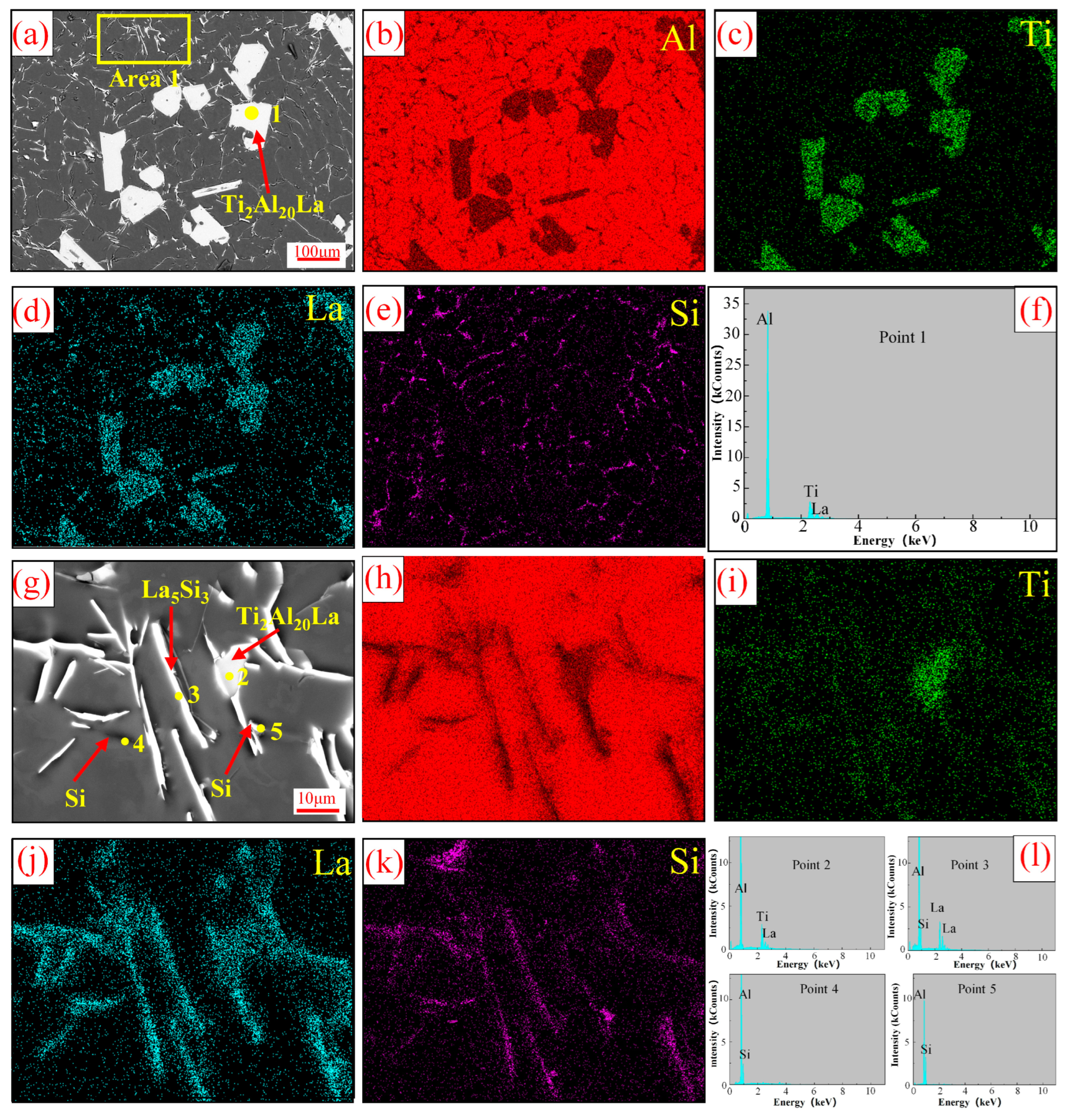
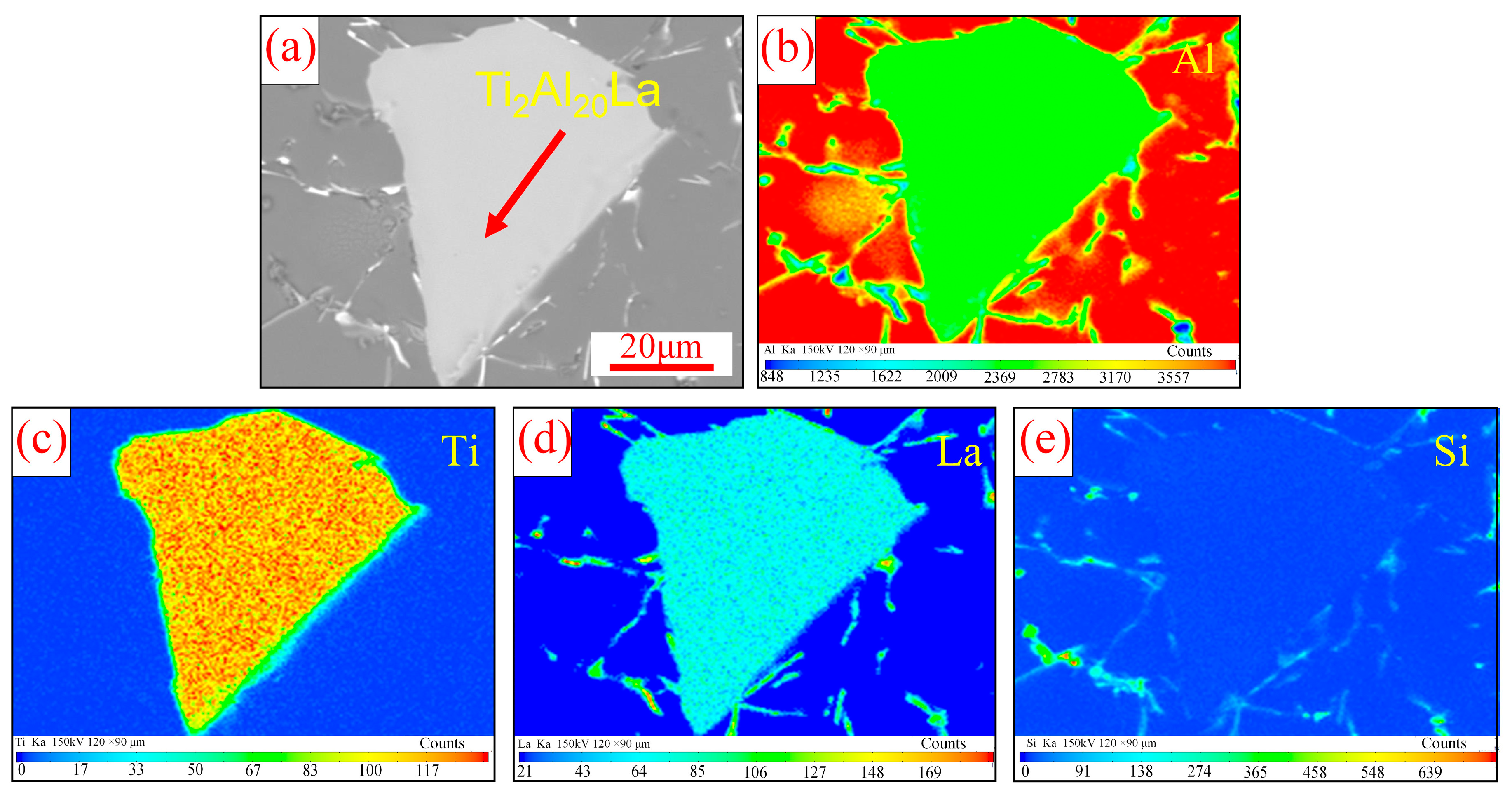
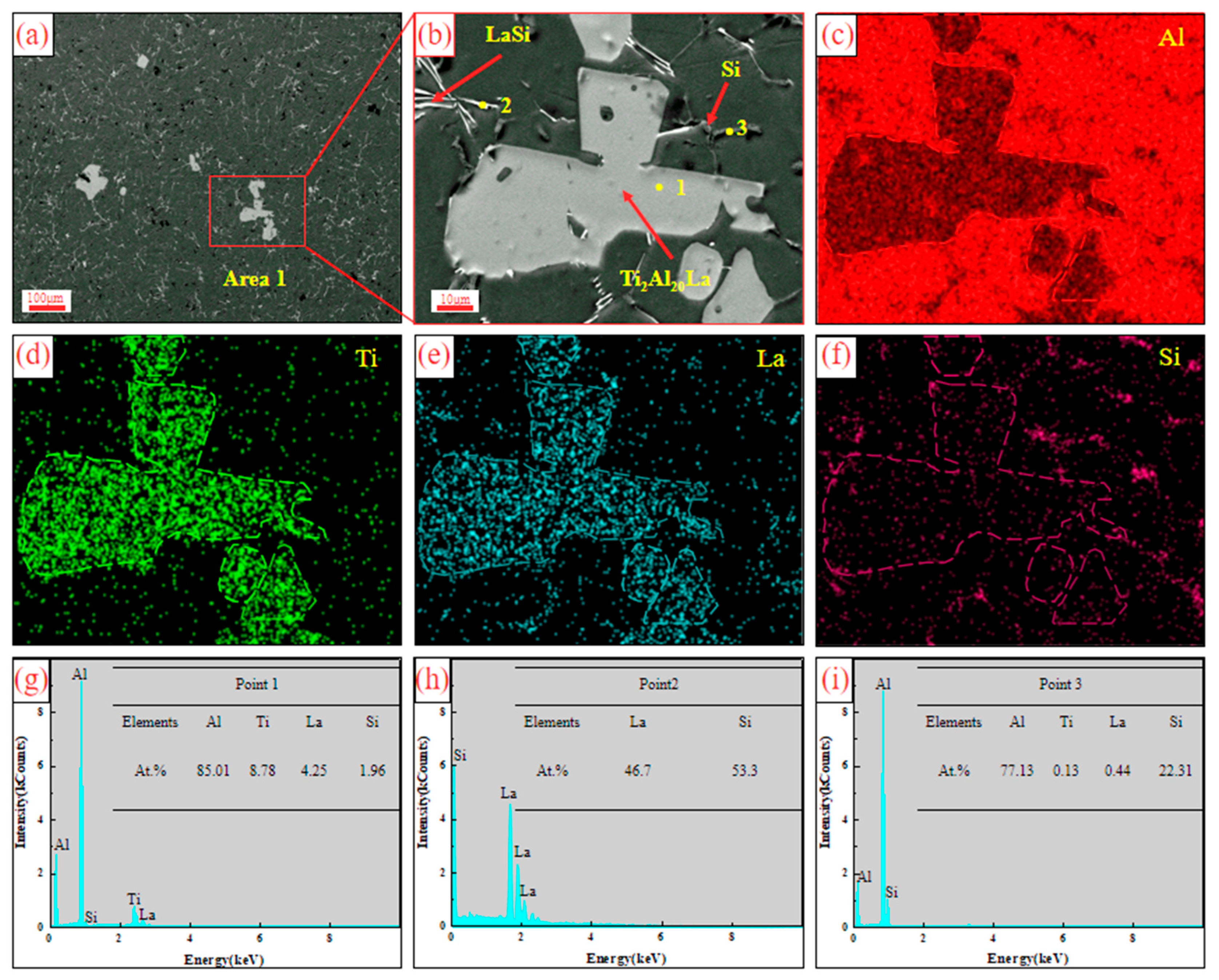
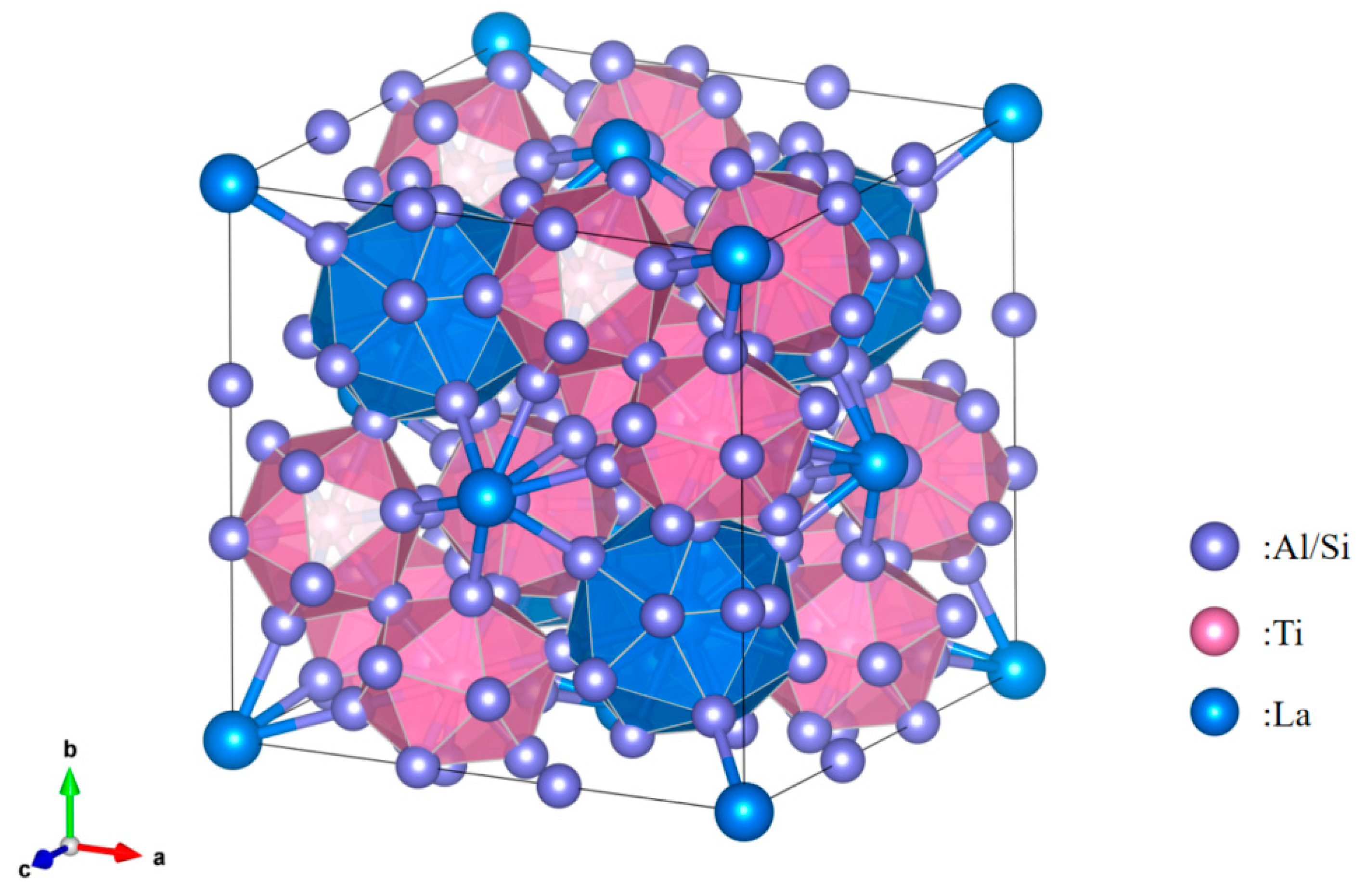
3.1. Analysis of Al-Ti-La Intermediate Alloys
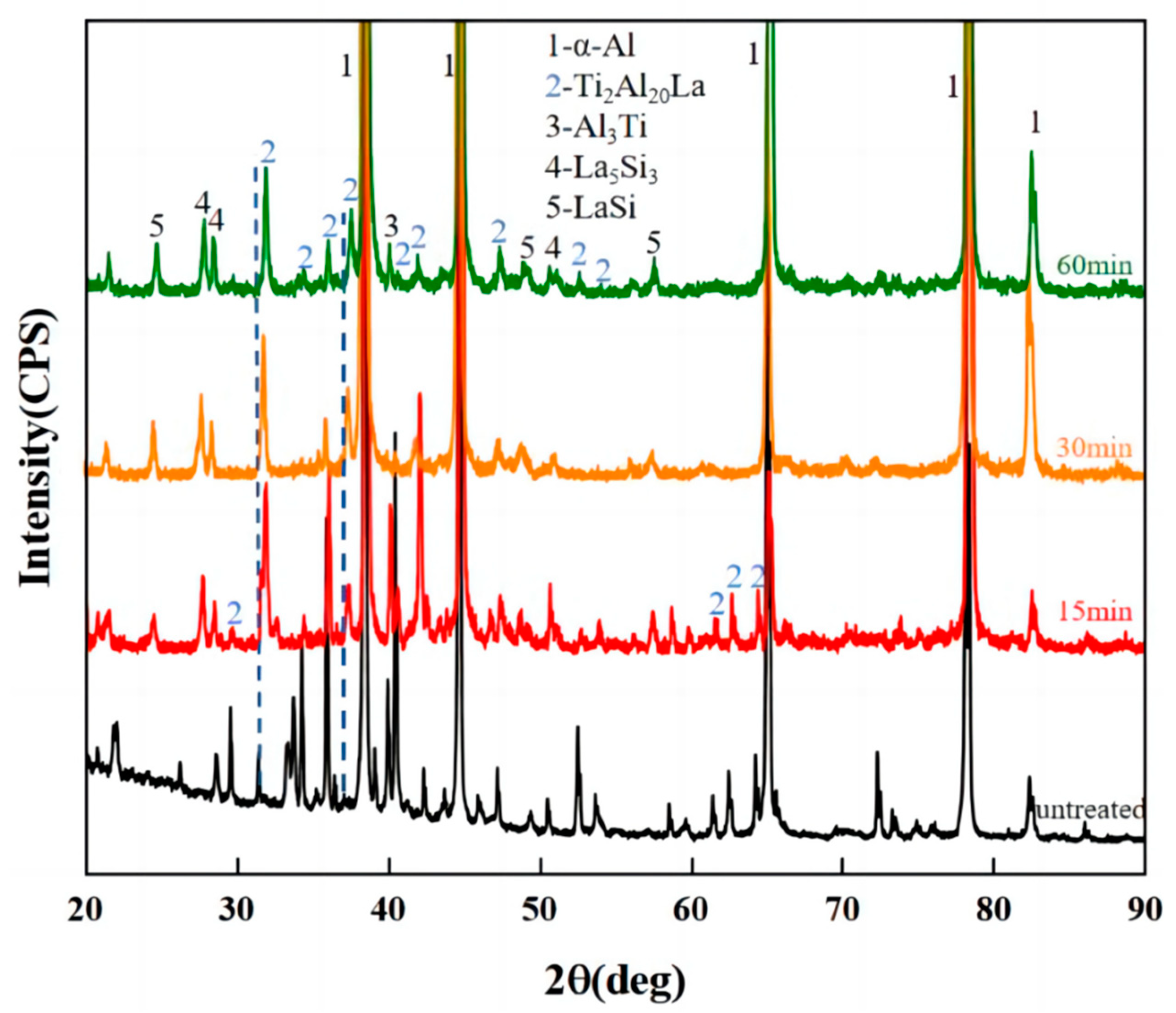
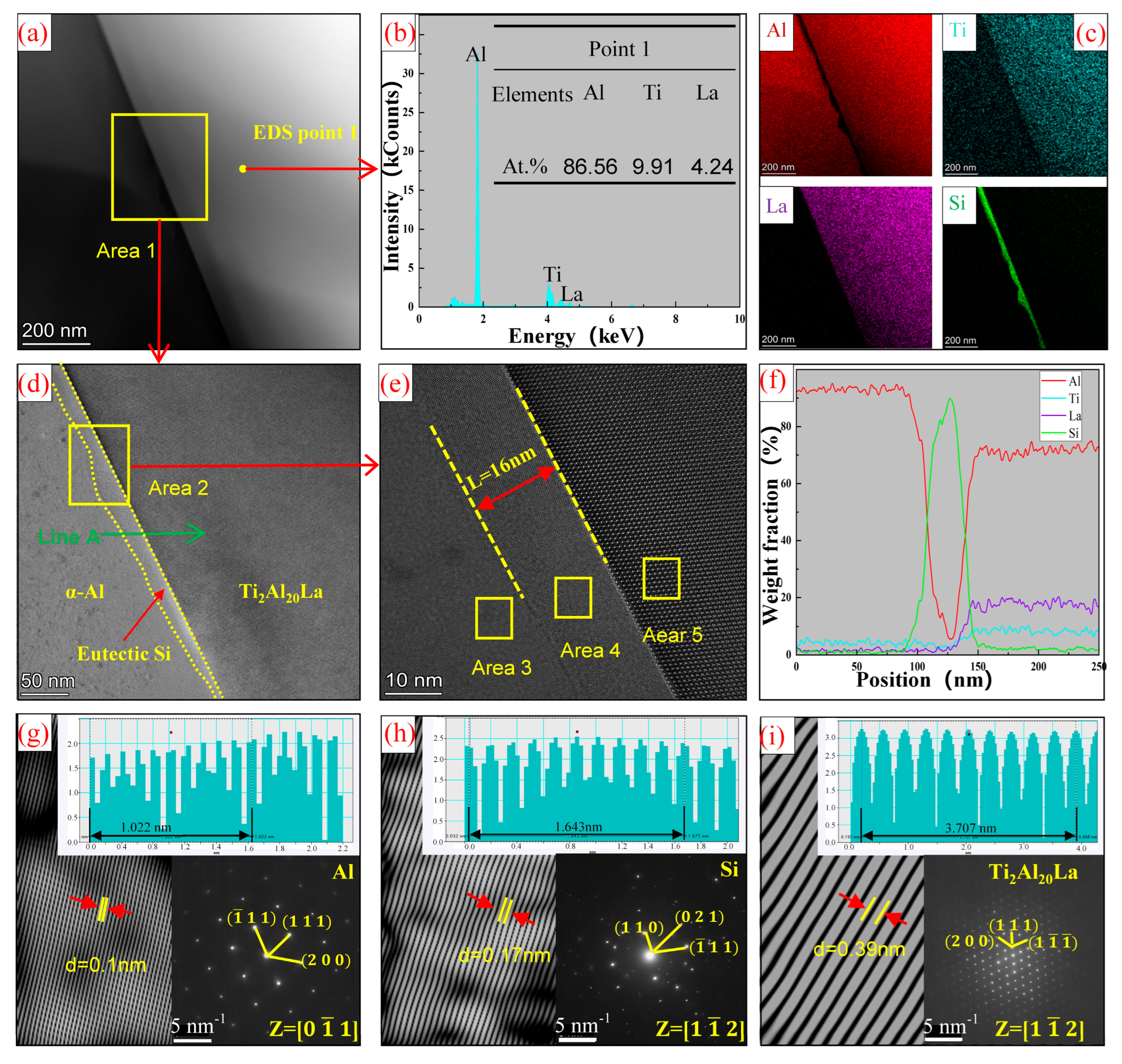
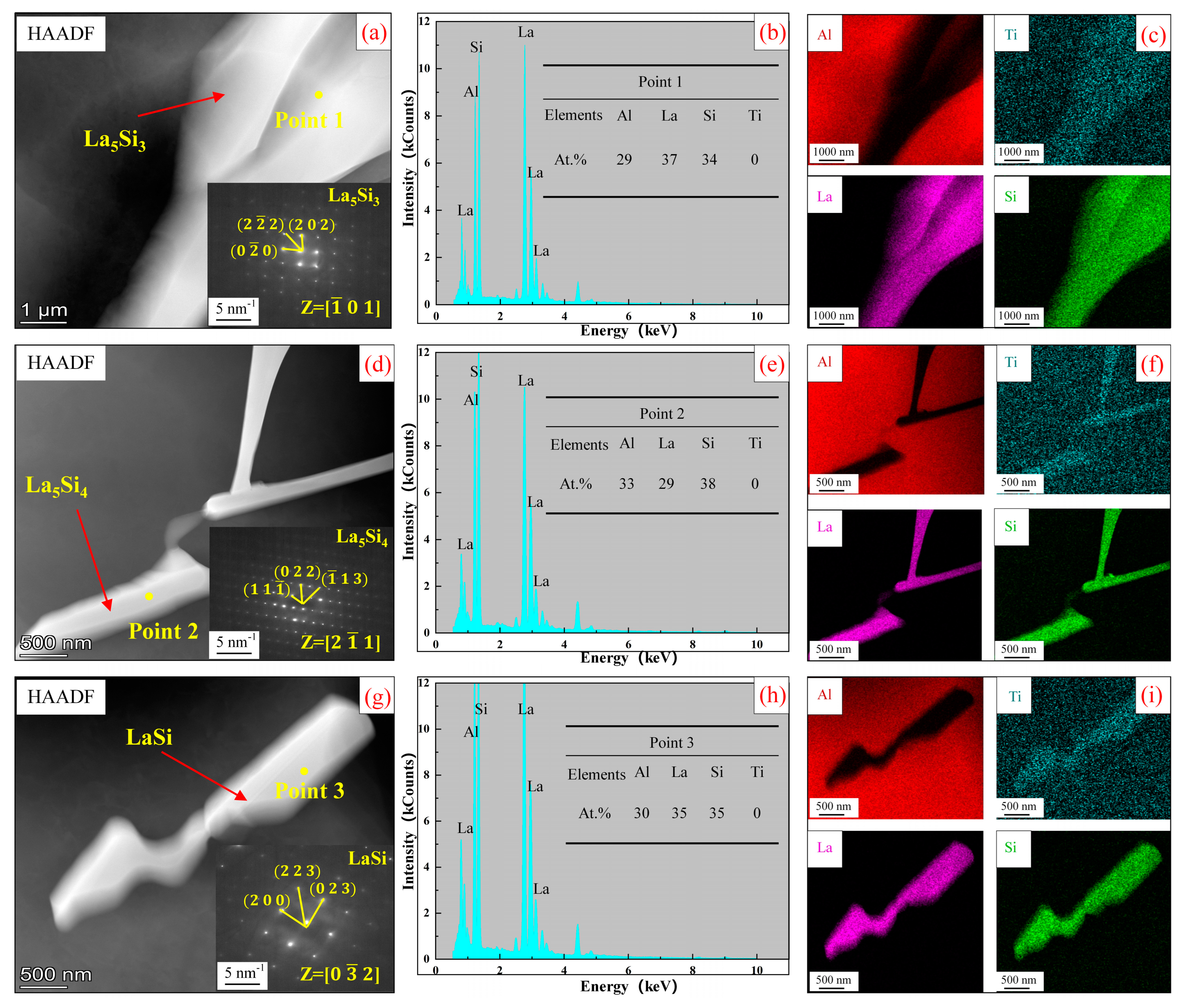
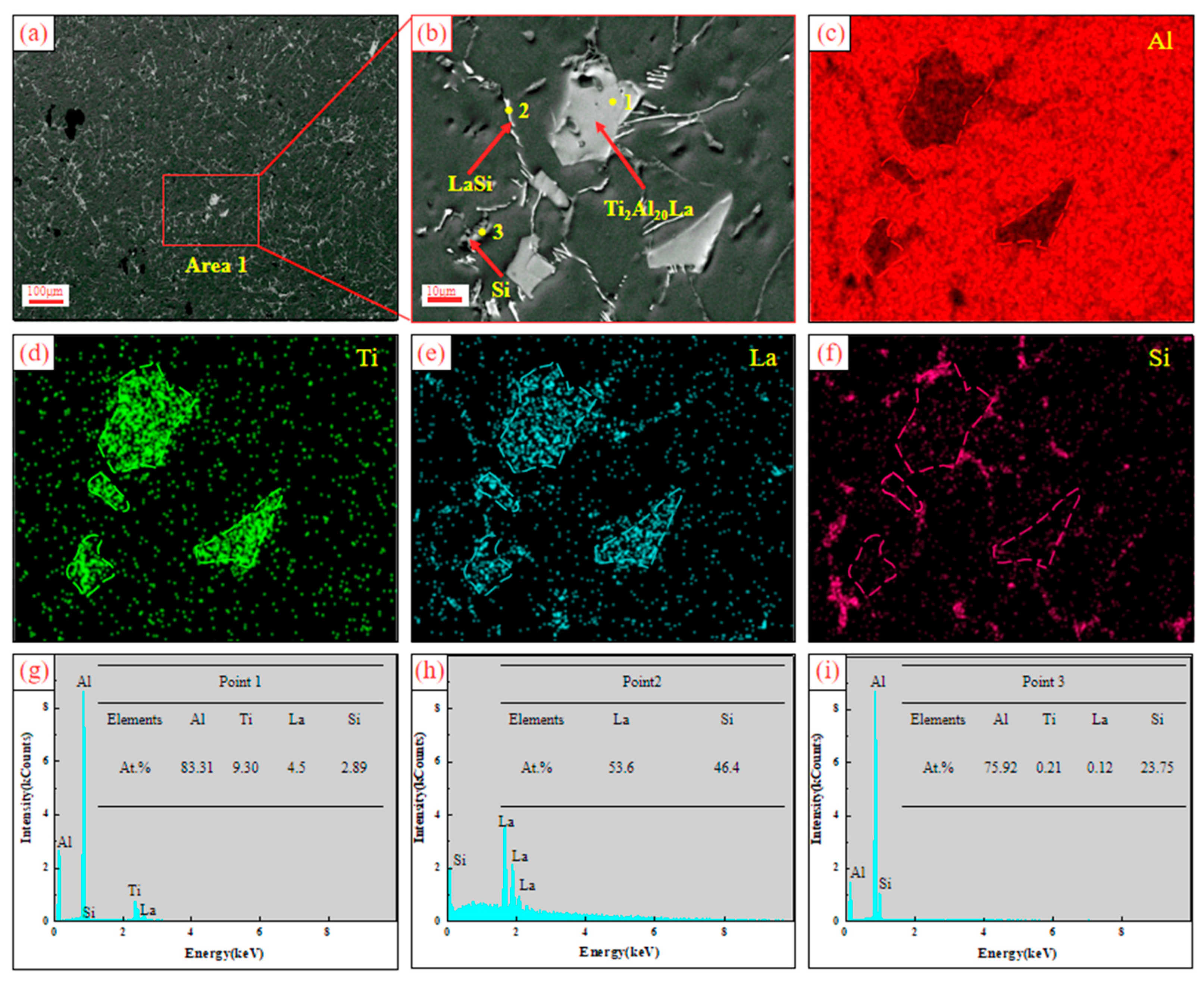
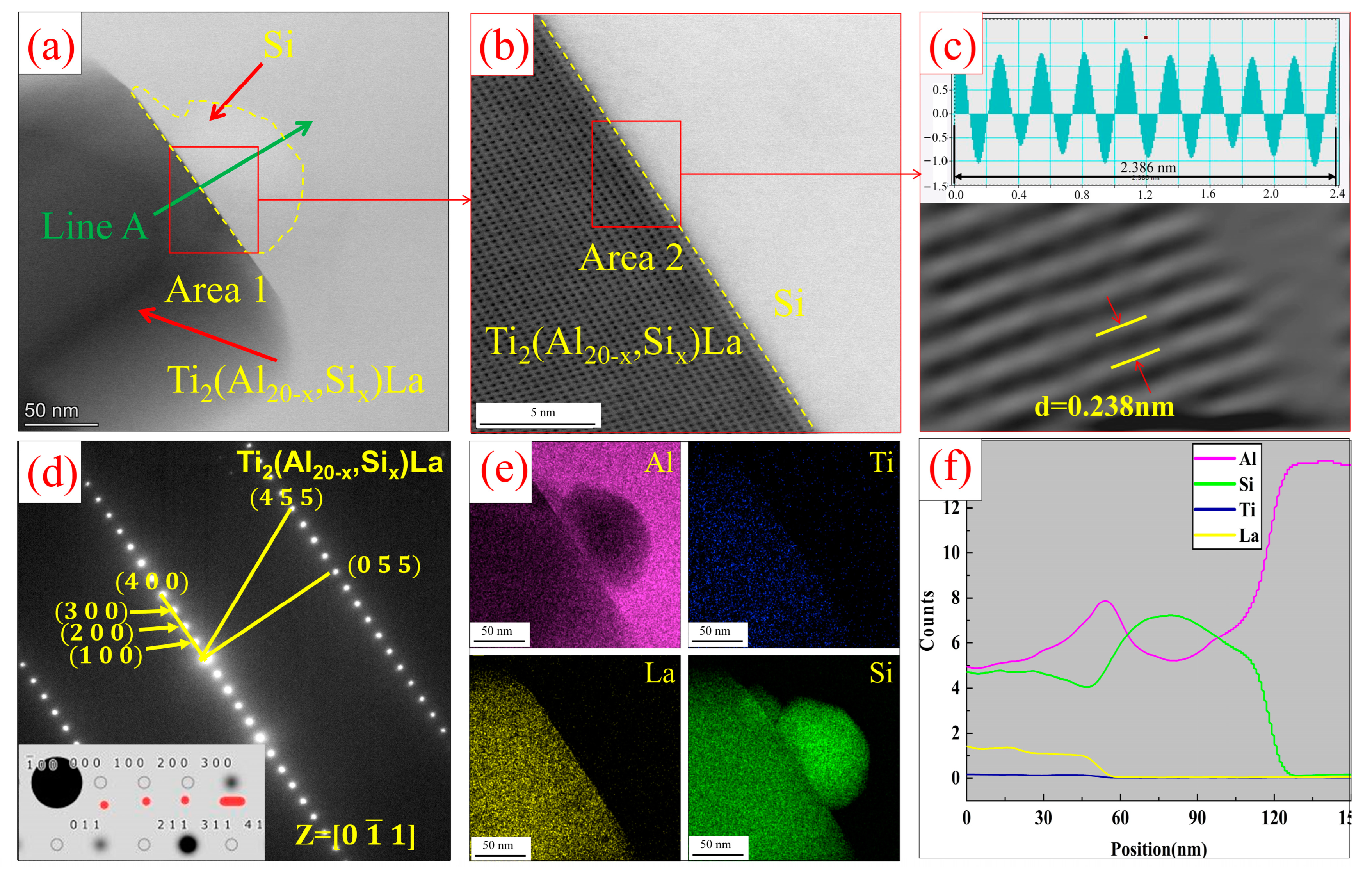
3.2. Analysis of Al-Ti-La-Si Alloy under Different Holding Time
4. Conclusions
- (1)
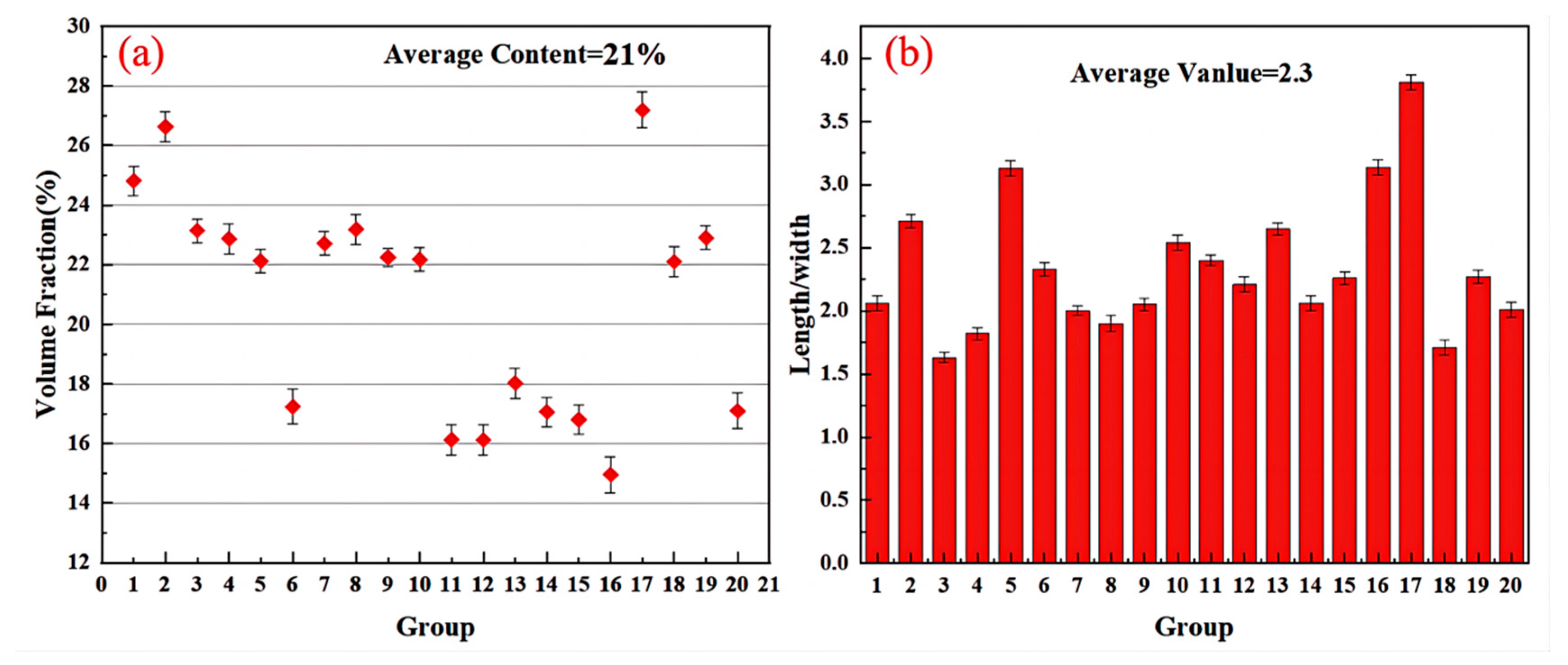
- The aspect ratio of Ti2Al20La phase in Al-3Ti-4.35 intermediate alloy with a holding time of 15 min at 750 °C is 2.0 (with 2.3 wt% Si addition), which has the efficient refining and modification effects, although the volume fraction of Ti2Al20La phase decreased significantly from 21% (without Si addition) to 4% (with 2.3 wt% Si addition), respectively.
- (2)
- The Si in the Al-Ti-La intermediate alloy will attach to the Ti2Al20La phase, which is the main reason for the avoidance of the “Si poisoning effect” with the addition of Al-Ti-La alloy to Al-7Si alloy. Meanwhile, some new rare earth phases La5Si3, La5Si4 and LaSi will form and distribute in the grain boundary of α-Al.
- (3)
- With the increase of holding time from 15 min to 60 min, Si will replace the positions of some low-energy Al atoms in Ti2Al20La, forming Ti2(Al20−x,Six)La phase, which has a similar structure to Ti2Al20La and has refining and modification effects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrov, I.; Shlyaptseva, A.; Ryakhovsky, A.; Medvedeva, E.; Tcherdyntsev, V. Effect of Rubidium on Solidification Parameters, Structure and Operational Characteristics of Eutectic Al-Si Alloy. Metals 2023, 13, 1398. [Google Scholar] [CrossRef]
- Dash, S.; Chen, D. A Review on Processing-Microstructure-Property Relationships of Al-Si Alloys: Recent Advances in Deformation Behavior. Metals 2023, 13, 609. [Google Scholar] [CrossRef]
- Pammer, M.; Pölzl, J.; Li, J. Silicon Poisoning and Effects of Tantalum on Al-Si Alloys. Metals 2023, 12, 1917. [Google Scholar] [CrossRef]
- Callegari, B.; Tiago, N.; Rodrigo, S. The Influence of Alloying Elements on the Microstructure and Properties of Al-Si-Based Casting Alloys: A Review. Metals 2023, 13, 1174. [Google Scholar] [CrossRef]
- Shlyaptseva, A.; Petrov, I.; Ryakhovsky, A.; Medvedeva, E.; Tcherdyntsev, V. Complex Structure Modification and Improvement of Properties of Aluminium Casting Alloys with Various Silicon Content. Metals 2021, 11, 1946. [Google Scholar] [CrossRef]
- Wang, Y.; Que, Z.; Hashimoto, T.; Zhou, X.; Fan, Z. Mechanism for Si poisoning of Al-Ti-B grain refiners in Al alloys. Metall. Mater. Trans. A 2020, 51, 5743. [Google Scholar] [CrossRef]
- Banerji, A.; Reif, W.; Feng, Q. Metallographic investigation of TiC nucleants in the newly developed Al-Ti-C grain refiner. J. Mater. Sci. 1994, 29, 1958. [Google Scholar] [CrossRef]
- Uludağ, M. Influence of Al-B grain refiner on porosity formation of directionally solidified Al-Si alloys. China Foundry 2020, 17, 372. [Google Scholar] [CrossRef]
- Powell, G.; Colligan, G.A. Silicon morphology in sodium modified Al-Si eutectic alloys. Mater. Res. Bull. 1970, 5, 431. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Z.; Li, J. Influence of alloying element mg on Na and Sr modifying Al-7Si hypoeutectic alloy. Materials 2022, 15, 1537. [Google Scholar] [CrossRef]
- Elgallad, E.M.; Ibranhim, M.F.; Doty, H.W. Microstructural characterisation of Al-Si cast alloys containing rare earth additions. Philos. Mag. 2018, 98, 1337. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Hu, B. Novel Al-Ti-Nb-B Grain Refiners with Superior Efficiency for Al-Si Alloys. Scr. Mater. 2020, 187, 262–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Shen, Y.; Chang, W. Grain refinement of hypoeutectic Al-7wt.% Si alloy induced by an Al-V-B master alloy. J. Alloys Compd. 2020, 812, 152022. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Zhang, Z.; Zhao, Y.; Mu, S. Study on the grain refinement of A356 alloy by Al-3wt.%VN master alloy. Mater. Sci. Technol. 2020, 36, 819. [Google Scholar] [CrossRef]
- Jiang, B.; Jiang, B.; Yang, W.; Wang, Y.; Xu, H. Efficient modification eutectic Si of Al-10Si alloy with Mg-Gd master alloy addition. Mater. Lett. 2024, 361, 136071. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Glavatskikh, M.V.; Makhov, S.V.; Napalkov, V.L. The synthesis of novel powder master alloys for the modification of primary and eutectic silicon crystals. Mater. Lett. 2014, 128, 325. [Google Scholar] [CrossRef]
- Cui, X.; Wu, Y.; Gao, T.; Liu, X. Preparation of a Novel Al–3B–5SR Master Alloy and Its Modification and Refinement Performance on A356 Alloy. J. Alloy. Compd. 2014, 615, 906–911. [Google Scholar] [CrossRef]
- Qiu, C.; Miao, S.; Li, X.; Xia, X.; Ding, J.; Wang, Y.; Zhao, W. Effect of Sr and La on the Microstructure and Mechanical Properties of A356.2 Alloy. Mater Des. 2017, 114, 563–571. [Google Scholar] [CrossRef]
- Zhao, H.; Bai, H.; Wang, J.; Guan, S. Preparation of Al-Ti-C-Sr Master Alloys and Their Refining Efficiency on A356 Alloy. Mater. Charact. 2009, 5, 377–383. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Muhammad, A.; Hanada, S.; Yamagata, H.; Wang, W.; Ma, C. Effects of Al-Ti-B-Re Grain Refiner on Microstructure and Mechanical Properties of al-7.0si-0.55mg Alloy. Trans. Nonferrous Met. Soc. 2014, 7, 22–50. [Google Scholar] [CrossRef]
- Quan, S.; Liu, C.; Jiang, W.; Zhang, X. First-Principles Investigation of the Mechanical, Anisotropic and Thermodynamic Properties of RET2AL20 (Re = La, Ce, Gd, T = Ti, V). Phys. B 2019, 554, 64–71. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, W.; Zhao, W.; Wang, W.; Hanada, S.; Yamagata, H.; Ma, C. Microstructure and Formation Mechanism of Grain-Refining Particles in Al-Ti-C-Re Grain Refiners. J. Rare Earth. 2015, 5, 53–60. [Google Scholar] [CrossRef]
- Ding, W.; Gou, L.; Hu, L.; Zhang, H.; Zhao, W.; Ma, J.; Qiao, J.; Li, X. Modification of Eutectic Si in Hypoeutectic Al-Si Alloy with Novel Al-3Ti-4.35La Master Alloy. J. Alloy. Compd. 2022, 929, 167350. [Google Scholar] [CrossRef]
- Pandee, P.; Uthaisangsuk, S. Structure-Mechanical Property Relationships of In-Situ A356/Al3Zr Composites. Mater. Sci. Eng. 2023, 886, 144673. [Google Scholar] [CrossRef]
- Li, C.; Pan, Y.; Lu, T.; Jiang, L.; Pi, J. Effects of Ti and La Additions on the Microstructures and Mechanical Properties of B-Refined and SR-Modified Al-11Si Alloys. Metal. Mater. Int. 2018, 5, 1133–1142. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.; Hautier, G.; Chen, W. Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation. APL Mater. 2013, 1, 2–11. [Google Scholar] [CrossRef]
- Jian, W.; Xie, Z.; Xu, S.; Su, Y.; Yao, X.; Beyerlein, I. Effects of Lattice Distortion and Chemical Short-Range Order on the Mechanisms of Deformation in Medium Entropy Alloy CoCrNi. Acta Mater. 2020, 199, 352–369. [Google Scholar] [CrossRef]
- Chen, H.; Benedetti, T.; Gonçales, V.; Bedford, N.; Scott, R.; Webster, R.; Cheong, S.; Gooding, J.; Tilley, R. Preserving the Exposed Facets of Pt3Sn Intermetallic Nanocubes during an Order to Disorder Transition Allows the Elucidation of the Effect of the Degree of Alloy Ordering on Electrocatalysis. J. Am. Chem. Soc. 2020, 6, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Atomic Radius in the Periodic Table of Elements. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/periodic-table/atomic-radius (accessed on 5 April 2024).

| Elements | Ti | La | Si | Cl | Zn | Fe | Al |
|---|---|---|---|---|---|---|---|
| Al-Ti-La | 2.883 | 4.277 | 0.019 | 0.038 | 0.035 | 0.037 | Bal. |
| Al-Ti-La-Si | 1.874 | 2.875 | 2.301 | 0.072 | 0.039 | 0.095 | Bal. |
| Point | Elements At.% | |||
|---|---|---|---|---|
| Al | Ti | La | Si | |
| 1 | 84.87 | 8.81 | 4.58 | 1.74 |
| 2 | 85.29 | 8.21 | 4.68 | 1.82 |
| 3 | 81.44 | 0.00 | 8.94 | 9.62 |
| 4 | 83.55 | 0.00 | 0.80 | 15.66 |
| 5 | 62.37 | 0.05 | 0.96 | 36.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da, H.; Tian, X.; An, J.; Ding, W.; Chen, J.; Yu, H.; Zhang, H. Effect of Si and Holding Time on Ti2Al20La Phase in Al-Ti-La Intermediate Alloy. Materials 2024, 17, 3134. https://doi.org/10.3390/ma17133134
Da H, Tian X, An J, Ding W, Chen J, Yu H, Zhang H. Effect of Si and Holding Time on Ti2Al20La Phase in Al-Ti-La Intermediate Alloy. Materials. 2024; 17(13):3134. https://doi.org/10.3390/ma17133134
Chicago/Turabian StyleDa, Hu, Xudong Tian, Jiazhi An, Wanwu Ding, Jianchao Chen, Haicun Yu, and Haixia Zhang. 2024. "Effect of Si and Holding Time on Ti2Al20La Phase in Al-Ti-La Intermediate Alloy" Materials 17, no. 13: 3134. https://doi.org/10.3390/ma17133134
APA StyleDa, H., Tian, X., An, J., Ding, W., Chen, J., Yu, H., & Zhang, H. (2024). Effect of Si and Holding Time on Ti2Al20La Phase in Al-Ti-La Intermediate Alloy. Materials, 17(13), 3134. https://doi.org/10.3390/ma17133134





